Abstract
Objective:
For persons in states of disordered consciousness (DoC) after severe traumatic brain injury (sTBI), we report cumulative findings from safety examinations, including serious adverse events (AEs) of a repetitive transcranial magnetic stimulation (rTMS) parameter protocol in 2 different studies.
Participants:
Seven persons in states of DoC after sTBI with widespread neuropathology, but no large lesions in proximity to the site of rTMS. One participant had a ventriculoperitoneal shunt with programmable valve.
Methods:
Two clinical trials each providing 30 rTMS sessions to the right or left dorsolateral prefrontal cortex, involving 300 to 600 pulses over 1 or 2 sessions daily. One study provided concomitant amantadine. Safety indicators monitored related to sleep, temperature, blood pressure, skin integrity, sweating, weight loss, infections, and seizure.
Results:
Average changes for monitored indicators were of mild severity, with 75 nonserious AEs and 1 serious AE (seizure). The participant incurring a seizure resumed rTMS while taking antieplieptics without further seizure activity.
Conclusions:
Considering elevated risks for this patient population and conservative patient selection, findings indicate a relatively safe profile for the specified rTMS protocols; however, potential for seizure induction must be monitored. Future research for this population can be broadened to include patients previously excluded on the basis of profiles raising safety concerns.
Keywords: amantadine, disorders of consciousness, seizure, severe traumatic brain injury, transcranial magnetic stimulation
NEUROMODULATION TREATMENTS using repetitive transcranial magnetic stimulation (rTMS) are being explored for many conditions. When provided within specified safety guidelines,1 there is generally a low incidence of serious adverse events (AEs). Developing rTMS as a neuromodulatory intervention for severe traumatic brain injury (sTBI) is particularly challenging because of a limited understanding of rTMS safety for persons who have sustained gross insults to their brain tissue. The most concerning rTMS-related AE is status epilepticus; thus, rigorous safety monitoring is critical. This is especially true considering (i) risk of seizure after TBI is as high as 20% or more in the years following TBI, depending on severity and other characteristics of the TBI,2 and (ii) persons remaining in states of disordered consciousness (DoC) after sTBI are more prone to a variety of infections that may further predispose them to seizures.
For persons remaining in states of DoC, our lab’s treatment development studies include investigating the therapeutic effects of rTMS concomitant with amantadine (AMA). AMA continues to be investigated as a therapeutic agent for improving level of consciousness and functional recovery for persons remaining in states of DoC.3–6 The seizure risk for rTMS paired with AMA is unknown; theoretically, AMA by itself may lower seizure threshold. A recent systematic review reports a 0.7% prevalence of seizure (2/259 patients) with 100 to 200 mg twice a day of AMA.7 High doses of AMA (ie, 800 mg/d) can cause epileptiform activity, but these high doses are unlikely to be used in daily practice as they are considered toxic.8
In addition to elevated seizure risk, rTMS for individuals with sTBI may be less precise; how this potential imprecision alters safety is unknown. Prior skull fractures, craniotomy, craniectomy, and missing tissue could affect the magnetic field such that electrical currents induced by rTMS are either lower in amplitude or more scattered and diffuse than intended.9 Posttraumatic structural changes could also alter neurophysiologic effects of rTMS. The consequences of these unintentional rTMS-induced neural effects are not well understood, in part, because few patients with complex neurotrauma have undergone rTMS.
Advancing therapeutic utility of rTMS with sTBI can be done only after or in conjunction with studying safety of this procedure within established safety guidelines. While there is an increase in the number of studies assessing different rTMS protocols for the treatment of DoC due to TBI, there remains a lack of published details on safety monitoring to sufficiently advance our understanding of rTMS safety.10–12 Our early detailed safety reports of a paired-pulse rTMS protocol13 continue to represent the majority of safety evidence.14,15
The purpose of this article is to describe serious AEs for 2 different studies, thereby elucidating safety factors critical to informing risk for rTMS-related AEs for persons remaining in states of DoC after sTBI. To do so, we report safety findings from 2 different studies that examine the same rTMS parameters13 but differ according to daily dose, site of stimulation,14,15 and whether rTMS was provided alone or in combination with AMA (NCT-02025439). While studies have explored potential benefits of AMA and rTMS in TBI populations, at this time, the use of these treatments is investigational/off-label.
METHODS
Data were derived from 7 patients who remained in states of DoC after an sTBI and were enrolled into one of 2 rTMS studies. For study 1, an open-label trial, 3 patients were enrolled and administered rTMS.14 For study 2, a baseline-controlled repeated-measures design study, 4 subjects were enrolled and randomized to one of 2 treatment orders: (i) AMA alone, followed by rTMS and amantadine (rTMS + AMA), or (ii) rTMS alone, followed by rTMS + AMA. Both the open-label and rTMS + AMA studies were approved by the institutional review boards (IRB) of Edward Hines VA Hospital and Northwestern University and the US Food and Drug Administration (FDA) via an Investigational Device Exemption (IDE G040195). Informed consent was obtained from each participant’s legally authorized representative.
