Abstract
Background
Controversy exists as to whether adjuvant chemotherapy improves survival in patients with invasive bladder cancer, despite a number of randomised controlled trials.
Objectives
To evaluate the effect of adjuvant chemotherapy in invasive bladder cancer. We conducted a systematic review and meta‐analysis of updated individual patient data from all available randomised controlled trials comparing local treatment plus adjuvant chemotherapy versus the same local treatment alone.
Search methods
MEDLINE and CancerLit searches were supplemented with information from registers and hand searching meeting proceedings and also by discussion with relevant trialists and organisations. They have been regularly updated until September 2004.
Selection criteria
Trials that aimed to randomise patients with biopsy proven invasive (i.e. clinical stage T2 to T4a) transitional cell carcinoma of the bladder to receive local definitive treatment with or without adjuvant chemotherapy were eligible for inclusion.
Data collection and analysis
We collected, validated and re‐analysed updated data on all randomised patients from all available randomised trials, including 491 patients from 6 RCTs. For all outcomes, we obtained overall pooled hazard ratios using the fixed effects model. To explore the potential impact of trial design, we pre‐planned analyses that grouped trials by important aspects of their design that might influence the treatment effect. To investigate any differences in effect by pre‐defined patient sub‐groups, we used a stratified logrank analysis on the primary endpoint of survival.
Main results
Analyses were based on 491 patients from six trials, representing 90% of all patients randomised in cisplatin‐based combination chemotherapy trials and 66% of patients from all eligible trials. The power of this meta‐analysis is clearly limited. The overall hazard ratio for survival of 0.75 (95% CI 0.60 to 0.96, P = 0.019) suggests a 25% relative reduction in the risk of death for chemotherapy compared to that on control. Cox regression suggests that small imbalances in patient characteristics do not bias the results in favour of chemotherapy. However, the impact of trials that stopped early, of patients not receiving allocated treatments or not receiving salvage chemotherapy is less clear.
Authors' conclusions
This IPD meta‐analysis provides the best evidence currently available on the role of adjuvant chemotherapy for invasive bladder cancer. However, at present there is insufficient evidence on which to reliably base treatment decisions. These results highlight the urgent need for further research into the use of adjuvant chemotherapy. The results of appropriately sized randomised trials, such as the ongoing EORTC‐30994 trial are needed before any definitive conclusions can be drawn.
Keywords: Humans; Chemotherapy, Adjuvant; Randomized Controlled Trials as Topic; Survival Analysis; Urinary Bladder Neoplasms; Urinary Bladder Neoplasms/drug therapy; Urinary Bladder Neoplasms/mortality; Urinary Bladder Neoplasms/radiotherapy; Urinary Bladder Neoplasms/surgery
Plain language summary
Adding chemotherapy after surgery or radiotherapy in patients with invasive bladder cancer
Standard treatments for invasive bladder cancer are either surgery (to remove the bladder and surrounding tissues) or radiotherapy (to kill the cancer cells). This review suggested that 54 out of every 100 patients who had chemotherapy after surgery were alive after three years, compared to 45 out of every 100 patients who received only surgery. Although these results are encouraging, there are not enough trials or patients for these results to be completely reliable. More randomised trials are needed. This review should encourage greater participation in ongoing randomised trials.
Background
Bladder cancer is the second most common cancer of the genito‐urinary system. Worldwide, more than 100,000 cases of muscle invasive or advanced disease are diagnosed per year (Parkin 1999), with around 80% occurring in men. Over the last 25 years a number of RCTs (RCTs) have compared local treatment plus adjuvant chemotherapy with local treatment alone (Bono 1997; Einstein 1984; Freiha 1996; Richards 1983; Skinner 1990; Stockle 1995; Studer 1994; Shearer 1988; Otto 2001 (unpublished); Omura (unpublished); Allen (unpublished)). Unfortunately, these trials have been small and lacked the statistical power to be able to reliably assess any effect of chemotherapy.
A previous systematic review of published trials (Parmar 1999) concluded that there was no good evidence to suggest that adjuvant chemotherapy improved the survival of patients with invasive bladder cancer. Furthermore, the reviewers noted a number of flaws in the design and reporting of the trials. A subsequent review of four trials that used cisplatin‐based combination chemotherapy (Bono 1997; Freiha 1996; Skinner 1990; Studer 1994) concluded that the trials provided insufficient evidence to support the routine use of this type of adjuvant chemotherapy in invasive bladder cancer (Sylvester 2000). Criticisms raised by these reviewers related to the design, analysis and reporting of the trials. Firstly, all of the individual trials were underpowered to detect moderate differences between the two arms. Some of the methods used to analyse the individual trials were questionable, for example: not using conventional log rank tests to compare treatment and control arms; including non‐randomised patients and excluding randomised patients, thereby not conducting an intention‐to‐treat analysis. Furthermore, some trials did not clearly define endpoints or report sufficient details of the survival analyses, focusing instead on subgroup analyses based on very small numbers of patients.
Therefore in June 2001, we initiated a systematic review and meta‐analysis of individual patient data (IPD), which involves the central collection, validation and re‐analysis of all randomised patients from all relevant trials. This meta‐analysis was initiated and coordinated by the Medical Research Council (UK) Clinical Trials Unit and was part of a larger project encompassing neoadjuvant chemotherapy and concurrent chemotherapy, the results of which have already been published (ABC MAC 2003). Use of data from individual patients has many advantages (Stewart 1995) that are particularly pertinent in this comparison. With IPD, the ability to carry out detailed data checking and conduct intention‐to‐treat analysis using appropriate statistical methodology may overcome problems relating to the quality of the original analyses and combining the results of all trials in a meta‐analysis will increase the power to detect realistic treatment differences. Therefore, using this methodology, we aimed to provide a better evidence base with which to judge the effect of adjuvant chemotherapy on invasive bladder cancer.
