Abstract
The activity of imipenem against Pseudomonas aeruginosa HUS-3 decreased by 16 times in the presence of substances eluted from siliconized latex urinary catheters (SLUCs). SLUCs did not inactivate imipenem or increase β-lactamase activity. The outer membrane of P. aeruginosa HUS-3 grown in the presence of eluate lacked an OprD-like protein and expressed a new 50-kDa protein. The decreased activity of imipenem against P. aeruginosa in the presence of SLUCs is related to the loss of an OprD-like protein and the expression of a new outer membrane protein.
Pseudomonas aeruginosa is an important cause of urinary tract infection in patients with urinary catheters (17). The organism is able to colonize the surface of the catheter, forming biofilms that interfere with the activity of antimicrobial agents (14). It has been shown that P. aeruginosa adheres in vitro more efficaciously to siliconized latex urinary catheters (SLUCs) than to other plastic biomaterials (7–9). SLUCs elute substances that can be used by P. aeruginosa to grow (8) but are toxic for Escherichia coli or human polymorphonuclear leukocytes (7). It was reported previously that MICs of meropenem (MPM) against P. aeruginosa increased by 8 to 16 times in the presence of SLUC segments (15). It was postulated that the decreased activity of MPM could be related to the elution of substances from SLUCs but other mechanisms were also considered.
Resistance to imipenem (IMP) in P. aeruginosa has been shown to be related to the loss of the outer membrane protein OprD (23) coupled with the production of chromosomal β-lactamase (6, 22). MPM is more active than IMP against this microorganism, which could be related to its greater stability in the presence of the chromosomal β-lactamase (22). More recently, it has been reported that resistance of P. aeruginosa to these and other agents could be also related to the elimination of drugs by efflux systems (4, 12, 18–20).
This study was undertaken to evaluate the role of outer membrane protein alterations and/or production of β-lactamase in the decreased activity of IMP against P. aeruginosa in the presence of eluates from SLUCs.
(This research was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September to 1 October 1997 [10].)
P. aeruginosa HUS-3 is a previously described clinical isolate (15). P. aeruginosa PAO1 and an OprD mutant were kindly provided by J. Trías (Microcide Pharmaceuticals Inc., Mountain View, Calif.). IMP-resistant mutants from P. aeruginosa HUS-3 were obtained on plates of Mueller-Hinton agar containing 8 μg of IMP/ml. One of these mutants was retained and was designated P. aeruginosa HUS-3/MUT2. Segments (eight segments 0.5 cm in length/ml of media) of SLUCs (pediatric siliconized latex Foley catheters; Euromedical, Kuala Lumpur, Malaysia) were incubated in sterile cation-adjusted Mueller-Hinton broth (MHB) at 37°C for 24 h. Catheter segments were removed, and the resulting broth containing the substances eluted from SLUCs (eluate) was used immediately. MICs of IMP, ceftazidime, cefepime, cefpirome, trimethoprim, tetracycline, and chloramphenicol were determined by a microdilution assay according to National Committee for Clinical Laboratory Standards guidelines (13) using both MHB and eluate as media. Eluate diluted in MHB in different proportions (1:2, 1:4, 1:8, and 1:16) was also used to determine the activities of IMP against P. aeruginosa HUS-3 and MUT2. To evaluate the possible inactivation of IMP by eluate, IMP at a concentration of 5,120 μg/ml was incubated at 37°C for 24 h in MHB and in eluate. MICs of IMP, preincubated in either MHB or eluate, for P. aeruginosa HUS-3 were determined in both MHB and eluate and compared with MICs of freshly prepared IMP determined in the same conditions. In another set of experiments, P. aeruginosa HUS-3 was grown in MHB and in eluate. The activity of IMP against bacteria grown in either medium was again determined in MHB and in eluate. Outer membrane proteins of P. aeruginosa HUS-3 grown in MHB or eluate were prepared as previously described (4), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using Laemm-li’s buffers (5), and stained with Coomassie blue. β-Lactamase activity was determined spectrophotometrically using the crude supernatants obtained after sonication of P. aeruginosa grown either in HMB or in eluate and cephaloridine as substrate. One unit of activity was defined as the amount of enzyme that hydrolyzed 1 μmol of substrate per minute at 37°C at 295 nm.