Study eligibility criteria unique to each study and common across both studies are listed in Table 1. For both studies, rTMS was provided in an acute hospital–based clinical research unit bed with 24/7 medical monitoring. For both studies, all nonstudy medications that could potentially modulate central nervous system function and that could be safely stopped were weaned at baseline. If a medication was provided for reasons other than neurostimulation, then this medication was kept stable during the treatment phase. For both studies, rTMS was provided using 2 Magstim 200 units joined via a Bistim Module (Magstim, Eden Prairie, Minnesota). Stimulation was provided to either the left or the right dorsolateral prefrontal cortex (DLPFC) via a figure-of-eight coil (70-mm diameter) positioned with the handle pointing posteriorly and parallel to the skull angle at the site of stimulation. Site of therapeutic stimulation and location of the primary hand motor cortex (for determining resting state motor threshold [MT]) were identified first by neuronavigation using Brain Sight (Rogue Research, Montreal, Quebec, Canada). Procedures to obtain MT and determine rTMS intensity are previously reported.15
TABLE 1.
Eligibility criteria for studies
| Inclusion criteria | |
|---|---|
| Unique to study 1 (n = 3) | Unique to study 2 (n = 4) |
| Age 18–80 y | Age 18–64 y |
| TBI at least 3 mo prior to enrollment | TBI at least 1 y prior to enrollment |
|
Common across both studies | |
| Severe brain injury of traumatic origin | |
| State of disordered consciousness at the time of enrollment (coma, VS, MCS) | |
| Exclusion criteria | |
| Unique to study 1 (n = 3) | Unique to study 2 (n = 4) |
|
| |
| Significant heart disease | Blunt force trauma injuries |
| Shunts placed within 5 cm of the right DLPFC or finger area of motor cortex | Using medication that may interfere with amantadine and cannot be safely titrated |
| Contraindications to amantadine, including estimated glomerular filtration rate of ≤60 (mL/min) or abnormal liver function test | |
|
Common across both studies | |
| Fully conscious at the time of enrollment | |
| Pregnant at the time of study enrollment | |
| MRI/TMS contraindications: History of claustrophobia, metal in eyes/face, etc | |
| Implanted cardiac pacemaker or defibrillator, cochlear implant, nerve stimulator, intracranial metal clips | |
| Documented history of previous TBI or psychiatric illness according to DSM criteria | |
| Incurred ischemic infarct subsequent to TBI | |
| Surgery on blood vessels of the brain and/or heart valves, heart valves with metallic materials | |
| Did not speak English prior to injury | |
| Programmable shunts located in any position other than the retroauricular position | |
| Brain injury due to anoxia, ischemic stroke, hemorrhagic stroke, cancer | |
| Increased intracranial pressure at the time of study enrollment | |
| Nontraumatic encephalopathy | |
| Ventilator dependent | |
| Taking seizure medications due to active seizures or cannot be weaned from seizure medications due to active seizures at the time of enrollment. | |
| Has had documented seizures within 3 mo of study enrollment | |
| For both studies, participants had to have suffered a severe brain injury of traumatic origin and be in a state of DoC at the time of enrollment. For inclusion criteria, differences between the 2 studies involved age at the time of enrollment and duration of states of DoC. States of DoC were classified, at the time of enrollment, according to current clinical consensus criteria distinguishing between VS16–18 and MCS.19 Individuals who exhibited behaviors consistent with recovery of consciousness were not eligible for enrollment in this study. Medical stability was insured through review of medical records. Patients were excluded if they had conditions placing them at a known or plausible elevated risk for adverse events. | |
Abbreviations: DLPFC, dorsolateral prefrontal cortex; DoC, disordered consciousness; DSM, Diagnostic and Statistical Manual of Mental Disorders; MCS, minimally conscious state; MRI, magnetic resonance imaging; TBI, traumatic brain injury; TMS, transcranial magnetic stimulation; VS, vegetative state.
For both studies, rTMS was delivered in trains of paired stimuli (100-μs pulse width, 100-ms interpulse interval, 5-second intertrain interval, total of 300 paired-pulse per treatment) at 110% of MT. In study 1, a total of 30 rTMS treatment sessions were provided. Treatments were given once a day (equivalent to 300 pulses a day), 5 days per week, over 6 weeks, with no treatment on weekends. In study 2, a total of 30 rTMS treatment sessions were provided twice a day (equivalent to 600 pulses a day) over approximately 4 weeks, 4 days per week, with no treatment on Wednesdays or weekends.
Site of stimulation differed by study. For study 1, the site of stimulation was right DLPFC; however, one participant received left DLPFC stimulation due to complicated right hemisphere neuropathology. For study 2, the site of stimulation was right DLPFC.