Objectives
We aimed to assess the effect of adjuvant chemotherapy plus standard local treatment (radical cystectomy, radical radiotherapy or preoperative radiotherapy and cystectomy) versus the same local treatment alone.
Methods
Criteria for considering studies for this review
Types of studies
To be included in the meta‐analysis, trials had to be properly randomised. Trials should be closed to patient accrual, with the aim of including all trials that had completed patient recruitment at the time of the final data collation.
Types of participants
Trials should also have aimed to randomise patients with biopsy proven, invasive (i.e. clinical stage T2 to T4a) transitional cell carcinoma of the bladder.
Types of interventions
Patients should have been randomised to receive local definitive treatment with or without adjuvant chemotherapy. The comparison had to be unconfounded by additional agents or interventions. The same local treatment should have been used on each arm, i.e. control and experimental arms had to differ only by the addition of chemotherapy.
Types of outcome measures
The primary endpoint of overall survival was defined as the time from randomisation until death. Patients still alive were censored at the date of last follow up. Overall disease‐free survival was defined as the time from randomisation until first recurrence or progression (after randomisation) or death, whichever occurred first. Locoregional disease‐free survival was defined as the time from randomisation to first locoregional recurrence or progression (after randomisation) or death. Metastases‐free survival was defined as the time from randomisation to first metastases (after randomisation) or death. In each case, patients alive without disease were censored on the date of last follow up. For all endpoints, death was defined as death by any cause.
Search methods for identification of studies
To limit publication bias, published and unpublished trials were included. Computerised bibliographic searches of MEDLINE and CancerLit were done using a version of the Cochrane Collaboration optimal search strategy (Dickersin 1994). These were supplemented by a search of the Cochrane Central Register of Controlled Trials and by hand searches of the reference lists of identified trials, bibliographies of relevant books and review articles. The National Cancer Institute PDQ (Physicians Data Query) Clinical Protocols, United Kingdom Coordinating Committee for Cancer Research trials register and the Current Controlled Trials metaRegister of trials were also searched to identify unpublished and ongoing trials. All trialists who took part in the meta‐analysis were asked to help to identify additional trials. Initial searches were completed for the period up to and including January 1st, 2001. These were revised regularly to identify any additional new material that had appeared by our final analyses in September 2004. Two reviewers independently assessed all titles identified by search strategies for relevance and full papers were obtained for all potentially relevant titles. Where there was uncertainty about the eligibility of a trial or particular treatment arms within a trial, this was discussed and resolved by consensus within the project Secretariat, the international Advisory Group and the members of the ABC Collaborators' Group.
PT=RANDOMIZED‐CONTROLLED‐TRIAL
RANDOMIZED‐CONTROLLED‐TRIAL.DE.
RANDOM‐ALLOCATION.DE.
DOUBLE‐BLIND‐METHOD.DE.
SINGLE‐BLIND‐METHOD.DE.
1 OR 2 OR 3 OR 4 OR 5
PT=CLINICAL‐TRIAL
CLINICAL‐TRIAL#.DE.
(CLIN$ WITH TRIAL$).AB, TI.
((SINGL$ OR DOUBL$ OR TREBL$ OR TRIPL$) WITH (BLIND$ OR MASK$)).AB,TI.
PLACEBO$.DE.
PLACEBO$.AB,TI.
RANDON$.AB,TI.
RESEARCH‐DESIGN.DE
7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14
CARCINOMA#.DE.
BLADDER‐NEOPLASMS.DE.
BLADDER ADJ CARCINOMA$.AB,TI.
BLADDER ADJ CANCER$.AB,TI
BLADDER ADJ NEOPLASM$.AB,TI.
(CANCER WITH BALDDER).AB,TI.
(CARCINOMA WITH BLADDER).AB,TI.
16 AND 17
OR 19 OR 20 OR 21 OR 22
23 OR 24
DRUG‐THERAPY#.DE
QS NEOPLASMS# WITH DT
26 OR 27
RADIOTHERAPY#.DE
QS NEOPLASMS# WITH RT
29 OR 30
SURGERY#.DE
QS NEOPLASMS# WITH SU
32 OR 33
28 OR 31 OR 34
SUPERFICIAL
6 OR 15
37 AND 25 AND 35
38 NOT 36
Data collection and analysis
Up‐to‐date individual patient information on date of randomisation, survival, local recurrence, metastases and date of last follow up was sought. Details of treatment allocated, age, sex, TNM category, grade, performance status, tumour diameter, renal function and pre‐treatment haemoglobin were also collected. To reduce potential bias (Tierney 2005), information was requested for all randomised patients including those who had been excluded from the investigators' original analyses. A number of standard checks were applied to all incoming trials, including checks for missing values, data validity and consistency across variables. To assess the randomisation integrity, we looked for unusual patterns in the sequencing of allocation or imbalances in baseline characteristics between treatment arms. Follow up of patients still alive was also assessed to ensure that it was balanced by treatment arm and as up‐to‐date as possible. Any queries were resolved and the final database entries verified by the responsible trial investigator or statistician. Analyses of all endpoints, subsets and subgroups were pre‐specified in the protocol and carried out on an intention‐to‐treat basis; that is, patients were analysed according to their allocated treatment, irrespective of whether they received that treatment. Analyses of all endpoints were stratified by trial, and the log rank expected number of deaths and variance used to calculate individual trial hazard ratios and overall pooled hazard ratios (HR) based on the fixed effect model (Yusuf 1985). Thus, the times to event (recurrence, progression or death) for individual patients were used within trials to calculate the HR, representing the overall risk of an event for those patients allocated to adjuvant chemotherapy compared with those allocated to no chemotherapy. (NB Hazard ratios entered via the IPD outcome type in RevMan are labelled as Peto ORs in all of the forest plots.)
