Abstract
Background
Benign prostatic hyperplasia (BPH), a non‐malignant enlargement of the prostate in aging men, can cause bothersome urinary symptoms (intermittency, weak stream, straining, urgency, frequency, incomplete emptying). Finasteride, a five‐alpha reductase inhibitor (5ARI), blocks the conversion of testosterone to dihydrotestosterone, reduces prostate size, and is commonly used to treat symptoms associated with BPH.
Objectives
To compare the clinical effectiveness and harms of finasteride versus placebo and active controls in the treatment of lower urinary tract symptoms (LUTS).
Search methods
We searched The Cochrane Library (which includes CDSR (Cochrane Database of Systematic Reviews), DARE (Database of Abstracts of Reviews of Effects), HTA (Heath Technology Assessments), and CENTRAL (Cochrane Central Register of Controlled Trials, and which includes EMBASE and MEDLINE), LILACS (Latin American and Caribbean Center on Health Sciences Information) and Google Scholar for randomized, controlled trials (RCTs). We also handsearched systematic reviews, references, and clinical‐practice guidelines.
Selection criteria
Randomized trials in the English language with placebo and/or active arms with a duration of at least 6 months.
Data collection and analysis
JT extracted the data, which included patient characteristics, outcomes, and harms. Our primary outcome was change in a validated, urinary symptom‐scale score, such as the AUA/IPSS. A clinically meaningful change was defined as 4 points. We also categorized outcomes by trial lengths of ≤ 1 year (short term) and > 1 year (long term).
Main results
Finasteride consistently improved urinary symptom scores more than placebo in trials of > 1 year duration, and significantly lowered the risk of BPH progression (acute urinary retention, risk of surgical intervention, ≥ 4 point increase in the AUASI/IPSS). In comparison to alpha‐blocker monotherapy, finasteride was less effective than either doxazosin or terazosin, but equally effective compared to tamsulosin. Both doxazosin and terazosin were significantly more likely than finasteride to improve peak urine flow and nocturia, versus finasteride. Versus tamsulosin, peak urine flow and QoL improved equally well versus finasteride. However, finasteride was associated with a lower risk of surgical intervention compared to doxazosin, but not to terazosin, while finasteride and doxazosin were no different for risk of acute urinary retention. Two small trials reported no difference in urinary symptom scores between finasteride and tamsulosin. Finasteride + doxazosin and doxazosin monotherapy improved urinary symptoms equally well (≥ 4 point improvement).
For finasteride, there was an increased risk of ejaculation disorder, impotence, and lowered libido, versus placebo. Versus doxazosin, finasteride had a lower risk of asthenia, dizziness, and postural hypotension, and versus terazosin, finasteride had a significant, lower risk of asthenia, dizziness, and postural hypotension.
Authors' conclusions
Finasteride improves long‐term urinary symptoms versus placebo, but is less effective than doxazosin. Long‐term combination therapy with alpha blockers (doxazosin, terazosin) improves symptoms significantly better than finasteride monotherapy. Finasteride + doxazosin improves symptoms equally ‐ and clinically ‐ to doxazosin alone. In comparison to doxazosin, finasteride + doxazosin appears to improve urinary symptoms only in men with medium (25 to < 40 mL) or large prostates (≥ 40 mL), but not in men with small prostates (25 mL).
Comparing short to long‐term therapy, finasteride does not improve symptoms significantly better than placebo at the short term, but in the long term it does, although the magnitude of differences was very small (from < 1.0 point to 2.2 points). Doxazosin improves symptoms better than finasteride both short and long term, with the magnitude of differences ∼2.0 points and 1.0 point, respectively. Finasteride + doxazosin improves scores versus finasteride alone at both short and long term, with mean differences ∼2.0 points for both time points. Finasteride + doxazosin versus doxazosin improves scores equally for short and long term.
Drug‐related adverse effects for finasteride are rare; nevertheless, men taking finasteride are at increased risk for impotence, erectile dysfunction, decreased libido, and ejaculation disorder, versus placebo. Versus doxazosin, which has higher rates of dizziness, postural hypotension, and asthenia, men taking finasteride are at increased risk for impotence, erectile dysfunction, decreased libido, and ejaculation disorder. Finasteride significantly reduces asthenia, postural hypotension, and dizziness versus terazosin. Finasteride significantly lowers the risk of asthenia, dizziness, ejaculation disorder, and postural hypotension, versus finasteride + terazosin.
Keywords: Humans; Male; 5‐alpha Reductase Inhibitors; Adrenergic alpha‐Antagonists; Adrenergic alpha‐Antagonists/therapeutic use; Disease Progression; Doxazosin; Doxazosin/therapeutic use; Drug Therapy, Combination; Drug Therapy, Combination/methods; Enzyme Inhibitors; Enzyme Inhibitors/adverse effects; Enzyme Inhibitors/therapeutic use; Finasteride; Finasteride/adverse effects; Finasteride/therapeutic use; Prostatic Hyperplasia; Prostatic Hyperplasia/drug therapy; Prostatism; Prostatism/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Finasteride provides relief of symptoms related to benign prostatic hyperplasia.
Finasteride, when compared to placebo and active comparators, improves long‐term urinary tract symptoms associated with benign prostatic hyperplasia.
Summary of findings
Summary of findings for the main comparison. Outcomes for validated symptom scores.
| Study (duration) | Score at entry | Mean change | Per cent change | P value |
| Finasteride vs placebo | ||||
| Abrams (1 year) (finasteride) | IPSS 19.4 | ‐4.8 | ‐13.7 | P > 0.05 |
| Abrams (1 year) (placebo) | IPSS 17.4 | ‐3.3 | ‐9.4 | |
| Andersen (2 years) (finasteride) | Boyarsky I 13.4 | ‐2.0 | ‐3.7 | P < 0.05 |
| Andersen (2 years) (placebo) | Boyarsky I 13.1 | 0.2 | 0.3 | |
| Byrnes (1 year) (finasteride) | AUASI ‐‐ | ‐4.8 | ‐‐ | P < 0.05 |
| Byrnes (1 year) (placebo) | AUASI ‐‐ | ‐3.4 | ‐‐ | |
| Gormley (1 year) (finasteride 1 mg) | Boyarsky II 10.6 | ‐‐ | ‐9.0 | (vs PLA) NS |
| Gormley (1 year) (finasteride 5 mg) | Boyarsky II 10.2 | ‐‐ | ‐21.0 | (vs PLA) P < 0.05 |
| Gormley (1 year) (placebo) | Boyarsky II 9.8 | ‐‐ | ‐2.0 | |
| Kirby '03 (1 year) (finasteride) | IPSS 17.1 | ‐6.6 | ‐18.9 | (vs PLA) P > 0.05 |
| Kirby '03 (1 year) (placebo) | IPSS 17.2 | ‐5.7 | ‐16.1 | |
| Marberger (2 years) (finasteride) | Boyarsky I 14.5 | ‐3.2 | ‐9.1 | P < 0.05 |
| Marberger (2 years) (placebo) | Boyarsky I 14.3 | ‐1.5 | ‐4.3 | |
| Marks (6 months) (finasteride) | IPSS 17.0 | ˜6.5 | ˜18.6 | NS |
| Marks (6 months) (placebo) | IPSS 16.0 | ˜4.5 | ˜12.8 | |
| McConnell '03 (4.5 years) (finasteride) | AUASI 17.6 | ‐5.6 | ‐16.0 | P < 0.05 |
| McConnell '03 (4.5 years) (placebo) | AUASI 16.8 | ‐4.9 | ‐14.0 | |
| Nickel (2 years) (finasteride) | Boyarsky I 15.8 | ‐2.1 | ‐3.9 | P < 0.05 |
| Nickel (2 years) (placebo) | Boyarsky I 16.6 | ‐0.7 | ‐1.3 | |
| Polat (1 year) (finasteride) | AUASI 11.6 | ‐4.6 | ‐13.1 | P < 0.05 |
| Polat (1 year) (placebo) | AUASI 14.1 | ‐3.2 | ‐9.2 | |
| Tenover (1 year) (finasteride) | AUASI 19.03 | ‐4.96 | ‐14.30 | P < 0.05 |
| Tenover (1 year) (placebo) | AUASI 18.35 | ‐3.71 | ‐10.60 | |
| Yu (6 months) (finasteride) | AUASI 19.45 | ‐5.98 | ‐30.00 | P < 0.05 |
| Yu (6 months) (placebo) | AUASI 16.68 | ‐2.36 | ‐12.00 | |
| Finasteride 1 mg vs 5 mg | ||||
| Gormley (1 yr) (finasteride 1 mg) | Boyarsky II 10.6 | ‐‐ | ‐9.0 | (vs PLA) NS (vs 5 mg FIN) P < 0.05 |
| Gormley (1 year) (finasteride 5 mg) | Boyarsky II 10.2 | ‐‐ | ‐21.0 | (vs PLA) P < 0.05 |
| Gormley (1 year) (placebo) | Boyarsky II 9.8 | ‐‐ | ‐2.0 | |
| Finasteride vs doxazosin | ||||
| Kirby '03 (1 year) (finasteride) | IPSS 17.1 | ‐6.2 | ‐17.8 | (vs PLA) P > 0.05 |
| Kirby '03 (1 year) (doxazosin) | IPSS 17.1 | ‐8.4 | ‐24.0 | (vs FIN) P < 0.05 |
| Kirby '03 (1 year) (finasteride + doxazosin) |
IPSS 17.3 | ‐8.6 | ‐24.5 | (vs FIN) P < 0.05 (vs PLA) P < 0.05 |
| Kirby '03 (1 year) (placebo) | IPSS 17.2 | ‐5.7 | ‐16.1 | |
| McConnell '03 (4.5 years) (finasteride) | AUASI 17.6 | ‐5.6 | ‐16.0 | (vs FIN + DOX) P < 0.05 (vs PLA) P > 0.05 |
| McConnell '03 (4.5 years) (doxazosin) | AUASI 17.0 | ‐6.6 | ‐18.9 | |
| McConnell '03 (4.5 years) (finasteride + doxazosin) |
AUASI 16.8 | ‐7.4 | ‐21.1 | (vs PLA) P < 0.05 |
| McConnell '03 (4.5 years) (placebo) | AUASI 16.8 | ‐4.9 | ‐14.0 | |
| Finasteride vs tamsulosin | ||||
| Lee (24 weeks) (finasteride) | IPSS 19.0 | ‐5.8 | ‐30.5 | P > 0.05 |
| Lee (24 weeks) (tamsulosin) | IPSS 19.9 | ‐6.9 | ‐34.7 | |
| Rigatti (26 weeks) (finasteride) | IPSS 16.9 | ‐5.7 | ‐32.0 | P > 0.05 |
| Rigatti (26 weeks) (tamsulosin) | IPSS 16.3 | ‐6.3 | ‐37.3 | |
| Finasteride vs terazosin | ||||
| Lepor (56 weeks) (finasteride) | AUASI 16.2 | ‐3.2 | ‐9.2 | (vs TER) P < 0.05 (vs FIN + TER) P < 0.05 (vs PLA) P > 0.05 |
| Lepor (56 weeks) (terazosin) | AUASI 16.2 | ‐6.1 | ‐17.4 | |
| Lepor (56 weeks) (placebo) | AUASI 15.8 | ‐2.6 | ‐7.4 | |
| Lepor (56 weeks) (finasteride + terazosin) |
AUASI 15.9 | ‐6.2 | ‐17.7 | (vs PLA) P < 0.05 (vs TER) P > 0.05 |
| Finasteride vs Permixon® | ||||
| Carraro (6 months) (finasteride) | IPSS 15.7 | ‐6.2 | ‐17.8 | P = 0.14 |
| Carraro (6 months) (Permixon®) | IPSS 15.7 | ‐5.8 | ‐16.6 | |
| Finasteride vs PRO 160/120 | ||||
| Sökeland (48 weeks) (finasteride) | IPSS 11.8 | ‐5.6 | ‐16.0 | NS |
| Sökeland (48 weeks) (PRO 160/120) | IPSS 11.3 | ‐4.8 | ‐13.7 | |
Background
Description of the condition
Lower urinary tract symptoms (LUTS) consistent with benign prostatic hyperplasia (BPH) may be evident in men beginning the third decade of life (Litwin, Saigal (editors) 2007). These symptoms can be both obstructive and irritative, and include voiding at night (nocturia), incomplete emptying, hesitancy, weak stream, and frequent and urgent urination. Around 40% of men in their fifties and 90% in their eighties have histologic evidence of BPH (Berry 1984). LUTS secondary to BPH are believed to be caused by bladder irritation or obstruction, which in turn are caused by prostatic enlargement or small muscle contractions within the bladder or prostate. Because most men are evaluated with histologic (i.e., by biopsy) evaluation of their prostate, BPH is often noted as BPO (benign prostatic obstruction) (Campbell‐Walsh Urology 2007).
Description of the intervention
The goal of treatment is to reduce bothersome and irritative urinary symptoms that negatively affect quality of life (QoL). Typically, men are first advised to make lifestyle changes (reduction of alcohol, caffeine) to relieve these symptoms. If still bothered, pharmacologic interventions such as five‐alpha reductase inhibitors, including dutasteride and finasteride, and alpha1‐adrenoreceptor antagonists (alpha blockers) are often recommended. Five‐alpha reductase inhibitors and alpha blockers, which include doxazosin, alfuzosin, tamsulosin and terazosin, may be used alone or in combination. An additional treatment option is phytotherapies (e.g., Serenoa repens). In this review we do not address surgical options, such as TURP (transurethral resection of the prostate), TUNA (transurethral needle ablation) or TUMT (transurethral microwave thermotherapy). It is known that finasteride and other 5ARIs reduce prostate volume by shrinking it and thus relieving pressure on the urethra. The process by which it accomplishes this is disputed.
How the intervention might work
The causes of progressive LUTS secondary to BPH are not yet known, although a combination of cellular proliferation and age‐related detrusor dysfunction are likely factors (Campbell‐Walsh Urology 2007). In the hyperplastic prostate, as compared to a healthy one, cell proliferation and cell death have achieved disequilibrium, causing a net increase of cells in the organ. This process is not well understood, but the newest evidence suggests a complex interplay among "[a]ndrogens, estrogens, stromal‐epithelial interactions, growth factors, and neurotransmitters[,] … either singly or in combination, in the etiology of the hyperplastic process" (Campbell‐Walsh Urology 2007).
Why it is important to do this review
Pharmacologic therapy is the most common treatment for men with moderate to severe LUTS and related bother. Five‐alpha reductase inhibitors are frequently prescribed to improve LUTS and reduce long‐term symptom progression, including the risk for acute urinary obstruction and the need for surgical intervention, or both. Determining the effectiveness and harms of finasteride (alone or in combination with other therapies) versus other 5ARI or alpha‐blockers provides clinicians, patients, and health policy makers useful healthcare information.
Objectives
We assessed the efficacy and harms of finasteride, alone or in combination, versus placebo or control, for the treatment of bothersome urinary symptoms consistent with BPH.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, controlled trials (RCTs) of 6 months or greater duration.
Types of participants
Men with symptomatic BPH as determined by urinary symptoms or symptom‐scale scores. We did not consider as eligible studies comprising men presenting with or treated for hematuria.
Types of interventions
Finasteride in comparison to placebo, active pharmacologic controls, and phytotherapies (Stoner 1992a).
Types of outcome measures
Primary outcomes
Our primary clinical outcome was improvement in urologic symptoms as assessed by validated symptom‐scale scores, such as the IPSS and the AUASI (range 0 to 35, with a higher score denoting worse symptoms). A clinically meaningful change is defined as a variance of 4 points from baseline (Barry 1995).
Secondary outcomes
Secondary clinical outcomes included BPH progression (defined as a ≥ 4 point increase from baseline to endpoint of the IPSS/AUASI; acute urinary retention; or need for surgical intervention), peak urine flow (measured in mL/s (millilitres per second), prostate size (measured in cc (cubic centimetres)), post‐void residual volume (cc), nocturia, quality of life (QoL), and harms (either drug‐related or all‐cause). We did not assess finasteride for the chemoprevention of prostate cancer.
Search methods for identification of studies
Electronic searches
We searched MEDLINE from 1950 to March 2010 using the following search string.
prostatic hyperplasia.mp. or exp Prostatic Hyperplasia/
bph.mp.
benign prostatic hyperplasia.mp.
lower urinary tract symptoms.mp.
luts.mp.
or/1‐5
finasteride.mp. or exp Finasteride/
proscar.mp.
5‐alpha reductase inhibitor$.mp.
or/7‐9
6 and 10
limit 11 to (controlled clinical trial or randomized controlled trial) [Limit not valid in ACP Journal Club; records were retained]
We also searched LILACS and Google Scholar for key words.
We used only English‐language RCTs.
Searching other resources
We handsearched relevant peer‐reviewed journals.
Data collection and analysis
Selection of studies
Two reviewers (JT, RM) independently searched the identified studies for eligibility in the review against a pre‐determined check list of inclusion criteria. If a title, or abstract, appeared to meet the eligibility criteria for inclusion in the review, a full text version of the article was obtained to assess it in detail. We did not include non‐ or quasi‐randomized trials. Excluded studies were listed with reasons for their exclusion. Consultation with a third reviewer was employed to resolve differences of opinion.
Data extraction and management
Two reviewers (JT, RM) decided trials' eligibility. Results of trial eligibility and data extraction were discussed with TW and HF. One reviewer (JT) assessed study characteristics and extracted data. Missing data was sought from authors. Data was extracted into Microsoft Excel spreadsheets and reviewed by JT and RM. Any discrepancies were resolved by discussion.
Assessment of risk of bias in included studies
We assessed methodological study quality and bias by the GRADE criteria (GRADE 2004).
Measures of treatment effect
Our statistical analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook 2008). The effect measures for dichotomous outcomes were expressed using relative risk (RR) or absolute risk reduction (RD), and for continuous outcomes, mean differences, with respective 95% CI (confidence intervals). Whenever we had unequal scales with changes from baseline and variances, we combined them using standardized mean differences (SMD).
Unit of analysis issues
We did not accept quasi randomized trials for inclusion.
Dealing with missing data
We noted all trials not using an intention‐to‐treat analysis (ITT), but conducted our analysis by this principal. We attempted to contact authors for missing data.
Assessment of heterogeneity
Statistical evidence of heterogeneity was assessed graphically and by the I² statistic. Minimal heterogeneity was defined as ≤ 10%, with a middle range between 11% and 50%. Anything over 50% was considered maximal heterogeneity. All combined outcomes were assessed by the random‐effects model.
Assessment of reporting biases
To minimize publication bias we conducted electronic searches of multiple databases, contacted authors, searched http://clinicaltrials.gov/, and handsearched references, clinical practice guidelines, and prior systematic reviews. From lack of resources we did not assess foreign‐language trials.
Data synthesis
We compared mean change from baseline to endpoint; otherwise, we compared endpoints.
We assessed for effect size inconsistency as well as clinical study design and statistical heterogeneity. We used a random‐effects model and reported continuous outcomes by comparing the weighted mean difference (WMD). For categorical effect measures, we used RR or MD. For both continuous and dichotomous outcomes we used 95% CI.
Subgroup analysis and investigation of heterogeneity
We attempted to conduct subgroup analyses of the following predefined groups:
prostate size (< 40 cc versus ≥ 40 cc) as measured by TRUS (transrectal ultrasound) or MRI (magnetic resonance imaging);
age (< 65 versus ≥ 65);
PSA (prostate‐specific antigen) (< 4 ng/mL (nanograms per millilitre) versus ≧ 4 ng/mL);
study duration (short = 6 to 12 months versus long = greater 12 months); and
baseline prostate symptom severity (mild (0 to 7) versus moderate (8 to 19) versus severe (20 to 35) symptom scores (IPSS/AUASI) or bother).
Sensitivity analysis
If "considerable" heterogeneity (I² > 50%) was detected using the random‐effects model, or if there was judged to be clinical or study design heterogeneity, we conducted a sensitivity analysis to assess the robustness of our pooled outcomes and conclusions.
Results
Description of studies
Our search strategy found 23 trials meeting inclusion criteria. Nineteen studies (20,821 men) were placebo controlled (Abrams 1999; Agrawal 2001; Andersen 1995; Beisland 1992; Byrnes 1995; Finasteride Study Group; Gormley 1992; Kirby 2003; Lepor 1996; Marberger 1998; Marks 1997; McConnell 1998; McConnell 2003; Nickel 1996; Polat 1997; Tammela 1995; Tempany 1993; Tenover 1997; Yu 1995). Gormley 1992 and Finasteride Study Group, both placebo controlled, compared 1 mg (milligram) to 5 mg finasteride, and another trial, Tempany 1993, also placebo controlled, combined both finasteride arms (1 mg and 5 mg) into one comparator. Two trials compared finasteride to phytotherapies (1614 men) (Carraro 1996 = Permixon®; Sökeland 2000 = PRO 160/120). Six trials (6119 men) compared finasteride to alpha‐adrenergic blocking agents (alpha blockers) (Kirby 2003 and McConnell 2003 = doxazosin; Lee 2002 and Rigatti 2003 = tamsulosin; Lepor 1996 and Agrawal 2001 = terazosin). Agrawal 2001, a four‐armed trial, also compared finasteride to allylestrenol, a progestational, synthetic steroid. Three trials compared finasteride and alpha blocker mono therapies to combination therapies (Kirby 2003 and McConnell 2003 = finasteride + doxazosin; Lepor 1996 = finasteride + terazosin). Trials ranged from 6 months to 4 years, with 22% (5/23) of greater than 1 year duration.
A total of 21,945 men were randomized (finasteride = 11,086; tamsulosin = 302; terazosin = 340; doxazosin = 1031; Permixon® = 553; PRO 160/120 = 261; finasteride + doxazosin = 1072; finasteride + terazosin = 309; placebo = 6956; allylestrenol = 35). Men were primarily young‐elderly, white race, had moderately severe lower urinary tract symptoms and flow abnormalities; mean prostate volumes were considered enlarged. Weighted mean age was 62.4 (20 trials), which ranged from 40 to 94 years (17 trials). Sixteen trials reported trial origination (US = 4; US and Canada = 2; US and Europe = 1; Canada = 1; Europe = 6; South Korea = 1; multinational = 2). Seven trials reported racial data, with 84.2% White, 7.8% Black, 4.8% Hispanic, 0.9% other, Asian/Pacific < 1%, and Native American < 1%. Study discontinuations ranged from 0% to 38%, with an overall of 21.9%.
Weighted, baseline mean IPSS/AUASI differed slightly by comparisons: finasteride (5 mg) = 18.2 points versus placebo = 17.0 points, 7 trials; finasteride (5 mg) = 15.7 points versus Permixon® = 15.7 points, 1 trial; finasteride (5 mg) = 11.8 points versus PRO 160/120 = 11.3 points, 1 trial; finasteride (5 mg) = 17.5 points versus doxazosin = 17.0 points, 2 trials; finasteride (5 mg) + doxazosin = 16.9 points versus finasteride = 17.5 points, 2 trials; finasteride (5 mg) + doxazosin = 16.9 points versus doxazosin = 17.0 points, 2 trials; finasteride (5 mg) = 17.6 points versus tamsulosin = 19.9 points, 2 trials; finasteride (5 mg) = 16.2 points versus terazosin = 16.2 points, 1 trial; finasteride (5 mg) = 16.2 points versus finasteride + terazosin = 15.9 points, 1 trial. Overall, at baseline (13 trials), the mean symptom severity was in the moderate range (IPSS/AUASI 8 to 19).
The weighted means for peak urine flow measures at baseline per comparison were: finasteride ( 5 mg) = 10.6 mL/s versus placebo = 10.5 mL/s, 15 trials; finasteride ( 1 mg) = 9.2 mL/s versus finasteride (5 mg) = 9.2 mL/s, 1 trial; finasteride ( 5 mg) = 10.8 mL/s versus Permixon® = 10.6 mL/s, 1 trial; finasteride ( 5 mg) = 12.7 mL/s versus PRO 160/120 = 12.7 mL/s, 1 trial; finasteride ( 5 mg) = 10.5 mL/s versus doxazosin = 10.3 mL/s, 2 trials; finasteride + doxazosin = 10.5 mL/s versus finasteride = 10.5 mL/s, 2 trials; finasteride + doxazosin = 10.5 mL/s versus doxazosin = 10.3 mL/s, 2 trials; finasteride ( 5 mg) = 10.4 mL/s versus tamsulosin = 10.3 mL/s, 2 trials; finasteride ( 5 mg) = 10.6 mL/s versus terazosin = 10.5 mL/s, 1 trial; finasteride ( 5 mg) = 10.6 mL/s versus finasteride + terazosin = 10.4 mL/s, 1 trial; finasteride ( 5 mg) = 7.0 mL/s versus allylestrenol = 8.4 mL/s, 1 trial.
The weighted means for prostate volume at baseline were: finasteride (5 mg) = 45.3 cc versus placebo = 46.0 cc, 14 trials; finasteride (5 mg) = 58.6 cc versus finasteride (1 mg) = 60.9 cc, 1 trial; finasteride (1 & 5 mg) = 61.7 cc versus placebo = 108.7 cc, 1 trial; finasteride (1 mg) = 54.8 cc versus placebo = 54.2 cc, 2 trials; finasteride (1 mg) = 54.8 cc versus finasteride (5 mg) = 53.3 cc, 2 trials; finasteride (5 mg) = 44.0 cc versus Permixon® = 43.0 cc, 1 trial; finasteride (5 mg) = 44.0 cc versus PRO 160/120 = 42.7 cc, 1 trial; finasteride (5 mg) = 36.9 cc versus doxazosin = 36.9 cc, 1 trial; finasteride (5 mg) = 30.9 cc versus tamsulosin = 28.7 cc, 1 trial; finasteride + doxazosin = 36.4 cc versus finasteride = 36.9 cc, 1 trial; finasteride + doxazosin = 36.4 cc versus doxazosin = 36.9 cc, 1 trial; finasteride = 36.3 cc versus terazosin = 36.4 cc, 2 trials; finasteride = 36.2 cc versus finasteride + terazosin = 37.2 cc, 1 trial; finasteride = 37.2 cc versus allylestrenol = 34.9 cc, 1 trial.
Ten trials had an active control (Tempany 1993 had finasteride arms of 1 and 5 mg but combined them in the comparison to placebo); of those, 6 had a placebo arm.
Results of the search
Our search of 5 February, 2009, found 303 references. Of those, and from subsequent handsearching, we identified 51 possible RCTs. Of those 51, 23 were unique studies that met inclusion criteria. The remainder (28 studies) either did not meet inclusion criteria, were open‐label adjunct papers to the included trials, were not randomized, had no clinical outcomes, or were reviews.
The original search was repeated on 4 March, 2010; one other trial was identified, resulting in a total of 23 unique studies meeting all inclusion criteria.
Included studies
Excluded studies
Risk of bias in included studies
We assessed risk of bias in eight domains.
Adequate sequence generation?
Allocation concealment?
Blinding?
Incomplete outcome data addressed?
Free of selective reporting?
Free of other bias?
Intention‐to‐treat analysis
Non‐industry funded
Each domain was answered by 'yes,' 'unclear,' or 'no,' and summarized in Figure 1.
1.
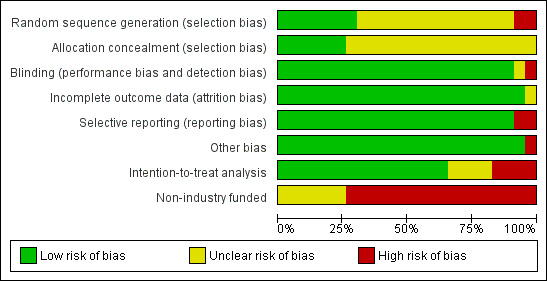
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Allocation concealment was adequate ('yes') in 6 trials and 'unclear' in 17 (Figure 2).
