Abstract
Pineal germ cell tumors (GCTs) are primarily seen in pediatric and Asian populations. These tumors are divided into germinomatous and non-germinomatous GCTs (NGGCTs). GCTs are thought to arise by misplacement of totipotent stem cells en route to gonads during embryogenesis. Intracranial GCTs display an affinity to develop along the pineal-suprasellar axis and have variable manifestations dependent upon the location of the tumor. Management and outcomes are driven by histopathologies. In this study, we highlight two cases of pineal GCTs and present a review of the literature with an emphasis on histopathologies and biomarkers.
Keywords: Germinoma, Germ cell tumor, Intracranial neoplasms, Pathology, Yolk sac tumor
1. Introduction
Pineal germ cell tumors (GCTs) are rare, comprising less than 5% and 18% of primary brain tumors in Western and Asian countries, respectively [1–8]. Pineal GCTs account for nearly 50% of intracranial GCTs (IGCTs) and are more common in males [8]. Histologically, these tumors are categorized as germinomatous or non-germinomatous [1]. Germinomatous GCTs include germinomas and germinomas with syncytiotrophoblastic giant cells (STGCs). Non-germinomatous GCTs (NGGCTs) include teratomas, embryonal carcinomas, yolk sac tumors, and choriocarcinomas [9].
IGCTs commonly develop along the pineal-suprasellar axis. Computed tomography (CT) demonstrates punctate hyperdensities, which indicate tumoral calcifications embedded in a hypodense, enhancing mass [10,11]. Magnetic resonance imaging (MRI) remains the diagnostic modality of choice, and reveals a T1- and T2-isointense vividly enhancing mass. Management and prognoses of these tumors are driven by histopathological subtype.
Advances in immunohistochemistry and next-generation sequencing have furthered our understanding of the origin and pathomolecular mechanisms underlying the development of GCTs. DNA damage response (DDR) signaling and the ATM-ChK2-p53 pathway, which are involved in the development of solid tumors, have been shown to be downregulated in germinomas and other subtypes of primary GCTs [12]. This pattern is also observed in testicular GCTs. Mutations in the KIT/RAS and AKT1/mTOR pathways, among others, have also been demonstrated in IGCTs [8]. Here we report two cases of pineal GCTs (yolk sac tumor and germinoma). The literature is reviewed with an emphasis on histopathologies and biomarkers.
2. Cases
All patients gave informed consent for the treatments described below. Institutional Review Boards at each respective institution did not require consent for this type of article. Nevertheless, details that might disclose the identities of the subjects are omitted. Permission to reproduce images was appropriately obtained. The slides depicted in this paper are not from the patients herein described and are for non-CNS GCTs.
2.1. Pineal germinoma (Case 1)
A 20-year-old male initially presented to an optometrist with a one-month history of decreased left eye visual acuity, headache, nausea, and erectile dysfunction. The patient was referred to an external neuro-ophthalmology department for further care.
Neurologic examination revealed decreased visual acuity and papilledema. Brain MRI showed mild hydrocephalus and a 1 × 1 × 1 cm round, heterogeneously enhancing pineal lesion. Diffusion-weighted imaging (DWI) demonstrated restricted diffusion at the anterolateral edge of the mass. Gradient recalled echo (GRE) images showed areas of hypointensity attributed to calcification. Several small lesions were present in the hypothalamus, left foramen of Monro, the right lateral recess of the fourth ventricle, floor of the fourth ventricle at the facial colliculus, and obex. Spine MRI demonstrated enhancing drop lesions in the sacral region (images unavailable). Serum α-fetoprotein (AFP) and β-human chorionic gonadotropin (β-hCG) were within normal limits.
The patient underwent placement of a ventriculoperitoneal shunt (VPS) and an endoscopic transventricular biopsy via a right frontal neuro-endoscopic approach. Histopathological diagnosis was germinoma and confirmed by immunohistochemistry (IHC) (slides unavailable). IHC was positive for placental alkaline phosphatase (PLAP), octamer-binding transcription factor 4 (Oct-4), and c-kit (CD117). Staining was negative for glial fibrillary acidic protein (GFAP), synaptophysin, and cytokeratins.
As the patient was on vacation in California from Great Britain, he requested to be transferred home for further management. His visual disturbances resolved and he was discharged in stable condition.
2.2. Pineal yolk sac tumor (Case 2)
A 23-year-old male with a rapidly enlarging pineal tumor presented at an outside hospital with hydrocephalus status post VPS. The patient subsequently developed fevers, mental status deterioration, respiratory failure, and hypotension. He was transferred to our institution for escalation of care. On exam, he intermittently followed commands. Given the concern for infection, the VPS was removed and ventriculostomy was subsequently performed. Cerebrospinal fluid (CSF) cultures were negative. Prolonged fevers and fluctuating serum white blood cell counts were attributed to a sympathetic storm.
Head CT revealed an enhancing, heterogeneously hyperdense, and partially calcified 3 × 4 × 4 cm irregularly shaped pineal mass. Brain MRI showed a heterogeneously enhancing pineal tumor containing multiple T1- and T2-hypointense foci consistent with calcifications (images unavailable). Obstructive hydrocephalus was attributed to aqueductal stenosis caused by the pineal mass. Elevated levels of CSF AFP (599 ng/mL, normal: 0.0–6.7) and lactate dehydrogenase (LDH) (278 U/L, normal: 91–223) were found and consistent with a diagnosis of yolk sac tumor. hCG levels were not reported.
Due to location and extent of involvement, the patient’s tumor was deemed unresectable. He was treated with chemotherapy (carboplatin and etoposide) to which he demonstrated radiographic response on follow-up imaging. The hydrocephalus persisted and he required repeat VPS. Neurological examination at discharge revealed spontaneous eye opening with the patient alert, following commands, and mouthing words. Strength remained severely diminished in all four extremities. He was discharged to an acute rehabilitation facility for local hematology/oncology follow-up.
3. Discussion
In this report, we described two cases of pineal GCTs. These patients provided the rationale for conducting a review of the literature with an emphasis on histopathologies and biomarkers. The PubMed database was queried using relevant search terms. A total of 298 IGCTs, comprised of 193 germinomas (64.8%), 52 teratomas (17.4%), 20 choriocarcinomas (6.7%), 19 yolk sac tumors (6.4%), and 14 embryonal carcinomas (4.7%) were identified (Table 1). Our analysis represents the aggregated data from the literature that investigated tumor marker expression patterns in serum and CSF (Table 2) and IHC staining of histologically pure GCTs (Table 3).
Table 1.
Quantitative summary of studies evaluating intracranial germ cell tumor markers.
| Authors and year [Ref] | Germinoma | Teratoma | Embryonal carcinoma | Yolk sac tumor | Choriocarcinoma |
|---|---|---|---|---|---|
|
| |||||
| Lv et al. (2010) [53] | – | – | – | – | 6 |
| Ngan et al. (2008) [60] | 5 | 4 | – | 3 | – |
| Kamakura et al. (2006) [40] | 13 | 4 | – | 2 | 2 |
| Nakamura et al. (2006) [58] | 25 | 2 | 1 | 2 | – |
| Hattab et al. (2005) [28] | 25 | – | – | – | – |
| Matsutani et al. (1997) [55] | 36 | 18 | 4 | 3 | 3 |
| Ho et al. (1992) [29] | 23 | 8 | – | 5 | 2 |
| Inoue et al. (1987) [32] | 39 | 6 | 4 | – | 2 |
| Yamagami et al. (1987) [85] | 9 | 7 | 2 | 3 | 2 |
| Bjornsson et al. (1985) [11] | 16 | 3 | 1 | 1 | 1 |
| Allen et al. (1979) [4] | 2 | – | 2 | – | 2 |
| Total, n (%) | 193 (64.8) | 52 (17.4) | 14 (4.7) | 19 (6.4) | 20 (6.7) |
Table 2.