To examine the potential impact of trial design and the treatments used, we prospectively planned analyses that grouped trials by important aspects that might influence the effect of chemotherapy. Groups were defined according to the type of the chemotherapy regimen and also by the local treatment. For each of these analyses, a pooled HR was calculated for each group of trials and for all trials together. A Chi2 test for interaction was used to test whether there were any substantial differences in the effect of adjuvant chemotherapy between the trial groups. The effects of chemotherapy within subgroups of patients were investigated using similar analyses. Analyses were performed for each pre‐specified subgroup, for example, comparing treatment and control for males and for females within each individual trial. These results were then combined to give overall HRs for males and for females. These analyses focused on the primary endpoint of overall survival. However they were conducted for the other endpoints to help support or refute any patterns found.
Results are also presented as absolute differences at three years, calculated using the overall HRs and the control arm event rate (Parmar 1995). Confidence intervals for absolute differences were calculated from the baseline event rate and the HR at the 95% confidence interval boundary values. Chi2 heterogeneity tests were used to test for statistical heterogeneity across trials. We also calculated the I2 statistic (Higgins 2003) to measure any inconsistency between the trials. Chi2 tests for interaction or trend were used to test for differences in outcome between subsets of trials or between subgroups of patients. Survival curves are presented as simple (non‐stratified) Kaplan‐Meier curves (Kaplan 1958). All P values quoted are two‐sided.
Exploratory analyses of survival In addition to the planned analyses described, we conducted supplementary analyses to investigate some of the previous criticisms of these trials in more detail. To assess whether modest imbalances impact on (a) the results of individual trials and (b) the pooled results over all trials, we performed Cox regression analyses, stratified by trial, including in the model terms for age, sex, grade, pT and pN categories. Because data on every variable were not available for all patients from each trial, a proportion of patients were necessarily lost from these analyses. Therefore, we conducted a second, unadjusted Cox regression analysis stratified by trial based on the same subset of patients, so that direct comparisons could be made.
To investigate whether the collection and analysis of updated follow up was able to counter any potential effects of early stopping in these trials, we estimated HRs from the trial publications using the reported statistics or from the survival curves (Parmar 1998) and compared these with HRs obtained from updated IPD.
Results
Description of studies
We identified 11 RCTs that had used adjuvant chemotherapy, all of which were potentially eligible for inclusion. We understand that one trial (Allen (unpublished)) failed to recruit any patients and was therefore considered ineligible. One further trial had given chemotherapy both before and after local treatment (Shearer 1988) and was therefore considered separately (ABC MAC 2003). This left 9 trials that were eligible for inclusion (see 'Table of included studies'). We were unable to locate data for two trials; one of 129 patients (Richards 1983), and one of 80 patients (Einstein 1984). A third trial (Omura (unpublished)) closed early due to a lack of funding after randomising 42 patients. No data were available for this trial. We therefore included 6 trials (Otto (unpublished) and Bono 1997; Freiha 1996; Freiha 1996; Skinner 1990; Stockle 1995; Studer 1994) that randomised 498 patients (see 'Table of included studies'). IPD were supplied for 493 of these patients because data on 5/43 patients, who had been excluded from the investigators' own analyses, could not be obtained. The 493 patients include 90% (402 patients) of all patients randomised in adjuvant cisplatin‐based combination chemotherapy trials and represent 66% of individuals from all known randomised trials.
Patient accrual for the individual trials ranged from 49 to 108. Design features of these trials are summarised in the 'Table of included studies'. For all of these trials, the planned local treatment was cystectomy and all trials used cisplatin‐based chemotherapy; one as a single agent (Studer 1994) and five in combination with one or more of methotrexate, vinblastine, cyclophosphamide and either doxorubicin or epirubicin. The planned cisplatin doses ranged from 90 mg/m2 per cycle for 2 cycles to 100 mg/m2 per cycle for 4 cycles, every 3 to 4 weeks.
Four of the six trials (293/493 patients) stopped early; three because the results of interim analyses favoured chemotherapy (Freiha 1996; Skinner 1990; Stockle 1995) and the fourth because interim results showed less benefit of chemotherapy than had been anticipated (Studer 1994). Updated follow up was supplied for all surviving patients for two of these four trials (Skinner 1990; Stockle 1995) and for a proportion of the surviving patients from one further trial (Studer 1994). Further details are provided in 'Table 1'. We found no good evidence that important patient characteristics such as age, grade or stage were imbalanced by arm for individual trials. For all trials together, there was a slight imbalance by age group, although the median age was comparable in both arms ('Table 2').
1. Summary of trial details (included trials only).
| Bono | Freiha | Skinner | Stockle | Studer | Otto | |
| Randomisation / allocation method | Central; blocked in groups of 10 | Simple randomisation, sealed envelope | Central telephone; minimisation | Stratified by nodal status | Locked randomisation list | Central telephone; permuted blocks |
| Patients randomised | 93 | 55 | 102 | 91 | 49 | 108 |
| Patients excluded by investigator | 12 | 5 | 11 | 14 | 0 | 0 |
| No. excluded patients reinstated in the meta‐analysis | 9 | 1 | 11 | 14 | n/a | n/a |
| Stopped early | No | Yes | Yes | Yes | Yes | No |
| Reason for stopping early | n/a | Patients in control arm performed better than anticipated therefore many more than 80 patients would be needed to show a survival benefit | Planned analysis after 75 patients showed a significant benefit of chemotherapy. Trial continued for further 2 years | Interim analysis of 80 patients showed smaller difference than anticipated. Accrual rate too slow therefore trial stopped | Significant advantage in favour of chemotherapy for recurrence free survival (P=0.0015) | n/a |
| Accrual period | Aug 1983 ‐ Oct 1987 | Mar 1986 ‐ Oct 1991 | Jul 1980 ‐ May 1989 | Jan 1984 ‐ May 1989 | May 1987 ‐ Aug 1990 | Jan 1993 ‐ Jun 1999 |
| Follow up updated for meta‐analysis | 20 patients (8.76 ‐ 18.44 years) | no | 28 patients (11.51 ‐ 20.25 years) | 6 patients (12.76‐17.29 years) | 10 patients (13.83‐16.13 years) | Not necessary as all up‐to‐date |
| Follow up not updated for meta‐analysis | 24 patients (0.57‐3.62 years) | 21 patients (2.17‐7.75 years) | n/a | 30 patients (3.06‐8.20 years) | n/a | 51 patients (0.65‐7.18 years) |
| Median follow up | 3.45 years | 5.08 years | 14.54 years | 6.09 years | 14.83 years | 3.62 years |