In a previous report, we showed that SLUCs interfere in vitro with the inhibitory activity of MPM against P. aeruginosa (15). In the present report, it is shown that SLUCs also affect the activity of IMP. It was previously postulated that SLUCs are degraded when immersed in a liquid phase independently of the presence of microorganisms and that the silicone layer covering urinary catheters may dissolve in vivo, thus exposing the inner latex layer to the environment, the latex being responsible for tissue toxicity (21). The results of this study suggest that the eluate from SLUCs contains some substance(s) which inhibits IMP activity against P. aeruginosa HUS-3. The activity of IMP against P. aeruginosa HUS-3 decreased by 16 times when it was measured in eluate from SLUCs (Table 1). This effect progressively disappeared when the eluate was diluted in MHB. Eluate kept at 4°C for up to 4 weeks maintained its activity against IMP (data not shown). MICs of IMP against P. aeruginosa HUS-3 were 8, 4, 1, and 1 μg/ml when eluate was diluted in MHB 1:2, 1:4, 1:8, and 1:16, respectively. MICs of IMP against MUT2 were the same (32 μg/ml) in both MHB and eluate (either pure or diluted 1:2 to 1:16 in MHB). Similarly, the MIC of IMP against P. aeruginosa PAO1 was 8 to 16 times higher than the corresponding value against the organism grown in MHB, while against the OprD deficient mutant both MICs were the same (16 μg/ml). Preincubation of IMP in eluate did not result in inactivation of the drug as determined by the bioassay with P. aeruginosa HUS-3. MICs of eluate-preincubated IMP were 2 μg/ml in MHB and 32 μg/ml in eluate, exactly the same values as obtained with IMP preincubated in MHB, and only one dilution step higher than those obtained with freshly prepared IMP. The MIC of IMP against P. aeruginosa HUS-3 grown in eluate was 16 μg/ml when performed in eluate and 1 μg/ml when performed in MHB; the corresponding values, determined in a parallel experiment with the organism grown in MHB were the same, as previously observed.
TABLE 1.
Susceptibility of P. aeruginosa strains to antimicrobial agents in MHB or MHB plus eluate from SLUCs
| Strain | MIC (μg/ml)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imipenem
|
Ceftazidime
|
Cefepime
|
Cefpirome
|
Trimethoprim
|
Tetracycline
|
Chloramphenicol
|
||||||||
| MHB | Ea | MHB | E | MHB | E | MHB | E | MHB | E | MHB | E | MHB | E | |
| HUS-3 | 1 | 16 | 32 | 32–64 | 8 | 32 | 64 | 128 | 128 | 256 | 16 | 16 | 128 | 256 |
| PAO1 | 1 | 8–16 | 1 | 2 | 1 | 2 | 2 | 4 | 128 | 128 | 16 | 16 | 32 | 64 |
| PAO1 OprD(−) | 16 | 16 | 1 | 4 | 1 | 2 | 2 | 4 | 128 | 128 | 16 | 16 | 32 | 64 |
E, MHB plus eluate from SLUCs.
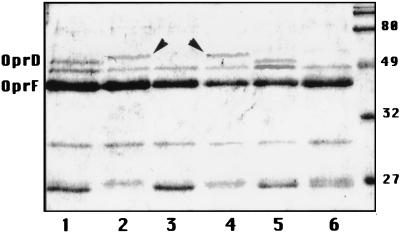
P. aeruginosa HUS-3 grown in eluate lost an outer membrane protein comigrating with OprD of P. aeruginosa PAO1 and expressed a new outer membrane protein of about 50 kDa (Fig. 1). Mutant MUT2 did not express OprD in MHB and, like its parental strain, expressed a new protein of ca. 50 kDa when grown in eluate. The outer membrane protein profile of P. aeruginosa PAO1 was similar to that of HUS-3, while that of the PAO1 OprD-deficient mutant was similar to that of MUT2 (data not shown). The expression of new proteins of around 50 kDa in the outer membrane of P. aeruginosa has been recently related to the expression of efflux systems (11). The pattern of outer membrane proteins of P. aeruginosa HUS-3 grown in the presence of eluate is similar to that of nfxC-type mutants (12). Köhler et al. (4) have recently reported that nfxC-type mutants express the MexE-MexF-OprN efflux system along with decreased OprD expression. Unfortunately, the position of the 50-kDa band cannot be used to distinguish between the three outer membrane proteins (OprM, OprJ, or OprN) associated with efflux systems of P. aeruginosa because of their similar mobilities during sodium dodecyl sulfate-polyacrylamide gel electrophoresis. It has been reported that MexE-MexF-OprN increases resistance to chloramphenicol and trimethoprim but not to tetracycline or cephalosporins (4). In order to evaluate the possible activation of MexE-MexF-OprN by eluates, the MICs of six antimicrobial agents (Table 1) against strains HUS-3, PAO1, and OprD-deficient PAO1 were determined in both MHB and eluate. The results showed in the table, however, are not conclusive. At this moment, it is not possible to establish that the new 50-kDa protein is any of the known outer membrane proteins associated with efflux systems or even a totally different one. New experiments with P. aeruginosa strains carrying mutations in the MexA-MexB-OprM, MexC-MexD-OprJ, and MexE-MexF-OprN systems would contribute to evaluation of their role, if any, in the resistance of P. aeruginosa induced by SLUCs.
FIG. 1.
Outer membrane proteins of P. aeruginosa grown in MHB (lanes 1, 3, 5, and 6) or in eluate (lanes 2 and 4). Lanes: 1 and 2, strain HUS-3; 3 and 4, mutant MUT2; 5, strain PAO1; 6, OprD-deficient mutant of PAO1. Molecular markers are shown at right. The ca. 50-kDa new protein expressed in eluate is marked with arrowheads.