Elevated serum and cerebrospinal fluid markers in cases reviewed.
| Histopathologies | Serum AFP, n (%) | CSF AFP, n (%) | Serum hCG, n (%) | CSF hCG, n (%) |
|---|---|---|---|---|
|
| ||||
| Germinoma | 1 (2) | 0 (0) | 13 (25)* | 8 (73) |
| Teratoma | 4 (17) | 0 (0) | 5 (22) | 0 (0) |
| Embryonal carcinoma | 5 (56) | 4 (80) | 4 (44) | 4 (80) |
| Yolk sac tumor | 3 (100) | – | 0 (0) | – |
| Choriocarcinoma | 1 (17) | 1 (33) | 11 (85) | 3 (100) |
Expression is associated with germinoma with syncytiotrophoblastic giant cells.
Table 3.
Immunohistochemical marker reactivities in cases reviewed.
| Histopathologies | AFP, n (%) | hCG, n (%) | PLAP, n (%) | CEA, n (%) | HPL, n (%) | Cytokeratin, n (%) | Oct-4, n (%) | c-kit, n (%) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Germinoma | 0 (0) | 14 (35)* | 82 (93) | 1 (2) | 1 (11)* | 2 (22) | 30 (100) | 38 (100) |
| Teratoma | 5 (29) | 0 (0) | 0 (0) | 20 (83) | 0 (0) | 8 (100) | 1 (25) | 3 (50) |
| Embryonal carcinoma | 4 (57) | 4 (57) | 1 (25) | 2 (29) | 1 (50) | – | 3 (100) | 0 (0) |
| Yolk sac tumor | 9 (100) | 0 (0) | 3 (60) | 4 (44) | 0 (0) | 5 (100) | 0 (0) | 0 (0) |
| Choriocarcinoma | 0 (0) | 5 (100) | 1 (50) | 3 (60) | 1 (50) | 2 (100) | – | 0 (0) |
AFP = α-fetoprotein, hCG = human chorionic gonadotropin, PLAP = placental alkaline phosphatase, CEA = carcinoembryonic antigen, HPL = human placental lactogen.
Expression is associated with germinoma with syncytiotrophoblastic giant cells.
IGCTs are thought to arise by misplacement of totipotent stem cells en route to gonads during embryogenesis [13,14]. According to this theory, IGCTs and gonadal GCTs are derived from a common cellular origin and thus share serological, histological, and immunohistochemical similarities [15,16]. Germinomas are the most common IGCTs, followed by teratomas, yolk sac tumors, embryonal carcinomas, and choriocarcinomas. Germinomas are prevalent in the suprasellar region whereas intracranial NGGCTs are mostly found in the pineal region [1].
3.1. Radiology
Radiologic profiles of IGCTs are relatively non-specific. However, subtle variations in imaging may help guide diagnoses. Germinomas are generally spherical and display calcifications when found in the pineal gland; NGGCTs are more irregularly shaped [17,18]. Germinomas appear hyperdense and enhance homogeneously on CT with contrast. Mature teratomas are well demarcated, variably dense, have larger cystic components, more areas of calcification, and rarely enhance. Malignant teratomas are poorly demarcated, display peritumoral edema, smaller cysts, less pronounced calcifications, and enhance [17]. Embryonal carcinomas are hyperdense and have variable patterns of enhancement. Yolk sac tumors display variable densities and enhancement patterns. Choriocarcinomas are hyperdense and enhance heterogeneously. Most IGCTs are hypo- to isointense and iso- to hyperintense on T1- and T2-weighted sequences, respectively. MRI of the entire neuro-axis and CSF cytological examination are also recommended because of the ability of GCTs to disseminate via CSF [14,19,20].
3.2. Management
Management is driven by histopathologies. Intracranial germinomas are highly radiosensitive and can be treated with radiation alone [21–29]. Intracranial germinomas have responded favorably to radiation doses of 40–55 Gy, but tumor response has also been demonstrated with doses as low as 20 Gy [30–33]. The optimal doses and fields have long been debated, but high success rates of radiation therapy for germinomas have enabled researchers to direct their efforts toward the reduction of radiation intensity [34–39]. Prior radiation may be a negative prognostic factor for surgical resection of residual pure germinoma [40].
In contrast, mature teratomas are thought to be radio-insensitive and surgical resection is considered the only cure [7,20,28,29,41–43]. Immature teratomas are generally treated with chemotherapy while residual tumors are often managed with surgical resection and/or radiation therapy [28,29]. Malignant teratomas are treated with surgical resection, chemotherapy, and radiation [19,20]. However, the safety and efficacy of these strategies have yet to be fully elucidated and therefore treatment protocols have not been defined. Furthermore, the role of other modalities, such as radiosurgery, has not been established.
NGGCTs have shown positive responses to chemotherapy, and thus, chemotherapy alone is recommended as the initial therapeutic strategy [30]. Radiation therapy has yielded variable results, providing complete regression in some cases, while having little effect in others [15,24]. NGGCTs may benefit from a multimodal approach (chemotherapy and radiation) [21,44–46]. Platinum-based chemotherapy (carboplatin or cisplatin) has been associated with improved progression-free (PFS) and overall survival (OS), as well as complete remission in few cases of NGGCT [28,47–54]. Other chemotherapeutic regimens used to treat IGCTs included JEB (carboplatin, etoposide, and bleomycin), BEP (bleomycin, etoposide, and cisplatin), EP (etoposide and cisplatin), and PVB (cisplatin, vinblastine, and bleomycin) [55–58]. Resection of NGGCTs has also conferred survival advantage; however, these tumors are often of mixed composition, which renders these results ambiguous [28,29].
3.3. Prognoses
Prognoses are dictated by histopathologies. In a study conducted on patients with IGCTs who had undergone adjuvant therapy, 5-year survival probabilities were reported as 95.4% for pure germinomas, 83.3% for germinomas with STGCs, 92.9% for pure mature teratomas, and 70.7% for teratomas with malignant transformation [57]. Embryonal carcinomas, yolk sac tumors, and choriocarcinomas exhibited the poorest outcomes with patients reported to have a 3-year survival of 27.3% [59]. A separate study of IGCTs demonstrated superior 5-year survival in mature teratomas, followed by germinomas and immature teratomas [22].
3.4. Histopathologies
Germinomas appear as a mixture of lymphocytes and undifferentiated large cells with clear cytoplasm, round nuclei, and conspicuous nucleoli (Fig. 1) [60]. Germinomas with STGCs are less radiosensitive compared to pure germinomas, and thus, have higher rates of recurrence after being irradiated [59]. Teratomas are composed of cells with components from all three germ layers. Mature teratomas (good prognosis) are completely differentiated with low mitotic indices (Fig. 2). This is in stark contrast to immature teratomas (intermediate prognosis), which contain poorly differentiated cells and have elevated mitotic indices (Fig. 3) [9,61,62].