Amantadine treatment (study 2 only)
For study 2, there were 2 treatment arms: (i) AMA alone, followed by rTMS + AMA (taking AMA for approximately 56 days), or (ii) rTMS alone, followed by rTMS + AMA (taking AMA for ~28 days). AMA was titrated up to 100 mg twice daily (200 mg/d) for 28 days. Titration occurred in increments of less than 50% of total dose on 4 consecutive days; AMA was weaned off similarly by increments of less than 50% of total dose on consecutive days and fully stopped, for 3 of the 4 participants, 24 hours prior to discharge to community for the follow-up phase. See Supplemental Digital Content Table 2 (available at: http://links.lww.com/JHTR/A382) for titration details.
Safety monitoring: Schedule and factors
For both studies, a 60-minute electroencephalogram (EEG) was obtained after the MT session. If no seizures or other AEs occurred over 24 hours, then rTMS was initiated. For study 1, on each treatment day, a 1-hour EEG was obtained before the TMS session and a 20-minute EEG was obtained after the rTMS session. For study 2, on each treatment day, a 20-minute EEG was obtained immediately prior to the first rTMS treatment and a 60-minute EEG was obtained after the second rTMS session. For both studies, additional EEGs were obtained if clinically indicated. All EEGs were reviewed by the study epileptologist for ictal rhythms and epileptiform discharges.
For both studies, structural magnetic resonance scans were collected at baseline and repeated periodically to monitor for hemorrhage and edema/toxic tissue. These were interpreted and monitored by the study neuroradiologist. For study 1, structural magnetic resonance imaging (MRI) was completed weekly throughout study participation. For study 2, structural MRI was repeated after 30 rTMS sessions or 28 doses of AMA at endpoint 1 (rTMS alone or AMA alone, respectively) and following the final rTMS session for both groups (rTMS + AMA) at endpoint 2. For both studies, structural MRI was repeated if clinically indicated.
Both studies were overseen by a Data Safety Monitoring Board (DSMB) whose oversight included a Data Safety Monitoring Plan (DSMP) (study 1 DSMP previously reported15; see Supplemental Digital Content Table 1 (available at: http://links.lww.com/JHTR/A381) for study 2 DSMP). The DSMPs each specify safety indicators to be monitored for change, frequency of monitoring, and a response plan. For each DSMP, the safety indicators were collected using a 2- to 6-point scale, depending on the potential for medical intervention. Higher numbers indicate more deleterious change from baseline. A change from baseline in any safety indicators constituted an AE (serious or nonserious), which was reported to medical monitors after patient stabilization. After the DSMB determined the relationship between rTMS and AEs, this decision and supporting documentation were submitted to each local IRB and the FDA for review and consensus.
There were some differences in the DSMPs between the 2 studies. Nystagmus was a safety indicator only for study 1. As reported previously,14 for study 1, we enrolled a patient with a shunt including a programmable valve; the DSMP was modified to account for (i) need to monitor valve pressure after each rTMS session and (ii) weekly skull radiographs to monitor for valve movement due to rTMS. For study 2, the Glasgow Coma Scale (GCS) (ie, GCS neurocheck safety indicator) was administered hourly during the 3-hour break between the 2 rTMS sessions. For participants in study 2 who received AMA alone in their community and before receiving the combination of rTMS + AMA, an abbreviated list of 6 safety indicators was collected in the participant’s community setting. These included fatigue (defined by the amount of sleep over 24-hour period), hypotension, hypertension, fever, general skin integrity, and sweating. These safety indicators were collected by family members, who were trained by study team personnel, and reported to researchers during weekly telephone monitoring.
RESULTS
Of the 7 participants, 6 were male, 6 were in the vegetative state (VS), and all were younger than 40 years at the time of study enrollment (see Table 2). Figure 1 illustrates the spectrum of pathologies for all 7 participants.
TABLE 2.
Characteristics of the sample
| State | Time postinjury | Age at the time of injury, y | Gender | Cause of TBI | Shunts | |
|---|---|---|---|---|---|---|
| S001 | VS | 8 mo | 26 | Male | MVC | None |
| S002 | VS | 6–7 mo | 54 | Male | Pedestrian struck | None |
| S003 | VS | 9 y | 23 | Male | Assault | Medtronic Strata II shunt valve |
| R001 | VS | 1.07 y | 28 | Male | MCC | None |
| R002 | VS | 1.13 y | 19 | Male | MCC | None |
| R003 | VS | 11 y | 28 | Male | MVC | None |
| R004 | MCS | 15 y | 37 | Female | MVC | None |
Abbreviations: MCC, motorcycle crash; MCS, minimally conscious state; MVC, motor vehicle collision; TBI, traumatic brain injury; VS, vegetative state.
Figure 1.