| No. patients lost to follow up | 3 | 0 | 0 | 10 | 0 | 0 |
| Follow up balanced by arm | Yes | Yes | Yes | Yes | Yes | Yes |
2. Characteristics of 491 analysed patients.
| Subgroup | Adj CT (246 pts) | Control (245 pts) | Total (491 pts) |
| Age | |||
| Median age (interquartile range) | 61.5 (55‐67) | 62.0 (57‐68) | 62.0 (56‐67) |
| Range | 23‐76 | 30‐85 | 23‐85 |
| <55 | 60 (24%) | 43 (18%) | 103 (21%) |
| 55‐64 | 92 (37%) | 105 (43%) | 197 (40%) |
| >=65 | 94 (38%) | 97 (40%) | 191 (39%) |
| Unknown | 0 | 0 | 0 (0%) |
| Sex | |||
| Male | 205 (83%) | 196 (80%) | 401 (82%) |
| Female | 41 (17%) | 49 (20%) | 90 (18%) |
| Unknown | 0 | 0 | 0 (0%) |
| pT category | |||
| T0‐1 | 10 (5%) | 8 (3%) | 18 (4%) |
| T2 | 25 (10%) | 35 (14%) | 60 (12%) |
| T3 | 144 (59%) | 147 (60%) | 291 (59%) |
| T4 | 37 (15%) | 33 (13%) | 70 (14%) |
| Unknown | 30 (12%) | 22 (9%) | 52 (11%) |
| pN category | |||
| N0 | 149 (61%) | 156 (64%) | 305 (62%) |
| N1‐2 | 85 (35%) | 81 (33%) | 166 (34%) |
| Nx / unknown | 12 (5%) | 8 (3%) | 20 (4%) |
| Grade | |||
| G0‐1 | 7 (3%) | 9 (4%) | 16 (3%) |
| G2 | 30 (12%) | 30 (12%) | 60 (12%) |
| G3 | 155 (63%) | 155 (63%) | 310 (63%) |
| G4 | 40 (16%) | 40 (16%) | 80 (16%) |
| Unknown | 14 (6%) | 11 (4%) | 25 (2%) |
Data on allocated treatment were missing for two patients from one trial (Bono 1997) and therefore these patients are excluded from the analyses. Patients' characteristics for the remaining 491 patients across all trials are shown in 'Table 2'. Data for age and sex were provided for all trials. Pathological T and N categories and grade were supplied for five trials. Performance status, tumour diameter and renal function could only be supplied in full for one trial, although two others were able to provide some data on renal function. Pre‐treatment haemoglobin was not supplied in full for any of the trials. Based on these available data, patients were mostly male with a median age of 62 years (range 23 to 85 years). They had tumours that were predominantly pT3, grade 3. The median follow up for all surviving patients was 5.2 years (range 0.1 to 14.8 years).
Risk of bias in included studies
All data were thoroughly checked for validity, consistency, plausibility and integrity of randomisation and follow up. Any queries were resolved and the final database entries verified by a responsible investigator, data manager or statistician.
Effects of interventions
Overall Survival Survival analyses were based on 283 events and 491 patients from 6 trials. The confidence intervals around the estimated HR for these trials are wide and so the individual results are inconclusive. There was no clear evidence of statistical heterogeneity or inconsistency between the trials (Chi2=2.25, P = 0.814; I2 = 0%). The overall hazard ratio of 0.75 (95% CI 0.60 to 0.96) represents a 25% relative decrease in the risk of death on chemotherapy compared with that on control. This is conventionally significant (P = 0.019), and is equivalent to an absolute improvement in survival of 9% (95% CI 1% to 16%) at three years. With this number of patients, it is possible to reliably detect an absolute effect in the order of 15% (80% power, 5% significance). The survival curve for these results is shown in 'Figure 1'.
1.
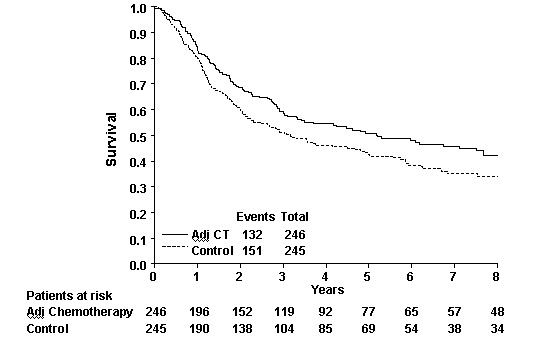
Survival Curve (all trials)
Pre‐planned analyses grouping trials according to whether cisplatin was used or not, or by the type of local treatment employed were not possible, as all trials used cystectomy as the local treatment and used a cisplatin‐based chemotherapy regimen. However, an analysis of trials grouped according to whether they had used single‐agent cisplatin or cisplatin‐based combination chemotherapy was possible, although it was limited by small numbers and the fact that only one trial used single agent cisplatin therapy. The hazard ratio (HR) for the one trial that gave cisplatin as a single agent was 1.02 (95% CI 0.57 to 1.84, P = 0.945). For the group of five trials (400 patients) that used cisplatin‐based combination chemotherapy the pooled HR of 0.71 (95% CI 0.55 to 0.92, P = 0.010) represents a 29% relative decrease in the risk of death on chemotherapy compared to that on control. There was no evidence of a difference in the effect of chemotherapy between these two groups of trials (interaction Chi2 = 1.20, P = 0.237).