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Blinding
Twenty‐one of twenty‐three trials were blinded or double blinded (91%), and thus marked adequate ('yes'). In Abrams 1999 only the assessors were blinded. The other, an unambiguously single‐blinded trial, Lee 2002 (finasteride versus tamsulosin), did not report who was blinded, although presumably it was the subjects. Three trials were probably single blinded (Carraro 1996; McConnell 2003; Tenover 1997); any ambiguity had to do with the term "double‐masked," which was sometimes described as "double‐blind." Tempany 1993 and Polat 1997 did not report blinding, but presumably were. Two trials, Marks 1997 and Nickel 1996, were described as blinding both patients and investigators. The overwhelming majority of trials did not describe who was blinded, making it impossible to discriminate between providers and assessors.
Incomplete outcome data
Twenty‐two of twenty‐three trials (96%) dealt adequately with incomplete outcome data.
Selective reporting
Twenty‐one trials were free of selective reporting. Beisland 1992 excluded men from analysis whose peak urine flow was < 150 mL during clinic visits. McConnell 2003 did not report losses to follow up for the placebo arm.
Other potential sources of bias
Twenty‐one trials were free of other potential sources of bias; one trial (Tempany 1993) combined two arms (finasteride 1 and 5 mg) into one. Seventy‐four per cent of our included trials (17/23) were funded by industry. The funding source of the remaining six trials was not clear.
Effects of interventions
See: Table 1
Finasteride versus placebo
Total symptom score
Nineteen trials compared finasteride monotherapy to a placebo arm, and thirteen reported baseline (and endpoint) values for validated symptom‐scale scores. The weighted mean score was 17.8 points for IPSS/AUASI (8 trials), 14.5 points for Boyarsky I (range 0 to 54, 3 trials), and 9.8 points for Boyarsky II (range 0 to 36, 1 trial). Higher numbers denoted worse symptoms. All were categorized as moderately symptomatic at baseline.
Follow‐up ≤ 1 year
(11 trials)
At 1 year, the MTOPS trial (McConnell 2003), the largest, highest quality of these trials and with the longest duration (4 years), found no statistically significant difference (median improvements of 4.0 points for both arms) between finasteride and placebo.
Yu 1995 (N = 50) reported a significant per cent difference in the AUASI favoring finasteride, but with the caveat that baseline scores were significantly different (MD ‐18.00%, 95% CI ‐27.44 to ‐8.56). The improvement in the finasteride arm was clinically significant (≥ 4 point decrease in the AUASI/IPSS) as well. In Abrams 1999 (N = 121) mean IPSS decreased 4.9 points in the finasteride arm, and 3.2 points in the placebo arm at 1 year, for a non‐significant mean treatment effect of 1.5 points (95% CI ‐4.1 to 1.1). A 3‐armed trial using Boyarsky II (range 0 to 36), Gormley 1992 compared 1 mg finasteride (as well as 5 mg finasteride) to placebo. At 1 year, Gormley's 5 mg arm was compared in a meta‐analysis of two symptoms scores (Gormley = Boyarsky II, Kirby = IPSS). The SMD was ‐0.19 (95% CI ‐0.31 to ‐0.07) (Analysis 1.1) and favored finasteride. Lepor 1996, in a 4‐armed trial (other comparators were terazosin and terazosin + finasteride) utilizing the AUASI (range 0 to 35), reported absolute mean changes at 1 year of ‐3.2 and ‐2.6 points for finasteride (n = 310) and placebo (n = 305), respectively. The comparison was not significant. Marks 1997, a small (N = 41), short‐term trial of 6 months, found improvements of ∼5.5 and ∼5.0 points for finasteride and placebo, respectively, for the IPSS total score. The comparison was not significant. At endpoint (1 year), Polat 1997 (N = 123), utilizing the AUASI, recorded a decrease of 4.6 points for finasteride (which was clinically significant as well), and 1.6 points for placebo. The inter group differences were significant from 3 months to endpoint. Tenover 1997, which randomized 2112 men for 1 year and also used the AUASI, reported significant mean differences favoring finasteride beginning at 6 months and continuing to 1 year (‐4.96, ‐3.71 points, respectively). This was also a clinically significant intra arm change for finasteride (adjusted mean change ‐4.96 points). The results from Tenover should be taken with some caution since age and symptom score were significantly different for the two arms at baseline. MTOPS, comparing the AUASI at 1 year, reported median changes of ‐4.0 for both finasteride (n = 686) and placebo (n = 656) (McConnell 2003). The comparison was not significant (P = 0.77). At 1 year Nickel 1996 (N = 613) reported a statistically significant difference favoring finasteride.
1.1. Analysis.
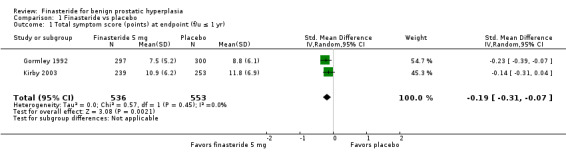
Comparison 1 Finasteride vs placebo, Outcome 1 Total symptom score (points) at endpoint (f/u ≤ 1 yr).
Follow‐up > 1 year
(4 trials)
Four large trials randomizing 600 to 2900 men, with endpoints from 2 to 4 years, found finasteride significantly better than placebo.
Andersen 1995 (N = 707), with a follow‐up of 2 years, reported a statistically significant difference favoring finasteride (MD ‐2.20 points, 95% CI ‐3.56 to ‐0.84). Marberger 1998 (N = 2902), utilizing Boyarsky I (range 0 to 54), found a statistically significant difference favoring finasteride (P ≤ 0.05). With longer follow‐up (4 years) MTOPS authors (N = 3047) reported a statistically significant difference favoring finasteride in the AUASI (P = 0.047) (McConnell 2003). Nickel 1996 (N = 613) used the Boyarsky I and found a statistically significant difference favoring finasteride at 2 years.
BPH progression (≥ 4 point increase)
Follow‐up > 1 year
(1 trial)
Progression for finasteride versus placebo was 8.5% versus 13.2%, respectively, with finasteride decreasing the absolute risk of progression by 5% at 4 year follow up (McConnell 2003) (RD ‐0.05, 95% CI ‐0.08 to ‐0.02).
BPH progression (acute urinary retention)
Follow‐up ≤ 1 year
(1 trial)
For acute urinary retention, the absolute risk difference was not significant (McConnell 2003) (RD ‐0.00, 95% CI ‐0.01 to 0.01).
Follow‐up > 1 year
(6 trials)
Finasteride significantly decreased absolute risk of acute urinary retention by 3% (Analysis 1.3). The analysis had significant heterogeneity (I2 = 87%) (a ratio of nearly 3:1 between events per arm) and for which we had no explanation.
1.3. Analysis.
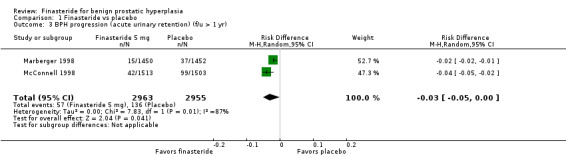
Comparison 1 Finasteride vs placebo, Outcome 3 BPH progression (acute urinary retention) (f/u > 1 yr).
BPH progression (need for surgical intervention)
Follow‐up ≤ 1 year
(6 trials)
The absolute risk of surgery was non significant (Analysis 1.4).
1.4. Analysis.
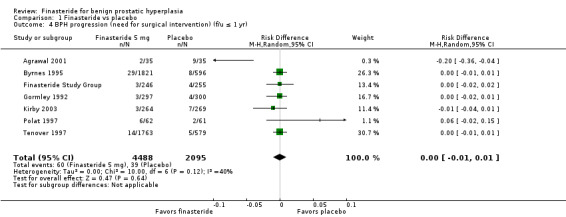
Comparison 1 Finasteride vs placebo, Outcome 4 BPH progression (need for surgical intervention) (f/u ≤ 1 yr).
Follow‐up > 1 year
(4 trials)
Finasteride decreased the absolute risk of surgery by 3% (Analysis 1.5). Heterogeneity was considerable (I2 = 87%), which we were unable to account for, neither by baseline data, nor trial duration.
1.5. Analysis.

Comparison 1 Finasteride vs placebo, Outcome 5 BPH progression (need for surgical intervention) (f/u > 1 yr).
PSA as a surrogate endpoint
Follow‐up > 1 year
To see if long‐term change in PSA, as a surrogate endpoint, was affected by the active intervention, MTOPS compared finasteride to placebo (McConnell 2003). At 4 years, finasteride decreased baseline PSA by a median 50%, and for placebo, increased by 15% (P < 0.001) (computed by the "Wei‐Lachin test of stochastic ordering computed for all follow‐up visit measurements").
Peak urine flow
Sixteen trials reported baseline peak urine flow measures for the finasteride and placebo comparison; the overall weighted baseline mean was 10.6 mL/s. One trial (Tempany 1993) reported overall measures of 9.4 mL/s, but did not give measures per arm.
Follow‐up ≤ 1 year
(12 trials)
Nine of ten trials with endpoints from 6 months to 1 year found finasteride significantly increased urinary flow versus placebo.
Abrams 1999 (N = 121) and Kirby 2003 (N = 1095) reported improved urinary flow for finasteride but not for placebo (MD 0.77 mL/s, 95% CI 0.09 to 1.46). Polat 1997, a small trial randomizing 123 men for 1 year, reported significant absolute improvement (2.8%) of finasteride over placebo. Beisland 1992 (N = 182) reported finasteride was significantly better than placebo at 6‐month endpoint (P = 0.02). In a meta‐analysis of endpoints of 5 trials, finasteride was significantly better than placebo (MD 1.36, 95% CI 0.26 to 2.47), but with considerable heterogeneity (I2 = 77%) (Analysis 1.16). Agrawal 2001, the source of the heterogeneity, has a point estimate of 3.20 (95% CI 2.08 to 4.32), whereas the other 4 trials had point estimates from 10 to 2 fold of Agrawal. When we eliminated Agrawal (n = 70 for these 2 arms), the aggregate point estimate was 0.88 mL/s (95% CI 0.20 to 1.57), in favor of finasteride, and with heterogeneity below threshold (I2 = 29%). Marks 1997 (N = 41) reported no significant difference at endpoint. McConnell 2003 found 1‐year, significant median changes favoring finasteride (n = 678) versus placebo (n = 653). The Finasteride Study Group reported significant differences favoring finasteride at 7 and 12 months (P = 0.025). This trial also reported, for 1 mg finasteride versus placebo, median changes favoring finasteride (P < 0.001).
1.16. Analysis.
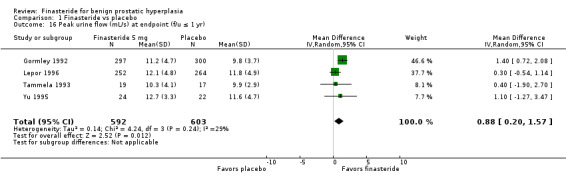
Comparison 1 Finasteride vs placebo, Outcome 16 Peak urine flow (mL/s) at endpoint (f/u ≤ 1 yr).
Follow‐up > 1 year
(5 trials)
Five trials with trial lengths of 2 to 4 years, consistently found finasteride significantly improved peak urine flows versus placebo.
McConnell 2003, comparing peak urine flow at 4 years, found finasteride (n = 551) significantly better than placebo (n = 519). Nickel 1996 (N = 613) reported maximum flow rates increased significantly for finasteride versus placebo through the 2‐year endpoint. Finasteride was significantly better than placebo at 1 year, with the gap widening at 2 years. Andersen 1995 (N = 707) reported a significant difference favoring finasteride (MD 1.80 mL/s, 95% CI 0.74 to 2.86). Marberger 1998 (N = 2902) reported significant differences between finasteride and placebo at 12, 20, and 24 months. In the trial by McConnell 1998 (N = 3040) by 4‐year endpoint finasteride significantly improved flows versus placebo, although the absolute magnitude was small (MD 1.7 mL/s, 95% CI 1.3 to 2.1).
Residual volume
Follow‐up ≤ 1 year
(3 trials)
With endpoints of 6 months to 1 year, these trials consistently reported finasteride improved residual volume versus placebo, although none of the comparisons were significant.
Polat 1997 (N = 123) reported non‐significant MD at 3, 6, 9 and 12 month follow‐up. For Tammela 1993 (N = 36), a 6‐month trial, a comparison of endpoints was not significant (MD ‐26.00, 95% CI ‐78.07 to 26.07). The Finasteride Study Group reported that "[r]esidual urine volume did not change appreciably in any of the treatment groups." Agrawal 2001, with a 6 month follow‐up, found no significant difference at endpoint (MD 3.90, 95% CI ‐3.04 to 10.84).
Prostate volume
Seventeen trials reported baseline prostate volumes for an accumulative weighted mean of 43.7 cc for the finasteride arm (5 mg), and 46.1 cc for the placebo arm. One trial reported prostate sizes of 61.7 for the combined finasteride 1 mg and 5 mg dose group and 108.7 cc for the placebo arm, respectively (Tempany 1993).
Follow‐up ≤ 1 year
(9 trials)
All nine trials reported statistically significant improvements for prostate volume for finasteride versus placebo at endpoints from 6 months to 1 year.
Gormley 1992, for 1 mg finasteride (n = 298) versus placebo (n = 300), found finasteride significantly improved volumes versus placebo (MD ‐10.70, 95% CI ‐17.09 to ‐6.31). Yu 1995 (N = 50) reported significant improvement in the finasteride arm versus placebo (MD ‐15.00, 95% CI ‐21.67 to ‐8.33). Three trials (Agrawal 2001; Gormley 1992; Tammela 1993) compared 5 mg finasteride to placebo at endpoint, which was a non‐significant difference (MD ‐5.64, 95% CI ‐18.87 to 7.59), but with considerable heterogeneity (I² = 93%). After we excluded the two smallest trials, Agrawal (n = 70 for these arms) and Tammela (N = 36), which favored placebo and finasteride, respectively, and kept the highest quality trial (Gormley, N = 597), the comparison significantly favored finasteride (MD ‐12.30, 95% CI ‐17.50 to ‐7.10). Marks 1997 (N = 41), with a 6 month follow‐up, found prostate volume decreased steadily in the finasteride arm versus placebo (P < 0.05). Tempany 1993 (N = 20), which combined finasteride doses (1 mg and 5 mg), found a significant difference favoring finasteride (MD ‐12.70 cc, 95% CI ‐21.44 to ‐3.96). Abrams 1999 (N = 121) reported a decrease for finasteride and an increase for placebo (P < 0.05). Polat 1997 (N = 123), with a 1 year follow‐up, noted improvements in prostate size for both arms to endpoint, with significant differences favoring finasteride at 3, 6, and 9 and 12 months. Lepor 1996 (N = 1229), comparing endpoints, found finasteride significantly better than placebo (MD ‐8.80, 95% CI ‐10.74 to ‐6.86). The Finasteride Study Group found per cent median improvements for finasteride (5 mg) and placebo, but which significantly favored finasteride at 3, 6, and 12 months (P < 0.001 for all 3 time points). The study also reported per cent median improvements for 1 mg finasteride and placebo, respectively, and which favored finasteride (P < 0.001).
Follow‐up > 1 year
(4 trials)
Comparing prostate volume, all trials reported statistically significant improvements for finasteride versus placebo at endpoints up to 4 years.
Andersen 1995 (N = 707), reporting per cent mean changes, found 5 mg finasteride significantly improved prostatic volumes versus placebo (MD ‐30.70, 95% CI ‐45.50 to ‐15.90). In a 2‐year trial, Nickel 1996 (N = 613) reported steady decreases in prostate volume for finasteride and volume increases for placebo (P < 0.05). Marberger 1998 (N = 2902), with 2 year follow‐up, found finasteride volume steadily declined, with significant differences with placebo at 12 and 24 months. In McConnell 1998 (N = 3040), prostate volume decreased for the finasteride arm to year 4; in the placebo arm prostate volume increased steadily for 4 years (MD 32%, 95% CI 28 to 36).
Nocturia
Follow‐up ≤ 1 year
(3 trials)
Finasteride did not significantly reduce nocturia versus placebo in three trials reporting this outcome.
Tammela 1993 (N = 36), with a 6 month follow‐up and comparing endpoints, found no difference of night‐time incidences between finasteride and placebo (MD 0.00, 95% CI ‐0.51 to 0.51). Johnson 2007, in a post hoc analysis of the included trial by McConnell 2003 (hereafter referenced as Johnson 2007 (McConnell 2003)), reported baseline incidences in men with "1 or more" episodes at baseline. At 1 year the finasteride (n = 496) and placebo arms (n = 459) were not significantly different. For men with "2 +" episodes at baseline, the mean changes were ‐0.60 and ‐0.61 incidents for finasteride (n = 496) and placebo (n = 459), respectively. Johnson 2003 (N = 1040), a secondary analysis of the included trial by Lepor 1996 (N = 1229) (hereafter referenced as Johnson 2003 (Lepor 1996)), in a report of men with nocturia at baseline and 1 year, reported that men taking finasteride (n = 252) and placebo (n = 254) had mean episodes of 2.1, respectively.
Follow‐up > 1 year
(1 trial)
For endpoints > 1 year, finasteride did not significantly reduce nocturia versus placebo.
Johnson 2007 (McConnell 2003) reported (men with "1 or more incidents at baseline") at year 4 a non‐significant comparison for finasteride (n = 385) versus placebo (n = 354). Men with "2 +" episodes at baseline improved by ‐0.68 and ‐0.66 nightly incidents for finasteride (n = 385) and placebo (n = 354), respectively; statistical significance was not reported.
Study discontinuations
Follow‐up ≤ 1 year
(9 trials)
There was no statistical difference between finasteride and placebo at these endpoints for study discontinuations.
We pooled 11 trials for a non‐significant comparison (RR 1.03, 95% CI 0.93 to 1.15) (Analysis 1.19). Gormley 1992 (N = 895) and the Finasteride Study Group compared 1 mg finasteride to placebo; the comparison was not significant (RR 0.91, 95% CI 0.56 to 1.47).
1.19. Analysis.

Comparison 1 Finasteride vs placebo, Outcome 19 Study discontinuations (f/u ≤ 1 yr).
Follow‐up > 1 year
(4 trials)
For 4 trials with endpoints > 1 year, there were significantly more discontinuations in the placebo arm than the finasteride arm.
For the finasteride arm, McConnell 2003 reported discontinuations of 184/768 (24%), but did not report numbers for the placebo arm. In a meta‐analysis of 4 trials, the comparison favored finasteride (RR 0.87, 95% CI 0.80 to 0.94) (Analysis 1.20).
1.20. Analysis.
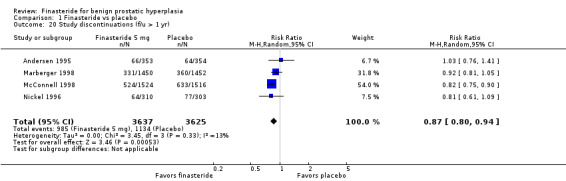
Comparison 1 Finasteride vs placebo, Outcome 20 Study discontinuations (f/u > 1 yr).
Adverse events/effects
We compared adverse effects ‐ events that were possibly causal by the active drug ‐ that were generally associated with each. So, for finasteride, we recorded erectile dysfunction (ED), impotence, ejaculation disorder, gynecomastia, and decreased libido. For alpha blockers, syncope (spontaneous loss consciousness from insufficient blood to the head), asthenia (abnormal loss of strength), fatigue, cardiovascular events, headaches, dizziness, and postural hypotension (a sudden decrease in blood pressure, which can cause syncope).
Follow‐up ≤ 1 year
(14 trials)
Although drug‐related effects were few, nevertheless, men taking finasteride were significantly at more risk for 'impotence', 'decreased libido', and 'ejaculation disorder'.
For 'any adverse event', we pooled 4 trials; the comparison was not significant (RR 1.02, 95% CI 0.94 to 1.11) (Analysis 1.6). In a meta‐analysis of 5 trials for 'withdrawals due to adverse events', the comparison was not significant (RR 1.15, 95% CI 0.92 to 1.45) (Analysis 1.7). We pooled serious adverse events ('patients reporting serious adverse events') for three trials but found significant heterogeneity (RR 1.03, 95% CI 0.72 to 1.47; I² = 63%). By removing Beisland 1992, a small trial (N = 182) with a 6 month follow‐up ‐ the other 2 trials followed men to 1 year ‐ we eliminated heterogeneity (RR 0.96, 95% CI 0.81 to 1.14; I² = 0%) (Analysis 1.8). The comparison was not significant. Tammela 1993 (N = 36), which reported no numbers for adverse effects, said "[f]inasteride . . . was tolerated well without any differences in side effects compared to the placebo group." Gormley 1992 and the Finasteride Study Group found 1 mg finasteride significantly increased risk for 'impotence' versus placebo (RR 4.01, 95% CI 1.43 to 11.25), and for 'ejaculatory disorders', the comparison just missed significance (RR 2.62, 95% CI 0.94 to 7.25), with more men taking 1 mg finasteride suffering from the disorder. Two trials (Byrnes 1995; Tenover 1997) were pooled for 'any adverse effects', and the comparison favored placebo (RR 1.54, 95% CI 1.28 to 1.85) (Analysis 1.9). Gormley, reporting 1 mg finasteride versus placebo for 'withdrawals due to adverse effects', found no significant difference (RR 0.78, 95% CI 0.40 to 1.55). Three trials were pooled (5 mg finasteride) (Byrnes; Gormley; Tenover) for 'withdrawals due to adverse effects'. The comparison was not significant (RR 1.31, 95% CI 0.89 to 1.93) (Analysis 1.10). Beisland 1992 and Byrnes 1995 reported finasteride significantly increased risk for serious adverse effects versus placebo (RR 5.42, 95% CI 1.00 to 29.40) (Analysis 1.11). Four trials were pooled for sexual adverse effects, and finasteride significantly increased risk versus placebo (RR 2.07, 95% CI 1.75 to 2.44) (Analysis 1.12). In a meta‐analysis, men in the finasteride arm were at significantly more risk than in the placebo arm for 'decreased libido' (RR 2.12, 95% CI 1.40 to 3.23), 'ejaculation disorder' (RR 2.86, 95% CI 1.79 to 4.56), and 'impotence' (RR 2.02, 95% CI 1.38 to 2.97) (Analysis 1.15).
1.6. Analysis.
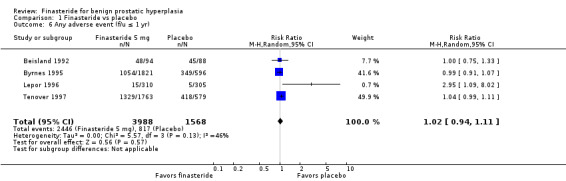
Comparison 1 Finasteride vs placebo, Outcome 6 Any adverse event (f/u ≤ 1 yr).
1.7. Analysis.
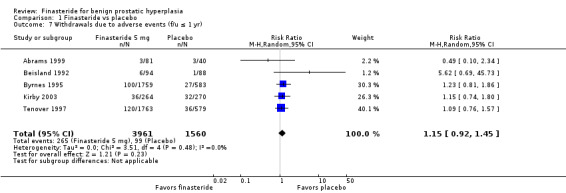
Comparison 1 Finasteride vs placebo, Outcome 7 Withdrawals due to adverse events (f/u ≤ 1 yr).
1.8. Analysis.
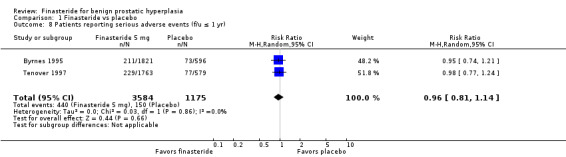
Comparison 1 Finasteride vs placebo, Outcome 8 Patients reporting serious adverse events (f/u ≤ 1 yr).
1.9. Analysis.

Comparison 1 Finasteride vs placebo, Outcome 9 Any adverse effects (f/u ≤ 1 yr).
1.10. Analysis.
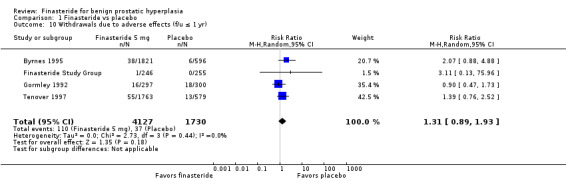
Comparison 1 Finasteride vs placebo, Outcome 10 Withdrawals due to adverse effects (f/u ≤ 1 yr).
1.11. Analysis.
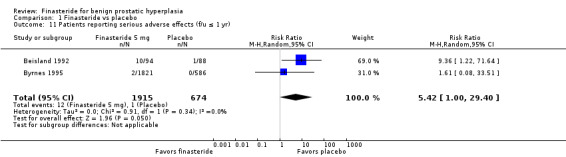
Comparison 1 Finasteride vs placebo, Outcome 11 Patients reporting serious adverse effects (f/u ≤ 1 yr).
1.12. Analysis.
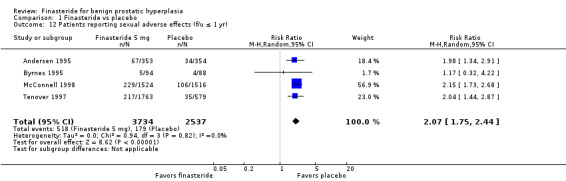
Comparison 1 Finasteride vs placebo, Outcome 12 Patients reporting sexual adverse effects (f/u ≤ 1 yr).
1.15. Analysis.
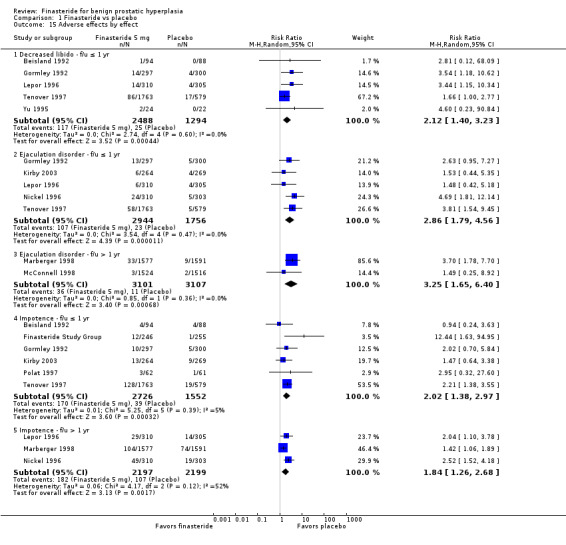
Comparison 1 Finasteride vs placebo, Outcome 15 Adverse effects by effect.
Follow‐up > 1 year
(13 trials)
For endpoints > 1 year, finasteride has significantly higher rates versus placebo of 'erectile dysfunction', 'decreased libido', and 'abnormal ejaculation'.