Lesion pathology relative to the site of rTMS. Each participant’s (A-G) site of stimulation by coordinates (top left of each panel) is depicted according to their (i) 3D sagittal image and (ii) axial image. For the 3D image, the anterior green dot is the site of rTMS and the posterior green dot is the motor threshold site. For the axial image, the green crosshairs with the red circle indicate the site of rTMS. In each panel, the bottom coronal slice(s) show the participant’s lesion pathology, labeled in yellow font, relative to the site of rTMS that is indicated with a blue arrow. The panels, collectively, indicate that all participants had widespread pathology, with one participant having lesions directly beneath the site of rTMS. The majority of participants had contusions (6/7) and/or microhemorrhages (5/7) and S001 (A) demonstrated T2 hyperintense foci in the subcortical white matter compatible with diffuse axonal injury lesions located directly beneath the site of rTMS. rTMS indicates repetitive transcranial magnetic stimulation.
Study 1: Safety findings from the open-label trial
Figure 2 illustrates average safety ratings per factor and the number of occurrences of each AE for the 3 participants in study 1. Across participants, 31 nonserious AEs and 1 serious AE were reported. For participants S001 and S002, no serious AEs were recorded during the intervention or follow-up phases. No EEGs showed epileptiform discharges. Weekly structural magnetic resonance scans indicated no anatomical changes. For nonserious AEs reported for S001 and S002, changes from baseline occurred with mild severity and medical attention was provided as needed; management and outcomes are previously described.14,15
Figure 2.
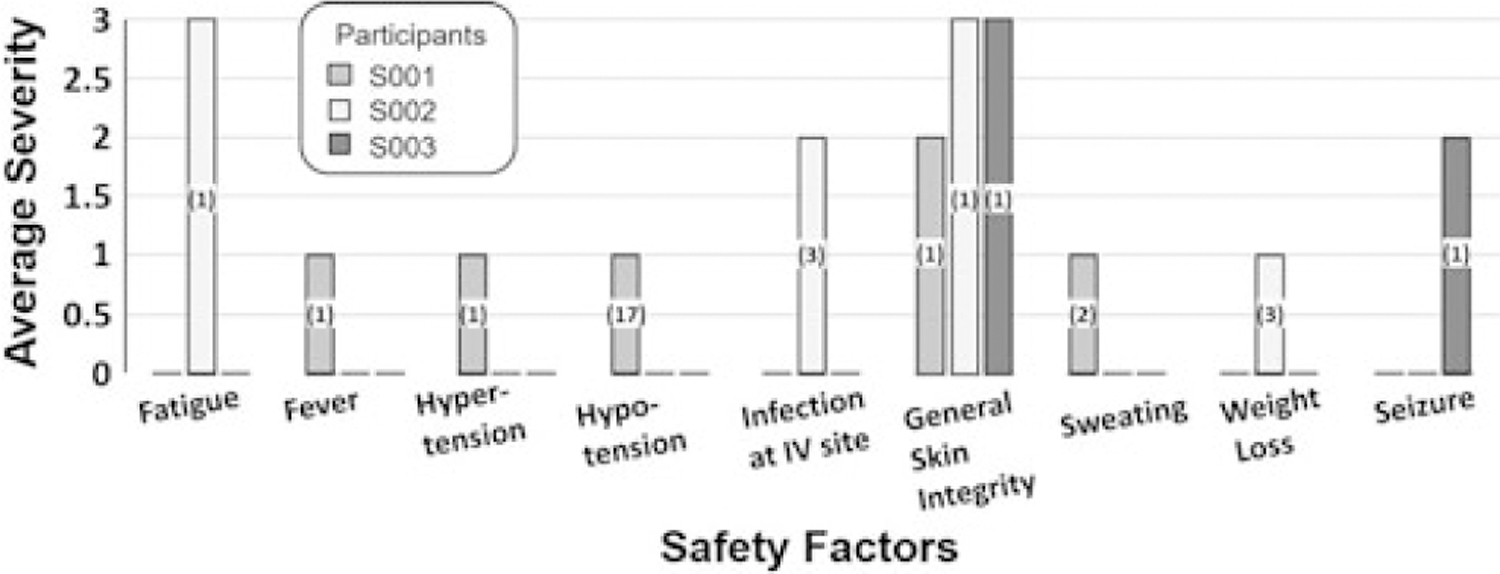
Average severity and incidence of safety factors per individual over 30 rTMS sessions for study 1. Safety factors are described in the study DSMP. Per participant, the average severity of change from baseline is reported. Safety factors not provided on the bar graph did not change from baseline for any of the 3 participants. Numbers in the bar indicate total number of incidences over 30 rTMS sessions for that participant. rTMS indicates repetitive transcranial magnetic stimulation; DSMP, Data Safety Monitoring Plan.