Disease‐free survival Data on overall disease‐free survival was supplied for five trials including 383 patients and 239 events. One trial could not provide data on recurrence or metastases and so could only be included in the analysis of overall survival (Otto (unpublished)). The overall HR of 0.68 (95% CI 0.53 to 0.89) represents a 32% relative decrease in the risk of recurrence or death on chemotherapy compared to that on control (P = 0.004). There was no clear evidence of statistical heterogeneity or inconsistency between the trials (Chi2 = 4.80, P = 0.308; I2 = 0%). This is equivalent to an absolute improvement in disease‐free survival of 12% (95% CI 4% to 19%) at three years. For the group of four trials (292 patients) that used cisplatin‐based combination chemotherapy the combined HR of 0.62 (95% CI 0.46 to 0.83, P = 0.001) represents a 38% relative decrease in the risk of recurrence or death on chemotherapy compared to that on control.
Data on locoregional disease‐free survival and metastases‐free survival were only available for 2 trials that included 192 patients, with 113 events (locoregional disease‐free survival) and 115 events (metastases‐free survival). These analyses were therefore extremely limited due to the low numbers of patients and are not presented here. Further details are available on request.
Subgroup analyses Predefined patient subgroups analyses were extremely limited due to the low numbers of patients and are therefore, exploratory in nature. Nevertheless, we found no evidence to suggest that chemotherapy was any more (or less) effective in any of the patient subgroups based on age, sex, grade, pT and pN category. Further pre‐planned analyses of performance status, pre‐treatment haemoglobin and tumour diameter were not possible because sufficient data were not available.
Exploratory analyses of survival
(i) Cox regression analyses Although based on fewer patients and events than the log rank tests, the results for the unadjusted Cox model were broadly similar to those from the log rank test, both for all trials and for the combination chemotherapy trials together ('Table 3'). Overall, there was a slight imbalance by age across all trials, with a slightly higher proportion of younger patients in the chemotherapy arm than the in the control arm. This meant that when the model was adjusted for age alone, the estimate of effect moved towards equivalence compared with the unadjusted analysis. However, when all of the baseline characteristics (age, sex, grade, pT and pN) were taken into account, the Cox regression survival analysis tended more in favour of adjuvant chemotherapy, suggesting that overall, the proportion of poor prognosis patients was greater in the chemotherapy arm.
3. Overall survival results from the Logrank test and Cox regression model.
| Events | Patients | HR | 95% CI | P‐value | |
| All trials | |||||
| Logrank test stratified by trial | 283 | 491 | 0.75 | 0.60‐0.96 | 0.019 |
| Unadjusted Cox model, stratified by trial | 238 | 418 | 0.71 | 0.55‐0.92 | 0.007 |
| Cox model, stratified by trial (including arm and age only) | 238 | 418 | 0.73 | 0.56‐0.95 | 0.017 |
| Cox model, stratified by trial (including arm, age, sex, grade, pT and pN categories) | 238 | 418 | 0.69 | 0.53‐0.89 | 0.005 |
| Combination CT trials only | |||||
| Logrank test stratified by trial | 238 | 400 | 0.71 | 0.55‐0.92 | 0.010 |
| Unadjusted Cox model, stratified by trial | 202 | 347 | 0.67 | 0.51‐0.89 | 0.006 |
| Cox model, stratified by trial (including arm and age only) | 202 | 347 | 0.70 | 0.53‐0.93 | 0.014 |
| Cox model, stratified by trial (including arm, age, sex, grade, pT and pN categories) | 202 | 347 | 0.65 | 0.49‐0.86 | 0.003 |
(ii) Comparison with published results For the three trials that stopped early and provided updated follow up (Freiha 1996; Skinner 1990; Stockle 1995) HRs based on the original reported analyses were estimated using data presented in the publications of the individual trials. These were compared with HRs calculated from updated IPD. For each of the three trials, the HRs estimated at the time of the original analysis were more strongly in favour of adjuvant chemotherapy than those obtained from the updated IPD ('Table 4').
4. Estimates of effect from publications and from IPD with updated follow‐up.
| Skinner | Studer (a) | Stockle (b) | |
| Endpoint analysed | Survival | Survival | Disease‐free survival* |
| % patients with updated follow up since published analysis** | 100 | 22 | 100 |
| HR derived from published statistics or survival curves | 0.65 | 0.86 | 0.39 |
| HR from IPD | 0.75 | 1.02 | 0.45 |
| (a) HR derived from published survival curve (b) Used single agent cisplatin * DFS shown as overall survival not reported **Where not reported, date of analysis estimated as 12 months prior to publication date |
Discussion
This meta‐analysis aimed to address the question of whether adjuvant chemotherapy improves survival of patients with invasive bladder cancer. We obtained IPD for six trials, including 90% of the total patients randomised in adjuvant cisplatin‐based combination chemotherapy trials (66% of the total randomised patients in all adjuvant chemotherapy trials). However, in spite of combining data from all of these trials, this meta‐analysis was limited by small numbers, with only 491 patients and 283 deaths. The overall hazard ratio for all trials of 0.75 suggests an absolute improvement in survival of 9% (95% CI 1% to 16%) at 3 years; 11% (95% CI 3% to 18%) for those trials that used cisplatin‐based combination chemotherapy. However, this analysis was further limited, to only 400 patients and 238 deaths and we are therefore unable to provide a definitive comment on the true effect of this therapy.