Fig. 1.
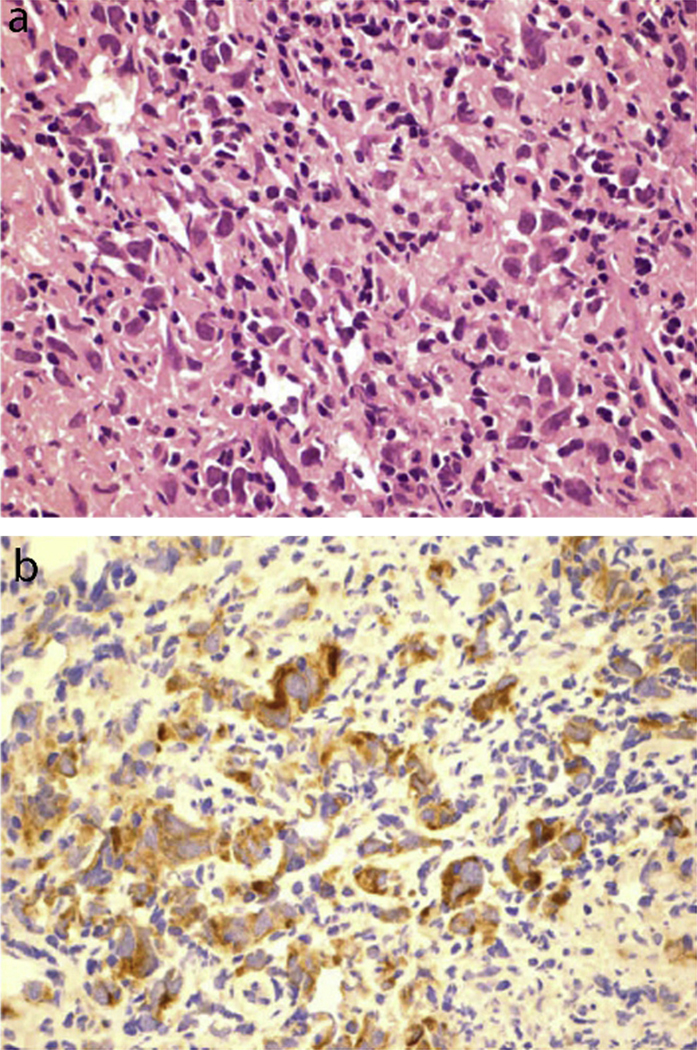
(a) Histopathology of a germinoma exhibiting large cells with clear cytoplasm and round nuclei, interspersed with lymphocytes. (b) Immunohistochemical stain positive for PLAP. This figure was reproduced with the kind permission of Elsevier. J Clin Neurosci 2009 Feb;16(2):321–5.
Fig. 2.
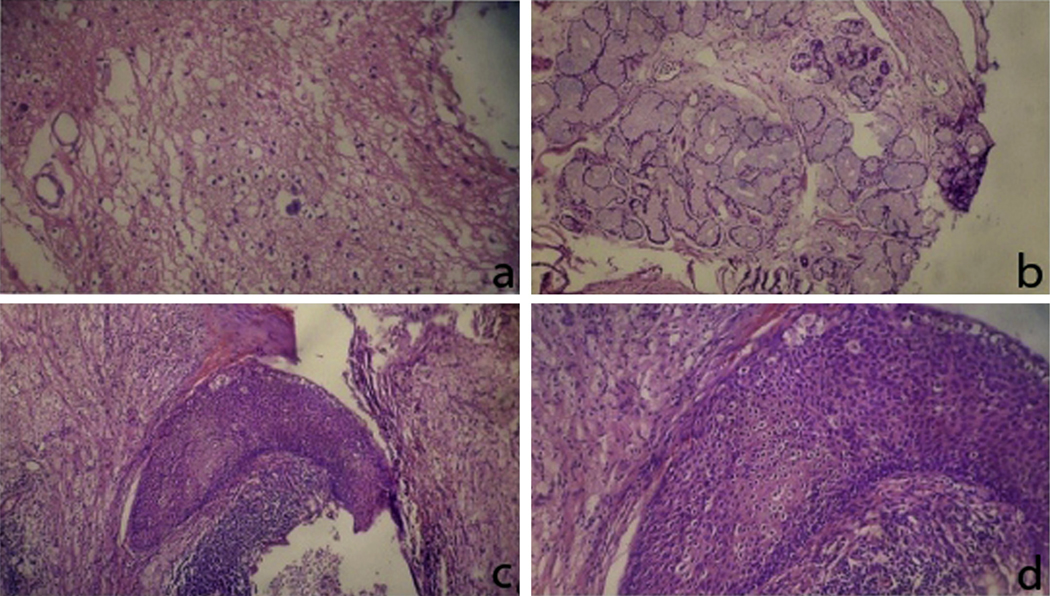
Histopathology of a mature teratoma composed of (a) mature glial cells, (b) mucous gland cells, and (c and d) squamous epithelium at different magnifications. This figure was reproduced with the kind permission of Elsevier. Urology 2011 Sep;78(3):689–91.
Fig. 3.
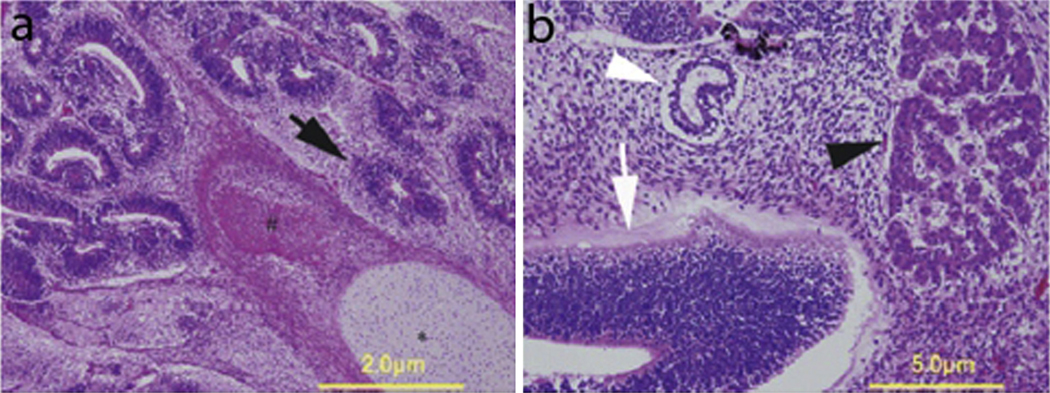
(a) Histopathology of an immature teratoma composed of brain tissue (solid arrow), bone (#), and cartilage (*). (b) Separate section of the tumor exhibiting elements of immature neuroepithelium (open arrowhead), enteric-type epithelium (open arrow), and liver tissue (solid arrowhead). This figure was reproduced with the kind permission of Wiley. J Obstet Gynaecol Res 2010 Dec;36(6):1252–5.