For 'any adverse event,' we pooled 2 trials. There was no significant difference between arms (RR 0.99, 95% CI 0.94 to 1.04; I² = 0%) (Analysis 1.13). One trial (Marberger 1998; N = 2902) found, for 'patients reporting serious adverse events', a marginally significant comparison favoring finasteride (RR 0.81, 95% CI 0.66 to 0.99). Three trials reported 'withdrawals due to adverse events'. Although the comparison was not significant (RR 0.99, 95% CI 0.77 to 1.27), there was significant heterogeneity (I² = 59%). The source of the heterogeneity was Marberger, which had about 4% to 5% fewer incidents in the finasteride arm than the other two trials. The re‐analysis also was not significant (RR 1.09, 95% CI 0.91 to 1.31), but heterogeneity was eliminated (I² = 0%) (Analysis 1.14). One trial, Marberger 1998, reported no difference for 'any adverse effects', although the comparison was marginal (RR 1.19, 95% CI 0.99 to 1.44). Marberger also found no difference for 'withdrawals due to adverse effects' (RR 1.01, 95% CI 0.64 to 1.58), or for serious adverse effects (RR 2.02, 95% CI 0.61 to 6.69). McConnell 1998 (N = 3040) found that sexual adverse effects converged (7% in both arms) between years 2 and 4 for a non‐significant comparison. Wessells 2003, a subsidiary study of McConnell 1998 (hereafter referenced as Wessells 2003 (McConnell 1998)), which reported post 1‐year effects, also found significant comparisons favoring finasteride versus placebo for "decreased libido" (∼6.5% versus ∼3.5%, respectively), "impotence" (∼8% versus ∼3.5%, respectively), and "decreased ejaculate volume" (∼3.7% versus ∼0.7%, respectively). By years 2, 3, and 4, only "decreased ejaculate volume" was significantly different, favoring placebo (P < 0.05). For 'ejaculation disorder', finasteride increased risk relative to placebo (RR 1.90, 95% CI 1.36 to 2.66) (Analysis 1.15). Nickel 1996 found no difference for 'decreased libido' between the two arms (RR 1.59, 95% CI 0.92 to 2.76). In the pooled analysis, the comparison favored placebo for 'ejaculation disorder' (RR 3.25, 95% CI 1.65 to 6.40). For 'impotence', the comparison again favored placebo, but with considerable heterogeneity (RR 1.56, 95% CI 1.06 to 2.29; I² = 73%). In a re‐analysis without McConnell 1998, a 4‐year trial (the other 3 were 1 and 2‐year trials), the comparison is the same, but with less heterogeneity (RR 1.84, 95% CI 1.26 to 2.68; I² = 52%). McConnell 2003, reporting the most frequent adverse effects (rate/100 person‐years of follow‐up) for finasteride and placebo, found significantly (P < 0.05) higher rates of:
1.13. Analysis.
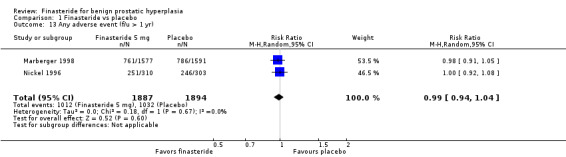
Comparison 1 Finasteride vs placebo, Outcome 13 Any adverse event (f/u > 1 yr).
1.14. Analysis.
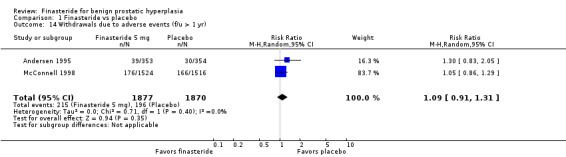
Comparison 1 Finasteride vs placebo, Outcome 14 Withdrawals due to adverse events (f/u > 1 yr).
ED (erectile dysfunction) (4.53/100 person‐years of follow‐up versus 3.32/100 person‐years of follow‐up);
decreased libido (2.36/100 person‐years of follow‐up versus 1.40/100 person‐years of follow‐up); and
abnormal ejaculation (1.78/100 person‐years of follow‐up versus 0.83/100 person‐years of follow‐up), respectively, for finasteride.
Quality of life
(4 trials)
Follow‐up ≤ 1 year
(4 trials)
There were quality‐of‐life improvements for finasteride but little evidence of statistically significant differences compared to placebo.
Lepor 1998, a secondary analysis of the included trial, Lepor 1996 (hereafter referenced as Lepor 1998 (Lepor 1996)), found small differences between men reporting "any improvement"* (69.3% versus 72%), and "marked or moderate improvement" (44% versus 39%), for finasteride and placebo, respectively. Lepor 1998 (Lepor 1996) also reported the validated Symptom Problem Index (range 0 to 28, with higher numbers worse symptoms; this scale is also known as the Symptom Problem Score), and found no statistical difference at 52 weeks. Byrnes 1995 (N = 2417), reporting a modified validated QoL instrument, the BPH‐specific interference‐with‐activities (BSIA), found modest changes for finasteride (‐2.65 points) and placebo (‐2.21 points) (range 0 to 42 ‐ higher numbers were worse symptoms). The comparison was not significant (MD ‐0.44, 95% CI ‐2.57 to 1.69). Byrnes and Tenover 1997, in a meta‐analysis using the validated BPH Impact Index (BII) (range 0 to 35 ‐ higher numbers denoted worse symptoms) also found no difference between finasteride and placebo (MD ‐0.36, 95% CI ‐0.87 to 0.15). This finding also confirmed Tenover's report of the BSIA (MD ‐0.44, 95% CI ‐2.57 to 1.69). In the tables below (Table 2 and Table 3), Johnson 2007 (McConnell 2003) found substantial improvements for finasteride from baseline (38.7%), but only modest changes for 1‐to‐4 year (38.7 to 42.6%), patient‐reported nocturia. Intra group comparisons for placebo were substantially the same.
| Table 2 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Finasteride (n = 653) |
14.2 | 47.0 | 38.7 |
| Placebo (n = 628) |
13.7 | 50.5 | 35.8 |
Follow‐up > 1 year
(1 trial)
Quality‐of‐life improvements were substantial for finasteride at endpoints from 1 to 4 years (Table 3), but nearly equivalent to placebo.
At both 1 and 4 years, more men reported finasteride was better than placebo (38.7% versus 35.8% and 42.6% versus 40.4%, respectively) at improving their nocturia. Intra group per cent changes from 1 to 4 years were modest as well (from 38.7% (1 year) to 42.6% (4 years), and 35.8% (1 year) to 40.4% (4 years), for finasteride and placebo, respectively). No statistical significances were given.
*"Any improvement" and "worse" were defined as a change of one or more episodes.
| Table 3 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Finasteride (n = 516) |
16.3 | 41.1 | 42.6 |
| Placebo (n = 488) |
17.2 | 42.4 | 40.4 |
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
Total symptom score
Follow‐up > 1 year
(2 trials)
For men with large prostates (> 40 cc), finasteride significantly, if modestly, improved symptom scores versus men taking finasteride and with small (≤ 40 cc) prostates.
Marberger 1998 compared Boyarsky I total scores in men with baseline prostates of ≤ 40 cc (n = 680) versus > 40 cc (n = 394). The study found placebo‐adjusted mean score improvements of ∼1.4 and ∼3.0 points for small and large prostates, respectively, at 2 years. The comparison was marginally significant (P = 0.053). Lepor 1998 (Lepor 1996) compared AUASI mean changes in men taking finasteride and placebo, respectively, with the following baseline prostate sizes:
≤ 40 cc = ‐3.2 versus ‐2.9 points (MD ‐0.30 points, 95% CI ‐1.41 to 0.81);
> 40 cc ≤ 50 cc = ‐2.1 versus ‐2.9 points (MD ‐0.30 points, 95% CI ‐1.41 to 0.81); and
> 50 cc = ‐3.6 versus ‐2.5 points (MD ‐1.10 points, 95% CI ‐3.33 to 1.13).
None of the subgroup cut points were significant.
BPH progression (≥ 4 points)
Follow‐up > 1 year
(1 trial)
At 4 years, for men with small (< 25 mL), medium (25 to < 40 mL), and large prostates (≥ 40 mL), finasteride consistently decreased the absolute risk of progression (≥ 4 points).
Kaplan 2006, a subsidiary study of the included trial, McConnell 2003 (hereafter referenced as Kaplan 2006 (McConnell 2003)), reported outcomes by baseline prostate subgroups of < 25 mL, 25 to < 40 mL, and ≥ 40 mL, at 4 years. As can be seen from Table 4 below, finasteride decreased the absolute risk of progression by 0.52%, 1.38%, and 3.67%, respectively. We also calculated MD and none where statistically significant.
| Table 4 | |||
| BPH progression (≥ 4 points) | |||
| Per cent progression/patient‐years (95% CI) |
|||
| < 25 mL | 25 to < 40 mL | ≥ 40 mL | |
| Finasteride (n = 765) |
2.54 (1.63 to 3.79) |
2.56 (1.70 to 3.70) |
3.67 (2.58 to 5.05) |
| Placebo (n = 737) |
3.06 (2.06 to 4.37) |
3.94 (2.84 to 5.33) |
7.34 (5.55 to 9.53) |
Peak urine flow
Follow‐up ≤ 1 year
(2 trials)
Men with large prostates and taking finasteride significantly improved symptom scores versus men with small prostates and taking finasteride or placebo.
Abrams 1999 (N = 121) compared peak urine flows at 1 year in men with baseline prostates of < 40 cc and ≥ 40 cc. In men with small prostates the between‐group difference (finasteride versus placebo) was 0.7 mL/s (95% CI ‐0.6 to 2.0) compared to men with large prostates, which was 1.6 mL/s (95% CI 0.2 to 3.0).
Lepor 1998 (Lepor 1996) compared peak urine flows by the subgroups:
≤ 40 cc = 1.4 versus 1.5 mL/s (MD ‐0.10 mL/s, 95% CI ‐0.93 to 0.73);
> 40 cc ≤ 50 cc = 0.9 versus 1.1 mL/s (MD ‐0.20 mL/s 95% CI ‐1.86 to 1.46); and
> 50 cc = 2.7 versus 0.6 mL/s (MD 2.10 mL/s, 95% CI 0.85 to 3.35).
Only in men with baseline prostate sizes > 50 cc was the comparison significant and favored finasteride.
Subgroup analysis: age (< 65 versus ≥ 65)
BPH progression (acute urinary retention and/or need for surgical intervention)
Follow‐up > 1 year
(1 trial)
At 4 years, versus placebo, taking finasteride significantly lowered risks of acute urinary retention and surgical intervention, or both, for younger men (45 to < 65 years) and older men (≥ 65 years); for men taking finasteride, older men had a greater risk of progression then did younger men.
Kaplan 2001 (McConnell 1998), combining two progression categories, found that treatment with finasteride led to significant reductions in both younger and older men (RR ‐51%, 95% CI 29 to 65 and RR ‐51%, 95% CI 34 to 64, respectively (P values were < 0.001 for both comparisons). Among men taking finasteride, older men had a greater 4‐year risk of progression than younger men (8% versus 5%).
Nocturia
Follow‐up ≤ 1 year
(1 trial)
For men aged ≥ 70 years, finasteride significantly lowered nocturnal incidents versus placebo.
Johnson 2007 (McConnell 2003) reported mean changes from baseline for men ‐ defined as subjects who completed at least 1 year of the trial and had nocturia at baseline ‐ aged < 70 versus ≥ 70 years old. For men younger than 70, at 1 year, there were changes of ‐0.43 and ‐0.41 episodes for the finasteride (n = 527) and placebo arms (n = 501), respectively (MD = 0.02). Johnson did not report if the comparison was significant or not. For men 70 years old or older, there were changes of ‐0.29 and ‐0.11 for finasteride (n = 126) and placebo (n = 127), respectively (MD = 0.18). The comparison was significant (P < 0.05).
Follow‐up > 1 year
(1 trial)
For men aged < 70 years, both the finasteride and placebo arms improved nocturia, although with nearly no difference; for men aged ≥ 70 years, the difference was more substantial, and favored finasteride.
For men younger than 70, at year 4, there were mean changes of ‐0.45 and ‐0.46 for finasteride (n = 417) and placebo (n = 389), respectively (MD = 0.01). For men 70 years old or older, there were mean changes of ‐0.29 and ‐0.08 for the finasteride (n = 99) and placebo arms (n = 99), respectively (MD = 0.21). Johnson did not report the significance of either comparison.
Prostate volume
Follow‐up > 1 year
(1 trial)
Versus placebo, men taking finasteride significantly had smaller prostate volumes versus placebo for both younger (< 65 years old) and older (≥ 65 years) men.
Kaplan 2001, a post hoc analysis of the included trial, McConnell 1998 (hereafter reported as Kaplan 2001 (McConnell 1998)), reported treatment with finasteride led to significant improvements in both younger (< 65 years old) and older (≥ 65 years) men (‐16.8% and ‐20.2% mean changes in volume, respectively) at 4‐year endpoint. That compares to mean changes of 14.1% and 13.7% in younger and older, placebo‐treated men, respectively. Intergroup comparisons were statistically significant as well (P < 0.001). The overall per cent of men who improved with finasteride relative to placebo also favored older men (30.9% versus 33.9%, respectively).
Adverse events/effects
Follow‐up > 1 year
(1 trial)
In the table below (Table 5), all drug‐related adverse effects were higher in the finasteride arm in both age cohorts, at year 1. By years 2 to 4, most adverse effects in the finasteride arm had decreased and were not significantly different than the placebo arm (Kaplan 2001 (McConnell 1998)).
| Table 5 | ||||
| Adverse effects by age | ||||
| Year 1 | Years 2 to 4 |
|||
| < 65 yrs | ≥ 65 yrs | < 65 yrs | ≥ 65 yrs | |
| Impotence | % | |||
| Finasteride | 8.8 | 7.4 | 5.5 | 4.6 |
| Placebo | 3.8 | 3.7 | 6.1 | 4.0 |
| Decreased libido | ||||
| Finasteride | 6.8 | 6.1 | 4.2 | 1.9 |
| Placebo | 4.5 | 2.3 | 3.2 | 1.8 |
| Ejaculation disorder | ||||
| Finasteride | 1.5 | 0.8 | 0.1 | 0.5 |
| Placebo | 0.3 | 0.1 | 0.0 | 0.1 |
| Gynecomastia | ||||
| Finasteride | 0.5 | 0.5 | 1.0 | 2.5 |
| Placebo | 0.0 | 0.1 | 0.8 | 1.5 |
Subgroup analysis: study duration (short (6 to 12 months) versus long (greater than 12 months))
Total symptom score
(1 trial)
At 1 year there was no difference in median improvements in the AUASI for finasteride and placebo; at 4 years there was a median difference of 1.0 point favoring finasteride.
McConnell 2003, reporting outcomes for the AUASI (but not variances) at years 1 and 4, found median improvements of 4.0 and 5.0 points, respectively, for finasteride, and 4.0 and 4.0 points, respectively, for placebo.
Peak urine flow
(1 trial)
Finasteride consistently improved urine flows versus placebo at 1 year and 4 year follow‐up.
There were changes at years 1 and 4 of 1.8 and 2.2 mL/s, respectively, for finasteride, and 1.3 and 1.4 mL/s, respectively, for placebo. Again, no SD were given (McConnell 2003).
Finasteride versus doxazosin
Total symptom score
Follow‐up ≤ 1 year
(2 trials)
At 1‐year endpoints, two trials reported clinically significant (≥ 4 point decrease in the AUASI/IPSS) mean and median improvements for finasteride and doxazosin, as well as statistically significant comparisons favoring doxazosin.
Kirby 2003 reported clinically significant mean changes of ‐6.6 and ‐8.3 points in the IPSS (range 0 to 35), for the finasteride (n = 239) and doxazosin (n = 250) arms, respectively, at 1 year follow‐up. The inter group mean difference was 1.70 points (95% CI 0.58 to 2.82) and favored doxazosin. At 1 year, McConnell 2003 noted median changes of ‐4.0 and ‐6.0 points in the AUASI (range 0 to 35) for finasteride and doxazosin, respectively. The inter group comparison was significant as well (P < 0.001).
Follow‐up > 1 year
(1 trial)
At 4 years, both finasteride and doxazosin improved urinary symptom scores clinically; head‐to‐head, the comparison was significant (P = 0.001) and favored doxazosin (McConnell 2003).
BPH progression (≥ 4 point increase)
(1 trial)
Follow‐up > 1 year
The absolute risk of progression was not significant (RD 0.01, 95% CI ‐0.02 to 0.04) (McConnell 2003).
BPH progression (acute urinary retention)
Follow‐up ≤ 1 year
(1 trial)
The absolute risk of acute urinary retention for finasteride versus doxazosin was not statistically significant (RD 0.01, 95% CI ‐0.01 to 0.02) (Kirby 2003).
Follow‐up > 1 year
(1 trial)
At 4 year follow‐up, for finasteride versus doxazosin, the absolute risk of acute urinary retention was not statistically significant (RD ‐0.00, 95% CI ‐0.01 to 0.01) (McConnell 2003).
BPH progression (need for surgical intervention)
Follow‐up ≤ 1 year
(1 trial)
The absolute risk of surgical intervention was not statistically significant (RD 0.01, 95% CI ‐0.01 to 0.02) (Kirby 2003).
Follow‐up > 1 year
(1 trial)
Finasteride significantly lowered the absolute risk of surgical intervention by 2% (RD ‐0.02, 95% CI ‐0.03 to ‐0.00) (McConnell 2003).
PSA as a surrogate endpoint
Follow‐up > 1 year
The MTOPS trial (McConnell 2003), to see if change in PSA, as a surrogate endpoint, was affected by active interventions, compared finasteride to doxazosin. At 4 years, finasteride decreased median baseline PSA by 50%; for doxazosin it was a 13% increase (P < 0.001).
Peak urine flow
Follow‐up ≤ 1 year
(2 trials)
Two trials reported doxazosin significantly improved peak urine flow versus finasteride.
For Kirby, peak flows increased 1.8 and 3.6 mL/s for finasteride and doxazosin, respectively, with the comparison favoring doxazosin (MD ‐1.80 mL/s, 95% CI ‐2.63 to ‐0.97). This outcome should be met with some caution, since baseline numbers were significantly different (10.2 versus 10.4 mL/s for finasteride and doxazosin, respectively; P = 0.09). At 1 year, McConnell noted median changes of 1.8 and 3.0 mL/s for the 2 arms, with the comparison favoring doxazosin (P < 0.001).
Follow‐up > 1 year
(1 trial)
At 4.5 year mean follow‐up doxazosin was significantly better than finasteride at improving peak urine flows.
At 4 years, McConnell 2003 reported median changes of 2.2 versus 2.5 mL/s, respectively, for finasteride versus doxazosin. The comparison just missed significance (P = 0.09). Over the study duration ‐ mean follow‐up 4.5 years ‐ the inter arm comparison was significant, and favored doxazosin (P = 0.03).
Nocturia
Follow‐up ≤ 1 year
(1 trial)
For self‐reported nocturia, doxazosin significantly lowered the risk of nocturia compared to finasteride.
Johnson 2007 (McConnell 2003) found self‐reported nocturia for men with one or more incidents at baseline, at 1 year. There were mean changes of ‐0.40% and ‐0.54% nightly incidents for finasteride (n = 653) and doxazosin (n = 649), respectively. The comparison was significant (P < 0.05). For men with two or more incidents at baseline, mean changes were ‐0.60% and ‐0.77% for finasteride (n = 496) and doxazosin (n = 484), respectively. The comparison was significant as well (P < 0.05).
Follow‐up > 1 year
(1 trial)
At 4‐year endpoint for self‐reported nocturia, and without statistical significances, doxazosin lowered risk versus finasteride.
Johnson also reported 4‐year outcomes for men with at least one or more incidents at baseline. There were mean changes of ‐0.42 and ‐0.53 incidents for finasteride (n = 516) and doxazosin (n = 533), respectively. For men with two or more incidents, mean changes were ‐0.68 and ‐0.77 for the finasteride (n = 385) and doxazosin arms (n = 393), respectively. Statistical significance was not reported.
Study discontinuations
Follow‐up ≤ 1 year
(1 trial)
Comparing study discontinuations, the comparison was not statistically significant (Kirby 2003) (RR 1.08, 95% CI 0.83 to 1.40).
Follow‐up > 1 year
(1 trial)
At 4‐year endpoint and comparing trial dropouts, the comparison between arms was not significant (McConnell 2003) (RR 0.89, 95% CI 0.75 to 1.05).
Adverse events/effects
Follow‐up ≤ 1 year
(1 trial)
Kirby reported no difference comparing 'withdrawals due to adverse effects' (RR 1.11, 95% CI 0.70 to 1.74).
Follow‐up > 1 year
(1 trial)
For finasteride and doxazosin, McConnell 2003 compared six "most frequent adverse [effects]," and found finasteride had higher rates for erectile dysfunction, decreased libido, and abnormal ejaculation (Table 6).
| Table 6 | |||
| Erectile dysfunction |
Decreased libido |
Abnormal ejaculation |
|
| rate/100 person‐years of follow‐up | |||
| Finasteride | 4.53/100 | 2.36/100 | 1.78/100 |
| Doxazosin | 3.56/100 | 1.56/100 | 1.10/100 |
The doxazosin arm had higher rates of dizziness, postural hypotension, and asthenia (Table 7).
| Table 7 | |||
| Dizziness | Postural hypotension |
Asthenia | |
| rate/100 person‐years of follow‐up | |||
| Finasteride | 2.33/100 | 2.56/100 | 1.56/100 |
| Doxazosin | 4.41/100 | 4.03/100 | 4.08/100 |
McConnell reported (Table 6, Table 7) only active comparisons to placebo and without statistical significances.
Quality of life
Follow‐up ≤ 1 year
(1 trial)
At 1 year follow‐up, considerably more men in the doxazosin arm reported improvement than in the finasteride arm.
In the tables below, Johnson 2007 (McConnell 2003) compared 1‐year (Table 8) and 4‐year outcomes ("worse," "no change," "improved") (Table 9) for patient‐reported nocturia. Changes ("improved" and "worse" were defined as a change of one or more episodes) from baseline were substantial, from 38.7% and 47.1% for finasteride and doxazosin, respectively, at 1 year. From years 1 to 4, changes were much more modest, at 38.7% to 42.6%, and 47.1% to 44.7%, for finasteride and doxazosin, respectively.
| Table 8 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Finasteride (n = 516) |
14.2 | 47.0 | 38.7 |
| Doxazosin (n = 649) |
9.6 | 43.3 | 47.1 |
Follow‐up > 1 year
(1 trial)
At 4 years more men in the doxazosin arm reported improvement of nocturia than did men in the finasteride arm, but the difference had narrowed considerably.
| Table 9 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Finasteride (n = 516) |
16.3 | 41.1 | 42.6 |
| Doxazosin (n = 533) |
11.4 | 43.9 | 44.7 |
No statistical significances were given.
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
BPH progression (≥ 4 points)
Follow‐up ≥ 1 year
(1 trial)
For men with small prostates and taking finasteride, there was a small absolute risk increase of progression. For men in the medium and large groups and taking doxazosin, there was a small absolute risk increase of progression.
Kaplan 2006 (McConnell 2003) reported clinical progression ‐ a ≥ 4 deterioration of the AUASI ‐ at 4 years by baseline prostate sizes of < 25 mL, ≥ 25 to < 40 mL, and ≥ 40 mL (Table 10). In men with small prostates and taking doxazosin, there was an absolute risk reduction of < 1%. For men with medium and large prostates and taking finasteride, there was an absolute risk increase of 8% (RD ‐0.08, 95% CI ‐4.14 to 3.98) and 2% (RD ‐0.02, 95% CI ‐3.54 to 3.50), respectively. Although Kaplan did not report statistical significances, by our calculations none were.
| Table 10 | |||
| BPH progression (≥ 4 points) | |||
| Per cent progression/patient‐years (95% CI) |
|||
| < 25 mL | 25 to < 40 mL | ≥ 40 mL | |
| Finasteride (n = 765) |
2.54 (1.63 to 3.79) |
2.56 (1.70 to 3.70) |
3.67 (2.58 to 5.05) |
| Doxazosin (n = 756) |
1.93 (1.18 to 2.98) |
2.64 (1.75 to 3.81) |
3.69 (2.60 to 5.08) |
Subgroup analysis: age (< 65 versus ≥ 65)
Nocturia
Follow‐up ≤ 1 year
(1 trial)
For men aged < 70 years, those taking doxazosin had significantly fewer events than those taking finasteride.
Johnson 2007 (McConnell 2003) found mean changes from baseline for men* aged < 70 versus ≥ 70 years old. For men younger than 70, at 1 year, there were changes of ‐0.43 and ‐0.56 incidences for finasteride (n = 527) and doxazosin (n = 521), respectively. The comparison was significant (P < 0.05). For men 70 years old or older, there were changes of ‐0.29 and ‐0.46 for finasteride (n = 126) and doxazosin (n = 128), respectively. Johnson did not report if the comparison was significant.
Follow‐up > 1 year
(1 trial)
For younger and older men at 4 year follow‐up, those taking doxazosin reported greater improvements in nocturia versus finasteride.
For men younger than 70, at year 4, there were mean changes of ‐0.45 and ‐0.52 for finasteride (n = 417) and doxazosin (n = 428), respectively. For men 70 years old or older, there were mean changes of ‐0.29 and ‐0.59 for the finasteride (n = 99) and doxazosin (n = 105), respectively. Johnson did not report if either comparison was significant.
*These men completed at least 1 year of the trial and had nocturia at baseline.
Finasteride + doxazosin versus finasteride
Total symptom score
Follow‐up ≤ 1 year
(2 trials)
At 1 year endpoint, combination therapy (finasteride + doxazosin) improved scores significantly versus finasteride monotherapy; both interventions improved scores clinically.
Kirby 2003 found clinically significant changes (≥ 4 point decrease in the AUASI/IPSS) of ‐8.5 and ‐6.6 points for combination therapy (n = 286) and finasteride (n = 264), respectively. The inter group comparison was significant as well, and favored combination therapy (MD ‐1.90, 95% CI ‐3.11 to ‐0.69). McConnell 2003 reported median improvements of 6.0 and 4.0 points for the combination (n = 786) and finasteride (n = 768) arms, respectively, at 1 year. The comparison was significant (P < 0.001).
Follow‐up > 1 year
At 4 years, the combination arm improved scores significantly versus finasteride. Improvements for both were clinically significant (≥ 4 point decrease in the AUASI/IPSS) as well.
McConnell 2003 reported median changes of ‐7.0 and ‐5.0 points at 4 years (P < 0.001), for the combination and finasteride arms, respectively.
BPH progression (≥ 4 point increase)
Follow‐up > 1 year
(1 trial)
Combination therapy reduced the absolute risk of progression 4% (McConnell 2003) (RD ‐0.04, 95% CI ‐0.06 to ‐0.01).
BPH progression (acute urinary retention)
Follow‐up ≤ 1 year
(1 trial)
The absolute risk of progression was not significant (Kirby 2003) (RD ‐0.01, 95% CI ‐0.03 to 0.00).
Follow‐up > 1 year
(1 trial)
The absolute risk of progression was not significant (McConnell 2003) (RD 0.00, 95% CI ‐0.01 to 0.01).
BPH progression (need for surgical intervention)
Follow‐up ≤ 1 year
(1 trial)
Combination therapy decreased ‐ insignificantly ‐ the absolute risk of surgical intervention by 1% (Kirby 2003) (RD ‐0.01, 95% CI ‐0.03 to 0.00).
Follow‐up > 1 year
(1 trial)
The absolute risk difference was 0% (McConnell 2003) (RD ‐0.00, 95% CI ‐0.02 to 0.01).
PSA as a surrogate endpoint
Follow‐up > 1 year
To see if long‐term change in PSA, as a surrogate endpoint, was affected by the active interventions, MTOPS compared combination therapy to finasteride monotherapy (McConnell 2003). At 4 years, both arms decreased median baseline PSA by 50% (P = 0.925).
Peak urine flow
Follow‐up ≤ 1 year
(2 trials)
In 2 trials finasteride + doxazosin were significantly better than finasteride at improving peak urine flows.
Kirby 2003 reported improvements of 3.8 and 1.8 mL/s for combination therapy and finasteride, respectively. The MD was 2.00 mL/s (95% CI 1.17 to 2.83) and favored the combination arm. At 1 year McConnell 2003 found significant (P < 0.001), similar, median changes of 3.6 and 1.8 mL/s for combination therapy and finasteride monotherapy, respectively.
Follow‐up > 1 year
(1 trial)
One trial found finasteride + doxazosin significantly improved urine flows versus finasteride.
At year 4, McConnell 2003 reported improvements (median change) of 3.7 and 2.2 mL/s, for combination therapy and finasteride, respectively. The comparison was significant (P < 0.001).