For S002, we report here additional information regarding 4 nonserious AEs. Fatigue (incident severity of 3) was reported as the participant slept 3 hours in a 24-hour period. The incident did not repeat and no action was indicated. General skin breakdown on bilateral anterior lower extremities (incident severity of 3) was reported. Duoderm was applied to promote healing; no further action was required. The participant’s weight gradually decreased by 5% over the course of the study (incident severity of 1). He was monitored, and his weight gradually returned to baseline with no action required. Finally, redness at the intravenous site (incident severity of 2) was reported. The site was rotated, and daily checks were performed. Since the study required all intravenous access for all participants, we revised the protocol to allow for peripherally inserted central catheters in lieu of peripheral intravenous sites.
For participant S003, management of a serious AE, a seizure, is previously described.14 Here, we report additional information on the seizure, as well as shunt monitoring. Available data at the time indicated that shunt valve pressure would require resetting for approximately 20% of all magnetic exposures and rTMS would not dislodge the valve.20 For our study, S003 had a Strata 2 programmable ventriculoperitoneal shunt. Valve pressure required resetting after every rTMS session and every magnetic resonance scan. Repeated skull radiographs indicated no valve movement.
S003 had no history of EEG-recorded ictal events; however, after the 21st rTMS session, an ictal event was recorded on EEG. The seizure stopped within 90 seconds without pharmacological intervention. Notably, S003 received rTMS to the left DLPFC because of severe right hemisphere encephalomalacia and the electrographic seizure arose in an area where the patient did not have substantive right hemisphere residual tissue (Figure 1C).
After S003’s seizure, rTMS was paused for 34 days. In consultation with the FDA and an external expert based at the National Institutes of Health, rTMS was restarted cautiously at a 2% lower intensity and with 100 fewer trains per session. It was thought that this would avoid neuronal overexcitation, particularly in areas that presented as having dormant or nonexistent structural neural connections at baseline. The participant continued to take levetiracetam (750 mg twice a day). No seizures occurred for the remaining 9 sessions. Ten additional rTMS sessions were subsequently provided; no serious AEs occurred. S003’s serious AE led to a change in future EEG protocols such that we included a 20-minute baseline EEG every morning prior to initiation of rTMS and a 60-minute EEG at the end of each rTMS day.
Study 2: Safety findings from the rTMS + AMA trial
Figure 3 illustrates average safety ratings and number of AEs, by type of AE, for the 3 treatment groups in study 2. During the interventions, 75 nonserious AEs and no serious AEs occurred. During follow-up, there was 1 serious AE not likely related to the interventions. Across treatment groups, rTMS + AMA yielded the greatest number of nonserious AEs (56/75; 75%), followed by AMA alone (12/75; 16%) and rTMS alone (7/75; 9%). For the group that received TMS alone, twice a day for a total of 30 sessions, the mild severity and number of incidences (n = 7) were similar to those reported for study 1. Changes from baseline ratings on fatigue, fever, hypertension, sweating, and GCS neurochecks were reported, all with a severity of 1. One participant spiked a fever (103°F) that resolved within 24 hours and was associated with increased respiratory suctioning. Chest radiographs showed opacities concerning for pneumonia; however, it is plausible that he experienced rebound hypersecretions due to AMA weaning and loss of anticholinergic effects. This was treated with aggressive respiratory therapy before feedings, adding acetylcysteine to daily nebulizers, switching antibiotics from vancomycin to levofloxacin, and additional oral suctioning. All other reported issues were monitored and resolved on their own. Structural magnetic resonance scans indicated no anatomical changes.
Figure 3.
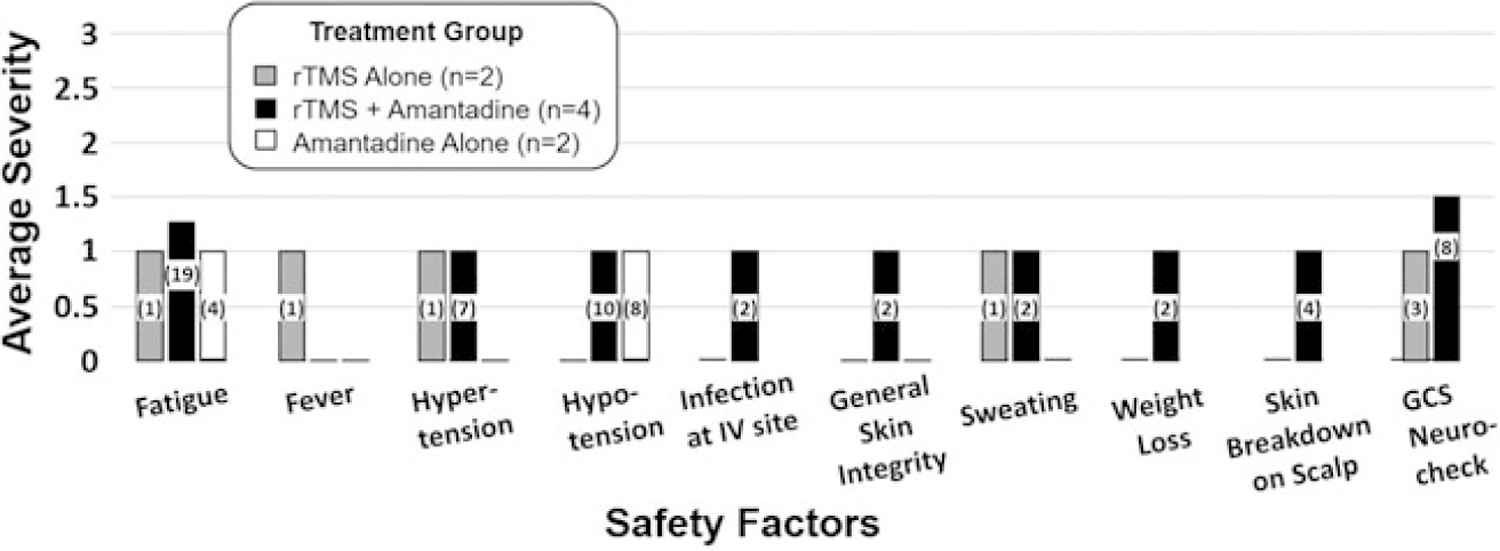