Embryonal carcinomas are characterized by epithelial cells containing large nuclei, increased mitotic activity, and areas of coagulative necrosis (Fig. 4) [9,63]. These cells demonstrate papillary or glandular architecture [9]. Yolk sac tumors are composed of under-developed epithelial cells with variable mitotic indices [9]. These cells often organize in reticular or sinusoidal patterns and may form Schiller-Duval bodies (Fig. 5) [9,64]. Some yolk sac tumor cells feature diagnostic eosinophilic and periodic acid Schiff (PAS) positive hyaline globules [9]. Choriocarcinomas are identified by the presence of mononucleate cytotrophoblastic and multinucleate syncytiotrophoblastic elements (Fig. 6) [65,66]. In addition, pools of erythrocytes and necrosis are diagnostic of choriocarcinomas [9].
Fig. 4.
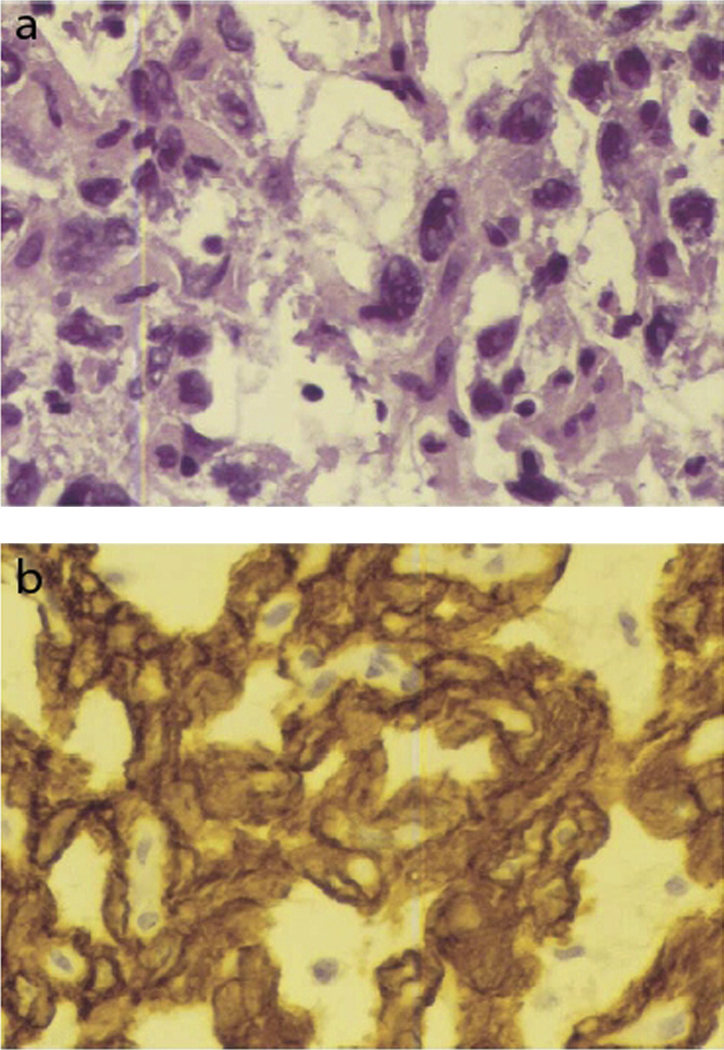
(a) Histopathology of an embryonal carcinoma exhibiting cells with large nuclei and irregular chromatin arrangement. (b) Immunohistochemical stain positive for cytokeratins. This figure was reproduced with the kind permission of Elsevier. Am J Ophthalmol 2005 Feb;139(2):380–1.
Fig. 5.
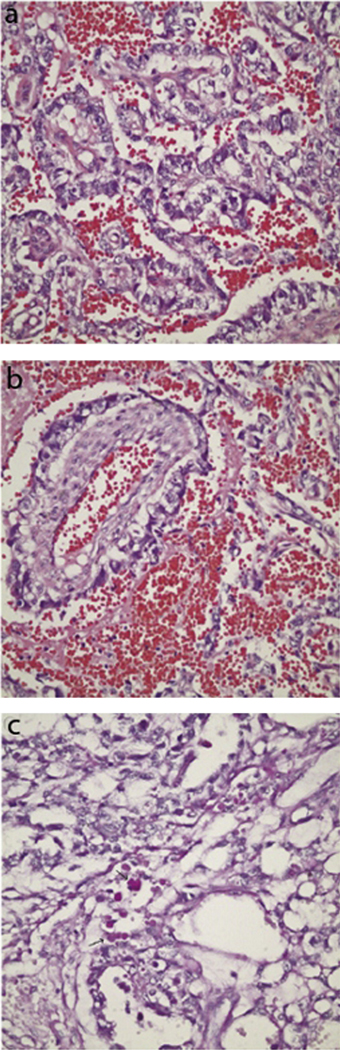
(a) Histopathology of a yolk sac tumor demonstrating tubular and polyvesicular vitelline arrangement of cells. (b) Characteristic Schiller-Duval body composed of a vascular space inside a cystic area and lined with tumor cells. (c) Appearance of hyaline globules. This figure was reproduced with the kind permission of Elsevier. J AAPOS 2008 Dec;12(6):623–5.
Fig. 6.
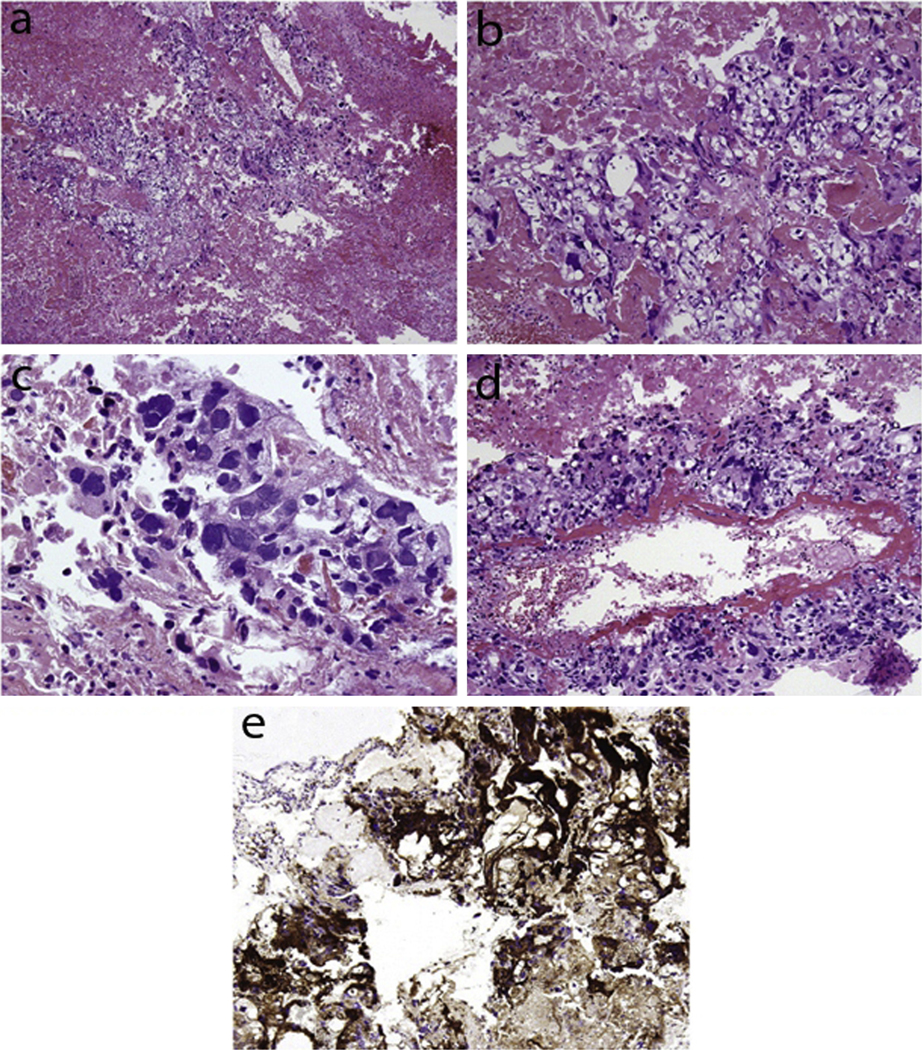
(a) Histopathology of a choriocarcinoma showing the presence of both cytotrophoblastic and syncytiotrophoblastic elements. (b) Increased magnification showing mononucleate cytotrophoblastic cells. (c) Increased magnification showing multinucleate syncytiotrophoblastic giant cells. (d) Cytotrophoblastic cells lining a villous-like structure. (e) Immunohistochemical stain of syncytiotrophoblastic cells positive for hCG. This figure was reproduced with the kind permission of Elsevier. Ann Diagn Pathol 2009 Apr;13(2):96–100.