Nocturia
Follow‐up ≤ 1 year
(1 trial)
At 1 year, men taking finasteride + doxazosin had significantly fewer incidents of nocturia than men taking finasteride alone.
Johnson 2007 (McConnell 2003) reported improvements for men with one or more incidents of nocturia at baseline, of 0.58% and 0.40% incidents for the combination (n = 653) and finasteride arms (n = 653), respectively. The comparison was significant (P < 0.05). In men with two or more baseline incidents, nocturia improved by ‐0.80 and ‐0.60 nightly incidents for combination therapy (n = 487) and finasteride (n = 496), respectively. The comparison was significant (P < 0.05).
Follow‐up > 1 year
(1 trial)
At 4 years, men on combination therapy reported greater improvement of nocturia than did men on finasteride monotherapy.
For men with one or more incidents at baseline, at year 4, Johnson also reported changes of ‐0.55 and ‐0.42 incidents for combination therapy (n = 528) and finasteride (n = 516), respectively. It was not reported if the comparison was significant. And for men with two or more incidents, mean changes were ‐0.79 and ‐0.68 and for combination therapy (n = 393) and finasteride (n = 385), respectively. Statistical significance was not reported (Johnson 2007 (McConnell 2003)).
Study discontinuations
Follow‐up ≤ 1 year
(1 trial)
Comparing study discontinuations, there was no statistical difference between combination therapy and finasteride alone (Kirby 2003) (RR 1.01, 95% CI 0.79 to 1.30).
Follow‐up > 1 year
(1 trial)
Significantly more men dropped out in the finasteride arm than the combination arm (McConnell 2003) (RR 0.75, 95% CI 0.62 to 0.91).
Adverse events/effects
Follow‐up ≤ 1 year
(1 trial)
For men taking combination therapy, the risk of asthenia, dizziness, and impotence was significantly increased versus monotherapy, and for men taking finasteride alone, they increased their risk for decreased libido versus combination therapy.
Kirby reported no difference between arms for 'withdrawals due to adverse events' (RR 1.01, 95% CI 0.79 to 1.30). Compared individually, the risk of 'asthenia' (RR 2.18, 95% CI 1.10 to 4.33), 'dizziness' (RR 1.71, 95% CI 1.04 to 2.84), and 'impotence' (RR 2.13, 95% CI 1.14 to 4.00) increased in the combination arm relative to the monotherapy arm. The combination arm had more men with events of 'syncope' (6 versus 0, respectively) (RR 12.00, 95% CI 0.68 to 212.04), 'postural hypotension' (8 events to 2) (RR 3.69, 95% CI 0.79 to 17.23) and 'ejaculation disorder' (7 events to 6) (RR 1.08, 95% CI 0.37 to 3.16), than finasteride alone, but none were significantly different. Finasteride significantly increased risk of 'decreased libido' than did the combination arm (RR 0.62, 95% CI 0.22 to 1.71).
Follow‐up > 1 year
(1 trial)
Comparing the most frequent, drug‐related adverse effects, McConnell found higher rates (rate/100 person‐years of follow‐up) in men in the combination arm for
asthenia (4.20/3832 versus 1.56/3600),
decreased libido (2.51/3832 versus 2.36/3600),
dizziness (5.35/3832 versus 2.33/3600),
ED (5.11/3832 versus 4.53/3600),
ejaculation disorder (3.05/3832 versus 1.78/3600), and
postural hypotension (4.33/3832 versus 2.56/3600),
than in the finasteride arm alone. McConnell did not give statistical significances for the active controls.
Quality of life
Follow‐up ≤ 1 year
(1 trial)
Johnson 2007 (McConnell 2003) (Table 11) found substantial improvement ‐ "improvement," as well as "worse," were defined as change of one or more episodes ‐ from baseline for combination therapy (47.9%) and finasteride monotherapy (38.7%), for self‐reported nocturia.
| Table 11 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Combination (n = 653) |
7.8 | 44.3 | 47.9 |
| Finasteride (n = 653) |
14.2 | 47.0 | 38.7 |
Follow‐up > 1 year
(1 trial)
For patient‐reported nocturia, changes from year 1 to 4 were modest: 47.9% to 47.3%, and 38.7% to 42.6%, for combination therapy and finasteride, respectively (Table 12) (Johnson 2007 (McConnell 2003)).
| Table 12 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Combination (n = 528) |
11.7 | 40.9 | 47.3 |
| Finasteride (n = 516) |
16.3 | 41.1 | 42.6 |
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
Total symptom score
Follow‐up > 1 year
(1 trial)
Kaplan 2006 (McConnell 2003) reported mean changes of the AUASI by baseline prostate subgroups of < 25 mL, 25 to < 40 mL, and ≥ 40 mL, at 4 years. Mean differences for all subgroups were significant and favored finasteride + doxazosin over finasteride monotherapy (P < 0.05).
BPH progression (≥ 4 points)
Follow‐up > 1 year
(1 trial)
As can be seen from Table 13 below, finasteride increased the absolute risk of progression (≥ 4 points) by 1.19%, 1.18%, and 1.90%, respectively. The relative risk of BPH progression for combination therapy versus monotherapy was not significant for men with small prostates (RR 0.54, 95% CI 0.27 to 1.09), but was significant and favorable for men taking combination therapy and with medium (RR 0.55, 95% CI 0.31 to 0.99) and large prostates (RR 0.48, 95% CI 0.27 to 0.85).
| Table 13 | |||
| Per cent progression (≥ 4 points)/ patient‐years (95% CI) |
|||
| Baseline | < 25 mL | 25 to < 40 mL | ≥ 40 mL |
| Combination (n = 783) |
1.35 (0.70 to 2.37) |
1.38 (0.83 to 2.15) |
1.77 (1.05 to 2.79) |
| Finasteride (n = 765) |
2.54 (1.63 to 3.79) |
2.56 (1.70 to 3.70) |
3.67 (2.58 to 5.05) |
BPH progression (need for surgical intervention)
Follow‐up > 1 year
(1 trial)
For all the three baseline subgroups, the relative risk of progression was not significant.
Kaplan defined progression as "invasive therapy," and which included TURP, transurethral incision of the prostate, transurethral microwave therapy, laser therapy, stenting, or open prostatectomy. The RR for all three subgroups was not significant.
Peak urine flow
Follow‐up > 1 year
(1 trial)
Kaplan also reported significant mean differences for all three subgroups that favored combination therapy.
Subgroup analysis: age (< 65 versus ≥ 65)
Nocturia
Follow‐up ≤ 1 year
(1 trial)
At 1 year, men aged < 70 years and taking combination therapy, had significantly less nocturia; men ≥ 70 years old and taking finasteride + doxazosin had fewer episodes of nocturia than did those taking monotherapy.
Johnson 2007 (McConnell 2003) reported mean changes from baseline for men* aged < 70 versus ≥ 70 years old. For men younger than 70, at 1 year, there were changes of ‐0.61 and ‐0.43 episodes for combination therapy (n = 539) and finasteride (n = 527), respectively. The comparison was significant (P < 0.05). For men 70 years old or older, there were changes of ‐0.42 and ‐0.29 for combination therapy (n = 114) and finasteride (n = 126), respectively. Johnson did not report if the comparison was significant.
Follow‐up > 1 year
(1 trial)
At 4 years of follow‐up, men taking combination therapy, and at both age cut points, had fewer nocturnal episodes than did men taking finasteride alone.
For men younger than 70, at year 4, there were mean changes of ‐0.58 and ‐0.45 for combination therapy (n = 442) and finasteride (n = 417), respectively. For men 70 years old or older, there were mean changes of ‐0.40 and ‐0.29 events for the combination arm (n = 89) and for finasteride (n = 99), respectively. Johnson did not report if either comparison was significant.
*These men completed at least 1 year of the trial and had nocturia at baseline.
Finasteride + doxazosin versus doxazosin
Total symptom score
Follow‐up ≤ 1 year
(2 trials)
From baseline, both combination therapy and doxazosin monotherapy provided similar and clinically significant improvements in urinary symptom scores.
At 1 year follow‐up, Kirby 2003 reported, for finasteride + doxazosin versus doxazosin monotherapy, mean changes from baseline of ‐8.5 and ‐8.3, for the IPSS. These were clinically meaningful changes, but were not significant head‐to‐head (MD ‐0.20, 95% CI ‐1.41 to 1.01). McConnell 2003, which used the AUASI, found median improvements of 6.0 for both arms (P = 0.077).
Follow‐up > 1 year
(2 trials)
Combination therapy and doxazosin alone clinically improved symptom scores; the inter group comparison was not significant (Kirby 2003) (MD ‐0.20, 95% CI ‐1.31 to 0.91). At 4 years McConnell reported median improvements of ‐7.0 and ‐6.0 for combination and monotherapy, respectively. The comparison was significant (P = 0.035).
BPH progression (≥ 4 point increase)
Follow‐up > 1 year
(1 trial)
Combination therapy decreased the absolute risk of progression by 4% (McConnell 2003) (RD ‐0.04, 95% CI ‐0.06 to ‐0.01).
BPH progression (acute urinary retention)
Follow‐up > 1 year
(1 trial)
The absolute risk reduction was not significant (McConnell 2003) (MD 0.01, 95% CI ‐0.00 to 0.02).
BPH progression (need for surgical intervention)
Follow‐up ≤ 1 year
(1 trial)
The absolute risk of progression was 0% (Kirby 2003) (RD ‐0.00, 95% CI ‐0.01 to 0.01).
Follow‐up > 1 year
(1 trial)
Doxazosin marginally, but significantly, decreased the absolute risk of surgical intervention by 1% (McConnell 2003) (RD 0.01, 95% CI 0.00 to 0.02).
PSA as a surrogate endpoint
Follow‐up > 1 year
MTOPS assessed whether long‐term change in PSA, as a surrogate endpoint, was affected by the active interventions (McConnell 2003). At 4 years, PSA levels decreased from baseline by a median of 50% for finasteride + doxazosin and increased by a median 13% for doxazosin monotherapy (P < 0.001).
Peak urine flow
Follow‐up ≤ 1 year
(1 trial)
One trial reported equivalent improvements for finasteride + doxazosin versus doxazosin, while another found combination therapy significantly better.
Kirby reported, for combination therapy versus finasteride monotherapy, mean changes of 3.8 and 3.6 mL/s, respectively. The comparison was not significant (MD 0.20, 95% CI ‐0.63 to 1.03). McConnell reported 1 year median changes of 3.6 and 3.0 mL/s for combination and monotherapy, respectively. The comparison was significant (P = 0.002).
Follow‐up > 1 year
(1 trial)
Finasteride + doxazosin were significantly better than doxazosin at improving urine flows.
At 4 years, McConnell reported median changes of 3.7 and 2.5 mL/s for combination versus doxazosin alone, respectively. The comparison was significant (P = 0.002).
Nocturia
Follow‐up ≤ 1 year
(1 trial)
At 1 year follow‐up, combination therapy and finasteride alone improved nocturia equivalently.
Johnson 2007 (McConnell 2003), for men with at least 1 episode (self‐reported) at baseline and who completed 1 year of follow‐up, found changes of ‐0.58% and ‐0.54% for combination (n = 653) and doxazosin monotherapy (n = 649), respectively, at 1 year. At 4 years, the study found changes of ‐0.80% and ‐0.77% for combination therapy (n = 487) and monotherapy (n = 484), respectively. No statistical significances were given.
Follow‐up > 1 year
(1 trial)
At 4 years follow‐up, combination therapy and finasteride reported nearly equivalent improvements for nocturia.
Johnson, also reporting 4‐year outcomes for at least 1 episode at baseline, found changes of ‐0.55% and ‐0.53% for combination therapy (n = 528) and doxazosin (n = 533) alone, respectively, and for 2‐or‐more episodes, changes of ‐0.79% and ‐0.77% for combination therapy (n = 393) and monotherapy (n = 393), respectively.
Study discontinuations
Follow‐up ≤ 1 year
(1 trial)
There was no significant difference between arms (Kirby) (RR 1.10, 95% CI 0.85 to 1.42).
Follow‐up > 1 year
(1 trial)
The risk of dropouts was significantly greater for monotherapy than for combination therapy.
There were 18% (141/786) and 27% (204/756) discontinuations in the combination and doxazosin arms, respectively (McConnell) (RR 0.66, 95% CI 0.55 to 0.80).
Adverse events/effects
Follow‐up ≤ 1 year
(1 trial)
For drug‐related adverse effects, finasteride + doxazosin marginally lowered the risk of postural hypotension versus finasteride monotherapy. Finasteride significantly increased the relative risk of ejaculation disorder (marginally) and impotence versus combination therapy.
Kirby reported no significant difference for 'withdrawals due to adverse events' (RR 1.05, 95% CI 0.67 to 1.65). Kirby found doxazosin had higher rates for
asthenia = 9.1% versus 10.5% (RR 0.87, 95% CI 0.58 to 1.30),
decreased libido = 2.1% versus 3.6% (RR 0.58, 95% CI 0.21 to 1.57),
dizziness = 13.6% versus 15.6% (RR 0.87, 95% CI 0.58 to 1.30), and
postural hypotension = 2.8% versus 5.8% (RR 0.48, 95% CI 0.21 to 1.11),
than combination therapy. Only postural hypotension was close to significance.
Finasteride + doxazosin had higher rates for
'ejaculation disorder' = 2.4% versus 0.4% (RR 6.73, 95% CI 0.83 to 54.35),
'impotence' = 10.5% versus 5.8% (RR 1.80, 95% CI 1.01 to 3.23), and
'syncope' = 2.1% versus 0.7% (RR 2.88, 95% CI 0.59 to 14.17),
than did monotherapy. Only 'impotence' was statistically significant. 'Ejaculation disorder' was marginally significant but had a very large CI.
Follow‐up > 1 year
(1 trial)
McConnell, reporting the "most frequent adverse [effects]," found higher rates (rate/100 person‐years of follow‐up) for combination therapy of
asthenia (4.20/3832 versus 4.08/3652),
decreased libido (2.51/3832 versus 1.56/3652),
dizziness (5.35/3832 versus 4.41/3652),
erectile dysfunction (5.11/3832 versus 3.56/3652),
ejaculation disorder (3.05/3832 versus 1.10/3652), and
postural hypotension (4.33/3832 versus 4.03/3652),
than for doxazosin monotherapy, respectively. McConnell reported only active comparisons to placebo; it is unknown whether any of the active comparisons were statistically significant.
Quality of life
Follow‐up ≤ 1 year
Johnson 2006 (an analysis of the included trial, McConnell 2003) found substantial, but nearly equal, improvements* of 47.9% and 47.1% for combination therapy and monotherapy, respectively (Table 14).
| Table 14 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Finasteride + doxazosin (n = 653) |
7.8 | 44.3 | 47.9 |
| Doxazosin (n = 649) |
9.6 | 43.3 | 47.1 |
Follow‐up > 1 year
(1 trial)
From years 1 to 4 (Table 15), improvements were virtually the same, changing from 47.9% to 47.3%, for combination therapy, and 47.1% to 44.7%, for monotherapy, respectively.
| Table 15 | |||
| Patient‐reported nocturia | |||
| Worse | No change | Improved | |
| % | |||
| Finasteride + doxazosin (n = 528) |
11.7 | 41.0 | 47.3 |
| Doxazosin (n = 533) |
11.4 | 43.9 | 44.7 |
*"Improved" and "worse" were defined as a change of one or more episodes.
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
Total symptom score
Follow‐up > 1 year
(1 trial)
Combination therapy significantly improved symptom scores for men with medium and large‐sized prostates versus doxazosin alone.
Kaplan 2006 (McConnell 2003) compared outcomes for men with baseline prostates of < 25 mL, 25 mL to < 40 mL, and ≥ 40 mL, and found no significant difference between arms for men with small prostates, but found significant differences favoring combination therapy for men with medium and large‐sized prostates (P < 0.05).
BPH progression (≥ 4 points)
Follow‐up > 1 year
(1 trial)
For men with medium and large prostates, combination therapy significantly decreased the risk of progression versus doxazosin alone.
For men with small prostates there was no significant difference between arms (RR 0.54, 95% CI 0.27 to 1.09) (Kaplan 2006 (McConnell 2003)). In men with medium and large‐sized prostates, the RR was 0.54 (95% CI 0.30 to 0.96) and 0.50 (95% CI 0.28 to 0.88), for combination therapy versus doxazosin alone, respectively, and favored combination therapy.
BPH progression (need for surgical intervention)
Follow‐up > 1 year
(1 trial)
For men with medium and large prostates and taking combination therapy, there were significant reductions of surgical interventions versus doxazosin alone.
Kaplan found no significant difference for men with small prostates. For men with medium and large prostates, however, there were significant risk reductions of about 60% to 80% for combination therapy (RR 0.19, 95% CI 0.05 to 0.65 and RR 0.38, 95% CI 0.17 to 0.85, respectively) versus doxazosin monotherapy.
Peak urine flow
Follow‐up > 1 year
(1 trial)
Combination therapy significantly decreased flows in men with medium and large prostates (Kaplan 2006 (McConnell 2003)) (P < 0.05). Kaplan reported a non‐significant difference for men with small prostates.
Finasteride + terazosin versus terazosin
Total symptom score
Follow‐up ≤ 1 year
(1 trial all outcomes)
Both combination therapy and terazosin alone improved the AUASI clinically, but were not significantly different (Lepor 1996) (P = 1.00).
BPH progression (need for surgical intervention)
The absolute risk difference was 0% (P > 0.05).
Peak urine flow
Urine flows improved for both finasteride + terazosin and terazosin monotherapy but were not significantly different (P = 0.15).
Prostate volume
Combination therapy significantly reduced prostate volume versus terazosin alone (Johnson 2003 (Lepor 1996)) (MD 7.80, 95% CI 6.14 to 9.46).
Nocturia
Men taking terazosin monotherapy had significantly fewer episodes at 1 year follow‐up than those taking combination therapy.
Of men with baseline nocturia, those taking terazosin had a mean of 1.8 episodes at endpoint; of those taking combination therapy, they had a mean of 2.0 episodes (P = 0.03) (Johnson 2003 (Lepor 1996)).
Study discontinuations
Relative risk was not significant (RR 0.90, 95% CI 0.64 to 1.28).
Adverse events/effects
Finasteride + terazosin was not significantly different than finasteride for 'asthenia', 'dizziness', 'headache', 'impotence', 'decreased libido', 'syncope', and 'postural hypotension'. Only for 'ejaculation disorder' did combination therapy significantly reduced risk versus terazosin alone (P < 0.05).
Quality of life
Lepor 1998 (Lepor 1996) reported no significant difference in the SPI (MD 0.30, 95% CI ‐0.53 to 1.13), but a significant difference in the BPH Impact Score (MD 0.40, 95% CI 0.13 to 0.67) that favored combination therapy.
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
Total symptom score
For men with small, medium, and large prostates, finasteride added to an alpha blocker did not significantly improve AUA scores versus terazosin alone:
≤ 40 cc = MD 0.00 points, 95% CI ‐1.25 to 1.25;
> 40 cc ≤ 50 cc = MD ‐0.60 points, 95% CI ‐3.09 to 1.89; and
> 50 cc = MD 1.00 points, 95% CI ‐1.36 to 3.36.
Peak urine flow
Lepor 1998 (Lepor 1996) also reported no significant advantage to adding finasteride to an alpha blocker:
≤ 40 cc = 3.1 versus 2.5 mL/s (MD ‐0.60 mL/s, 95% CI ‐1.71 to 0.51);
> 40 cc ≤ 50 cc = 3.4 versus 2.4 mL/s (MD ‐1.00 mL/s, 95% CI ‐3.08 to 1.08); and
> 50 cc = 3.7 versus 3.6 mL/s (MD ‐0.10 mL/s, 95% CI ‐2.49 to 2.29).
Finasteride versus tamsulosin
Total symptom score
Follow‐up ≤ 1 year
(2 trials all outcomes)
Two small trials reported clinically significant improvements (≥ 4 point decrease in the AUASI/IPSS) for finasteride and tamsulosin.
Two small trials (Lee 2002, N = 205; Rigatti 2003, N = 403), with 24‐week and 26‐week endpoints, respectively, compared finasteride to tamsulosin, but without placebo arms. Both trials reported the validated IPSS score. Lee reported improvements of 5.8 (‐30.5%) and 6.9 points (‐34.7%) for finasteride and tamsulosin, respectively. The intercurrent comparison of endpoints was not significant (MD 0.10 points, 95% CI ‐1.91 to 2.11). Rigatti 2003, comparing mean change, reported improvements of 5.7 and 6.3 points, for finasteride and tamsulosin, respectively. Head‐to‐head, the comparison was not significant (MD 0.60 points, 95% CI ‐0.50 to 1.70); nevertheless there were responders (≥ 50% improvement in score) at 26 weeks (35.6% and 42.5% for finasteride and tamsulosin, respectively), with an 6.9% absolute improvement favoring tamsulosin.
Peak urine flow
Two trials, reporting finasteride versus tamsulosin, reported equivalent improvements of about 2 mL/s for peak urine flows.
Lee reported improvements of 2.2 (22.2%) and 2.2 mL/s (23.9%) for finasteride and tamsulosin, respectively, but no difference between arms at endpoint (MD 0.20 mL/s, 95% CI ‐0.84 to 1.24). Rigatti found changes of 1.9 (21.7%) and 2.4 mL/s (30.7%) for finasteride and tamsulosin, respectively, but no significant difference in mean change (MD ‐0.50 mL/s, 95% CI ‐1.59 to 0.59).
Study discontinuations
In the pooled analysis there was a significant difference between arms favoring finasteride (RR 0.77, 95% CI 0.59 to 1.00).
Adverse events/effects
For drug‐related adverse effects, there was no statistically significant difference for finasteride versus tamsulosin.
For 'any adverse event', Rigatti reported no difference between arms (RR 0.92, 95% CI 0.68 to 1.23), as well as for 'withdrawals due to adverse events' (RR 0.66, 95% CI 0.33 to 1.29). For 'patients reporting adverse effects' we pooled the two trials; the comparison was not significant (RR 2.16, 95% CI 0.33 to 14.04), but inexplicably, had significant heterogeneity (I² = 92%). If we drop Lee, a 6 month trial, and keep the 1 year trial (Rigatti), there is still no significant difference (RR 0.92, 95% CI 0.68 to 1.23) between arms. Rigatti also reported 'patients reporting serious adverse events', and found no significant difference between arms (RR 0.96, 95% CI 0.48 to 1.91). Also reported by Rigatti were 'ejaculation disorder' and 'impotence', but with no significant comparison for either (RR 0.32, 95% CI 0.07 to 1.57, and RR 1.12, 95% CI 0.38 to 3.28, respectively). Lee reported 'withdrawals due to adverse effects' and found no difference between arms, although just barely (RR 6.06, 95% CI 0.74 to 49.44).
Quality of life
Both trials reported the IPSS QoL score (range 0 to 6), but Lee reported endpoints (MD 0.30 points, 95% CI ‐0.06 to 0.66) and Rigatti mean differences (0.10, 95% CI ‐0.14 to 0.34). Neither was significant.
Finasteride versus terazosin
Total symptom score
Follow‐up ≤ 1 year
(2 trials all outcomes)
At 1 year, terazosin significantly improved symptom scores versus finasteride, and was clinically significant (≥ 4 point decrease in the AUASI/IPSS) as well.
Lepor 1996 found absolute changes in the AUASI of ‐3.2 and ‐6.1 points for finasteride (n = 310) and terazosin (n = 305), respectively (P < 0.05), but only terazosin was clinically significant (≥ 4 point improvement) at 52 weeks.
BPH progression (need for surgical intervention)
(1 trial)
For terazosin, there was an (non significant) absolute risk reduction of 1% (Lepor 1996) (RD 0.01, 95% CI ‐0.01 to 0.03).
Peak urine flow
(2 trials)
Two trials found terazosin significantly improved flows versus finasteride.
Lepor reported, at all follow‐up visits (2, 4, 13, 26, 39, 52 weeks) peak urine flow for terazosin was significantly higher than finasteride (P ≤ 0.05, with Bonferroni's adjustment). Agrawal 2001, a 6‐month trial comparing endpoints, reported 12.4 and 14.0 mL/s for finasteride and terazosin, respectively. Mean difference was ‐1.60 (95% CI ‐3.09 to ‐0.11) and favored terazosin.
Residual volume
(1 trial)
One trial found 6‐month, equivalent improvements of residual volume for finasteride and terazosin.
Agrawal 2001 found improvements at 6 months of 43.4% and 57.0% for finasteride and terazosin, respectively. However, the endpoint comparison was not significant (MD 2.30 mL, 95% CI ‐5.64 to 10.24).
Prostate volume
(1 trial)
Two trials found contradictory results, but the largest and longest of these found finasteride significantly shrank the prostate relative to terazosin.
Lepor found volumes changed ‐6.1 cm3 and 0.5 cm3 for finasteride (nadir 26 weeks) and terazosin, at 52 weeks, respectively (P ≤ 0.05). Only finasteride decreased mean volumes significantly from baseline. Agrawal reported changes of ‐22.0% and 2.8% for finasteride versus terazosin, respectively, but endpoint comparisons were not significant (MD 1.30, 95% CI ‐6.13 to 8.73).
Nocturia
(1 trial)
For a ≥ 50% reduction in nocturia, terazosin reduced the absolute risk of nocturia by 14%.
Johnson 2003 (Lepor 1996) found decreases ‐ from a baseline of 2.5 nightly episodes ‐ of 0.4 and 0.7 episodes for finasteride (n = 252) and terazosin (n = 226) at 1 year, respectively. The comparison was significant (P = 0.0001). For a man to achieve a ≥ 50% reduction, terazosin had an 14% absolute advantage over finasteride.
Study discontinuations
(1 trial)
Lepor found no significant difference between arms (RR 1.35, 95% CI 0.96 to 1.88).
Adverse events/effects
(2 trials)
For drug‐related adverse effects, finasteride significantly decreased risks for asthenia, postural hypotension, and dizziness versus terazosin.
There was no significant difference for 'withdrawals due to adverse events' (RR 0.89, 95% CI 0.34 to 2.34). For 'ejaculation disorder' (RR 5.90, 95% CI 0.71 to 48.74), 'headache' (RR 1.04, 95% CI 0.56 to 1.94), 'impotence' (RR 1.59, 95% CI 0.90 to 2.79), 'syncope' (RR 0.98, 95% CI 0.20 to 4.84), and 'decreased libido' (RR 1.72, 95% CI 0.73 to 4.05), there was no significant difference. Finasteride was significantly better than terazosin for 'asthenia' (RR 0.54, 95% CI 0.33 to 0.87), 'postural hypotension' (RR 0.28, 95% CI 0.13 to 0.63), and 'dizziness' (RR 0.32, 95% CI 0.21 to 0.49) (Analysis 3.1).
3.1. Analysis.
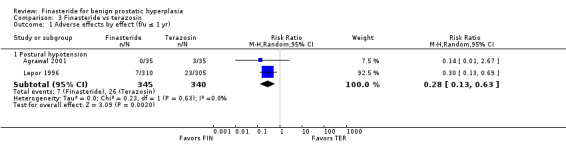
Comparison 3 Finasteride vs terazosin, Outcome 1 Adverse effects by effect (f/u ≤ 1 yr).
Quality of life
(1 trial)
Men reported terazosin significantly improved QoL versus finasteride.
Lepor 1998 (Lepor 1996) reported endpoints for quality of life data using the validated Symptom Problem Index (range 0 to 28) and BPH Impact Score (range 0 to 13) (higher scores mean greater dissatisfaction). Both comparisons favored terazosin (MD 1.90, 95% CI 1.07 to 2.73; MD 0.60, 95% CI 0.32 to 0.88, respectively). Patients reporting "any improvement" were 69.3% and 80.2%, and for "marked or moderate improvement," 44% and 61%, for finasteride and terazosin, respectively.