Average severity and incidence of safety factors per treatment phase during intervention for study 2. Safety factors are described in the study DSMP (see Supplemental Digital Content Table 1, available at: http://links.lww.com/JHTR/A381). Per treatment phase, the average severity of change from baseline is reported. Safety factors not provided on the bar graph did not change from baseline for any of the 3 treatment phases. Numbers in the bar indicate total number of incidences over treatment phase for that group. Note that during the amantadine-alone treatment phase, weight loss, skin breakdown on the scalp, GCS neurocheck, and infection at the intravenous site were not monitored. DSMP indicates Data Safety Monitoring Plan; GCS, Glasgow Coma Scale.
For the group that received AMA alone in the community, caregivers reported fatigue 4 times (average incident severity of 1) and hypotension 8 times (average incident severity of 1). The team monitored the issues, and all resolved without active intervention.
For the group that received rTMS + AMA, change from baseline was reported for 9 safety factors, and 6 of these were ranked with a severity of 1. Nineteen incidents of change in fatigue occurred (average incident severity of 1.3). The team monitored sleep-wake cycles and issues resolved on their own. Eight incidents of change in GCS neurochecks occurred (average incident severity of 1.5). Half the incidents had a severity of 2—requiring consultation with the research physician. These declines in neurobehavioral function were monitored, and it was noted that these fluctuations were generally due to changes related to fatigue.
During the intervention phase, no serious AEs occurred. During the follow-up phase, 1 serious AE was recorded for R002 that was unlikely due to rTMS treatment. He suffered acute hypoxemic respiratory failure. After he stabilized in the medical intensive care unit, he returned to his home and all issues resolved.
Participants’ families maintained contact with the research team after study completion. Family of R001 contacted researchers to report occurrence of seizures 46 days after final study treatment. A seizure occurred at home, and a second seizure was observed by the responding emergency medical technician. The subject was taking AMA at the time of seizures. After the seizure, AMA dose was reduced from 200 to 100 mg per day (50 mg, twice per day). He was prescribed the antiepileptic medication, levetiracetam, 1000 mg twice daily. Family of R002 contacted researchers to report the occurrence of a possible seizure 54 days after final study treatment. While reported as a seizure by nonstudy personnel, it is unclear whether what was witnessed constitutes a seizure. EEG was not obtained at the time. The subject was not receiving any neurostimulants (including AMA) at the time of the episode. These poststudy seizure events resulted in a change to the protocol where safety follow-up was extended from 6 to 12 weeks.
DISCUSSION
This report advances an understanding of rTMS safety for persons with complex and heterogeneous brain damage resulting from sTBI. Findings are based on a comprehensive set of critical safety factors monitored during 30 sessions of rTMS performed on 7 participants enrolled in 2 clinical trials. Each trial was designed with a conservative approach to patient selection. No patient had large lesions in proximity to the site of rTMS; however, all participants had widespread neuropathology. This report provides a safety profile for the specified rTMS protocol, provided within established safety guidelines, where 300 to 600 pulses were provided over 1 to 2 sessions per day. Considering elevated health risks inherent to DoC after sTBI and a medically conservative selection of patients in states of DoC, the safety findings indicate a relatively safe profile for the specified rTMS protocol.
Findings indicate that 1 serious AE, a seizure, may have been due to the rTMS. The seizure was detected only by EEG and was atypical for 2 reasons. First, seizures due to rTMS typically occur during or soon after cessation of stimulation; this seizure occurred 40 minutes after treatment. Second, rTMS-related seizures typically originate at the site of stimulation; this seizure focus was within the nonstimulated right hemisphere. These circumstances question whether this seizure was due to rTMS. Regardless, the DSMB recommended a revised protocol for the subsequent rTMS + AMA study (study 2) to include a 60-minute EEG after every second treatment of the day.