3.5. Biomarkers
Serum and CSF concentrations of AFP and hCG have been traditionally evaluated [19,20]. These biomarkers may be elevated in several other disorders (e.g., gastrointestinal, pancreatic, biliary, and hepatocellular carcinomas), which limit specificity. Nevertheless, diagnostic sensitivity for IGCTs remains high [14,67]. The use of additional markers such as PLAP, human placental lactogen (HPL), Oct-4, and c-kit (CD117) has recently been introduced. Evaluation of biomarkers can enhance diagnostic accuracy and provide a way to monitor treatment response. Table 2 describes the results of serum and CSF evaluation of AFP and hCG among the cases reviewed. Table 3 details the marker reactivities of the cases reviewed.
Germinomas rarely demonstrate serum and CSF AFP elevations: serum and CSF AFP were elevated in 2% and 0% of the germinoma cases, respectively. AFP and CEA immunoreactivities were observed in 0% and 2% of the germinoma cases, respectively. This suggests that non-elevated AFP and non-immunoreactivity for AFP and CEA may characterize germinomas [11,16,59,68–70]. Immunoreactivities for c-kit, Oct-4, and PLAP are strongly indicative of germinomas and were observed in Case 1 (germinoma) [9,71,72]. Immunoreactivities for c-kit, Oct-4, and PLAP were found in the germinoma cases reviewed [69–71,73–75]. Cytokeratin immunoreactivity was less than expected with only 22% of germinomas demonstrating immunoreactivity [9,70]. Germinomas with STGCs have been associated with elevated serum hCG and immunoreactivities for hCG and HPL [9]. Serum hCG elevation was observed in 25%, hCG immunoreactivity in 35% and HPL immunoreactivity in 11% of germinomas [11,16,59,68,69]. hCG levels were not available in Case 2 and we acknowledge that if elevated, the diagnosis would have favored mixed GCT.
Teratomas express a characteristic marker profile [9]. Elevations in serum AFP and hCG were reported in 17% and 22% of cases, respectively [59,69]. AFP immunoreactivity was reported in 29%, CEA in 83%, Oct-4 in 25%, and c-kit in 50% [11,16,69–71,73,75]. None of the cases reported elevations in CSF AFP and hCG, nor immunoreactivities for hCG, PLAP, or HPL [11,16,69]. Unlike germinomas, cytokeratin was consistently expressed in all teratomas reviewed [70].
Embryonal carcinomas display variable levels of serum and CSF AFP and hCG expression. Elevated serum AFP was seen in 56%, serum hCG in 44%, CSF AFP in 80%, and CSF hCG in 80% of embryonal carcinoma [59,68,69]. Embryonal carcinomas demonstrated positive immunoreactivities for Oct-4 and PLAP, and negative immunoreactivity for c-kit in a previous study [9]. In line with this report, 100% of embryonal carcinomas exhibited Oct-4 immunoreactivity. Only 25% of cases reported PLAP immunoreactivity [69] and none exhibited c-kit immunoreactivity [73,75]. AFP, hCG, CEA, and HPL immunoreactivities were variable [11,16,69]. There was a paucity of data regarding cytokeratin expression in intracranial embryonal carcinomas. Nevertheless, studies have linked gonadal and extra-gonadal embryonal carcinomas with positive cytokeratin immunoreactivity [76,77].
Yolk sac tumors have been linked to elevated serum and CSF concentrations of AFP, with concentrations correlated to tumor size [11,70,78,79]. Our analysis demonstrated that all previously examined yolk sac tumor cases reported elevations in serum AFP without elevations in serum hCG [59]. Elevated serum LDH concentrations have been associated with gonadal yolk sac tumors and other gonadal GCTs, but there is limited data regarding LDH concentrations in intracranial yolk sac tumors [80–87]. Elevated CSF LDH in Case 2 (yolk sac tumor) coincides with previous findings. The connection between yolk sac tumors and elevated AFP levels was observed in Case 2 (yolk sac tumor). Positive AFP and negative Oct-4 and c-kit immunoreactivities have also been associated with yolk sac tumor histology [9]. Our analysis of markers by IHC support these observations [11,16,70,71,73,75]. Furthermore, all cases were reactive to cytokeratin, while none were reactive for HPL or hCG [11,59,70]. These results corroborated previous findings which suggested that non-elevated serum hCG levels, positive cytokeratin immunoreactivity, negative hCG and HPL immunoreactivities are typical of yolk sac tumors [9]. PLAP and CEA immunoreactivities in yolk sac tumors were variable with 60% and 44% of cases having demonstrated immunoreactivities, respectively [11,16,70].
Choriocarcinomas often display serum and CSF hCG elevations: serum and CSF hCG levels were elevated in 85% and 100% of cases, respectively [59,68,69,88]. Serum and CSF AFP elevations were reported in 17% and 33%, respectively [59,68,69]. Positive hCG and HPL immunoreactivities are typical of choriocarcinomas [9]. All choriocarcinoma cases were reactive for hCG, but only half were reactive for HPL [11,16,70]. We attribute this discrepancy to the small sample size. Cytokeratin immunoreactivity was demonstrated in all cases, without immunoreactivities for AFP or c-kit [11,16,70,71]. These results were consistent with prior statements that immunoreactivity for cytokeratin and nonimmunoreactivities for AFP and c-kit were typical of choriocarcinomas [9]. PLAP and CEA immunolabeling were inconsistent with 50% and 60% demonstrating immunoreactivity, respectively [11,16,70]. Insufficient data regarding Oct-4 expression limited quantitative synthesis. However, studies investigating gonadal and other extra-gonadal choriocarcinomas report negative Oct-4 expression to be characteristic [89,90].