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
Total symptom score
(1 trial)
For men with small, medium, and large prostates, terazosin significantly improved AUA scores versus finasteride.
Lepor 1998 (Lepor 1996) reported mean changes in the AUA total score by men with baseline prostate sizes of:
≤ 40 cc = MD 2.60 points, 95% CI 1.49 to 3.71;
> 40 cc ≤ 50 cc = MD 4.40 points, 95% CI 1.91 to 6.89; and
> 50 cc = MD 2.40 points, 95% CI 0.04 to 4.76.
Peak urine flow
(1 trial)
For men with small prostates, terazosin significantly improved urine flows versus finasteride.
Lepor 1998 (Lepor 1996) also reported, for finasteride versus terazosin, changes in peak urine flows by the same cut points.
≤ 40 cc = 1.4 versus 2.5 mL/s (MD ‐1.10 mL/s, 95% CI ‐2.08 to ‐0.12)
> 40 cc ≤ 50 cc = 0.9 versus 2.4 mL/s (MD ‐1.50 mL/s, 95% CI ‐3.31 to 0.31)
> 50 cc = 2.7 versus 3.6 mL/s (MD ‐0.90 mL/s, 95% CI ‐3.09 to 1.29)
All comparisons favored terazosin but only the first was statistically significant.
Finasteride versus finasteride + terazosin
Total symptom score
Follow‐up ≤ 1 year
(1 trial all outcomes)
Finasteride + terazosin significantly improved the AUASI versus finasteride alone.
Lepor 1996 reported absolute mean changes in the AUASI of ‐3.2 and ‐6.2 points for finasteride versus combination therapy, respectively (P < 0.001), but only combination therapy was clinically significant (≥ 4 point decrease).
BPH progression (need for surgical intervention)
There was a non‐significant, absolute risk reduction for combination therapy of 1%.
There were 2% (5/243) and 0.8% (2/254) of men with an "absolute indication for surgery" in the finasteride and combination arms, respectively. The comparison was not significant (RD 0.01, 95% CI ‐0.01 to 0.03).
Peak urine flow
Finasteride + terazosin improved flows significantly than finasteride alone.
At all follow‐up cut points (weeks 2, 4, 13, 26, 39, 52) mean flow rates were significantly higher in the combination arm versus finasteride (P < 0.05).
Prostate volume
Finasteride and combination therapy improved prostate volumes equally.
Significant volumes decreases of 6.1 and 7.0 cc were reported in the finasteride and combination arms (nadir 26 weeks for both), respectively. The between‐group comparison was not significant.
Nocturia
Men taking combination therapy significantly experienced ≥ 50% reductions in nocturia versus those taking finasteride alone.
There were decreases of 2.1 and 2.0 episodes per night for finasteride and combination therapy, respectively (Johnson 2003 (Lepor 1996), P = 0.004). Of men with 2 or more episodes at baseline, 25% (52/205) and 32% (63/195) in the finasteride and combination arms, respectively, experienced 50% or greater reductions. The intra group comparison was significant.
Study discontinuations
There was no significant difference between arms (RR 1.21, 95% CI 0.88 to 1.67).
Adverse events/effects
Finasteride, for drug‐related adverse effects, significantly lowered the risk of asthenia, dizziness, ejaculation disorder, and postural hypotension, versus combination therapy.
For 'withdrawals due to adverse events', the comparison was not significant (RR 0.62, 95% CI 0.33 to 1.16). The risk of 'asthenia' (RR 0.53, 95% CI 0.33, 0.86), 'dizziness' (RR 0.39, 95% CI 0.26 to 0.60), 'ejaculation disorder' (RR 0.28, 95% CI 0.12 to 0.70), and 'postural hypotension' (RR 0.26, 95% CI 0.11 to 0.58), was significantly less for finasteride versus combination therapy. There were no significant differences for 'decreased libido' (RR 0.93 [0.46, 1.89), headache (RR 1.18, 95% CI 0.62 to 2.26), 'impotence' (RR 1.00, 95% CI 0.61 to 1.63), and 'syncope' (RR 0.60, 95% CI 0.14 to 2.48).
Quality of life
Combination therapy significantly improved QoL versus finasteride alone.
Both arms experienced improvements (mean change ‐1.7 versus ‐4.2 points for finasteride and combination therapy, respectively) of the validated Symptom Problem Index (range 0 to 28), but the comparison of endpoints favored combination therapy (MD 2.20, 95% CI 1.37 to 3.03). The endpoint comparison for the BPH Impact Score also favored combination therapy (MD 1.00, 95% CI 0.72 to 1.28).
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
Total symptom score
Combination therapy significantly improved symptom scores for men with small, medium, and large prostates, versus finasteride alone.
Lepor 1998 (Lepor 1996) reported AUA mean changes, for finasteride and combination therapy, for men with baseline prostates of:
≤ 40 cc = ‐3.2 versus ‐5.8 points, respectively (MD 2.60 points, 95% CI 1.35 to 3.85);
> 40 cc ≤ 50 cc = ‐2.1 versus ‐7.1 points, respectively (MD 5.00 points, 95% CI 2.51 to 7.49); and
> 50 cc = ‐3.6 versus ‐7.0 points, respectively (MD 3.40 points, 95% CI 0.91 to 5.89).
Peak urine flow
Combination therapy significantly improved peak urine flows for men with small and medium‐sized prostates, versus finasteride monotherapy.
Lepor 1998 (Lepor 1996) also reported improvements in peak urine flows, for finasteride versus combination therapy, by the same cut points:
≤ 40 cc = 1.4 versus 3.1 mL/s, respectively (MD ‐1.70 mL/s, 95% CI ‐2.68 to ‐0.72);
> 40 cc ≤ 50 cc = 0.9 versus 3.4 mL/s, respectively (MD ‐2.50 mL/s, 95% CI ‐4.46 to ‐0.54); and
> 50 cc = 2.7 versus 3.7 mL/s, respectively (MD ‐1.00 mL/s, 95% CI ‐2.69 to 0.69).
All comparisons favored combination therapy but only the first two were significant.
Finasteride versus Permixon®
Permixon® is the proprietary name of an extract from the Serenoa repens (Saw palmetto) berry. Its distinction from other Serenoa repens phytotherapeutic extracts is the way it is manufactured: Permixon® uses a hexane extraction method; many others use either ethanol or CO₂.
Total symptom score
Follow‐up ≤ 1 year
(1 trial all outcomes)
At half year, one trial found clinically significant (≥ 4 point decrease in the AUASI/IPSS), and nearly equivalent, improvements, for finasteride and Permixon®.
Carraro 1996 (N = 1098) reported clinically significant improvements of 6.2 (39%) and 5.8 points (37%) of the IPSS for finasteride and Permixon®, respectively, at 6 month follow‐up. The inter group comparison of endpoints was not significant (MD ‐0.40, 95% CI ‐1.04 to 0.24).
BPH progression (need for surgical intervention)
The absolute risk difference was 0% (RD ‐0.00, 95% CI ‐0.01 to 0.00).
Peak urine flow
Finasteride and Permixon® improved peak urine flows equally.
At 6 months flow measures improved 30% and 25% for finasteride and Permixon®, respectively. There was no difference in the endpoint comparison (MD 0.70, 95% CI ‐0.14 to 1.54).
Prostate volume
Prostate volume was significantly smaller for finasteride than Permixon® (MD ‐4.80, 95% CI ‐8.18 to ‐1.42).
Nocturia
There was no difference in the comparison at endpoint (MD ‐0.05, 95% CI ‐0.49 to 0.39).
Study discontinuations
Finasteride significantly lowered the risk of dropping out of the trial versus Permixon® .
There were 61/545 (11.2%) in the finasteride arm, and 86/553 (15.6%) in the Permixon® arm. The comparison just reached significance (RR 0.72, 95% CI 0.53 to 0.98).
Adverse events/effects
For drug‐related adverse effects ‐ decreased libido and impotence ‐ there was no significant difference between finasteride versus Permixon®.
For adverse effects mostly associated with finasteride, there was no difference for 'decreased libido' (RR 1.36, 95% CI 0.65 to 2.84) or 'impotence' (RR 1.91, 95% CI 0.81 to 4.46) versus Permixon®, and for 'withdrawals due to adverse events', the comparison favored finasteride (RR 0.51, 95% CI 0.27 to 0.95).
Quality of life
For the IPSS QoL, both finasteride and Permixon® experienced nearly equal improvements; however, in the Sexual Function Score, Permixon® significantly improved versus finasteride.
In the IPSS QoL (range 0 to 6), 73% and 69% of men in the finasteride and Permixon® arms, respectively, reported their quality of life had improved (≥ 1 point decrease) by the end of 26 weeks of follow‐up. The inter group comparison was not significant. In the Sexual Function Score (range 0 to 20), finasteride experienced a significant deterioration (9% increase) versus Permixon® (6% decrease). The intercurrent comparison was significant at 6 and 26 weeks, and favored Permixon®.
Finasteride versus PRO 160/120
PRO 160/120, a phytotherapy otherwise known as Prostagutt® forte, is a combination of Serenoa repens and (160 mg) and Urtica dioica extracts (120 mg). Urtica dioica is a herbaceous, flowering perennial found on most of Earth. It is also known as the stinging nettle.
Total symptom score
Follow‐up ≤ 1 year
(1 trial all outcomes)
Both finasteride and PRO 160/120 improved symptom scores clinically.
Sökeland 2000 (N = 516) reported clinically significant changes from baseline of ‐5.6 and ‐4.8 points of the IPSS for finasteride and PRO 160/120, respectively, at 48 weeks. The inter group comparison was not significant (MD ‐0.05, 95% CI ‐0.24 to 0.13).
Peak urine flow
Finasteride and PRO 160/120 improved flows to about 2 mL/s.
Both arms improved flows 2.4 and 1.9 mL/s for finasteride and PRO 160/120, respectively, but were not significantly different (MD 0.50 mL/s, 95% CI ‐0.58 to 1.58).
Prostate volume
Volumes improved 6.8 and 0.4 mL at 48 weeks for finasteride versus PRO 160/120, respectively.
Study discontinuations
Discontinuations were not statistically significant (RR 0.86, 95% CI 0.39 to 1.88).
Adverse events/effects
Finasteride increased risk of adverse events versus PRO 160/120.
More men in the finasteride arm (41.4%, 96/232) than in the PRO 160/120 (31.8%, 74/233) suffered adverse events. The comparison favored PRO 160/120 and just reached significance (RR 1.30, 95% CI 1.02 to 1.66).
Subgroup analysis: prostate size (< 40 cc versus ≥ 40 cc)
Total symptom score
For men with small and large prostates at baseline and taking finasteride versus PRO 160/120, respectively, endpoint symptom scores were not statistically different.
For men with prostates ≤ 40 mL (n = 227), Sökeland 2000 reported IPSS mean endpoints of 6.3 and 7.0 points for finasteride versus PRO 160/120, respectively, but were not significantly different (MD ‐0.70, 95% CI ‐2.21 to 0.81). For men with prostates > 40 mL (n = 202), endpoints were 5.6 and 6.3 points, and not significantly different as well (MD ‐0.70, 95% CI ‐1.96 to 0.56). Intracurrent comparisons for finasteride and PRO 160/120 were not significant (MD 0.70, 95% CI ‐0.55 to 1.95, and MD 0.70, 95% CI ‐0.82 to 2.22, respectively).
Peak urine flow
For men with small and large prostates at baseline, peak urine flows improved equally for those taking finasteride versus PRO 160/120.
For men with prostates ≤ 40 mL (n = 215), respective urine flows improved 2.7 and 1.6 mL/s for finasteride and PRO 160/120, but were not significantly different (MD 1.10, 95% CI ‐0.69 to 2.89). For men with prostates > 40 mL (n = 193), flows improved 2.7 and 2.1 mL/s, respectively. The comparison was not significant (MD 1.10, 95% CI ‐0.69 to 2.89). In the finasteride arm at 48 weeks, men with prostates ≤ 40 mL and > 40 mL had mean changes of 2.7 mL and 2.7 mL, respectively (MD 0.00, 95% CI ‐1.78 to 1.78). At 48 weeks in the PRO 160/120 arm, men with prostates ≤ 40 mL and > 40 mL had improved flows of 1.6 and 2.1 mL, respectively. The comparison was not significant (MD ‐0.50, 95% CI ‐2.25 to 1.25).
Finasteride versus allylestrenol
Follow‐up ≤ 1 year
(1 trial all outcomes)
BPH progression (need for surgical intervention)
The absolute risk difference was 0% (RD 0.00, 95% CI ‐0.11 to 0.11).
Peak urine flow
Both finasteride and allylestrenol reported per cent improvements, but the difference was not statistically significant.
Agrawal 2001 found improvements, at 6 months, of 77% and 60.1% for finasteride and allylestrenol, respectively. The comparison of endpoints was not significant (MD ‐1.10, 95% CI ‐2.48 to 0.28).
Residual volume
Both arms increased residual volumes, but the comparison was not significant.
Residual volumes improved 43.4% and 61.1% for finasteride and allylestrenol, respectively. The endpoint comparison was not significant (MD 3.30, 95% CI ‐4.93 to 11.53).
Prostate volume
Volume improvements were 22.0% and 23.5% for finasteride and allylestrenol, respectively. The endpoint comparison was not significant (MD 2.30, 95% CI ‐3.43 to 8.03).
Adverse effects/events
For 'decreased libido', the comparison was not significant (RR 0.14, 95% CI 0.01 to 2.67).
Discussion
Summary of main results
This review analyzed 23 trials and 21,945 men. Follow‐up ranged from 6 to 48 months. Ninety‐one per cent of the trials (21/23) were described as blinded. Two trials were unequivocally single blinded, with three others probably single blinded. Fourteen trials were described as double blinded, but with no other description. Only two trials described patients and investigators as being blinded. Two placebo‐controlled trials did not mention blinding at all. Allocation concealment was adequate in 26% (6/23) and uncertain in 74% (17/23) of included trials.
Finasteride versus placebo
At endpoints of < 1 year or less, there is little evidence that finasteride improved symptom scores versus placebo, although this may be partially attributable to a progressive, long‐term placebo effect (Rief 2002). Of 4 trials with endpoints from 2 to 4 years, only 1 trial showed finasteride improved scores clinically, although all 4 found finasteride significantly improved scores versus placebo. Finasteride also decreased, at endpoints > 1 year, the absolute risk of progression (≥ 4 point increase), as well as acute urinary retention, and the absolute risk of surgery. For men with large prostates (> 40 cc), finasteride significantly improved symptom scores versus men taking finasteride and with small (≤ 40 cc) prostates. For men with small (< 25 mL), medium (25 to < 40 mL), and large (≥ 40 mL) prostates, finasteride modestly decreased the absolute risk of progression (≥ 4 point increase). At 4 years, versus placebo, finasteride significantly lowered risks of acute urinary retention and surgical intervention, or both, for younger men (45 to < 65 years) and older men (≥ 65 years); for men taking finasteride, older men had a greater risk of progression then did younger men. Comparing short‐term (6 to 12 months) to long‐term (> 1 year) therapy, MTOPS reported no difference in median improvements of the AUASI at 1 year, but a median significant difference at 4 years. Drug‐related adverse effects for finasteride were rare; nevertheless, men taking finasteride were at increased risk for impotence, erectile dysfunction, decreased libido, and ejaculation disorder, versus placebo.
Finasteride versus doxazosin
The alpha‐blocker doxazosin improved symptom scores significantly versus finasteride at 1 and 4‐year endpoints. Both doxazosin and finasteride improved scores clinically at both endpoints. At 1 year there was no significant difference in absolute risk of acute urinary retention and surgical intervention. Significantly, at 4 years, finasteride lowered the absolute risk of surgical intervention by 2%, but there was a non‐significant absolute risk of progression (≥ 4 point increase). For men with small prostates and taking finasteride, there was a small absolute risk increase of progression. For men in the medium and large groups and taking doxazosin, there was a small absolute risk increase of progression. Drug‐related adverse effects were rare; none were greater than 5 per 100 person‐years of follow‐up. Finasteride had higher rates of erectile dysfunction, decreased libido, and abnormal ejaculation, whilst doxazosin had higher rates of dizziness, postural hypotension, and asthenia.
Finasteride versus tamsulosin
Two small, 6 month trials reported clinically significant improvements (≥ 4 point decrease in the AUASI/IPSS) for both finasteride and tamsulosin, but no difference intercurrently. Drug‐related adverse effects were rare and not significantly different.
Finasteride versus terazosin
At 1 year, terazosin significantly improved symptom scores versus finasteride, and was clinically significant as well (≥ 4 point decrease in the AUASI/IPSS). There was no significant difference in absolute risk of surgical intervention. Terazosin, for men with small (≤ 40 mL), medium (> 40 to ≤ 50 mL) and large (> 50 mL) prostates, significantly reduced AUA total scores versus finasteride. For men with small prostates, terazosin significantly improved peak urine flows versus finasteride. Finasteride significantly reduced drug‐related adverse effects (asthenia, postural hypotension, dizziness) versus terazosin.
Finasteride + doxazosin versus finasteride
At 1 year, there was no difference in absolute risk of acute urinary retention and (risk of) surgical intervention. For drug‐related adverse effects, men taking combination therapy significantly increased their risk of asthenia, dizziness, and impotence, versus monotherapy, and for men taking finasteride alone, the risk of lowered libido increased versus combination therapy.
At 4 years, combination therapy significantly improved symptom scores versus finasteride alone; improvements in both arms were clinically significant as well (≥ 4 point decrease in the AUASI/IPSS). Combination therapy also reduced the absolute risk of progression (≥ 4 point increase) by 4%, but the absolute risk of surgery was 0%. For men with prostates of < 25 mL, 25 to < 40 mL, and ≥ 40 mL, combination therapy significantly improved symptom scores versus finasteride alone. For men with medium and large prostates, combination therapy significantly decreased the risk of progression (≥ 4 point increase) versus finasteride. Drug‐related effects were rare, with no more than 5.4 (dizziness) per 100 person‐years of follow‐up. Combination therapy was associated with higher rates of asthenia, decreased libido, dizziness, erectile dysfunction, ejaculation disorder, and postural hypotension, versus finasteride monotherapy. For men with medium and large prostates, finasteride significantly increased the absolute risk of progression (≥ 4 point increase) by 1.18% and 1.90%, respectively.
Finasteride + doxazosin versus doxazosin
At 1 year follow‐up, combination therapy and doxazosin alone improved scores clinically; the intercurrent comparison was not significant.
At 4 years, both monotherapy and combination therapy improved scores clinically. Combination therapy decreased the absolute risk of progression (≥ 4 point increase) by 4%. Doxazosin alone had fewer incidents of acute urinary retention (∼0.5%) than combination therapy (∼2.0%) at a mean follow‐up of 4.5 years. Doxazosin decreased the absolute risk for surgical intervention by 1%. For men with medium (25 mL to < 40 mL) and large (≥ 40 mL) prostates at baseline, combination therapy significantly improved symptom scores, lowered the risk of surgical intervention, and decreased the risk of progression (≥ 4 point increase), versus doxazosin alone. Drug‐related adverse effects were greater in the combination arm for asthenia, decreased libido, dizziness, erectile dysfunction, ejaculation disorder, and postural hypotension, versus doxazosin alone. Statistical significances were not given.
Finasteride + terazosin versus finasteride
Combination therapy significantly improved symptoms versus monotherapy, and was clinically significant (≥ 4 point decrease in the AUASI/IPSS) as well. For drug‐related effects, finasteride significantly lowered the risk of asthenia, dizziness, ejaculation disorder, and postural hypotension, versus combination therapy.
Finasteride + terazosin versus terazosin
Both finasteride + terazosin and terazosin monotherapy improved symptom scores clinically, but were not significantly different. For drug‐related harms, combination therapy significantly reduced the risk of ejaculation disorder versus terazosin alone.
Overall completeness and applicability of evidence
There are sufficient trials comparing finasteride to placebo and doxazosin. What is lacking is a tamsulosin trial with a placebo arm that is sufficiently powered and of at least a year's duration. Also lacking are phytotherapeutic comparators (e.g., Permixon®, PRO 160/120) with placebo arms. The standard prescribed dose of finasteride is 5 mg. Another dose comparison trial, with a longer follow‐up (> 1 year), a placebo arm, and utilizing the IPSS/AUASI, would be helpful in confirming, or denying, Gormley's finding of little or no efficacy for 1 mg finasteride. Another missing comparison is dutasteride.
Current clinical practice of prescribing 5 mg finasteride is validated by the evidence presented here: finasteride is clinically efficacious and has few sexual adverse effects that dissipate past 1 year follow‐up.
Quality of the evidence
In general, the included trials adequately addressed the PICO criteria (patients, intervention, comparison, outcomes) (Richardson 1995), which analyzed the constituents of a well‐focused, clinical question. The men of the included trials were symptomatic for LUTS and were about 60 years old, an age when about half of men have histologic evidence of BPH. The usual dose of 5 mg of finasteride was used in all but 3 trials. Presumably this was because 1 mg was not thought to be clinically efficacious. The data for this conclusion is inconclusive: two trials compared 1 mg to 5 mg finasteride and placebo (and used validated and non‐validated scores, respectively) and found no significant difference to placebo at 1 year; however, there was a persistent placebo effect for both trials. A longer trial with 1 mg would put this to rest. The interventions compared finasteride to placebo, alpha‐blockers, and combination therapies (alpha‐blockers + finasteride). The only missing comparator was dutasteride, another commonly used 5ARI. Most trials (70%) used validated symptom scores, which was useful for reporting our primary clinical endpoints. These were generally well designed and adequately powered trials, although with the caveat that allocation concealment (70%) and blinding (87%) were not adequately described.
Potential biases in the review process
There were three main realms of bias in this review: most of the trials ‐ 75% ‐ were industry funded; although 91% of the trials were blinded, the overwhelming majority did not describe who were blinded (assessors, subjects, providers). A third bias was the use of English‐only trials, which sprang from a lack of resources and the deficiencies of the main author.
Agreements and disagreements with other studies or reviews
Boyle (Boyle 1996) and we agree that, for men with large baseline prostates (≥ 60 cc and ≥ 40 cc, respectively) finasteride significantly improved symptom scores versus placebo. Boyle reported a significant difference in peak urine flows for men with baseline prostates of ≥ 60 cc and favoring finasteride versus placebo; we found the same, but with baseline subgroups of ≥ 40 cc and > 50 cc. Byrnes and we also found a significant difference favoring placebo for 'decreased libido', and 'ejaculation disorder'. Roehrborn (Roehrborn 1998) found a positive correlation between symptom scores and peak urine flows (i.e., improved symptom scores correlated with improved urine flows), a finding we confirmed. Edwards found greater improvements for finasteride for symptom scores, peak urine flows, and prostate volume, versus placebo. We found greater improvements ‐ and significant differences ‐ for finasteride versus placebo for urine flows and prostate volume, but no difference for symptom scores. Edwards (Edwards 2002) wrote "Significantly more sexual dysfunction, impotence, ejaculation disorder and decreased libido occurred with finasteride at 12 months," results which we confirmed as well. In a sensitivity analysis, she found finasteride efficacious regardless of baseline prostate size. Our evidence was mixed. In one trial we found finasteride significantly improved symptom scores versus placebo for men with large prostates (> 40 cc). In another, comparing progression (≥ 4 points), and with baseline prostates sizes of < 25 mL, 25 to < 40 mL, and ≥ 40 mL, we found no significant mean differences between finasteride and placebo. We also found, in 2 trials, a significant difference in symptom scores favoring men taking finasteride and with large prostates (> 50 cc and ≥ 40 cc, respectively) versus men taking finasteride or placebo and with small (≤ 40 cc) prostates. And in another trial, in men with large prostates (> 50 cc), finasteride significantly improved peak urine flows versus placebo.
Boyle 1996, a Bayesian meta‐analysis of individual patient data from five of our included trials (Andersen 1995; Finasteride Study Group; Gormley 1992; Lepor 1996; Nickel 1996), as well as other data from Merck Research Labs, presented outcomes (peak urine flow, quasi IPSS) for baseline prostate volume cut points of < 20 cc, 20 to 29 cc, 30 to 39 cc, 40 to 49 cc, 50 to 59 cc, and ≥ 60 cc. By conflating the Boyarsky, IPSS/AUASI, and changing the scale (0 to 30), Boyle created a quasi IPSS. The review found improvements of 1.80, 1.64, 2.32, 2.52, 2.55, and 2.82 points, respectively, for each cut point, but only the last (≥ 60 cc) was significant and favored finasteride. His findings generally mirror our own: Lepor 1998 (Lepor 1996) reported improvements in the AUASI of 3.2, 2.1, and 3.6 points for the baseline prostate cut points ≤ 40 cc, > 40 cc ≤ 50 cc, and > 50 cc, but none were statistically significant versus placebo. Marberger, on the other hand, found placebo‐adjusted, significant mean improvements of 1.4 and ∼3.0 points (Boyarsky I), for small (< 40 cc) and large (≥ 40 cc) prostates, respectively (P = 0.053).
For peak urine flow Boyle reported changes from baseline of 0.89, 1.32, 1.53, 1.19, 1.39, and 1.84 cc/s (cubic centimetres per second) for baseline prostate sizes < 20 cc, 20 to 29 cc, 30 to 39 cc, 40 to 49 cc, 50 to 59 cc, and ≥ 60 cc, respectively, but only the last (≥ 60 cc) was significant and favored finasteride. We reported, for men with prostates > 50 cc, a significant comparison favoring finasteride as well (Lepor 1998 (Lepor 1996)). Similarly, the included trial Abrams 1999 (N = 121), with a 1 year follow‐up, found no significant difference between finasteride and placebo for men with prostates < 40 cc (MD 0.7 mL/s, 95% CI 0.6 to 2.0), but a significant difference (MD 1.6 mL/s, 95% CI 0.2 to 3.0) for men with prostates ≥ 40 cc. And in Lepor 1998 (Lepor 1996) improvements for finasteride were recorded of 1.4, 0.9, and 2.7 mL/s for baseline prostate sizes of ≤ 40 cc, > 40 cc ≤ 50 cc, and > 50 cc, respectively. Only in men with prostates > 50 cc was finasteride significantly better than placebo (P = 0.001).
Byrnes 1997, which analyzed two included trials (Byrnes 1995; Tenover 1997) using data unavailable to us, reported outcomes for the AUASI and BII (a validated symptom score), for subgroups of men aged < 65 years and ≥ 65 years. In younger men, AUASI baseline mean scores were 19.2 and 18.7, for finasteride and placebo, respectively; in older men, they were 18.4 and 18.0 years. At end of follow‐up ‐ 1 year ‐ , the review reported comparable adjusted mean changes (‐5.12 and ‐4.43 points) for the finasteride arms in the small and large groups, respectively. Intercurrent comparisons for both groups were significantly different (P < 0.01).
Overall, for the BII (a validated, QoL score), Byrnes, in the systematic review, as well in her trial, reported a significant difference (P = 0.046) between point estimates that favored finasteride. In our meta‐analysis of Byrnes and Tenover, we found no significant difference between finasteride and placebo (Analysis 1.18). Using data unavailable to us, Byrnes reported a significant statistical difference favoring finasteride in younger men, but not for older men.
1.18. Analysis.

Comparison 1 Finasteride vs placebo, Outcome 18 QoL (BII ‐ points) WMD (f/u ≤ 1 yr).
Byrnes also analyzed adverse effects/events. Overall, she found no difference between arms, which agrees with our analysis for 'any adverse events' (Analysis 1.6) and 'patients reporting serious adverse events' (Analysis 1.8). She, as well as we, found significant differences favoring the placebo arm for drug‐related adverse effects ('any adverse effects') (Analysis 1.9), 'decreased libido', and 'ejaculation disorder' (Analysis 1.15). Byrnes found a significant difference ‐ we did not ‐ favoring placebo for 'withdrawals due to adverse effects' (Analysis 1.10).