Because we have analysed IPD, we have been able to address some of the prior criticisms of these trials. For example, use of inappropriate or non‐standard statistical tests, not reporting overall survival results and over‐emphasing subgroup analyses based on very small numbers of patients. Furthermore, we found no clear imbalances in known prognostic factors such as age, pathological stage or grade between the arms of individual trials. Minor imbalances did not seem to bias the results in favour of adjuvant chemotherapy. However, even with the collection and re‐analysis of IPD, there are some issues that we have not been able to address. In two trials (Stockle 1995; Skinner 1990) around a quarter of patients randomised to receive chemotherapy did not receive it; many received no chemotherapy at all and others received regimens other than those described in the trial protocol. The most likely influence of this on our results would be to dilute the apparent effect of chemotherapy. In contrast, four trials (Stockle 1995; Freiha 1996; Skinner 1990; Bono 1997) did not specify salvage chemotherapy for patients on the control arm whose disease progressed or recurred, with a likely consequence of exaggerating the estimate in favour of chemotherapy. It should be noted though, that where such data were available, we found that many patients did in fact receive additional salvage treatments, including chemotherapy ('Figure 2'). It is difficult therefore to assess the extent to which these conflicting factors could be influencing the results of this meta‐analysis. Systematic removal of any of these patients could introduce other biases into the analysis.
2.
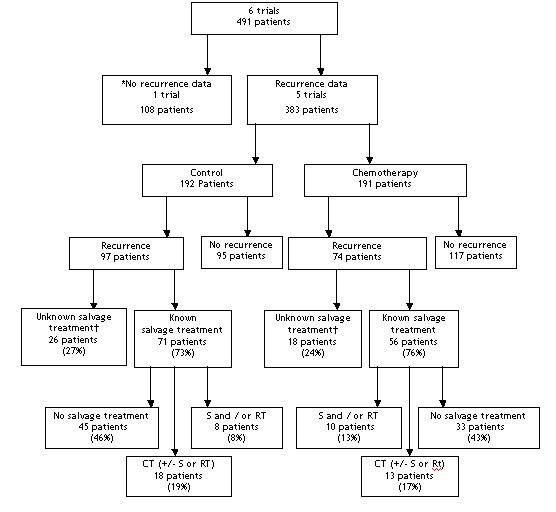
Recurrence and treatment for recurrence flow diagram * Otto et al (unpublished) supplied overall survival data only. Patients who recurred on the control arm received 2 cycles of MVEC (PJ Goebell, pers comm.). † Treatment on recurrence data not available for 3 trials [4‐6]. However, for one trial [6] patients randomised to control were treated with standard CMV on first evidence of recurrence and for another trial [5] there was no restriction on patients randomised to control arm receiving chemotherapy on recurrence / progression. For the final trial [4] patients randomised to the control arm were not recommended to receive chemotherapy on recurrence.
Trials that stop early following favourable interim analyses can unduly influence the results of a meta‐analysis, although obtaining updated follow up may go some way to redressing the effects of trials that stopped on a 'random high', even without additional accrual into the trial (Green 1987). However, if early treatment effects reflect differences between patients in the two treatment arms or some other type of selection bias, then extended follow up is unlikely to make a difference. Any inflated benefits seen in early analyses are likely to persist. In this meta‐analysis, three trials stopped early because of favourable interim results. The underlying reasons why the results of these trials led to their being stopped earlier than planned remain unclear. We have been able to show that in all of the individual trials, the arms are balanced at least in terms of known prognostic factors, such as age, sex, grade and pT category. However, other subtle imbalances in the known or perhaps more importantly, in unknown prognostic factors could exist. For those trials with extended follow up a comparison of the results estimated from the trial reports (Parmar 1998) with the results obtained from updated IPD showed that the latter tended more towards equivalence with the estimate of treatment effect being reduced.
In interpreting these results, we should also consider that IPD for three further eligible trials (251 patients) were unavailable. Despite the potential problems of using information from published analyses when IPD is not available, we thought it important to consider how the results of the unavailable trials might impact on these findings. However, one trial was never published (Omura (unpublished)) and another did not publish survival data (Einstein 1984). Therefore, we were only able to estimate a HR (Parmar 1998) for one trial of 129 patients (Richards 1983). The inclusion of this estimate (HR = 0.97) in a sensitivity analysis had little impact on the pooled HR estimate for the meta‐analysis, changing it from 0.75 to 0.77. It should be acknowledged that this trial used a different local treatment (radiotherapy) to all of the other trials and was also unique in using a non‐cisplatin based regimen. It is also worth noting that even if IPD had been available from all of the unavailable trials, we would have still fallen short of the 900 events needed to reliably detect a 9% absolute survival benefit with 80% power (5% significance).
Authors' conclusions
Implications for practice.
Despite being limited by small numbers and by the caveats described, this IPD meta‐analysis of all available data, using gold standard methodology, provides the best information currently available on the role of adjuvant chemotherapy for invasive bladder cancer. We conclude that the current evidence is clearly limited with too few trials and too few patients on which to base reliable treatment decisions.
Implications for research.
It is clear that the results of additional appropriately sized RCTs are required before a definitive answer can be obtained. Ongoing studies such as the EORTC‐30994 trial and the USC p53 trial are therefore of particular importance. If these reach their recruitment targets, around 2000 additional patients will have taken part in relevant RCTs, and will provide the power needed to detect realistic treatment effects reliably. However, we recognise that adjuvant chemotherapy is already being used in the treatment of patients with invasive bladder cancer. The results presented here should encourage this only in the context of ongoing trials. We hope that the results draw the attention of the urological oncology community to consider the need for extensive participation in such ongoing and future randomised trials on this subject.
What's new
| Date | Event | Description |
|---|---|---|
| 15 March 2011 | Amended | Minor edits |
History
Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
| 22 February 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to the British Medical Research Council for funding this work. We would like to thank all those patients who took part in these trials and contributed to this research. The meta‐analysis would not have been possible without the collaborating institutions that kindly supplied their trial data or without the help of those responsible for maintaining, updating and preparing trial data.