We also described here 2 poststudy events suspicious for seizure; however, because of their timing, these were not directly related to rTMS. It is possible, however, that seizure threshold is altered for a sustained duration following treatment with rTMS and/or AMA. A confound to the interpretation is that risk of seizure is already heightened in survivors of sTBI and underlying medical conditions may contribute to seizure induction. To expand our window of observation, the follow-up protocol for the last 2 participants increased by 6 weeks. Families were advised to not start neurostimulants for at least 3 months after study participation.
Other nonserious AEs identified were consistent with what would be expected in individuals with diminished mobility secondary to DoC. These include skin integrity issues, which occur when an individual is confined to a bed, and sleep disturbances due to disruptions in homeostatic and circadian factors due to the injury. Intravenous site infections and infiltrations are also not unusual, particularly during prolonged hospitalizations.
At the outset of this research, we considered the presence of a ventriculoperitoneal shunt with a programmable valve to be an exclusionary criterion, due to the risk of altering the valve’s pressure setting with the magnetic field. A significant proportion of patients being screened were excluded because of this criterion. Thus, we created protocols to include patients with shunts with programmable valves. This protocol involved understanding the implanted valve model and preferred programming setting. If this information could not be obtained (as these patients had been through many institutions and may not have had the shunt evaluated in a long time), then a skull radiograph was obtained. We also ensured availability from on-site neurosurgeons during rTMS treatment and magnetic resonance scans. The shunt was checked after every TMS session and after every magnetic resonance scan; setting readjustments were made as needed. There were no shunt malfunctions and no hydrocephalus symptoms during the short periods when the valve setting was altered during rTMS.
In conclusion, the findings indicate the specified rTMS protocols are relatively safe for medically stable patients remaining in states of DoC after sTBI who have (i) widespread neuropathology but no large lesions in close proximity to the site of rTMS and (ii) who do not have known seizure activity but are known to be at an elevated risk for seizures. While this advances knowledge of safety, this is a small sample and the reported findings should not be interpreted as advocating for any less rigorous monitoring of safety. It is critical to continue informing the medical research community of safety findings and comprehensive reporting in peer-reviewed literature. For example, current guidelines exclude patients taking antiepileptic medications for known or suspected seizure activity. However, we found that a patient who experienced a seizure successfully continued receiving rTMS while taking antiepileptic medications and with a reduction in intensity of pulses as well as number of trains per session. Considering that persons with severe TBI are, without rTMS, at an elevated risk for seizures, the rate of seizures due to rTMS is important for future research reports. Importantly, however, risk of seizures from rTMS while receiving antiepileptics to control active seizures can only be computed according to data based on rTMS placebo-controlled trials. In addition, for persons in states of DoC after sTBI, future research should investigate whether rTMS alone, or when paired with AMA, alters seizure threshold for a prolonged period of time. During this time, consideration should be given to aggressive treatments of infections and fevers and to avoid medications that lower the seizure threshold. Given that this population is fairly rare, and thus difficult to conduct a randomized controlled trial with a large sample size, collaborations and data sharing are critical to advance the field. For example, future research that pools data across studies that include placebo rTMS is needed to estimate seizure risk according to known and unknown seizure risk factors. This will allow us to better understand who is most at risk of central nervous system–related serious AEs and better understand what factors could be employed to decrease risk. Advanced neuroimaging—while difficult to achieve high-quality resolution due to common movement for persons in states of DoC—should be considered for future studies. Structural and functional connectivity changes occur beyond the site of injury for persons with sTBI21; understanding changes in connectivity could provide a means to elucidate potential mechanisms of adverse events, such as a seizure. Moving forward, research with comprehensive safety monitoring to examine rTMS safety is possible with a broader population of persons remaining in DoC after sTBI. Our findings suggest the study population could be broadened by lesion pathology. Our findings also indicate safety monitoring should include a minimum 60-minute EEG after the last rTMS session each day.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.headtraumarehab.com).
The authors declare no conflicts of interest.
Contributor Information
Sandra L. Kletzel, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois.
Alexandra L. Aaronson, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois; The Department of Veterans Affairs Mental Health Service Line, Hines VA, Hines, Illinois; The Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine Chicago, Illinois.
Ann Guernon, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois; Marianjoy Rehabilitation Hospital/Northwestern Medicine, Wheaton, Illinois.
Christina Carbone, Battle Creek VA Medical Center, Kalamazoo, Michigan.
Noor Chaudhry, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois; University of Illinois at Chicago, Chicago, Illinois.
Elyse Walsh, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois.
Mark Conneely, Chicago Medical School, Rosalind Franklin University of Science and Medicine, North Chicago, Illinois.
Vijaya Patil, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois; Loyola University Chicago, Stritch School of Medicine, Maywood, Illinois.