Roehrborn 1998 meta‐analyzed the included trials Gormley, the Finasteride Study Group, Andersen, Nickel, Lepor, as well as Bonilla 1997, a trial that compared intra‐ and interobserver prostate volume measurements. All were of at least a year duration. The review presented a positive correlation between point estimates and mean prostate sizes across 6 trials for the quasi IPSS and peak urine flow, a conclusion we confirmed (for peak urine flow) in Abrams 1999 (N = 121), and partially confirmed, in Lepor 1998 (Lepor 1996).
Edwards 2002 analyzed 15 included trials and 2 that compared finasteride and devices (urethral stent, balloon dilation) to placebo (plus devices). Edward's wrote "Over 48 months finasteride produced greater improvements in total symptom score, maximum urinary flow rate, and prostate volume. Significantly more sexual dysfunction, impotence, ejaculation disorder and decreased libido occurred with finasteride at 12 months . . . Significantly fewer men treated with finasteride experienced acute retention or had surgery at 24 or 48 months than with placebo." We confirmed her conclusions. She also wrote "Sensitivity analyses showed benefit with finasteride 5 mg to be constant irrespective of the initial prostate volume." In a trial she did not use ‐ Abrams ‐ , our analysis of subgroups did find significant efficacy (peak urine flow) favoring finasteride versus placebo in men with prostates ≥ 40 cc, and in Lepor 1998 (Lepor 1996), in men with prostates > 50 cc, those taking finasteride had significantly improved flow rates (2.7 and 0.6 mL/s, respectively) than those taking placebo.
Authors' conclusions
Implications for practice.
Finasteride provides moderate relief of symptoms, especially after 1 year follow‐up, with few adverse effects that dissipate over time. Finasteride decreases the absolute risk of progression (≥ 4 point increase) and acute urinary retention, but increases the absolute risk of surgery. For men with large prostates (> 40 cc), finasteride significantly improves symptom scores versus men taking finasteride and with small (≤ 40 cc) prostates. At 4 years, versus placebo, finasteride significantly decreases the risks of acute urinary retention and surgical intervention, or both, for younger men (45 to < 65 years) and older men (≥ 65 years); for men taking finasteride, older men have a greater risk of progression then do younger men. Comparing short‐term (6 to 12 months) to long‐term (> 1 year) therapy, there was no difference in median improvements of the AUASI at 1 year, but a median difference of 1.0 point favoring finasteride, at 4 years. Drug‐related adverse effects for finasteride are rare; nevertheless, men taking finasteride are at increased risk for impotence, erectile dysfunction, decreased libido, and ejaculation disorder, versus placebo.
The alpha‐blocker doxazosin significantly improves symptom scores versus finasteride at 1 and 4‐year endpoints. Both doxazosin and finasteride improves scores clinically at both endpoints. Significantly, at 4 years, finasteride lowers the absolute risk of surgical intervention. For men with small prostates (< 25 mL) and taking finasteride, there is a small absolute risk increase of progression (≥ 4 point increase). For men in the medium (25 to < 40 mL) and large (≥ 40 mL) groups and taking doxazosin, there is a small absolute risk increase of progression. Drug‐related adverse effects are rare, although finasteride has higher rates of erectile dysfunction, decreased libido, and abnormal ejaculation, whilst doxazosin had higher rates of dizziness, postural hypotension, and asthenia.
Both finasteride and tamsulosin improved IPSS scores equally, as well as clinically. Drug‐related adverse effects are rare for both and not significantly different.
Terazosin significantly improves AUA scores versus finasteride, and is clinically significant (≥ 4 point decrease in the AUASI/IPSS) as well. Terazosin, for men with small (≤ 40 mL), medium (> 40 to ≤ 50 mL) and large (> 50 mL) prostates, significantly reduces symptom scores versus finasteride. For men with small prostates (≤ 40 mL), terazosin significantly improves peak urine flows versus finasteride. Finasteride significantly reduces drug‐related adverse effects (asthenia, postural hypotension, dizziness) versus terazosin.
Combination therapy (finasteride + doxazosin) significantly improves symptom scores versus finasteride alone. Combination therapy also reduces the absolute risk of progression (≥ 4 point increase) by 4%. For men with prostates of < 25 mL, 25 to < 40 mL, and ≥ 40 mL, combination therapy significantly improves symptom scores versus finasteride alone. For men with medium and large prostates, combination therapy significantly decreases the risk of progression (≥ 4 point increase) versus finasteride. Drug‐related effects are rare, although combination therapy is associated with higher rates of asthenia, decreased libido, dizziness, erectile dysfunction, ejaculation disorder, and postural hypotension, versus finasteride monotherapy. For men with baseline prostates of < 25 mL, 25 to < 40 mL, ≥ 40 mL, combination therapy significantly improves symptom scores for all groups, versus finasteride alone. For men with medium and large prostates, finasteride significantly increases the absolute risk of progression (≥ 4 point increase) by 1.18% and 1.90%, respectively.
Finasteride + doxazosin significantly improve symptom scores versus doxazosin monotherapy. Both monotherapy and combination therapy improve scores clinically as well. Combination therapy decreases the absolute risk of progression (≥ 4 point increase) by 4%. Doxazosin decreases the absolute risk of surgical intervention by 1%. For men with medium (25 mL to < 40 mL) and large (≥ 40 mL) prostates at baseline, combination therapy significantly improves symptom scores, lowers the risk of surgical intervention, and decreases the risk of progression (≥ 4 point increase), versus doxazosin alone. Drug‐related adverse effects are greater in the combination arm for asthenia, decreased libido, dizziness, erectile dysfunction, ejaculation disorder, and postural hypotension, versus doxazosin alone.
Finasteride + terazosin significantly improve the AUASI versus finasteride alone. For drug‐related effects, finasteride significantly lowers the risk of asthenia, dizziness, ejaculation disorder, and postural hypotension, versus combination therapy.
Implications for research.
Evidence of symptom‐score efficacy for finasteride, especially at endpoints ≤ 1 year, is somewhat equivocal, especially with the persistence of a long‐term placebo effect. Stating that, it may be impossible to answer definitively. The comparative effectiveness of finasteride versus alpha blockers is fairly well established, with the exception of long‐term, placebo‐controlled, tamsulosin and terazosin trials. Also needed is a high quality, comparative effectiveness trial with dutasteride.
Feedback
Herxheimer, 3 March 2013
Summary
This huge and heroic review does not address some crucial questions related to adverse effects (AEs). A recent review from the Institute of Safe Medication Practices (ISMP) alerted me to the problem (*).
Nothing is said on how AEs were elicited or ascertained in each trial, or how the trial reports described them. For example, were participants asked specific or open questions at particular time points, and what questions? Or were they merely invited to report a problem when it arose? These approaches will determine the types and number of reports, giving widely differing results. Could the current methods of assessing bias detect this?
The review mentions 'serious' AEs. How did the included trial reports use and define 'serious', and how did the review team define it? Were any included patients asked whether they considered an AE serious, or was this the clinician's opinion?
Do some AEs wane and disappear when finasteride is stopped, whereas others persist? Sexual function matters greatly to many men – what should they be told about this?
*ISMP 2012. Finasteride and possibly persistent sexual side effects. Quarterwatch 2012Q2: 7‐9.
Reply
Thank you, Dr Herxheimer, for your remarks.
We will respond to your comments in order.
-
The reporting of adverse events in the RCTs was quite varied.
Adverse events were recorded during follow‐up for four studies (Kirby 2003; Lepor 1996; Marberger 1998; Tenover 1997), but it was not always possible to tell if providers elicited the information or if they relied on patients to report them (Kirby 2003).
Wessells 2003, a secondary report of the included trial, McConnell 1998, reported that providers elicited sexual dysfunction information from participants at a screening visit, but relied on spontaneous self‐report during the treatment period.
Lepor 1996 reported men were interviewed at each visit about adverse effects, but not whether that included a baseline assessment. A better narrative description of AEs could have highlighted the inherent biases of these studies, but a bespoke assessment tool for detecting reporting bias of AEs would have been better.
We did not, a priori, define 'serious AEs', although it would have been helpful if we had. Instead, we relied on reporting in the trials, which generally were not defined in the trials.
Yes, incidents of sexual AEs fluctuate over time (Stoner 1992b). A new review with expanded scope on the topic of 5‐alpha reductase inhibitors for lower urinary tract symptoms secondary to benign prostatic obstruction (García‐Perdomo 2015), which will replace this review, will include a discussion on this important point.
Contributors
Feedback: Andrew Herxheimer
Response: James Tacklind, Howard A Fink, Roderick MacDonald, Indy Rutks, Timothy J Wilt
What's new
| Date | Event | Description |
|---|---|---|
| 7 December 2015 | Feedback has been incorporated | Feedback and review authors' response added. |
| 7 December 2015 | Amended | Published note added. Contact person's contact details updated and author affiliations aligned. Minor formatting changes made to headings in the Results section 'Effects of interventions' and to headings in Tables 2 to 15. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 10 January 2012 | Amended | Added "Editorial support in part was funded by Grant no. 5R01DK63300‐4" under 'Acknowledgements'. |
| 17 December 2010 | Amended | In the protocol under 'Types of interventions', we changed the sentence "Finasteride in comparison to placebo, active pharmacologic controls, phytotherapy, surgery (TURP), and minimally invasive interventions (e.g., TUNA and TUMT)." to " Finasteride in comparison to placebo, active pharmacologic controls, and phytotherapies." |
| 3 April 2009 | Amended | This review has been abandoned by authors Tello Royloa C, Cao Avellandeda E, Lopez Cubillana P, and Rigabert Montiel M. It will be finished by Tacklind J, Fink H, MacDonald R, Rutks I, and Wilt TJ. There were no changes to the scope of the review. |
| 8 November 2008 | Amended | Converted to new review format. |
Notes
This review will be replaced by a new review with expanded scope on the topic of 5‐alpha reductase inhibitors for lower urinary tract symptoms secondary to benign prostatic obstruction.
Acknowledgements
Editorial support in part was funded by Grant no. 5R01DK63300‐4.
Data and analyses
Comparison 1. Finasteride vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total symptom score (points) at endpoint (f/u ≤ 1 yr) | 2 | 1089 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.31, ‐0.07] |
| 2 BPH progression (acute urinary retention) (f/u ≤ 1 yr) | 4 | 4048 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 3 BPH progression (acute urinary retention) (f/u > 1 yr) | 2 | 5918 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.00] |
| 4 BPH progression (need for surgical intervention) (f/u ≤ 1 yr) | 7 | 6583 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 5 BPH progression (need for surgical intervention) (f/u > 1 yr) | 4 | 8038 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.00] |
| 6 Any adverse event (f/u ≤ 1 yr) | 4 | 5556 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.94, 1.11] |
| 7 Withdrawals due to adverse events (f/u ≤ 1 yr) | 5 | 5521 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.92, 1.45] |
| 8 Patients reporting serious adverse events (f/u ≤ 1 yr) | 2 | 4759 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.14] |
| 9 Any adverse effects (f/u ≤ 1 yr) | 2 | 4759 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.28, 1.85] |
| 10 Withdrawals due to adverse effects (f/u ≤ 1 yr) | 4 | 5857 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.89, 1.93] |
| 11 Patients reporting serious adverse effects (f/u ≤ 1 yr) | 2 | 2589 | Risk Ratio (M‐H, Random, 95% CI) | 5.42 [1.00, 29.40] |
| 12 Patients reporting sexual adverse effects (f/u ≤ 1 yr) | 4 | 6271 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.75, 2.44] |
| 13 Any adverse event (f/u > 1 yr) | 2 | 3781 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.94, 1.04] |
| 14 Withdrawals due to adverse events (f/u > 1 yr) | 2 | 3747 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.91, 1.31] |
| 15 Adverse effects by effect | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Decreased libido ‐ f/u ≤ 1 yr | 5 | 3782 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.40, 3.23] |
| 15.2 Ejaculation disorder ‐ f/u ≤ 1 yr | 5 | 4700 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [1.79, 4.56] |
| 15.3 Ejaculation disorder ‐ f/u > 1 yr | 2 | 6208 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [1.65, 6.40] |
| 15.4 Impotence ‐ f/u ≤ 1 yr | 6 | 4278 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.38, 2.97] |
| 15.5 Impotence ‐ f/u > 1 yr | 3 | 4396 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.26, 2.68] |
| 16 Peak urine flow (mL/s) at endpoint (f/u ≤ 1 yr) | 4 | 1195 | Mean Difference (IV, Random, 95% CI) | 0.88 [0.20, 1.57] |
| 17 Peak urine flow (mL/s) WMD (f/u ≤ 1 yr) | 2 | 598 | Mean Difference (IV, Random, 95% CI) | 0.77 [0.09, 1.46] |
| 18 QoL (BII ‐ points) WMD (f/u ≤ 1 yr) | 2 | 3890 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.04, 0.25] |
| 19 Study discontinuations (f/u ≤ 1 yr) | 11 | 7523 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.93, 1.15] |
| 20 Study discontinuations (f/u > 1 yr) | 4 | 7262 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.80, 0.94] |
1.2. Analysis.
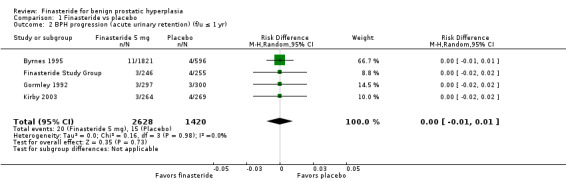
Comparison 1 Finasteride vs placebo, Outcome 2 BPH progression (acute urinary retention) (f/u ≤ 1 yr).
1.17. Analysis.
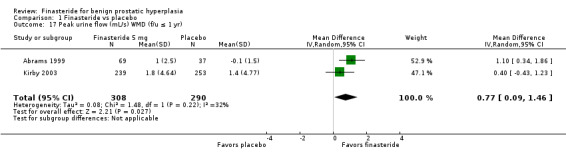
Comparison 1 Finasteride vs placebo, Outcome 17 Peak urine flow (mL/s) WMD (f/u ≤ 1 yr).
Comparison 2. Finasteride vs tamsulosin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Study discontinuations | 2 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.59, 1.00] |
2.1. Analysis.
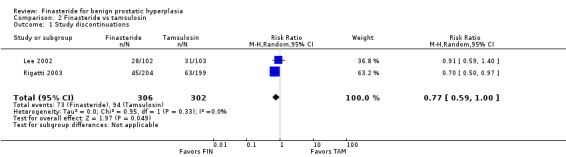
Comparison 2 Finasteride vs tamsulosin, Outcome 1 Study discontinuations.
Comparison 3. Finasteride vs terazosin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects by effect (f/u ≤ 1 yr) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Postural hypotension | 2 | 685 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abrams 1999.
| Methods | A multi center, multinational, randomized, single blind (assessors), placebo‐controlled trial. | |
| Participants | Geographic region: US and Europe Study setting: NA N = 121 (finasteride n = 81; placebo n = 40) Baseline IPSS: finasteride = 19.4 points; placebo = 17.4 points Baseline peak urine flow: finasteride = 6.7 mL/s; placebo = 7.0 mL/s Baseline prostate volume: finasteride = 45.4 cc; placebo = 44.8 cc Baseline PSA: NA Mean age (range): 67.9 years (NA) Race: NA Inclusion: > 55 years old; ambulatory; in good general and mental health; clinically diagnosed with benign prostatic obstruction by LUTS and an enlarged prostate on digital exam Exclusion: PSA > 10 ng/mL; need of immediate surgery; post‐void residual volume ≥ 300 mL; bladder outlet obstruction due to causes other than BPH; known or suspected neurogenic bladder, bacterial prostatitis; acute urinary tract infection; history of recurrent urethral strictures; testicular or prostate surgery; suspected or confirmed prostate cancer on digital exam; chronic and current use of antiandrogens, alpha‐agonists, alpha‐blockers, plant extracts; history of drug and/or alcohol abuse; evidence of renal and/or hepatic impairment; history of recurrent renal bladder or prostatic calculi Study discontinuations: n = 11 (finasteride n = 8 (9.9%); placebo n = 3 (7.5%)) Study duration: 12 months |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Outcomes for IPSS were assessed but with the caveat that the study did not have statistical power to detect changes in symptom score. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | assessors only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 subjects dropped out or had incomplete data |
| Selective reporting (reporting bias) | Low risk | no selective reporting |
| Other bias | Low risk | no other bias detected |
| Intention‐to‐treat analysis | High risk | not described |
| Non‐industry funded | High risk | funded by Merck Research Laboratories |
Agrawal 2001.
| Methods | A prospective, double‐blind, randomized controlled trial. | |
| Participants | Geographic region: NA Study setting: NA N = 140 (finasteride n = 35; terazosin n = 35; allylestrenol n = 35; placebo n = 35) Baseline Boyarsky (range 0 to 36): finasteride = 14.2 points; terazosin = 12.0 points; allylestrenol = 11.9 points; placebo = 14.0 points Baseline peak urine flow: finasteride = 7.0 mL/s; terazosin = 10.5 mL/s; allylestrenol = 8.4 mL/s; placebo = 8.6 mL/s Baseline prostate volume: finasteride = 37.2 mL; terazosin = 26.9 mL; allylestrenol = 34.9 mL; placebo = 22.8 mL Baseline PSA: NA Mean age (range): NA Race: NA Inclusion: aged 45 to 80 years; symptomatic prostatism (Boyarsky > 8); peak urine flow < 15 mL/s; prostate enlargement. Exclusion: prostate cancer; acute or chronic urine retention; recurrent urinary infection; hematuria; changes in upper tract; bladder calculi; urethra stricture; neurogenic bladder; significant comorbid illnesses; drug taking that affect voiding; previous bladder neck or prostate surgery. Study discontinuations: n = 29 (finasteride n = 8 (5.7%); terazosin n = 4 (2.9%); allylestrenol n = 7 (5.0%); placebo n = 10 (7.1%)) Study duration: 6 months |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | all arms received a single tab except allylestrenol, which was given twice daily; not sure if terazosin, which was titrated, was a single tab or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all dropouts were accounted for |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Unclear risk | unclear |
| Non‐industry funded | Unclear risk | unclear |
Andersen 1995.
| Methods | A multi center, multinational, randomized, double‐blind, placebo‐controlled trial, with moderate symptoms of BPH. | |
| Participants | Geographic region: 5 Scandinavian countries Study setting: NA N = 707 (finasteride n = 353; placebo n = 354) Baseline modified Boyarsky (Boyarsky I) (range 0 to 54): finasteride = 13.4 points; placebo = 13.1 points Baseline peak urine flow: finasteride = 10.2 mL/s; placebo = 10.5 mL/s Baseline prostate volume: finasteride = 40.6 cc; placebo = 41.7 cc Baseline PSA: NA Mean age (range): 65.5 years (46 to 80) Race: NA Inclusion: age ≤ 80 yrs; Max urinary flow rate ≥ 5 and ≤ 15 cc/s at screening or at start of placebo run‐in; subjects had to have 2 symptoms indicating moderate BPH, but not more than 2 severe symptoms; enlarged prostate by DRE; serum PSA ≤ 10 ng/mL; post‐void residual volume ≤ 150 cc. Exclusion: hematuria associated with untreated active urinary tract infection, prostatitis, or urinary bladder carcinoma; use of drugs with antiandrogenic properties; serum creatinine > 150mmol/L or liver function tests ≥ 50% above ULN; previous conditions predisposing patients to urethral strictures; chronic bacterial prostatitis; previous prostate surgery or other invasive procedures; evidence of suggestion of prostate cancer; neurogenic bladder dysfunction; ≥ catheterizations for acute urinary retention in the previous 2 yrs; significant abnormalities in pre study clinical exam or lab measures. Study discontinuations: n = 130 (finasteride n = 66 (18.7%); placebo n = 64 (18.1%)) Study duration: 24 months (plus a 1 month run‐in period) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Outcomes are for 24 months, but there were also outcomes for month 12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded. Presumably patients were blinded; not sure if the second blinded group were providers or assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All randomized patients with efficacy measurements at baseline and follow‐up were analyzed." |
| Selective reporting (reporting bias) | Low risk | no selective reporting |
| Other bias | Low risk | no other bias detected |
| Intention‐to‐treat analysis | Low risk | "Patients who withdrew were included by using the last observation on treatment for all time points subsequent to withdrawal." |
| Non‐industry funded | Unclear risk | not described |
Beisland 1992.
| Methods | A multi center, parallel, double‐blind, placebo‐controlled study. | |
| Participants | Geographic region: NA Study setting: NA N = 182 (finasteride n = 94; placebo n = 88) Baseline modified Boyarsky* (range 0 to 36): finasteride = 8.8 points; placebo = 7.8 points Baseline peak urine flow: finasteride = 8.0 mL/s; placebo = 7.6 mL/s Baseline prostate volume: finasteride = 44.2 cc; placebo = 43.8 cc Baseline PSA: NA Mean age (range): 67.3 years (46 to 80) Race: NA Inclusion: men between ages 40 and 80 in good physical; mental health with symptoms of urinary obstruction and peak urine flow < 15 mL/s (2 measurements at screening); enlarged prostate diagnosed by palpation Exclusion: clinical and lab abnormalities Study discontinuations: finasteride n = 6; placebo n = 3 Study duration: 24 weeks (plus a 4‐week, single‐blind, placebo run‐in) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | *The modified Boyarsky used by Beisland does not seem to have been validated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" but no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline data for symptom score not given per arm |
| Selective reporting (reporting bias) | High risk | men with peak urine flow < 150 mL were excluded from analysis |
| Other bias | Low risk | no other bias detected |
| Intention‐to‐treat analysis | High risk | not all patients were evaluated |
| Non‐industry funded | Unclear risk | possibly funded by Merck |
Byrnes 1995.
| Methods | A prospective, double‐blind, placebo‐controlled, randomized trial of men with moderate‐to‐severe BPH. | |
| Participants | Geographic region: US Study setting: Community‐based urology clinics N = 2417 (finasteride n = 1821; placebo n = 596) Baseline AUASI (range 0 to 35): NA Baseline peak urine flow: NA Baseline prostate volume: NA Baseline PSA: NA Mean age (range): 65.0 years (42 to 91) Race: Caucasians/others n = 1623 (67.1%); Blacks n = 380 (15.7%); Hispanics n = 339 (14.0%) Inclusion: ≥ 45 year old; clinical diagnosis of BPH based on moderate‐to‐severe symptoms; prostate enlargement on digital exam; PSA ≤ 10 ng/mL Exclusion: evidence of urethra stricture; previous prostatectomy or other invasive procedures to treat BPH: pelvic radiotherapy; recurrent urine retention; chronic prostatitis; neurogenic bladder; recurrent urinary tract infection; current use of alpha‐adrenergic receptor antagonists; use of hormone therapy affecting the prostate; evidence of prostate cancer Study discontinuations: n = 475 (finasteride n = 353 (19.4%); placebo n = 122 (20.5%)) Study duration: 12 months (plus a 1 month, single‐blind, run‐in period) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" but no description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Merck |
Carraro 1996.
| Methods | A multi center, double‐blind, double‐dummy randomized trial | |
| Participants | Geographic region: Europe Study setting: Urology centers N = 1098 (finasteride + placebo n = 545; Permixon® + placebo n = 553) Baseline IPSS: finasteride + placebo = 15.7 points; Permixon® + placebo = 15.7 points Baseline peak urine flow: finasteride + placebo = 10.8 mL/s; Permixon® + placebo = 10.6 mL/s Baseline prostate volume: finasteride + placebo = 44.0 mL; Permixon® + placebo = 43.0 mL Baseline PSA: finasteride + placebo = 3.23 ng/mL; Permixon® + placebo = 3.26 ng/mL Mean age (range): 64.5 years (49 to 88) Race: NA Inclusion: BPH diagnosed by digital rectal examination and not requiring surgery; International Prostate Symptom Score (IPSS) > 6; maximum urinary flow between 4 to 15 mL/s for a urine volume of at least 150 mL, with a post voiding residual of < 200 mL; prostate volume > 25 mL; serum prostate‐specific antigen (PSA) < l0 ng/mL for prostates ≤ 60 mL, and < 15 ng/mL for prostates > 60 mL (measured before or 3 days after rectal examination and transrectal ultrasound); good physical and mental condition Exclusion: cancer of the prostate; known history of bladder disease (cancer, surgery of the bladder neck, or neurogenic disturbances); lower urinary tract pathology or infection; any disease potentially affecting micturition; Abnormal liver function (twice the ULN of serum aminotransferases and/or bilirubin, creatinine > 160 µmol/L); diuretics or drugs with antiandrogenic or alpha‐receptor properties administered during the preceding 3 months for non‐urological diseases (hypertension, or cerebrovascular insufficiency); prior treatment with either finasteride or Permixon® Study discontinuations: n = 147 (finasteride + placebo n = 61 (11.2%); Permixon® + placebo n = 86 (15.6%)) Study duration: 6 months |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomization code |
| Allocation concealment (selection bias) | Low risk | all men took a total of 3 pills daily |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "To guarantee the double‐blind design, patients received either Permixon® plus placebo bid, or finasteride plus placebo (morning) with two placebos (evening)." This is probably a single blind, double dummy design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Pierre Fabre Médicament |
Finasteride Study Group.
| Methods | A multi center, double‐blind, parallel randomized trial | |
| Participants | Geographic region: International Study setting: multicenter N = 750 (finasteride 1 mg n = 249; finasteride 5 mg n = 246; placebo n = 255) Baseline modified Boyarsky (range 0 to 36): finasteride 1 mg = 18.7 points; finasteride 5 mg = 18.6 points; placebo = 18.2 points Baseline peak urine flow: finasteride 1 mg = 8.8 mL/s; finasteride 5 mg = 9.2 mL/s; placebo = 8.6 mL/s Baseline prostate volume: finasteride 1 mg = 47.5 cm3; finasteride 5 mg = 47.0 cm3; placebo = 46.3 cm3 Baseline PSA: finasteride 1 mg = 5.5 ng/mL; finasteride 5 mg = 5.8 mg/mL; placebo = 5.7 ng/mL Mean age (range): 65.7 years (46 to 83) Race: NA Inclusion: aged 40 to 80 years; good mental and physical health; peak urine flow < 15 mL/s documented twice; prostate volume ≥ 30 cm3; symptomatic urinary tract obstruction Exclusion: suspicion of prostate cancer; bacterial prostatitis; previous testicular or prostate surgery; PSA ≥ 40 ng/mL; residual volume > 350 mL; suspicion of neurogenic bladder; repeated catheterizations; use of antiandrogenic drugs Study discontinuations: n = 42 (finasteride 1 mg n = 15 (2.0%); finasteride 5 mg n = 15 (2.0%); placebo n = 12 (1.6%)) Study duration: 1 year (plus 2 screening visits and 2‐week placebo run‐in) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Only flow rates from voided volumes ≥ 150 mL were included in analysis. A clinical response was considered a priori to be a peak urine flow of ≥ 3 mL/s. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" only description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Merck |
Gormley 1992.