Data and analyses
Comparison 1. Adjuvant chemotherapy + local treatment vs. local treatment alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall Survival | 6 | 491 | Peto Odds Ratio (95% CI) | 0.75 [0.60, 0.96] |
| 1.1 Single agent cisplatin | 1 | 91 | Peto Odds Ratio (95% CI) | 1.02 [0.57, 1.84] |
| 1.2 Cisplain‐based combination chemotherapy | 5 | 400 | Peto Odds Ratio (95% CI) | 0.71 [0.55, 0.92] |
| 2 Disease‐free survival | 5 | 382 | Peto Odds Ratio (95% CI) | 0.68 [0.52, 0.88] |
| 2.1 Single agent cisplatin | 1 | 90 | Peto Odds Ratio (95% CI) | 1.02 [0.57, 1.82] |
| 2.2 Cisplatin based combination chemotherapy | 4 | 292 | Peto Odds Ratio (95% CI) | 0.61 [0.46, 0.81] |
1.1. Analysis.
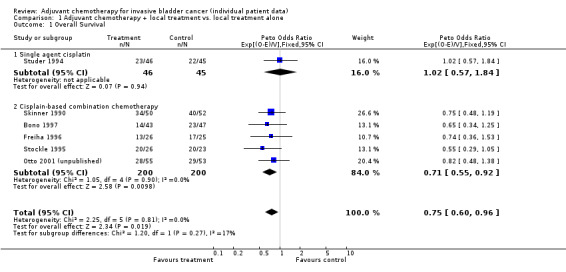
Comparison 1 Adjuvant chemotherapy + local treatment vs. local treatment alone, Outcome 1 Overall Survival.
1.2. Analysis.
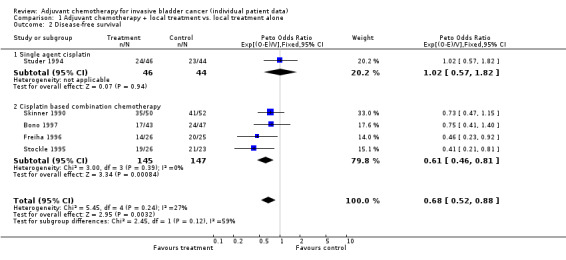
Comparison 1 Adjuvant chemotherapy + local treatment vs. local treatment alone, Outcome 2 Disease‐free survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bono 1997.
| Methods | RCT 1983‐1987 | |
| Participants | 93 T2‐T4a, N0, M0 | |
| Interventions | Surgery + Adjuvant CT vs. Surgery alone Cisplatin 70mg/m2 Methotrexate 40 mg/m2 4 cycles |
|
| Outcomes | Survival Disease‐free survival | |
| Notes | 9/12 patients excluded by investigator have been reinstated in the meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Freiha 1996.
| Methods | RCT 1986‐1991 | |
| Participants | 55 T3b‐T4, any N, M0 | |
| Interventions | Surgery + Adjuvant CT vs. Surgery alone Cisplatin 100mg/m2 Methotrexate 30mg/m2 Vinblastine 4mg/m2 4 x 3‐weekly cycles |
|
| Outcomes | Survival Disease‐free survival | |
| Notes | Stopped early 1/5 patients excluded by investigator have been reinstated in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Otto 2001 (unpublished).
| Methods | RCT 1993‐1999 | |
| Participants | 108 T3, N1‐2, M0 | |
| Interventions | Surgery + Adjuvant CT vs. Surgery alone Cisplatin 70mg/m2 Methotrexate 30mg/m2 Vinblastine 3mg/m2 Epirubicin 45mg/m2 3 x 4‐weekly cycles |
|
| Outcomes | Survival | |
| Notes | Unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Skinner 1990.
| Methods | RCT 1980‐1989 | |
| Participants | 102 T3‐T4, N+, M0 | |
| Interventions | Surgery + Adjuvant CT vs. Surgery alone Cisplatin 100mg/m2 Cyclophosphamide 600mg/m2 4 x 4‐weekly cycles |
|
| Outcomes | Survival Disease‐free survival | |
| Notes | Stopped early 11/11 patients excluded by investigator have been reinstated in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Stockle 1995.
| Methods | RCT 1987‐1990 | |
| Participants | 49 T3b‐T4a | |
| Interventions | Surgery + Adjuvant CT vs. Surgery alone Cisplatin Methotrexate Vinblastine Doxorubicin 3 cycles |
|
| Outcomes | Survival Disease‐free survival | |
| Notes | Stopped early | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Studer 1994.
| Methods | RCT 1984‐1989 | |
| Participants | 91 T1 (grade 2) ‐ T4 | |
| Interventions | Surgery + Adjuvant CT vs. Surgery alone Cisplatin 90mg/m2 2 x 4‐weekly cycles |
|
| Outcomes | Survival Disease‐free survival | |
| Notes | Stopped early Single agent cisplatin chemotherapy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen (unpublished) | Trial failed to recruit any patients |
| Einstein 1984 | Unable to locate data |
| Omura (unpublished) | No data available |
| Richards 1983 | Unable to locate data |
| Shearer 1988 | Chemotherapy was given before and after local treatment therefore trial considered separately |
Contributions of authors
All aspects of the meta‐analysis were carried out under the auspices of the ABC group. A V Bono, P J Goebell, S Groshen, J Lehmann, U Studer and F M Torti collated and supplied the individual patients data, contributed to the discussions of the results and commented on the drafts of the report. H Abol‐Enein, P Bassi, M Boyer, C M L Coppin, E Cortesi, R R Hall, A Horwich, P‐U Malmström, J A Martinez‐Piñeiro, L Sengeløv, A Sherif and D M A Wallace contributed to the discussions of the results and commented on the drafts of the report. The project was organised by the Advisory Group, N W Clarke, J T Roberts and R Sylvester and the Secretariat, M K B Parmar, L A Stewart, J F Tierney and C L Vale, who were responsible for formulating the questions, developing the protocol and discussing the preliminary results. The secretariat, MKB Parmar, L A Stewart, J F Tierney and C L Vale, were responsible for receiving, checking and analysing data. C L Vale managed the project and drafted the report, with detailed input from J F Tierney, L A Stewart and M K B Parmar. None of these authors have declared any conflict of interest in connection with this research.
Sources of support
Internal sources
Medical Research Council, UK.