Elliott Roth, Department of Physical Medicine and Rehabilitation, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Monica Steiner, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois; Loyola University Chicago, Stritch School of Medicine, Maywood, Illinois.
Marilyn Pacheco, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois; The Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine Chicago, Illinois.
Joshua Rosenow, Department of Physical Medicine and Rehabilitation, Northwestern University Feinberg School of Medicine, Chicago, Illinois; Departments of Neurosurgery and Neurology, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Theresa L. Bender Pape, The Department of Veterans Affairs (VA), Research Service, Edward Hines Jr VA Hospital, Hines, Illinois; Marianjoy Rehabilitation Hospital/Northwestern Medicine, Wheaton, Illinois; Department of Physical Medicine and Rehabilitation, Northwestern University Feinberg School of Medicine, Chicago, Illinois.
REFERENCES
- 1.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120(12):2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter AC, Wagner AK, Fabio A, et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: a Traumatic Brain Injury Model Systems study. Epilepsia 2016;57(12): 1968–1977. [DOI] [PubMed] [Google Scholar]
- 3.Giacino JT, Whyte J, Bagiella E, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N EnglJ Med 2012; 366(9):819–826. [DOI] [PubMed] [Google Scholar]
- 4.Meythaler JM, Brunner RC, Johnson A, Novack TA. Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: a pilot double-blind randomized trial. J Head Trauma Rehabil 2002;17(4):300–313. [DOI] [PubMed] [Google Scholar]
- 5.Ghalaenovi H, Fattahi A, Koohpayehzadeh J, et al. The effects of amantadine on traumatic brain injury outcome: a double-blind, randomized, controlled, clinical trial. Brain Inj 2018;32(8):1050–1055. [DOI] [PubMed] [Google Scholar]
- 6.Abbasivash R, Valizade Hasanloei MA, Kazempour A, Mahdkhah A, Shaaf Ghoreishi MM, Akhavan Masoumi G. The effect of oral administration of amantadine on neurological outcome of patients with diffuse axonal injury in ICU. J Exp Neurosci 2019; 13:1179069518824851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loggini A, Tangonan R, El Ammar F, et al. The role of amantadine in cognitive recovery early after traumatic brain injury: a systematic review. Clin Neurol Neurosurg 2020;194:105815. [DOI] [PubMed] [Google Scholar]
- 8.Ma HM, Zafonte RD. Amantadine and memantine: a comprehensive review for acquired brain injury. Brain Inj 2020;34(3): 299–315. [DOI] [PubMed] [Google Scholar]
- 9.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil 2012;27(4):274–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia X, Bai Y, Zhou Y, et al. Effects of 10 Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in disorders of consciousness. Front Neurol 2017;8:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legostaeva L, Poydasheva A, Iazeva E, et al. Stimulation of the angular gyrus improves the level of consciousness. Brain Sci 2019; 9(5):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cincotta M, Giovannelli F, Chiaramonti R, et al. No effects of 20 Hz-rTMS of the primary motor cortex in vegetative state: a randomised, sham-controlled study. Cortex 2015;71:368–376. [DOI] [PubMed] [Google Scholar]
- 13.Pape TL, Rosenow J, Lewis G. Transcranial magnetic stimulation: a possible treatment for TBI. J Head Trauma Rehabil 2006;21(5): 437–451. [DOI] [PubMed] [Google Scholar]
- 14.Pape TL, Rosenow JM, Patil V, et al. rTMS safety for two subjects with disordered consciousness after traumatic brain injury. Brain Stimul 2014;7(4):620–622. [DOI] [PubMed] [Google Scholar]
- 15.Louise-Bender Pape T, Rosenow J, Lewis G, et al. Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul 2009;2(1):22–35. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Neurology. Practice Parameters: Assessment and Management of Patients in the Persistent Vegetative State. Report of the Quality Standards Sub-committee of the American Academy of Neurology Minneapolis, MN: American Academy of Neurology; 1995. [DOI] [PubMed] [Google Scholar]
- 17.Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (1). N EnglJ Med 1994;330:1499–1508. [DOI] [PubMed] [Google Scholar]
- 18.Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state (2). N EnglJ Med 1994;330:1572–1579. [DOI] [PubMed] [Google Scholar]
- 19.Giacino J, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58(3):349–353. [DOI] [PubMed] [Google Scholar]
- 20.LeFranc M, Ko J, Peltier J, et al. Effect of transcranial magnetic stimulation on four types of pressure-programmable valves. Acta Neurochir 2010;162(4):689–697. [DOI] [PubMed] [Google Scholar]
- 21.Bender Pape T, Livengood S, Blabas B, et al. Treatment induced changes in structural connectivity occurring in parallel with neurobehavioral recovery from disordered consciousness after TBI. Front Neurol doi: 10.3389/fneur.2020.01027. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