| Methods | A multi center, randomized, double‐blind, placebo‐controlled trial. | |
| Participants | Geographic region: Canada and the US Study setting: NA N = 895 (finasteride 1 mg n = 298; finasteride 5 mg n = 297; placebo n = 300) Baseline modified Boyarsky (Boyarsky II) (range 0 to 36): finasteride 1 mg = 10.6 points; finasteride 5 mg = 10.2 points; placebo = 9.8 points Baseline peak urine flow: finasteride 1 mg = 9.2 mL/s; finasteride 5 mg = 9.6; placebo = 9.6 mL/s Baseline prostate volume: finasteride 1 mg = 60.9 mL; finasteride 5 mg = 58.6 mL; placebo = 61.0 mL Baseline PSA: finasteride 1 mg = 3.8 µg/L; finasteride 5 mg = 3.6 µg/L; placebo = 4.1 µg/L Mean age (range): 64 years (40 to 83) Race: White n = 856 (96%); Black n= 27 (3%); other n = 12 (1%) Inclusion: Symptoms of urinary obstruction; enlarged prostate on digital exam; maximum urinary flow rate of < 15 mL/s with a voided volume ≥ 150 mL (men with very low flow rates were not excluded unless they were at risk for total urinary obstruction) Exclusion: any man whose rectal exam required a biopsy and had a positive result; post‐void residual volume of > 350 mL; a serum PSA ≥ 40 µg/L; evidence of prostate cancer; urinary tract infection; chronic prostatitis; neurogenic bladder Study discontinuations: n = 105 (finasteride 1 mg n = 28 (9.4%); finasteride 5 mg n = 40 (13.5%); placebo n = 37 (12.3%)) Study duration: 52 weeks (plus a 2‐week, single‐blind, placebo run‐in period) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blind, but not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | Unclear risk | some contributors were from Merck |
Kirby 2003.
| Methods | A multi center, prospective, double‐blind, placebo‐controlled trial. | |
| Participants | Geographic region: Europe Study setting: NA N = 1095 (finasteride n = 264; doxazosin n = 275; combination n = 286; placebo n = 270) Baseline IPSS: 17.2 (finasteride = 17.1 points; doxazosin = 17.1 points; combination = 17.3 points; placebo = 17.2 points) Baseline peak urine flow: 10.5 mL/s (finasteride = 10.2 mL/s; doxazosin = 10.4; combination = 10.4 mL/s; placebo = 10.8 mL/s Baseline prostate volume (by DRE): finasteride = 36 g; doxazosin = 36 g; combination = 37 g; placebo = 36 g Baseline PSA: 2.6 ng/mL (finasteride = 2.6 ng/mL; doxazosin = 2.5 ng/mL; combination = 2.7 ng/mL; placebo = 2.6 ng/mL) Mean age (range): 63.5 years (50 to 80) Race: NA Inclusion: men aged 50 to 80 years with BPH and a total International Prostate Symptom Score (IPSS) of 12 or greater; peak urine flow of 5 mL/s or greater but 15 mL/s or less in a total voided volume of 150 mL or greater; and an enlarged prostate as determined by DRE (prostate volume was estimated by the DRE to the nearest 5 g) Exclusion: previous prostate surgery or other invasive procedures for treating BPH or who had prostate cancer or a PSA level exceeding 10 ng/mL; men with a PSA of 4.1 to 10 ng/mL had to provide at least two forms of documentation (a) negative DRE findings (within the past 3 months) (b) negative transrectal ultrasound findings (within the past 3 months) or (c) negative biopsy findings (within the past 4 weeks) or negative results on all three tests if all were performed; lower urinary tract symptoms or reduced urinary flow rates resulting from a condition other than BPH; large bladder diverticulum, bladder stones, recurrent urinary tract infection, or two or more episodes of AUR requiring catheterization within the year before study entry; residual urine volumes greater than 200 mL; or active urinary tract infection; diagnoses of serious diseases or a history of drug or alcohol abuse were excluded, as were those with a history of sensitivity to alpha‐adrenergic blocking agents, quinazolines, or finasteride; hypotension (sitting BP less than 95/60mmHg) or orthostatic hypotension (greater than a 20‐mm Hg decrease in systolic BP (SBP) when changing from a supine to standing position) Study discontinuations: n = 324 (finasteride n = 81 (30.7%); doxazosin n = 78 (28.4%); combination n = 89 (31.1%); placebo n = 76 (28.1%)) Study duration: 52 weeks (plus a single‐blind, 2‐week, placebo run‐in) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The baseline characteristics were not significantly different statistically among the four treatment groups except for Qmax [peak urine flow]." |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | stated "double blind" but no description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Pfizer and Merck |
Lee 2002.
| Methods | A randomized, single‐blind trial of men with moderate‐to‐severe symptomatic BPH. | |
| Participants | Geographic region: South Korea Study setting: NA N = 205 (finasteride n = 102; tamsulosin n = 103) Baseline IPSS: finasteride = 19.0 points; tamsulosin = 19.9 points Baseline peak urine flow: finasteride = 9.6 mL/s; tamsulosin 9.2 mL/s Baseline prostate volume: finasteride = 30.9 mL; tamsulosin = 28.7 mL Baseline PSA: finasteride = 2.2 ng/mL; tamsulosin = 1.8 ng/mL Mean age (range): 64.7 years (51 to 80) Race: NA Inclusion: total IPSS > 8, peak urine flow 5 to 15 mL/s, residual urine < 150 mL/s Exclusion: prostate cancer, serum PSA > 10 ng/mL; prostatitis; neurogenic bladder; bladder cancer; bladder stones; urethral stricture; neurological conditions that might interfere with normal voiding; subjects with BPH who had undergone TURP or experienced urinary retention; cardiac, renal, hepatic disorders Study discontinuations: n = 59 (finasteride n = 28 (27.5%); tamsulosin n= 31 (30.1%)) Study duration: 24 weeks |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | single blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | No indication of industry funding |
Lepor 1996.
| Methods | A multi center, randomized, double‐blind, placebo‐controlled trial. | |
| Participants | Geographic region: US Study setting: Veterans Affairs medical centers N = 1229 (finasteride n = 310; placebo n = 305; terazosin n = 305; combination therapy n = 309) Baseline AUASI: finasteride = 16.2 points; placebo = 15.8 points; terazosin = 16.2; combination = 15.9 Baseline peak urine flow: finasteride = 10.6 mL/s; placebo = 10.4 mL/s; terazosin 10.5 mL/s; combination = 10.4 mL/s Baseline prostate volume: finasteride = 36.2 cc; placebo = 38.4 cc; terazosin = 37.5 cc; combination = 37.2 cc Baseline PSA: finasteride = 2.2 ng/mL; placebo = 2.4 ng/mL; terazosin = 2.2 ng/mL; combination = 2.3 ng/mL Mean age (range): 65 years (45 to 80) Race: White n = 1069 (87%); Black n= 135 (11.0%); Asian/Pacific Islanders n = 12 (1.0%); Native Americans n = 6 (0.5%) Inclusion: mean AUA of at least 8; a mean peak urinary flow rate of ≤ 15 mL/s and ≥ 4 mL/s, with a minimal voided volume of 125 mL, and a mean post‐void residual volume after voiding of < 300 mL (there was no threshold for prostatic enlargement) Exclusion: [Men] "unwilling or unable to give informed consent or if they had taken an experimental drug less than four weeks before being screened; if they had taken an alpha‐adrenergic–agonist drug, a cholinergic agonist or antagonist drug, a topical beta‐adrenergic–antagonist drug for glaucoma, or any antihypertensive drug except a diuretic or an angiotensin‐converting–enzyme inhibitor within two weeks before the lead‐in period; or if they had taken an estrogen, androgen, or drug causing androgen inhibition within the preceding three months. Other criteria for exclusion were an episode of unstable angina pectoris, a myocardial infarction, a transient ischemic attack, or a cerebrovascular accident in the past six months; insulin‐dependent diabetes mellitus; orthostatic hypotension (defined as a difference of more than 20 mm Hg between the systolic blood pressure measured when the man was standing and that measured when he was supine, independent of concomitant changes in pulse or symptoms of postural hypotension) or a history of syncope; a blood pressure of less than 90/70 mm Hg when the man was sitting; a history of carcinoma of the prostate, pelvic irradiation, or urethral stricture; surgery for benign prostatic hyperplasia or bladder‐neck obstruction; current evidence of prostatic carcinoma; active urinary tract disease, cystoscopy, or biopsy of the prostate within the previous two weeks; a history of recurrent urinary tract infections or an infection of the urinary tract, including asymptomatic bacteriuria, within the preceding two months; prior pelvic surgery that was likely to interfere with normal bladder function; any progressive disorder that might prevent the evaluation of drug efficacy and safety; clinically important renal or hepatic impairment (as evidenced by a serum creatinine concentration greater than 2.0 mg per deciliter (177 mmol/L) or a serum alanine aminotransferase concentration more than 1.5 times the upper limit of normal); and a serum concentration of prostate‐specific antigen above 10 ng/mL." Study discontinuations: n = 222 (finasteride n = 67 (21.6%); placebo n = 51 (16.7%); terazosin n = 49 (16.0%); combination n = 55 (17.8%)) Study duration: 52 weeks (including a 4‐week, single‐blind, lead‐in period where participants received placebos and a complete medical history) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "men at each site were randomly assigned by a central computer in equal proportions" |
| Allocation concealment (selection bias) | Low risk | described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blinded, but not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | "statistical analyses were based on the intention‐to‐treat principle; that is, the results for all men for whom any follow‐up data were available were included in the analyses of the treatment groups to which the men had been assigned" |
| Non‐industry funded | Unclear risk | Merck a possible funding agent |
Marberger 1998.
| Methods | A multi center, randomized, double‐blind, placebo‐controlled trial for men with moderate symptomatic BPH. | |
| Participants | Geographic region: Multinational Study setting: NA N = 2902 in the efficacy analysis (finasteride n = 1450; placebo n = 1452) (enrolled = 3270 men) Baseline modified Boyarsky (Boyarsky I) (range 0 to 54): finasteride = 14.5 points; placebo = 14.3 points Baseline peak urine flow: finasteride = 11.2 mL/s; placebo = 10.9 mL/s Baseline prostate volume: finasteride = 38.7 cc; placebo = 39.2 cc Baseline PSA: NA Mean age (range): 63.2 years (50 to 75) Race: NA Inclusion: diagnosis of BPH; age of 50 to 75 years and in good general health; maximal urinary flow rate of 5 to 15 mL/s with a voided volume of 150 mL or more, documented by two measurements, both at screening and month 21 visits; at least two urinary symptoms indicating moderate BPH, but not more than two severe symptoms, based on a modified Boyarsky scale; enlarged prostate gland detected by DRE; PSA level < 10 ng/mL; post void residual urine volume < 150 mL Exclusion: a history of any illness that might confound the results of the study or confer additional risk; dysuria, hematuria, or UTI (a thorough examination, including urine cytology, rule out active urinary tract infection, prostatitis, or urinary bladder carcinoma); abnormalities on clinical examination or in laboratory tests; liver function tests 50% above the ULN; multiple and/or severe allergies; treatment with any other investigational drug during the previous 3 months, or chronic/concurrent use of antiandrogenic drugs, alpha‐blockers, clonidine, or plant extracts; history of drug or alcohol abuse; history of predisposing conditions to urethral strictures; definitive diagnosis of chronic bacterial prostatitis; (10) previous prostatectomy or other invasive surgical procedures for the treatment of BPH; evidence or suggestion of prostate cancer; a history suggestive of neurogenic bladder; urinary catheterization for acute urinary retention (AUR) at least twice during the last 2 years; poor compliance (less than 80%) with placebo during the run‐in phase; planned fatherhood Study discontinuations: n = 691 (finasteride n = 331 (22.8%); placebo n = 360 (24.8%)) Study duration: 2 years (plus a 1‐month, placebo run‐in) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Low risk | "Patients and investigators were unaware of treatment allocation" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo and finasteride tablets were identical in appearance and taste." This was probably a single blind, double‐dummy design. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Merck Research Labs |
Marks 1997.
| Methods | A randomized, double‐blind, placebo‐controlled trial of symptomatic men. | |
| Participants | Geographic region: Los Angeles, California Study setting: Private urology practice N = 41 (finasteride n = 26; placebo n = 15) Baseline IPSS (range 0 to 35): finasteride = 17 points; placebo = 16 points Baseline peak urine flow: finasteride = 13 cc/s; placebo = 12 cc/s Baseline prostate volume: finasteride = 37 cc; placebo = 37 cc Baseline PSA: finasteride = 2.7 ng/mL; placebo = 3.3 ng/mL Mean age (range): 64.5 years (45 to 70) Race: White n = 26 (66.7%); Black n= 7 (18.0%); Hispanic n = 3 (7.7%); Asian n = 3 (7.7%) Inclusion: "ambulatory men 45 to 75 years old in good general physical and mental health who had chronic symptoms of bladder outlet obstruction, some degree of prostate enlargement on rectal examination, an International Prostate Symptom Score (I‐PSS) of 9 or more and a serum prostate specific antigen (PSA) of < 10 ng./mL." Exclusion: "Patients . . . using alpha‐adrenergic blocking agents or any form of therapy that could affect the pituitary‐gonadal axis, or if they had a history of a neurogenic bladder or urethral stricture, active urinary infection or any invasive therapy for BPH." Study discontinuations: n = 2 (finasteride n = 0 (0.0%); placebo n = 2 (13.3%)) Study duration: 6 months (plus a 1‐month, single‐blind placebo run‐in) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Low risk | yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double blinded: patients and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Unclear risk | no description |
| Non‐industry funded | High risk | no |
McConnell 1998.
| Methods | A multi center, randomized, double‐blind, placebo‐controlled trial of men with moderate‐to‐severe symptoms of urinary obstruction. | |
| Participants | Geographic region: North America Study setting: NA N = 3040 (finasteride n = 1524; placebo n = 1516) Baseline quasi AUASI: finasteride = 15 points; placebo = 15 points Baseline peak urine flow: finasteride = 11 mL/s; placebo = 11 mL/s Baseline prostate volume: finasteride = 54 mL; placebo = 55 mL Baseline PSA: finasteride = 2.8 ng/mL; placebo = 2.8 ng/mL Mean age (range): 64 years (45 to 78) Race: White n = 2894 (95.2%); Black n= 91 (3%); other n = 55 (1.8%) Inclusion: moderate‐to‐severe symptoms (by validated questionnaire), peak urine flow < 15 mL/s with a voided volume of ≥ 150 mL, and enlarged prostate by digital exam Exclusion: received alpha‐adrenergic‐antagonists, antiandrogens, history of chronic prostatitis, recurrent urinary tract infections, prostate or bladder cancer or surgery, PSA ≥ 10 ng/mL Study discontinuations: n = 1157 (finasteride n = 524 (34.4%); placebo n = 633 (41.8%)) Study duration: 4 years (plus a 1 month, single‐blind, placebo‐controlled run‐in) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated schedule |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded. Not clear if providers or assessors were blinded, or both. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Merck |
McConnell 2003.
| Methods | A multi center, prospective, randomized, double‐blind, placebo‐controlled trial of men with moderate‐to‐severe symptomatic BPH. | |
| Participants | Geographic region: US Study setting: Clinical centers N = 3047 (finasteride n = 768; doxazosin n = 756; placebo n = 737; combination n = 786) Baseline AUASI: finasteride = 17.6 points; doxazosin = 17.0 points; placebo = 16.8 points; combination = 16.8 points Baseline peak urine flow: finasteride = 10.5 mL/s; doxazosin = 10.3 mL/s; placebo = 10.5 mL/s; combination = 10.6 mL/s Baseline prostate volume: finasteride = 36.9 mL; doxazosin = 36.9 mL; placebo = 35.2 mL; combination = 36.4 mL Baseline PSA: finasteride = 2.4 ng/mL; doxazosin = 2.4 ng/mL; placebo = 2.3 ng/mL; combination = 2.3 ng/mL Mean age (range): 62.6 years (NA) Race: White n = 2509 (82.3%); Black n= 270 (8.9%); Hispanic n = 223 (7.3%); other n = 45 (1.5%) Inclusion: men at least 50 years old; moderate to severe symptoms of BPH (AUA symptom index 8 to 30); peak urinary flow rate 4 to 15 mL/s with a voided volume ≥125 mL Exclusion
Study discontinuations: n = 529 (not including the placebo arm) (finasteride n = 184 (24.0%); doxazosin n = 204 (27.0%); placebo n = NA; combination n = 141 (18.0%)) Study duration: mean follow‐up 4.5 years |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There were no significant differences among groups in baseline demographic characteristics. |
| Allocation concealment (selection bias) | Low risk | yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | participants were "double‐masked" to intervention; providers and assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | High risk | losses to follow‐up for placebo were not reported |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Merck |
Nickel 1996.
| Methods | A multi center, randomized, double‐blind, parallel‐group, placebo‐controlled prospective trial. | |
| Participants | Geographic region: Canada Study setting: NA N = 613 (finasteride n = 310; placebo n = 303) Baseline modified Boyarsky (Boyarsky I) (range 0 to 54): finasteride = 15.8 points; placebo = 16.6 points Baseline peak urine flow: finasteride = 11.1 mL/s; placebo = 10.9 mL/s Baseline prostate volume: finasteride = 44.1 cc; placebo = 45.8 cc Baseline PSA: NA Mean age (range): 63.2 years (46 to 80) Race: NA Inclusion: age ≤ 80 years old; maximum urinary flow rate of 5 to 15 mL/s at screening ‐ or start of placebo run‐in period, or both, with total voided volume of at least 150 mL; at least two moderate symptoms of BPH (e.g., increased frequency of urination or difficulty urinating), but no more than two severe symptoms; enlarged prostate gland detected by digital rectal examination (DRE); serum prostate‐specific antigen (PSA) level ≤ 10 ng/mL; post void residual urine volume ≤ 150 mL Exclusion: evidence or suggestion of prostate cancer; neurogenic bladder dysfunction; history of acute urinary retention necessitating two or more catheterizations in the previous 2 years; history of prostate surgery or other invasive procedures (e.g., transurethral microwave thermotherapy, urethral stenting, balloon urethroplasty); history of condition predisposing patient to urethral strictures: chronic bacterial prostatitis; serum creatine level > 150 mmol/L, or results of liver function tests > 50% above ULN; use of drugs with antiandrogenic properties; hematuria associated with untreated active urinary tract infection, prostatitis or bladder cancer; any condition jeopardizing patient's ability to complete the study Study discontinuations: n = 141 (finasteride n = 64 (20.6%); placebo n = 77 (25.4%)) Study duration: 2 years (plus a 1‐month run‐in of placebo therapy) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated schedule" |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Patients and investigators were blind to treatment allocation. The placebo tablets were made to be identical in appearance and taste to the finasteride tablets." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | funded by Merck Frosst Canada, Inc. |
Polat 1997.
| Methods | A randomized, controlled trial of men who were mildly symptomatic. | |
| Participants | Geographic region: NA Study setting: NA N = 123 (finasteride n = 62; placebo n = 61) Baseline AUASI: finasteride = 15.1 points; placebo = 15.3 points Baseline peak urine flow: finasteride = 9.9 mL/s; placebo = 10.1 mL/s Baseline prostate volume: finasteride = 39.1 cc; placebo = 38.2 cc Baseline PSA: finasteride = 2.2 ng/mL; placebo = 2.3 ng/mL Mean age (range): 60.0 years (44 to 80) Race: NA Inclusion: aged 50 to 80 years; good health status; prostate volume > 30 cc; peak urine flow < 15 mL/s; appropriate for regular follow‐up; no suspicion of prostate cancer; "mildly symptomatic" Exclusion: NA Study discontinuations: n = 24 (finasteride n = 11(17.7%); placebo n = 13 (21.3%)) Study duration: 12 months |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | High risk | no |
| Non‐industry funded | High risk | no |
Rigatti 2003.
| Methods | A multi center, double‐blind, double‐dummy, parallel, randomized trial. | |
| Participants | Geographic region: Italy Study setting: "Centres" N = 403 (finasteride n = 204; tamsulosin n = 199) Baseline IPSS: finasteride = 16.9 points; tamsulosin = 16.3 points Baseline SPI: finasteride = 14.0 points; tamsulosin = 13.6 points Baseline peak urine flow: finasteride = 10.8 mL/s; tamsulosin = 10.8 mL/s Baseline prostate volume: 39 mL Baseline PSA: NA Mean age (range): 63.0 years (NA) Race: NA Inclusion: men between 50 and 80 years with symptomatic LUTS/BPH, as diagnosed by an International Prostate Symptom Score (IPSS) ≥13; peak urine flow between 4 and 15 mL/s and a total Symptom Problem Index (SPI) score ≥7; post‐void residual volume was < 400 mL and PSA < 3 or 3 to 10 ng/mL (provided that prostate cancer was ruled out by the investigator according to the usual procedure in the centre) Exclusion: known history or a diagnosis of urological disturbances, cardiovascular diseases, neurological diseases, hepatic or renal insufficiency were excluded; those with clinically significant abnormalities of haematological and biochemical tests; patients taking an a1‐AR antagonist or phytotherapy in the 6 weeks prior to the study or finasteride in the 6 months prior to the study Study discontinuations: n = 108 (finasteride n = 45; tamsulosin n = 63) Study duration: 52 weeks (plus a 2‐week, single‐blind, placebo run‐in period) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Most reported outcomes were for 26 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Low risk | described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" stated but no description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | High risk | Boehringer Ingelheim Italy SpA sponsored the MICTUS trial |
Sökeland 2000.
| Methods | A multi center, double‐blind, double‐dummy, placebo‐controlled randomized study. | |
| Participants | Geographic region: Germany Study setting: "medical practices" N = 516 (finasteride n = 255; PRO 160/120 n = 261) Baseline IPSS: finasteride = 11.8 points; PRO 160/120 = 11.3 points Baseline peak urine flow: finasteride = 12.7 mL/s; PRO 160/120 = 12.7 mL/s Baseline prostate volume: finasteride = 44.0 mL; PRO 160/120 = 42.7 mL Baseline PSA: NA Mean age (range): NA (50 to 88) Race: NA Inclusion: early symptomatic HPH (I to II Alken stage); baseline peak urine flow < 20 mL/s and a voided volume of > 150 mL Exclusion: concomitant diseases; receiving additional treatment that could interfere with the trial procedure or the outcome evaluation Study discontinuations: n = 27 Study duration: 48 weeks (plus a 2‐week, placebo run‐in) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Subgroup outcomes by prostate size (≤ 40 mL vs > 40 mL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not described |
| Allocation concealment (selection bias) | Unclear risk | not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" and "double dummy" but not otherwise described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | yes |
| Non‐industry funded | Unclear risk | no description |
Tammela 1993.
| Methods | A double‐blind, placebo‐controlled randomized trial of men with bladder outlet obstruction due to BPH. | |
| Participants | Geographic region: Finland Study setting: NA N = 36 (finasteride n = 19; placebo n = 17) Baseline symptom score: NA Baseline peak urine flow: finasteride = 7.7 mL/s; placebo = 8.8 mL/s Baseline prostate volume: finasteride = 50 cc; placebo = 48 cc Baseline PSA: finasteride = 5.4 ng/mL; placebo = 4.0 ng/mL Mean age (range): 65.0 years (54 to 80) Race: NA Inclusion: ambulatory; good general physical and mental health with moderate‐to‐severe symptoms urinary obstruction; enlarged prostate gland; peak urine flow < 15 mL/s documented by two physiological free flow uroflowmetry measurements whole voiding at least 150 mL; a urodynamic pattern of bladder outlet obstruction Exclusion: definitive diagnosis of chronic bacterial prostatitis; history of urethral strictures; presence of urinary tract infection; previous prostate or testicular surgery; evidence of prostate cancer by digital exam; PSA > 40 ng/mL; prostate biopsies were taken in patients with PSA > 10 ng/mL before they were excepted into the trial; patients with residual urine volume of > 350 mL and those had catheterization for acute urinary retention were excluded; chronic concurrent use of barbiturates, heparin, warfarin, theophyllamine, antiarrhythmic agents and drugs with antiandrogenic properties were not allowed; patients with a serum creatinine of > 1.5 mg/dL of liver function tests outside the normal range were excluded Study discontinuations: n = 0 Study duration: 6 months |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" but no description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Unclear risk | unclear |
| Non‐industry funded | High risk | no description |
Tempany 1993.
| Methods | A randomized, placebo‐controlled, double‐blind trial for men with symptomatic BPH. | |
| Participants | Geographic region: NA Study setting: NA N = 20 (finasteride 1 mg n = 6; finasteride 5 mg n = 6; placebo n = 8) Baseline modified Boyarsky: 9.6 points Baseline peak urine flow: 9.4 mL/s Baseline prostate volume: finasteride 1mg and 5 mg = 61.7 cc; placebo = 108.7 cc Baseline PSA: NA Mean age (range): NA Race: NA Inclusion: NA Exclusion: NA Study discontinuations: n = 0 Study duration: 12 months |
|
| Interventions |
|
|
| Outcomes | Prostate volume | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Difference in the finasteride group vs the placebo group was 61.7 cc and 108.7 cc, respectively. |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" but no description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | High risk | The authors combined the two finasteride doses for the outcomes. |
| Intention‐to‐treat analysis | Unclear risk | unclear |
| Non‐industry funded | High risk | funded by Merck Research Laboratories |
Tenover 1997.
| Methods | A multi center, randomized, prospective, double‐blind, placebo controlled trial of men with moderate‐to‐severe symptomatic BPH. | |
| Participants | Geographic region: NA Study setting: Primary care centers N = 2315 (finasteride n = 1736; placebo n = 579) Baseline AUASI: finasteride 19.03 points; placebo 18.35 points Baseline BII: finasteride = 4.76 points (n = 1538); placebo = 4.67 points (n = 515) Baseline BSIA: finasteride = 12.70 points (n = 1531); placebo = 12.75 points (n = 512) Baseline peak urine flow: NA Baseline prostate volume: NA Baseline PSA: NA Mean age (range): 63.4 years (45 to 94) Race: White/other n = 1955 (92.6%); Black n= 104 (4.9%); Hispanic n = 53 (2.5%) Inclusion: age ≥ 45 years; symptomatic and with clinical diagnosis of BPH; moderate‐to‐severe symptoms (AUASI 9 to 35); prostate enlargement on digital exam; PSA ≤ 10 ng/mL Exclusion: evidence of urethral stricture; previous prostatectomy or other invasive procedure for BPH: history of repeated catheterizations; previous pelvic radiotherapy; recurrent episodes of urinary retention; chronic prostatitis; neurogenic bladder; recurrent or active UTI; current use of alpha‐adrenergic receptor antagonists; use of high‐dose ketoconazole; use of hormonal therapy affecting the prostate (subjects suspected of prostate cancer on DRE or PSA ≥ 4 ng/mL were excluded until cancer was ruled out by biopsy or other means) Study discontinuations: n = 388 Study duration: 12 months (plus 1 month, single‐masked placebo run‐in (subjects had to be compliant (≥ 80%) in order to make it into randomization) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Significant differences at baseline (P < 0.05) in regard to age and AUASI mean score. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Significant differences at baseline (P < 0.05) in regard to age and AUASI mean score. |
| Allocation concealment (selection bias) | Unclear risk | unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐masked" but no description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | Low risk | stated |
| Non‐industry funded | High risk | funded by Merck & Co. |
Yu 1995.