4. Conclusion
GCTs are rare tumors and more common in Asians and males. These tumors display an affinity to develop along the pineal-suprasellar axis. We herein presented two cases of pineal GCTs (germinoma and yolk sac tumor), which inspired a review of the literature on IGCTs with an emphasis on histopathologies and biomarkers. Germinomas are most common and appear as a mix of lymphocytes and undifferentiated large cells with clear cytoplasm, round nuclei, and prominent nucleoli. Germinomas rarely demonstrate serum CSF AFP elevation. Immunoreactivity for c-kit, Oct-4, and PLAP is strongly indicative of germinomas. Yolk sac tumors classically display the Schiller-Duval bodies. Yolk sac tumors have been linked to elevated serum and CSF concentrations of AFP, with concentrations correlated to tumor size. Positive AFP and negative Oct-4 and c-kit immunoreactivity have also been associated with yolk sac tumor histology. Diagnostic sensitivity of traditional biomarkers (AFP and hCG) remains high and may provide a way to monitor treatment response.
Acknowledgements
Carlito Lagman is supported by a Gurtin Skull Base Research Fellowship. Lawrance K. Chung is supported by an AMA Foundation Seed Grant and an AΩA Carolyn L. Kuckein Student Research Fellowship. Timothy T. Bui is a recipient of the David Geffen Medical Scholarship. Seung J. Lee is a recipient of the American Academy of Neurology Medical Student Summer Research Scholarship. Isaac Yang (senior author) is partially supported by a Visionary Fund Grant, an Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research UCLA Scholars in Translational Medicine Program Award, the Jason Dessel Memorial Seed Grant, the UCLA Honberger Endowment Brain Tumor Research Seed Grant, and the Stop Cancer (US) Research Career Development Award.
Footnotes
Conflicts of interest/disclosures
None.
References
- [1].Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 1985;63:155–67. [DOI] [PubMed] [Google Scholar]
- [2].Eberts TJ, Ransburg RC. Primary intracranial endodermal sinus tumor. Case report. J Neurosurg 1979;50:246–52. [DOI] [PubMed] [Google Scholar]
- [3].Jellinger K. Primary intracranial germ cell tumours. Acta Neuropathol 1973;25:291–306. [DOI] [PubMed] [Google Scholar]
- [4].Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg 1991;74:545–51. [DOI] [PubMed] [Google Scholar]
- [5].Lin IJ, Shu SG, Chu HY, et al. Primary intracranial germ-cell tumor in children. Chin Med J 1997;60:259–64. [PubMed] [Google Scholar]
- [6].Kuratsu J, Ushio Y. Epidemiological study of primary intracranial tumors: a regional survey in Kumamoto Prefecture in the southern part of Japan. J Neurosurg 1996;84:946–50. [DOI] [PubMed] [Google Scholar]
- [7].Ueki K, Tanaka R. Treatments and prognoses of pineal tumors–experience of 110 cases. Neurol Med Chir 1980;20:1–26. [DOI] [PubMed] [Google Scholar]
- [8].Wang L, Yamaguchi S, Burstein MD, et al. Novel somatic and germline mutations in intracranial germ cell tumours. Nature 2014;511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].WHO Classification of Tumours of the Central Nervous System, 4th ed., Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- [10].Liang L, Korogi Y, Sugahara T, et al. MRI of intracranial germ-cell tumours. Neuroradiology 2002;44:382–8. [DOI] [PubMed] [Google Scholar]
- [11].Yamagami T, Handa H, Yamashita J, et al. An immunohistochemical study of intracranial germ cell tumours. Acta Neurochir 1987;86:33–41. [DOI] [PubMed] [Google Scholar]
- [12].Bartkova J, Hoei-Hansen CE, Krizova K, et al. Patterns of DNA damage response in intracranial germ cell tumors versus glioblastomas reflect cell of origin rather than brain environment: implications for the anti-tumor barrier concept and treatment. Mol Oncol 2014;8:1667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoei-Hansen CE, Sehested A, Juhler M, et al. New evidence for the origin of intracranial germ cell tumours from primordial germ cells: expression of pluripotency and cell differentiation markers. J Pathol 2006;209:25–33. [DOI] [PubMed] [Google Scholar]
- [14].Packer RJ, Cohen BH, Cooney K. Intracranial germ cell tumors. Oncologist 2000;5:312–20. [PubMed] [Google Scholar]
- [15].Murovic JA, Ongley JP, Parker JC Jr, et al. Manifestations and therapeutic considerations in pineal yolk-sac tumors. Case report. J Neurosurg 1981;55:303–7. [DOI] [PubMed] [Google Scholar]
- [16].Bjornsson J, Scheithauer BW, Okazaki H, et al. Intracranial germ cell tumors: pathobiological and immunohistochemical aspects of 70 cases. J Neuropathol Exp Neurol 1985;44:32–46. [PubMed] [Google Scholar]
- [17].Fujimaki T, Matsutani M, Funada N, et al. CT and MRI features of intracranial germ cell tumors. J Neurooncol 1994;19:217–26. [DOI] [PubMed] [Google Scholar]
- [18].Chang T, Teng MM, Guo WY, et al. CT of pineal tumors and intracranial germcell tumors. AJNR Am J Neuroradiol 1989;10:1039–44. [PMC free article] [PubMed] [Google Scholar]
- [19].Kyritsis AP. Management of primary intracranial germ cell tumors. J Neurooncol 2010;96:143–9. [DOI] [PubMed] [Google Scholar]
- [20].Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist 2008;13:690–9. [DOI] [PubMed] [Google Scholar]
- [21].Haas-Kogan DA, Missett BT, Wara WM, et al. Radiation therapy for intracranial germ cell tumors. Int J Radiat Oncol Biol Phys 2003;56:511–8. [DOI] [PubMed] [Google Scholar]
- [22].Sawamura Y, Ikeda J, Shirato H, et al. Germ cell tumours of the central nervous system: treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer 1998;34:104–10. [DOI] [PubMed] [Google Scholar]
- [23].Calaminus G, Bamberg M, Baranzelli MC, et al. Intracranial germ cell tumors: a comprehensive update of the European data. Neuropediatrics 1994;25: 26–32. [DOI] [PubMed] [Google Scholar]
- [24].Wolden SL, Wara WM, Larson DA, et al. Radiation therapy for primary intracranial germ-cell tumors. Int J Radiat Oncol Biol Phys 1995;32:943–9. [DOI] [PubMed] [Google Scholar]
- [25].Dearnaley DP, A’Hern RP, Whittaker S, et al. Pineal and CNS germ cell tumors: Royal Marsden Hospital experience 1962–1987. Int J Radiat Oncol Biol Phys 1990;18:773–81. [DOI] [PubMed] [Google Scholar]