External sources
No sources of support supplied
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Bono 1997 {published and unpublished data}
- Bono AV, Benvenuti C, Gibba A, Guazzeri S, Cosciani‐Cunico S, Anselmo G, Martini E, Parma G, Ferrari P, Viggiano G. Adjuvant chemotherapy in locally advanced bladder cancer. Final analysis of a controlled multicentre study. Acta Urol Ital 1997;11(1):5‐8. [Google Scholar]
Freiha 1996 {published and unpublished data}
- Freiha F, Reese J, Torti F.M. A Randomised Trial of Radical Cystectomy versus Radical Cystectomy plus Cisplatin, Vinblastine and Methotrexate Chemotherapy for Muscle Invasive Bladder Cancer. Journal of Urology 1996;155:495‐500 4495‐500. [PubMed] [Google Scholar]
Otto 2001 (unpublished) {unpublished data only}
- Otto T, et al. University of Essen RCT of adjuvant chemotherapy in bladder cancer. Unpublished 2001.
Skinner 1990 {published and unpublished data}
- Skinner DG, Daniels JR, Russel CA, Lieskovsky G, Boyd SD, Krailo M, Groshen S. Adjuvant chemotherapy following cystectomy benefits patients with deeply invasive bladder cancer. Seminars in Urology 1990;8(4):279‐284. [PubMed] [Google Scholar]
Stockle 1995 {published and unpublished data}
- Stöckle M, Meyenburg W, Wellek S, Voges GE, Rossman M, Gertenbach U, Thüroff JW, Huber C, Hohenfellner R. Adjuvant polychemotherapy of non‐organ confined bladder cancer after radical cystectomy revisited: long term results of a controlled prospective study and further clinical experience. Journal of Urology 1995;153:47‐52. [DOI] [PubMed] [Google Scholar]
Studer 1994 {published and unpublished data}
- Studer UE, Bacchi M, Biedermann C, Jaeger P, Kraft R, Mazzucchelli L, Markwalder R, Senn E, Sonntag RW. Adjuvant Cisplatin Chemotherapy following cystectomy for bladder cancer: Results of a prospective randomised trial. Journal of Urology 1994;152:81‐84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen (unpublished) {published data only (unpublished sought but not used)}
- Allen TD. Phase III Randomized Evaluation of Postoperative Chemotherapy with CACP for Invasive Nonmetastatic Transitional Cell Carcinoma of the Bladder (NCI‐D78‐044‐145). Unpublished.
Einstein 1984 {published data only}
- Einstein AB, Shipley W, Coombs J, Cummings KB, Soloway MS, Hawkins I, for the National Bladder Cancer Cooperative Group. Cisplatin as adjunctive treatment for invasive bladder carcinoma: Tolerance and toxicities. Urology 1984;23(4 (suppl)):110‐117. [DOI] [PubMed] [Google Scholar]
Omura (unpublished) {published data only (unpublished sought but not used)}
- Omura G. Phase III Adjuvant Chemotherapy with CTX/ADR/CACP for Resected Transitional Cell Bladder Carcinoma (SEG‐78BL339). Unpublished.
Richards 1983 {published data only}
- Richards B, Bastable JRG, Freedman L, Glashan RW, Harris G, Newling DWW, Robinson MRG, Smith PH. chemotherapy with doxorubicin, and 5‐florouracil in T3, NX, MO bladder cancer treated with radiotherapy. British Journal of Urology 1983;55:386‐391. [DOI] [PubMed] [Google Scholar]
Shearer 1988 {published data only (unpublished sought but not used)}
- Shearer RJ, Chilvers CED, Bloom HJG, Bliss JM, Horwich A, Babiker A. Chemotherapy in T3 Carcinoma of the Bladder A prospective trial: preliminary report. British Journal of Urology 1988;62:558‐564. [DOI] [PubMed] [Google Scholar]
Additional references
ABC MAC 2003
- Advanced Bladder Cancer (ABC) Meta‐analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta‐analysis. Lancet 2003;361:1927‐1934. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. British Medical Journal 1994;309:1286‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 1987
- Green SJ, Fleming TR, Emerson S. Effects of overviews of early stopping rules for clinical trials. Statistics in Medicine 1987;6:361‐367. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 1958
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statistical Association 1958;53:457‐481. [Google Scholar]
Parkin 1999
- Parkin DM, Pisani P, Ferlay J. Global Cancer Statistics. CA: a Cancer Journal for Clinicians 1999;49:33‐64. [DOI] [PubMed] [Google Scholar]
Parmar 1995
- Parmar, Mahesh K. B, Machin, David. Survival analysis: a practical approach. John Wiley & Sons Ltd, 1995. [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Parmar 1999
- Parmar Mahesh K. B, Burdett Sarah. Neoadjuvant and adjuvant chemotherapy. In: Hall RR editor(s). Clinical management of bladder cancer. 1. London: Arnold, 1999:249‐263. [Google Scholar]
Stewart 1995
- Stewart LA, Clarke MJ, on behalf of the Cochrane Working Party Group on Meta‐analysis using Individual Patient Data. Practical methodology of meta‐analyses (overviews) using updated individual patient data. Statistics in Medicine 1995;14:2057‐2079. [DOI] [PubMed] [Google Scholar]
Sylvester 2000
- Sylvester R, Sternberg C. The role of adjuvant combination chemotherapy after cystectomy in locally advanced bladder cancer: what we do not know and why. Annals of Oncology 2000;11:851‐856. [DOI] [PubMed] [Google Scholar]
Tierney 2005
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34:79‐87. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Progress in Cardiovascular Disease 1985;27:335‐371. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
ABCMAC 2005
- Advanced Bladder Cancer (ABC) Meta‐analysis Collaboration. Adjuvant Chemotherapy in Invasive Bladder Cancer: A Systematic Review and Meta‐analysis of Individual Patient Data. European Urology 2005;In press. [DOI] [PubMed] [Google Scholar]