| Methods | A double‐blind, placebo‐controlled trial for men with enlarged prostates who complained of bothersome symptoms. | |
| Participants | Geographic region: NA Study setting: NA N = 50 (finasteride n = 25; placebo n = 25) Baseline AUASI: finasteride = 19.45 points; placebo = 16.68 points Baseline peak urine flow: finasteride = 11.19 mL/s; placebo = 11.44 mL/s Baseline prostate volume: finasteride = 26.70 mL; placebo = 20.12 mL Baseline PSA: finasteride = 2.76 ng/dL; placebo = 2.95 ng/dL Mean age (range): 65.8 years (50 to 82) Race: NA Inclusion: enlarged prostate and bothersome symptoms Exclusion: evidence of prostate cancer; prostatitis; urethral stricture; bladder neck contracture; bladder stones; severe renal or hepatic insufficiency Study discontinuations: n = 4 (finasteride n = 1(4%); placebo n = 3 (12%)) Study duration: 6 months (plus a 2‐week washout period) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Author was contacted to delineate 6‐month AUA score for finasteride. The author did not respond. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. It is difficult to know if subjects were properly randomized. Baseline demographics are for the per protocol group only. |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" but no description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | yes |
| Selective reporting (reporting bias) | Low risk | yes |
| Other bias | Low risk | yes |
| Intention‐to‐treat analysis | High risk | no |
| Non‐industry funded | High risk | no |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andriole 1998 | This study reports partial outcomes for the included trial McConnell 1998. |
| Baldwin 2001 | Not an RCT. |
| Bruskewitz 1999 | This was a report to an AUA Annual Meeting. |
| Ekman 1995 | Not an RCT. |
| Ekman 1996 | Secondary analysis to included trial Andersen 1995. |
| Geller 1995 | Secondary analysis to included trial Gormley 1992 |
| Girman 1996 | Not an RCT. |
| Gormley 1994 | Review of Gormley 1992 and Finasteride Study Group 1993. |
| Grino 1993 | Comparison of visual and automatic uroflowmetry. |
| Grino 1994 | This is a combination of 2 included trials, Gormley 1992 and the Finasteride Study Group 1993. |
| Jeong 2009 | Comparator groups are indefinitely defined: "group 1 received finasteride 5 mg plus alfuzocin 10 mg or tamsulosin." |
| Kaplan 2000 | A re‐analysis of the included trial, McConnell 1998. Subgroups based on a non‐validated, quasi‐AUASI. |
| Kaplan 2008 | A post hoc analysis of the included trial, McConnell 2003. |
| Kirby 1992 | Follow‐up < 6 months. |
| Lam 2003 | Not an RCT |
| Lowe 2003 | Open label extension to included trial Gormley 1992. |
| Marberger 2000 | Pooled results of 3 RCTs |
| Marks 1999 | Follow‐up of the finasteride arm only (see Marks 1997). |
| Moore 1995 | Not an RCT |
| Nacey 1995 | A subset of the included trial Gormley 1994. |
| Paick 2005 | Not original research |
| Perimenis 2002 | No outcomes |
| Roehrborn 2000 | Secondary to included trial McConnell 1998 |
| Roehrborn 2000b | Not original research |
| Roehrborn 2002 | Analysis of placebo‐treated patients only from the PLESS trial |
| Roehrborn 2004 | Reports 6‐year outcomes for included trial McConnell 1998. At this point all men are on finasteride. |
| Schäfer 1999 | Not original research |
| Siami 2007 | No comparison to finasteride |
| Stoner 1992a | Follow‐up < 6 months |
| Stoner 1994a | Secondary analysis to included trial Gormley 1992 |
| Stoner 1994b | Not an RCT |
| Tammela 1995 | Secondary analysis to included trial Tammela 1993 |
| Tewari 1995 | Not original research |
| Thompson 2003 | Endpoint is development of prostate cancer |
| Vaughan 2002 | A review |
Differences between protocol and review
Under Abstract/Background we changed "can cause constrictive symptoms . . . straining" to "can cause bothersome urinary symptoms . . . straining, urgency, frequency, incomplete emptying." We changed "which in part causes the hyperplastic prostate, contributing to" reduces prostate size and is commonly used to treat bothersome." Under Abstract/Background/Objectives we changed "To compare the clinical effectiveness of finasteride versus other active" to "To compare the clinical effectiveness and harms of finasteride versus placebo and active." We deleted the phrase "secondary to BPH." Under 'Selection criteria' we changed "Randomized trials with placebo" to "Randomized trials in the English language with placebo." Under Data collection and analysis to the sentence "Our primary . . . worse symptoms)" we added the clause "worse symptoms), and in particular clinically significant change (≤ or ≥ 4 points in the IPSS/AUASI)." We deleted the sentence ""We also categorized outcomes by trial lengths ≤ 1 year and > 1 year." Under Measures of treatment effect we added the sentence "Whenever we have unequal scales with changes from baseline and variances, we will combine them using standardized mean differences." Under Methods/Types of studies, we changed "Randomized, controlled clinical trials (RCTs) of greater than 6 months duration" to "Randomized, controlled trials (RCTs) of 6 months or greater duration." Under Types of interventions, we changed the sentence "Because of finasteride's relatively slow clinical effect, trials had a minimal duration of > 6 months (Stoner 1992a)" to "Because of finasteride's relatively slow clinical effect, trials had a minimal duration of ≥ 6 months (Stoner 1992a)." Under Types of outcome measures in the sentence "Our primary outcome was improvement," we changed to "Our primary clinical outcome was improvement." Under Secondary outcomes in the sentence "Secondary outcomes included peak urine flow, measured in mL/s (millilitres per second), prostate size, measured in cc (cubic centimetres), BPH progression (defined as a ≥ 4 point increase from baseline to endpoint of the IPSS/AUASI; acute urinary retention; or need for surgical intervention), post‐void residual volume (cc), nocturia, adverse events and effects (or both), and quality of life (QoL). We did not assess finasteride and the chemoprevention of prostate cancer" we changed to "Secondary clinical outcomes included BPH progression (defined as a ≥ 4 point increase from baseline to endpoint of the IPSS/AUASI; acute urinary retention; or need for surgical intervention) and adverse events and effects (or both). Other outcomes were peak urine flow, measured in mL/s (millilitres per second), prostate size, measured in cc (cubic centimetres), post‐void residual volume (cc), nocturia, and quality of life (QoL). We did not assess finasteride for the chemoprevention of prostate cancer." Under Data synthesis, we changed the sentence "For categorical effect measures, we used RR" to "For categorical effect measures, we used RR or RD." In the section Measures of treatment effect we changed "The effect measures for dichotomous outcomes were expressed using relative risk (RR)" to "The effect measures for dichotomous outcomes were expressed using relative risk (RR) or absolute risk reduction (RD)".
Contributions of authors
JT conducted the analysis and wrote the report. IR wrote the search string. HF, RD, TW, and JT were responsible for the review's concept and refinement.
Sources of support
Internal sources
-
Minneapolis VA Hospital, USA.
This material is the result of work supported with resources and the use of facilities at the Minneapolis VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
External sources
No sources of support supplied
Declarations of interest
None reported.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Abrams 1999 {published data only}
- Abrams P, Schafer W, Tammela TLJ, Barrett DM, Hedlund H, Rollema HJ, et al. Improvement of pressure flow parameters with finasteride is greater in men with large prostates. The Journal of Urology 1999;161(5):1513‐7. [PubMed] [Google Scholar]
Agrawal 2001 {published data only}
- Agrawal MS, Aron M. Prospective double‐blind randomized controlled trial of terazosin, finasteride and allylestrenol in the management of benign prostatic hyperplasia. Indian Journal of Urology [serial online] 2001 [cited 2010 Mar 4]; Vol. 17, issue 2:132‐40. Available from: http://www.indianjurol.com/text.asp$2001/17/2/132/21043.
Andersen 1995 {published data only}
- Andersen JT, Ekman P, Wolf H, Beisland HO, Johansson JE, Kontturi M, Lehtonen T, Tveter K, and The Scandinavian BPH Study Group. Can Finasteride reverse the progress of benign prostatic hyperplasia? A two‐year placebo‐controlled study. Urology 1995;46(5):631‐7. [DOI] [PubMed] [Google Scholar]
Beisland 1992 {published data only}
- Beisland HO, Binkowitz B, Brekkan E, Ekman P, Kontturi M, Letonen T, et al. Scandinavian clinical study of finasteride in the treatment of benign prostatic hyperplasia. European Urology 1992;22:271‐7. [DOI] [PubMed] [Google Scholar]
Byrnes 1995 {published data only}
- Byrnes CA, Morton AS, Liss CL, Lippert MC, Gillenwater JY on behalf of the CUSP investigators. Efficacy, tolerability, and effect on health‐related quality of life of finasteride versus placebo in men with symptomatic benign prostatic hyperplasia: a community‐based study. Clinical Therapeutics 1995;17(5):956‐69. [DOI] [PubMed] [Google Scholar]
Carraro 1996 {published data only}
- Carraro J‐C, Raynaud J‐P, Koch G, Chisholm GD, Silverio F, Teillac P, et al. Comparison of phytotherapy (Permixon®) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. The Prostate 1996;29:231‐40. [DOI] [PubMed] [Google Scholar]
Finasteride Study Group {published data only}
- The Finasteride Study Group. Finasteride (MK‐906) in the treatment of benign prostatic hyperplasia. The Prostate 1993;22:291‐9. [DOI] [PubMed] [Google Scholar]
Gormley 1992 {published data only}
- Gormley GJ, Stoner E, Bruskewitz RC, Imperato‐McGinley J, Walsh PC, McConnell JD, et al. The effect of finasteride in men with benign prostatic hyperplasia. The New England Journal of Medicine 1992;327(17):1185‐91. [DOI] [PubMed] [Google Scholar]
Kirby 2003 {published data only}
- Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, Sweeney M, and Grossman EB for the PREDICT Study Investigators. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology 2003;61:119‐26. [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee E. Comparison of tamsulosin and finasteride for lower urinary tract symptoms associated with benign prostatic hyperplasia in Korean patients. The Journal of International Medical Research 2002;30:584‐90. [DOI] [PubMed] [Google Scholar]
Lepor 1996 {published data only}
- Johnson, II TM, Jones K, Williford WO, Kutner MH, Issa MM, Lepor H. Changes in nocturia from medical treatment of benign prostatic hyperplasia: secondary analysis of the Department of Veterans Affairs Cooperative Study Trial. The Journal of Urology 2003;170:145‐8. [DOI] [PubMed] [Google Scholar]
- Lepor H, Jones K, Williford W. The mechanism of adverse events associated with terazosin: an analysis of the Veterans Affairs Cooperative Study. The Journal of Urology 2000;163:1134‐7. [PubMed] [Google Scholar]
- Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, Haakenson C, Machi M, Narayan P, Padley RJ, for the Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. The efficacy of terazosin, finasteride, or both n benign prostatic hyperplasia. The New England Journal of Medicine 1996;335(8):533‐9. [DOI] [PubMed] [Google Scholar]
- Lepor H, Williford WO, Barry MJ, Haakenson C, Jones K. The impact of medical therapy on bother due to symptoms, quality of life and global outcome, and factors predicting response. Journal of Urology 1998;160(4):1358‐67. [PubMed] [Google Scholar]
Marberger 1998 {published data only}
- Marberger MJ, on behalf of the PROWESS Study Group. Long‐term effects of finasteride in patients with benign prostatic hyperplasia: a double‐blind, placebo‐controlled, multicenter study. Urology 1998;51:677‐86. [DOI] [PubMed] [Google Scholar]
Marks 1997 {published data only}
- Marks LS, Partin AW, Gormley GJ, Dorey FJ, Shery ED, Garris JB, et al. Prostate tissue composition and response to finasteride in men with symptomatic benign prostatic hyperplasia. The Journal of Urology 1997;157(6):2171‐8. [PubMed] [Google Scholar]
McConnell 1998 {published data only}
- Kaplan S, Garvin D, Gilhooly P, Koppel M, Labasky R, Milsten R, et al. Impact of baseline symptom severity on future risk of benign prostatic hyperplasia‐related outcomes and long‐term response to finasteride. Urology 2000;56:610‐6. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, Holtgrewe HL, Bruskewitz R, Saltzman B, Mobley D, Narayan P, et al. Comparison of the efficacy and safety of finasteride in older versus younger men with benign prostatic hyperplasia. Urology 2001;57:1073‐7. [DOI] [PubMed] [Google Scholar]
- McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. New England Journal of Medicine 1998;338(9):557‐63. [DOI] [PubMed] [Google Scholar]
- Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, et al. Incidence and severity of sexual adverse experiences in finasteride and placebo‐treated men with benign prostatic hyperplasia. Urology 2003;61:579‐84. [DOI] [PubMed] [Google Scholar]
McConnell 2003 {published data only}
- Johnson, II TM, Burrows PK, Kusek JW, Nyberg LM, Tenover JL, Lepor H, et al. The effect of doxazosin, finasteride and combination therapy on nocturia in men with benign prostatic hyperplasia. The Journal of Urology 2007;178:2045‐51. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, McConnell JD, Roehrborn CG, Meehan AG, Lee MW, Noble WR, et al. Combination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 Ml or greater. The Journal of Urology 2006;175:217‐21. [DOI] [PubMed] [Google Scholar]
- McConnell JD, Roehrborn CG, Bautista OM, Andriole, Jr GL, Dixon CM, Kusek JW, et al. The long‐term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. The New England Journal of Medicine 2003;349(25):2387‐98. [DOI] [PubMed] [Google Scholar]
Nickel 1996 {published data only}
- Nickel JC, Fradet Y, Boake RC, Pommerville PJ, Perreault J‐P, Afridi SK, et al. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2‐year randomized controlled trial (the PROSPECT Study). Canadian Medical Association Journal 1996;155(9):1251‐9. [PMC free article] [PubMed] [Google Scholar]
Polat 1997 {published data only}
- Polat Ő, Őzbey I, Gűl O, Demirel A, Bayraktar Y. Pharmacotherapy of benign prostatic hyperplasia: inhibitor of 5 Alpha‐reductase. International Urology and Nephrology 1997;293(3):323‐30. [DOI] [PubMed] [Google Scholar]
Rigatti 2003 {published data only}
- Rigatti P, Brausi M, Scarpa RM, Porru D, Schumacher H, and Rizzi CA for the MICTUS Study Group. A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Prostate Cancer and Prostatic Diseases 2003;6:315‐23. [DOI] [PubMed] [Google Scholar]
Sökeland 2000 {published data only}
- Sökeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. British Journal of Urology International 2000;86:439‐42. [DOI] [PubMed] [Google Scholar]
Tammela 1993 {published data only}
- Tammela TLJ, Kontturi MJ. Urodynamic effects of finasteride in the treatment of bladder outlet obstruction due to benign prostatic hyperplasia. The Journal of Urology 1993;149:342‐4. [DOI] [PubMed] [Google Scholar]
Tempany 1993 {published data only}
- Tempany CMC, Partin AW, Zerhouni EA, Zinreich SJ, Walsh PC. The influence of finasteride on the volume of the peripheral and periurethral zones of the prostate in men with benign prostatic hyperplasia. The Prostate 1993;22:39‐42. [DOI] [PubMed] [Google Scholar]
Tenover 1997 {published data only}
- Tenover JL, Pagano GA, Morton AS, Liss CL, Byrnes CA, on behalf of the Primary Care Investigator Study Group. Efficacy and tolerability of finasteride in symptomatic benign prostatic hyperplasia: a primary care study. Clinical Therapeutics 1997;19(2):243‐58. [DOI] [PubMed] [Google Scholar]
Yu 1995 {published data only}
- Yu H‐J, Chiu T‐Y, Lai M‐K. Therapeutic effects of finasteride in benign prostatic hyperplasia: a randomized double‐blind controlled trial. Journal of the Formosan Medical Association 1995;94(1/2):37‐41. [PubMed] [Google Scholar]
References to studies excluded from this review
Andriole 1998 {published data only}
- Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, et al. Treatment with finasteride preserves usefulness of prostate‐specific antigen in the detection of prostate cancer: results of a randomized, double‐blind, placebo‐controlled clinical trial. Urology 1998;52:195‐202. [DOI] [PubMed] [Google Scholar]
Baldwin 2001 {published data only}
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha‐blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001;58:203‐9. [DOI] [PubMed] [Google Scholar]
Bruskewitz 1999 {published data only}
- Bruskewitz R, Girman CJ. Effect of finasteride on bother and health‐related quality of life associated with benign prostatic hyperplasia. The Journal of Urology 1999;161(4S Supplement):268. [DOI] [PubMed] [Google Scholar]
Ekman 1995 {published data only}
- Ekman P. Endocrine therapy for benign prostatic hyperplasia. Journal d'Urologie 1995;101(1):22‐5. [PubMed] [Google Scholar]
Ekman 1996 {published data only}
- Ekman P, Andersen JT, Wolf H. Finasteride in the treatment of benign prostatic hyperplasia. Acta Urologica Belgica 1996;64(1):IX‐XII. [PubMed] [Google Scholar]
Geller 1995 {published data only}
- Geller J. Five‐year follow‐up of patients with benign prostatic hyperplasia treated with finasteride. European Urology 1995;27:267‐73. [DOI] [PubMed] [Google Scholar]
Girman 1996 {published data only}
- Girman CJ, Kolman C, Liss CL, Bolognese JA, Binkowitz BS, Stoner E, Finasteride Study Group. Effects of finasteride on the health‐related quality of life in men with symptomatic benign prostatic hyperplasia. The Prostate 1996;29:83‐90. [DOI] [PubMed] [Google Scholar]
Gormley 1994 {published data only}
- Gormley GJ, Stoner E. Clinical results with finasteride. Progress in Clinical and Biological Research 1994;386:205‐7. [PubMed] [Google Scholar]
Grino 1993 {published data only}
- Grino PB, Bruskewitz R, Blaivas JG, Siroky MB, Andersen JT, Cook T, Stoner E. Maximum urinary flow rate by uroflowmetry: automatic or visual interpretation. The Journal of Urology 1993;149:339‐41. [DOI] [PubMed] [Google Scholar]
Grino 1994 {published data only}
- Grino P, Stoner E, and the Finasteride Study Group. Finasteride for the treatment and control of benign prostatic hyperplasia: summary of Phase III controlled studies. European Urology 1994;25(suppl 1):24‐8. [DOI] [PubMed] [Google Scholar]
Jeong 2009 {published data only}
- Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5a‐reductase inhibitors on prostate volume and symptoms in men with BPH: A prospective study. Urology 2009;73:802‐6. [DOI] [PubMed] [Google Scholar]
Kaplan 2000 {published data only}
- Kaplan S, Garvin D, Gilhooly P, Koppel M, Labasky R, Milsten R, et al. Impact of baseline symptom severity on future risk of benign prostatic hyperplasia‐related outcomes and long‐term response to finasteride. Urology 2000;56:610‐6. [DOI] [PubMed] [Google Scholar]
Kaplan 2008 {published data only}
- Kaplan SA, Roehrborn CG, McConnell JD, Meehan AG, Surynawanshi S, Lee JY, et al. Long‐term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in men enrolled in the MTOPS Trial. The Journal of Urology 2008;180:1030‐3. [DOI] [PubMed] [Google Scholar]
Kirby 1992 {published data only}
- Kirby RS, Bryan J, Eardley I, Christmas TJ, Liu S, Holmes SAV, et al. Finasteride in the treatment of benign prostatic hyperplasia. A dynamic evaluation. British Journal of Urology 1992;70:65‐72. [DOI] [PubMed] [Google Scholar]
Lam 2003 {published data only}
- Lam JS, Romas NA, Lowe FC. Long‐term treatment with finasteride in men with symptomatic benign prostatic hyperplasia: 10‐year follow‐up. Urology 2003;61:354‐58. [DOI] [PubMed] [Google Scholar]
Lowe 2003 {published data only}
- Lowe FC, McConnell JD, Hudson PB, Romas NA, Boake R, Lieber M, et al. Long‐term 6‐year experience with finasteride in patients with benign prostatic hyperplasia. Urology 2003;61:791‐6. [DOI] [PubMed] [Google Scholar]
Marberger 2000 {published data only}
- Marberger MJ, Andersen JT, Nickel JC, Malice M‐P, Gabriel M, Pappas F, et al. Prostate volume and serum prostate‐specific antigen as predictors of acute urinary retention. European Urology 2000;38:563‐8. [DOI] [PubMed] [Google Scholar]
Marks 1999 {published data only}
- Marks LS, Partin AW, Dorey FJ, Gormley GJ, Epstein JI, Garris JB, et al. Long‐term effects of finasteride on prostate tissue composition. Urology 1999;53:574‐80. [PubMed] [Google Scholar]
Moore 1995 {published data only}
- Moore E, Bracken B, Bremner W, Geller J, Imperato‐McGinley J, McConnell J, et al. Proscar®: Five‐year experience. European Urology 1995;28:304‐9. [DOI] [PubMed] [Google Scholar]
Nacey 1995 {published data only}
- Nacey JN, Mefan PJ, Delahunt B. The effect of finasteride on prostate volume, urinary flow and symptom score in men with benign prostatic hyperplasia. Australia New Zealand Journal of Surgery 1995;65:35‐9. [DOI] [PubMed] [Google Scholar]
Paick 2005 {published data only}
- Paick SH, Meehan A, Lee M, Penson DF, Wessells H. The relationship among lower urinary tract symptoms, prostate specific antigen and erectile dysfunction in men with benign prostatic hyperplasia: results from the PROSCAR long‐term efficacy and safety study. The Journal of Urology 2005;173:903‐7. [DOI] [PubMed] [Google Scholar]
Perimenis 2002 {published data only}
- Perimenis P, Gyftopoulos K, Markou S, Barbalias G. Effects of finasteride and cyproterone acetate on hematuria associated with benign prostatic hyperplasia: a prospective, randomized, controlled study. Urology 2002;59:373‐7. [DOI] [PubMed] [Google Scholar]
Roehrborn 2000 {published data only}
- Roehrborn CG, Bruskewitz R, Nickel GC, Glickman S, Cox II C, Anderson R, et al. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years. European Urology 2000;37:528‐36. [DOI] [PubMed] [Google Scholar]
Roehrborn 2000b {published data only}
- Roehrborn CG, McConnell J, Bonilla J, Rosenblatt S, Hudson PB, Malek GH, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. The Journal of Urology 2000;163:13‐20. [PubMed] [Google Scholar]
Roehrborn 2002 {published data only}
- Roehrborn CG, McConnell JD, Saltzman B, Bergner D, Gray T, Narayan P, et al. Storage (irritative) and voiding (obstructive) symptoms predictors of benign prostatic hyperplasia progression and related outcomes. European Urology 2002;42:1‐6. [DOI] [PubMed] [Google Scholar]
Roehrborn 2004 {published data only}
- Roehrborn CG, Bruskewitz R, Nickel JC, McConnell JD, Saltzman B, Gittelman MC, et al. Sustained decrease in incidence of acute urinary retention and surgery with finasteride for 6 years in men with benign prostatic hyperplasia. The Journal of Urology 2004;171:1194‐8. [DOI] [PubMed] [Google Scholar]
Schäfer 1999 {published data only}
- Schäfer W, Tammela TLJ, Barrett DM, Abrams P, Hedlund H, Rollema HJ, et al. Continued improvement in pressure‐flow parameters in men receiving finasteride for 2 years. Urology 1999;54:278‐83. [DOI] [PubMed] [Google Scholar]
Siami 2007 {published data only}
- Siami P, Roehrborn CG, Barkin J, Damiao R, Wyczolkowski M, Duggan A, et al. Combination therapy with dutasteride and tamsulosin in men with moderate‐to‐severe benign prostatic hyperplasia and prostate enlargement: the CombAT (Combination of Avodart® and Tamsulosin) trial rationale and study design. Contemporary Clinical Trials 2007;28:770‐9. [DOI] [PubMed] [Google Scholar]
Stoner 1992a {published data only}
- Stoner E, Finasteride Study Group. The clinical effects of 5a‐reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Journal of Urology 1992;47:1298‐1302. [DOI] [PubMed] [Google Scholar]
Stoner 1994a {published data only}
- Stoner E, members of the Finasteride Study Group. Three‐year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology 1994;43(3):284‐94. [DOI] [PubMed] [Google Scholar]
Stoner 1994b {published data only}
- Stoner E, and the Finasteride Study Group. Maintenance of clinical efficacy with finasteride therapy for 24 months in patients with benign prostatic hyperplasia. Archives of Internal Medicine 1994;154:83‐8. [PubMed] [Google Scholar]
Tammela 1995 {published data only}
- Tammela TLJ, Kontturi MJ. Long‐term effects of finasteride on invasive urodynamics and symptoms in the treatment of patients with bladder outflow obstruction due to benign prostatic hyperplasia. The Journal of Urology 1995;154:1466‐9. [PubMed] [Google Scholar]
Tewari 1995 {published data only}
- Tewari A, Shinohara K, Narayan P. Transition zone volume and transition zone ratio: predictor or uroflow response to finasteride therapy in benign prostatic hyperplasia patients. Urology 1995;45(2):258‐65. [DOI] [PubMed] [Google Scholar]
Thompson 2003 {published data only}
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. The New England Journal of Medicine 2003;349(3):215‐24. [DOI] [PubMed] [Google Scholar]
Vaughan 2002 {published data only}
- Vaughan D, Imperato‐McGinley J, McConnell J, Matsumoto AM, Bracken B, Roy J, et al. Long‐term (7 to 8‐year) experience with finasteride in men with benign prostatic hyperplasia. Urology 2002;60:1040‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Barry 1995
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker‐Corkery E, Lepor H. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients?. Journal of Urology 1995;154(5):1770‐5. [DOI] [PubMed] [Google Scholar]
Berry 1984
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. The Journal of Urology 1984;132:474‐9. [DOI] [PubMed] [Google Scholar]
Bonilla 1997
- Bonilla J, Stoner E, Grino P, Binkowitz B, Taylor A. Intra‐ and interobserver variability of MRI prostate volume measurements. The Prostate 1997;31:98‐102. [DOI] [PubMed] [Google Scholar]
Boyle 1996
- Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta‐analysis of randomized clinical trials. Urology 1996;48:398‐405. [DOI] [PubMed] [Google Scholar]
Byrnes 1997
- Byrnes CA, Liss CL, Tenover JL, Lippert MC, Gillenwater JY. Combined analysis of two multicenter studies of finasteride 5 mg in the treatment of symptomatic benign prostatic hyperplasia. Prostate Cancer and Prostatic Diseases 1997;1:26‐31. [DOI] [PubMed] [Google Scholar]
Campbell‐Walsh Urology 2007
- Roehrborn CG, McConnell JD. Chapter 86 ‐ Benign Prostatic Hyperplasia: Etiology, Pathophysiology, Epidemiology, and Natural History. In: Kavoussi LR, Novick AC, Partin AW, Peters CA editor(s). Campbell‐Walsh Urology. 9. Vol. 3, Philadelphia: Saunders Elsevier, 2007. [Google Scholar]
Cochrane Handbook 2008
- Higgins JPT, Green S, editors. Rationale for concern about bias. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. www.cochrane.org/resources/handbook/hbook.htm (accessed 17 February 2009).
Edwards 2002
- Edwards JE, Moore RA. Finasteride in the treatment of clinical benign prostatic hyperplasia: A systematic review of randomised trials. BMC Urology 2002;2(14):17 pages. [PUBMED: 12477383] [DOI] [PMC free article] [PubMed] [Google Scholar]
García‐Perdomo 2015
- García‐Perdomo HA, Lopez HE, Tacklind J. 5‐alpha‐reductase inhibitors for lower urinary tract symptoms secondary to benign prostatic obstruction. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011928] [DOI] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328:1‐8. [Google Scholar]
Litwin, Saigal (editors) 2007
- Wei JT, Calhoun EA, Jacobsen SJ. Benign prostatic hyperplasia. In: Litwin MS, Saigal CS editor(s). Urologic Diseases of America. Washington, DC: US Government Publishing Office, 2007:45‐69. [Google Scholar]
Richardson 1995
- Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well‐built clinical question: a key to evidence‐based decisions. ACP Journal Club 1995;123(3):A12‐3. [PubMed] [Google Scholar]
Rief 2002
- Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG. Meta‐analysis of the placebo response in antidepressant trials. Journal of Affective Disorders 2009;118:1‐8. [DOI] [PubMed] [Google Scholar]
Roehrborn 1998
- Roehrborn CG. Meta‐analysis of randomized clinical trials of finasteride. Urology 1998;51(Suppl 4A):46‐9. [DOI] [PubMed] [Google Scholar]
Stoner 1992b
- Stoner E, Finasteride Study Group. The clinical effects of 5a‐reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Journal of Urology 1992;47:1298‐1302. [DOI] [PubMed] [Google Scholar]
Wessells 2003
- Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, et al. Incidence and severity of sexual adverse experiences in finasteride and placebo‐treated men with benign prostatic hyperplasia. Urology 2003;61:579‐84. [DOI] [PubMed] [Google Scholar]