- [26].Linstadt D, Wara WM, Edwards MS, et al. Radiotherapy of primary intracranial germinomas: the case against routine craniospinal irradiation. Int J Radiat Oncol Biol Phys 1988;15:291–7. [DOI] [PubMed] [Google Scholar]
- [27].Sung DI, Harisliadis L, Chang CH. Midline pineal tumors and suprasellar germinomas: highly curable by irradiation. Radiology 1978;128:745–51. [DOI] [PubMed] [Google Scholar]
- [28].Balmaceda C, Finlay J. Current advances in the diagnosis and management of intracranial germ cell tumors. Curr Neurol Neurosci Rep 2004;4:253–62. [DOI] [PubMed] [Google Scholar]
- [29].Weiner HL, Finlay JL. Surgery in the management of primary intracranial germ cell tumors. Childs Nerv Syst 1999;15:770–3. [DOI] [PubMed] [Google Scholar]
- [30].Amendola BE, McClatchey K, Amendola MA. Pineal region tumors: analysis of treatment results. Int J Radiat Oncol Biol Phys 1984;10:991–7. [DOI] [PubMed] [Google Scholar]
- [31].Shibamoto Y, Abe M, Yamashita J, et al. Treatment results of intracranial germinoma as a function of the irradiated volume. Int J Radiat Oncol Biol Phys 1988;15:285–90. [DOI] [PubMed] [Google Scholar]
- [32].Fields JN, Fulling KH, Thomas PR, et al. Suprasellar germinoma: radiation therapy. Radiology 1987;164:247–9. [DOI] [PubMed] [Google Scholar]
- [33].Rich TA, Cassady JR, Strand RD, et al. Radiation therapy for pineal and suprasellar germ cell tumors. Cancer 1985;55:932–40. [DOI] [PubMed] [Google Scholar]
- [34].Jensen AW, Laack NN, Buckner JC, et al. Long-term follow-up of dose-adapted and reduced-field radiotherapy with or without chemotherapy for central nervous system germinoma. Int J Radiat Oncol Biol Phys 2010;77:1449–56. [DOI] [PubMed] [Google Scholar]
- [35].Cho J, Choi JU, Kim DS, et al. Low-dose craniospinal irradiation as a definitive treatment for intracranial germinoma. Radiother Oncol 2009;91:75–9. [DOI] [PubMed] [Google Scholar]
- [36].Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol 2005;6:509–19. [DOI] [PubMed] [Google Scholar]
- [37].Yen SH, Chen YW, Huang PI, et al. Optimal treatment for intracranial germinoma: can we lower radiation dose without chemotherapy? Int J Radiat Oncol Biol Phys 2010;77:980–7. [DOI] [PubMed] [Google Scholar]
- [38].Nakamura H, Takeshima H, Makino K, et al. Recurrent intracranial germinoma outside the initial radiation field: a single-institution study. Acta Oncol (Stockholm, Sweden) 2006;45:476–83. [DOI] [PubMed] [Google Scholar]
- [39].Aydin F, Ghatak NR, Radie-Keane K, et al. The short-term effect of low-dose radiation on intracranial germinoma. A pathologic study. Cancer 1992;69:2322–6. [DOI] [PubMed] [Google Scholar]
- [40].Sawamura Y, de Tribolet N, Ishii N, et al. Management of primary intracranial germinomas: diagnostic surgery or radical resection? J Neurosurg 1997;87: 262–6. [DOI] [PubMed] [Google Scholar]
- [41].Sawamura Y, Kato T, Ikeda J, et al. Teratomas of the central nervous system: treatment considerations based on 34 cases. J Neurosurg 1998;89:728–37. [DOI] [PubMed] [Google Scholar]
- [42].Ogawa K, Toita T, Nakamura K, et al. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer 2003;98: 369–76. [DOI] [PubMed] [Google Scholar]
- [43].Noudel R, Vinchon M, Dhellemmes P, et al. Intracranial teratomas in children: the role and timing of surgical removal. J Neurosurg Pediatr 2008;2:331–8. [DOI] [PubMed] [Google Scholar]
- [44].Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial nongerminoma germ cell tumors with multimodality therapy. J Neurooncol 1997;32:71–80. [DOI] [PubMed] [Google Scholar]
- [45].Calaminus G, Bamberg M, Jurgens H, et al. Impact of surgery, chemotherapy and irradiation on long term outcome of intracranial malignant non germinomatous germ cell tumors: results of the German Cooperative Trial MAKEI 89. Klin Padiatr 2004;216:141–9. [DOI] [PubMed] [Google Scholar]
- [46].Kochi M, Itoyama Y, Shiraishi S, et al. Successful treatment of intracranial nongerminomatous malignant germ cell tumors by administering neoadjuvant chemotherapy and radiotherapy before excision of residual tumors. J Neurosurg 2003;99:106–14. [DOI] [PubMed] [Google Scholar]
- [47].Patel SR, Buckner JC, Smithson WA, et al. Cisplatin-based chemotherapy in primary central nervous system germ cell tumors. J Neurooncol 1992;12:47–52. [DOI] [PubMed] [Google Scholar]
- [48].Itoyama Y, Kochi M, Kuratsu J, et al. Treatment of intracranial nongerminomatous malignant germ cell tumors producing alpha-fetoprotein. Neurosurgery 1995;36:459–64 [discussion 64–6]. [DOI] [PubMed] [Google Scholar]
- [49].Itoyama Y, Kochi M, Yamamoto H, et al. Clinical study of intracranial nongerminomatous germ cell tumors producing alpha-fetoprotein. Neurosurgery 1990;27:454–60. [DOI] [PubMed] [Google Scholar]
- [50].Yoshida J, Sugita K, Kobayashi T, et al. Prognosis of intracranial germ cell tumours: effectiveness of chemotherapy with cisplatin and etoposide (CDDP and VP-16). Acta Neurochir 1993;120:111–7. [DOI] [PubMed] [Google Scholar]
- [51].Allen JC, Bosl G, Walker R. Chemotherapy trials in recurrent primary intracranial germ cell tumors. J Neurooncol 1985;3:147–52. [DOI] [PubMed] [Google Scholar]
- [52].Allen JC, Kim JH, Packer RJ. Neoadjuvant chemotherapy for newly diagnosed germ-cell tumors of the central nervous system. J Neurosurg 1987;67:65–70. [DOI] [PubMed] [Google Scholar]
- [53].Schild SE, Scheithauer BW, Haddock MG, et al. Histologically confirmed pineal tumors and other germ cell tumors of the brain. Cancer 1996;78:2564–71. [DOI] [PubMed] [Google Scholar]
- [54].Mann JR, Raafat F, Robinson K, et al. The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol 2000;18:3809–18. [DOI] [PubMed] [Google Scholar]
- [55].Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation–a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 1996;14:2908–15. [DOI] [PubMed] [Google Scholar]
- [56].Grimison PS, Stockler MR, Thomson DB, et al. Comparison of two standard chemotherapy regimens for good-prognosis germ cell tumors: updated analysis of a randomized trial. J Natl Cancer Inst 2010;102:1253–62. [DOI] [PubMed] [Google Scholar]