It is difficult to assess the relative importance of OprD deficiency and of the 50-kDa protein expression induced by eluate in the resistance of P. aeruginosa HUS-3. In the case of IMP, it may be hypothesized that the loss of OprD is more important than the expression of the 50-kDa protein, as MICs of IMP against mutants MUT2 and OprD-deficient PAO1 are the same in both MHB and eluate, although when both mutants grow in eluate they also express the 50-kDa protein. Outer membrane protein changes and resistance to IMP reverted when organisms grown in the presence of eluate were subsequently cultured in MHB, which indicates that eluate regulates, by an unknown mechanism, the physiology of P. aeruginosa rather than selects for rare mutants.
β-Lactamase activities in P. aeruginosa HUS-3 and MUT-2 grown in eluate (707 and 825 mU/mg of protein, respectively) were similar to those observed after growing the organism in MHB (809 and 832 mU/mg of protein, respectively). The high level of enzyme produced by P. aeruginosa HUS-3 may contribute to the observed resistance to IMP in the presence of eluate. It is well known that the expression of chromosomal β-lactamase in OprD-deficient strains determines resistance to IMP in P. aeruginosa (6, 22).
The exact chemical nature of the materials used for making SLUCs is not known. Preliminary chromatographic characterization of the eluate resulted in the identification of several major peaks, including N,N-dibutylformamide; 1,1,3-trimethyl-3-phenylindane; ethane-1,1-2-di-3,4-xylil; and phthalate derivatives. The activity of IMP against P. aeruginosa HUS-3 remained unaltered in the presence of N,N-dimethylformamide; dimethyl-phthalate; or diethyl-phthalate in concentrations ranging between 0.5 and 40 μg/ml (data not shown). New experiments to evaluate the role of other SLUC components as a cause of resistance of P. aeruginosa HUS-3 to IMP are planned.
The clinical importance of these findings is unknown. Assuming that the concentrations of substances eluted from SLUCs could be high in the microenvironment of bacterial biofilm, we may speculate that this is an advantageous situation for P. aeruginosa attached to SLUCs because of its ability to grow using the eluate as a nutrient and to evade the activity of some antimicrobial agents.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Dinh T, Paulsen I T, Saier M H., Jr A family of extracytoplasmic proteins that allow transport of large molecules across the outer membrane of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda H, Hosaka M, Hirai K, Iyobe S. New norfloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990;34:1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köhler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechère J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:343–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 5.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 6.Livermore D M. Interplay of impermeability and chromosomal β-lactamase activity of imipenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:2046–2048. doi: 10.1128/aac.36.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López-López G, Pascual A, Martínez-Martínez L, Perea E J. Effect of a siliconized latex urinary catheter on bacterial adherence and human neutrophil activity. Diagn Microbiol Infect Dis. 1991;14:1–6. doi: 10.1016/0732-8893(91)90078-t. [DOI] [PubMed] [Google Scholar]
- 8.Martínez-Martínez L, Pascual A, Perea E J. Effect of three plastic catheters on survival and growth of Pseudomonas aeruginosa. J Hosp Infect. 1990;16:311–318. doi: 10.1016/0195-6701(90)90003-7. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Martínez L, Pascual A, Perea E J. Kinetics of adherence of mucoid and non-mucoid Pseudomonas aeruginosa to plastic catheters. J Med Microbiol. 1991;34:7–12. doi: 10.1099/00222615-34-1-7. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Martínez L, Pascual A, Conejo M C, Picabea L, Perea E J. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Siliconized latex urinary catheters (SLUC) induce imipenem (IMP) resistance and outer membrane proteins (OMP) alterations in P. aeruginosa, abstr. C-40; p. 52. [Google Scholar]
- 11.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakae T. Multiantibiotic resistance caused by active drug extrusion in Pseudomonas aeruginosa and other gram-negative bacteria. Microbiol SEM. 1997;13:273–284. [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 14.Nickel J, Ruseska I, Wright J B, Costerton J W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual A, Martínez-Martínez L, Ramírez de Arellano E, Perea E J. Susceptibility to antimicrobial agents of Pseudomonas aeruginosa attached to siliconized latex urinary catheters. Eur J Clin Microbiol Infect Dis. 1993;12:761–766. doi: 10.1007/BF02098464. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen I, Brown T, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack M. Pseudomonas aeruginosa. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1990. pp. 1980–2002. [Google Scholar]
- 18.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 19.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of siderophore pyoverdine. Mol Microbiol. 1993;10:529–554. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 20.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruutu M, Alfthan O, Talja M, Anderson L C. Cytotoxicity of latex urinary catheters. Br J Urol. 1985;57:82–87. doi: 10.1111/j.1464-410x.1985.tb08992.x. [DOI] [PubMed] [Google Scholar]
- 22.Satake S, Yoneyama H, Nakae T. Role of OmpD2 and chromosomal β-lactamase in carbapenem resistance in clinical isolates of Pseudomonas aeruginosa. J Antimicrob Chemother. 1991;28:199–207. doi: 10.1093/jac/28.2.199. [DOI] [PubMed] [Google Scholar]
- 23.Trías J, Nikaido H. Outer membrane protein D2 catalyzes facilitated diffusion of carbapenem and penems through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990;34:52–57. doi: 10.1128/aac.34.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]