- [57].Bosl GJ, Geller NL, Bajorin D, et al. A randomized trial of etoposide + cisplatin versus vinblastine + bleomycin + cisplatin + cyclophosphamide + dactinomycin in patients with good-prognosis germ cell tumors. J Clin Oncol 1988;6: 1231–8. [DOI] [PubMed] [Google Scholar]
- [58].Einhorn LH, Williams SD, Troner M, et al. The role of maintenance therapy in disseminated testicular cancer. N Engl J Med 1981;305:727–31. [DOI] [PubMed] [Google Scholar]
- [59].Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 1997;86:446–55. [DOI] [PubMed] [Google Scholar]
- [60].Yang DT, Rozen WM, Rickert CH, et al. Primary pontomedullary germinoma in a 12 year old boy. J Clin Neurosci 2009;16:321–5. [DOI] [PubMed] [Google Scholar]
- [61].Li Y, Zhong Z, Zhao X. Primary mature teratoma presenting as an adrenal tumor in a child. Urology 2011;78:689–91. [DOI] [PubMed] [Google Scholar]
- [62].Koizumi K, Abe E, Kusanagi Y, et al. Giant immature intracranial teratoma with antenatal cranial perforation. J Obstet Gynaecol Res 2010;36:1252–5. [DOI] [PubMed] [Google Scholar]
- [63].Rodriguez SL, Kostick DA, Hered RW, et al. Primary embryonal carcinoma of the orbit in a 10-month-old female: a seven-year follow-up. Am J Ophthalmol 2005;139:380–1. [DOI] [PubMed] [Google Scholar]
- [64].Kiratli H, Erkan Balci K, Guler G. Primary orbital endodermal sinus tumor (yolk sac tumor). J AAPOS 2008;12:623–5. [DOI] [PubMed] [Google Scholar]
- [65].Bell DM, Porras G, Tortoledo ME, et al. Primary sinonasal choriocarcinoma. Ann Diagn Pathol 2009;13:96–100. [DOI] [PubMed] [Google Scholar]
- [66].Yamagami T, Handa H, Takeuchi J, et al. Choriocarcinoma arising from the pituitary fossa with extracranial metastasis: a review of the literature. Surg Neurol 1983;19:469–80. [DOI] [PubMed] [Google Scholar]
- [67].Murray MJ, Nicholson JC. Alpha-Fetoprotein. Arch Dis Childhood Educ Pract Ed 2011;96:141–7. [DOI] [PubMed] [Google Scholar]
- [68].Allen JC, Nisselbaum J, Epstein F, et al. Alphafetoprotein and human chorionic gonadotropin determination in cerebrospinal fluid. An aid to the diagnosis and management of intracranial germ-cell tumors. J Neurosurg 1979;51:368–74. [DOI] [PubMed] [Google Scholar]
- [69].Inoue HK, Naganuma H, Ono N. Pathobiology of intracranial germ-cell tumors: immunochemical, immunohistochemical, and electron microscopic investigations. J Neurooncol 1987;5:105–15. [DOI] [PubMed] [Google Scholar]
- [70].Ho DM, Liu HC. Primary intracranial germ cell tumor. Pathologic study of 51 patients. Cancer 1992;70:1577–84. [DOI] [PubMed] [Google Scholar]
- [71].Kamakura Y, Hasegawa M, Minamoto T, et al. C-kit gene mutation: common and widely distributed in intracranial germinomas. J Neurosurg 2006;104:173–80. [DOI] [PubMed] [Google Scholar]
- [72].Shinoda J, Miwa Y, Sakai N, et al. Immunohistochemical study of placental alkaline phosphatase in primary intracranial germ-cell tumors. J Neurosurg 1985;63:733–9. [DOI] [PubMed] [Google Scholar]
- [73].Nakamura H, Takeshima H, Makino K, et al. C-kit expression in germinoma: an immunohistochemistry-based study. J Neurooncol 2005;75:163–7. [DOI] [PubMed] [Google Scholar]
- [74].Hattab EM, Tu PH, Wilson JD, et al. OCT4 immunohistochemistry is superior to placental alkaline phosphatase (PLAP) in the diagnosis of central nervous system germinoma. Am J Surg Pathol 2005;29:368–71. [DOI] [PubMed] [Google Scholar]
- [75].Ngan KW, Jung SM, Lee LY, et al. Immunohistochemical expression of OCT4 in primary central nervous system germ cell tumours. J Clin Neurosci 2008;15:149–52. [DOI] [PubMed] [Google Scholar]
- [76].Suster S, Moran CA, Dominguez-Malagon H, et al. Germ cell tumors of the mediastinum and testis: a comparative immunohistochemical study of 120 cases. Hum Pathol 1998;29:737–42. [DOI] [PubMed] [Google Scholar]
- [77].Cheville JC, Rao S, Iczkowski KA, et al. Cytokeratin expression in seminoma of the human testis. Am J Clin Pathol 2000;113:583–8. [DOI] [PubMed] [Google Scholar]
- [78].Arita N, Bitoh S, Ushio Y, et al. Primary pineal endodermal sinus tumor with elevated serum and CSF alphafetoprotein levels. Case report. J Neurosurg 1980;53:244–8. [DOI] [PubMed] [Google Scholar]
- [79].Green DM. The diagnosis and treatment of yolk sac tumors in infants and children. Cancer Treat Rev 1983;10:265–88. [DOI] [PubMed] [Google Scholar]
- [80].Kinumaki H, Takeuchi H, Nakamura K, et al. Serum lactate dehydrogenase isoenzyme-1 in children with yolk sac tumor. Cancer 1985;56:178–81. [DOI] [PubMed] [Google Scholar]
- [81].von Eyben FE. A systematic review of lactate dehydrogenase isoenzyme 1 and germ cell tumors. Clin Biochem 2001;34:441–54. [DOI] [PubMed] [Google Scholar]
- [82].von Eyben FE. Biochemical markers in advanced testicular tumors: serum lactate dehydrogenase, urinary chorionic gonadotropin and total urinary estrogens. Cancer 1978;41:648–52. [DOI] [PubMed] [Google Scholar]
- [83].von Eyben FE. Lactate dehydrogenase and its isoenzymes in testicular germ cell tumors: an overview. Oncodev Biol Med 1983;4:395–414. [PubMed] [Google Scholar]
- [84].von Eyben FE, Skude G, Fossa SD, et al. Serum lactate dehydrogenase (S-LDH) and S-LDH isoenzymes in patients with testicular germ cell tumors. Mol Gen Genet 1983;189:326–33. [DOI] [PubMed] [Google Scholar]
- [85].Gilligan TD, Seidenfeld J, Basch EM, et al. American Society of Clinical Oncology Clinical Practice Guideline on uses of serum tumor markers in adult males with germ cell tumors. J Clin Oncol 2010;28:3388–404. [DOI] [PubMed] [Google Scholar]
- [86].Lippert MC, Javadpour N. Lactic dehydrogenase in the monitoring and prognosis of testicular cancer. Cancer 1981;48:2274–8. [DOI] [PubMed] [Google Scholar]
- [87].Ito M, Taki T, Mitsuoka A, et al. Lactate dehydrogenase isoenzyme-1 in the mediastinal yolk sac tumor. Jpn J Surg 1988;18:419–22. [DOI] [PubMed] [Google Scholar]
- [88].Lv XF, Qiu YW, Zhang XL, et al. Primary intracranial choriocarcinoma: MR imaging findings. AJNR Am J Neuroradiol 2010;31:1994–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jones TD, Ulbright TM, Eble JN, et al. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 2004;28:935–40. [DOI] [PubMed] [Google Scholar]
- [90].Liu A, Cheng L, Du J, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am J Surg Pathol 2010;34:697–706. [DOI] [PubMed] [Google Scholar]