In this issue of Blood, Lee et al report a retrospective analysis of the effects of SARS-CoV-2 vaccination on thrombocytopenia in patients with immune thrombocytopenia (ITP).1 This study shows that SARS-CoV-2 vaccines are safe in general for patients with preexisting ITP. Although the data presented should encourage the administration of both the doses of 2-dose vaccines to counter the virus, the authors also observed that thrombocytopenia exacerbations may occur, and, hence, careful monitoring is required. The authors emphasize the need for more careful postvaccination tracking of vulnerable patient groups, such as those who have had splenectomy and those who have received 5 or more therapies for ITP because they have a higher risk for worsening thrombocytopenia.
A major breakthrough was achieved in the ongoing COVID-19 pandemic by the rapid development, emergency rollout, and administration of several vaccines against SARSCoV-2 coronavirus.2 There has also been a global push to understand potential side effects, especially in patients with comorbidities. Although SARS-CoV-2 vaccines are largely effective and safe, with the increasing number of vaccinated individuals across the world, there have been reports of thrombocytopenia after vaccinations with SARS-CoV-2 vaccines.3
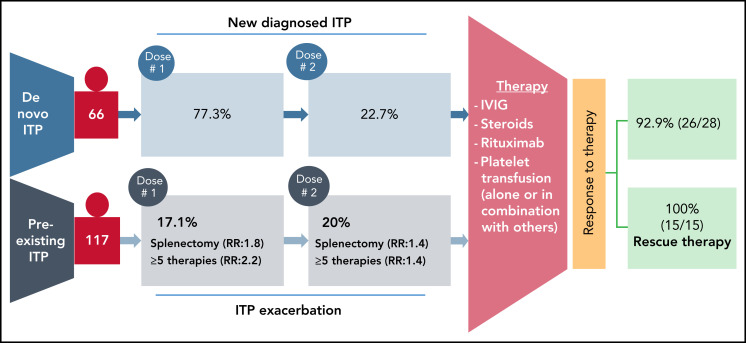
The goal of the study by Lee et al was to track the development of de novo ITP in patients after COVID-19 vaccination and to track postvaccination exacerbation in preexisting patients with ITP. The authors used 4 different data sets to look for thrombocytopenia post-SARS-CoV-2 vaccination. They used Vaccine Adverse Events Reporting System for estimating the cases of a de novo ITP. Furthermore, for patients with preexisting ITP, data from a 10-center retrospective study, and surveys distributed by the Platelet Disorder Support Association (PDSA) and the United Kingdom (UK) ITP Support Association. From the Vaccine Adverse Events Reporting System dataset, 77 cases of de novo ITP were identified at ∼1 week postvaccination. Of these, 92.9% (26/28 of available data) responded well to treatment with corticosteroids and/or intravenous immunoglobulin, and/or platelet transfusions (see figure).
Summary of thrombocytopenia events after SARS-CoV-2 vaccination. Individuals without a previously reported platelet disorders and suspected de novo ITP were identified in the Vaccine Adverse Events Reporting System. In patients with preexisting ITP, splenectomy and/or 5 more prior therapies led to a greater risk of exacerbation. Both groups of patients responded well to therapy.
In 109 patients identified with preexisting ITP who received a SARS-CoV-2 vaccine, approximately 20% experienced an ITP exacerbation following the first dose with 14 of 70 patients having an exacerbation after the second dose. Data from the Platelet Disorder Support Association (57 patients) and UK surveys (43 patients) confirmed the absence of severe bleeding but identified splenectomy as a risk factor for an ITP exacerbation. Along with splenectomy, 5 or more prior lines of therapy for ITP was also shown to increase risk of exacerbation. Response to treatment and outcomes were also favorable in the patients with preexisting ITP, and no major bleeds were reported after vaccination. Therefore, the authors concluded that ITP might worsen in some patients with preexisting ITP, or may occur de novo post-SARS-CoV-2 vaccination. However, under both circumstances, patients respond well to treatment regimes. Most importantly, although vaccinations were applied intramuscularly, no local vaccination-related hematomas were reported in this study. Authors recommend, however, a proactive monitoring of platelet counts for patients with known ITP, and especially those in high-risk groups including postsplenectomy and more refractory disease. Cases of de novo ITP were reported previously in healthy recipients following SARS-CoV-2 vaccination followed with a broad global attention.4,5 Although rare but serious adverse events have also been reported with a higher fatality rate after ChAdOx1 nCoV-19 (AstraZeneca) vaccination.6,7 These thrombotic events associated with thrombocytopenia after vaccination against SARS-CoV-2 are collectively referred as vaccine-induced immune thrombotic thrombocytopenia or thrombosis with thrombocytopenia syndrome.8 Cases of thrombosis, including cerebral venous sinus thrombosis, associated with severe thrombocytopenia have also been reported after administration of Ad26.COV2.S vaccine (Johnson & Johnson/Janssen).9 This important study comes in a timely manner because of considerably growing vaccine hesitancy and uncertainty among high-risk patient groups, general population, and physicians caring for high-risk patient groups. Therefore, reassuring data regarding successful treatment of de novo as well as preexisting ITP will have significant impact on patient management and care for ITP patients after vaccination.
Supplementary Material
Footnotes
Conflict-of-interest disclosure: T.B. has received research funding from CoaChrom Diagnostica GmbH, DFG, Robert Bosch GmbH, Stiftung Transfusionsmedizin und Immunhämatologie e.V.: Ergomed, Surrey, DRK Blutspendedienst, Deutsche Herzstiftung, Ministerium fuer Wissenschaft, and Forschung und Kunst Baden-Wuerttembergm; has received lecture honoraria from Aspen Germany GmbH, Bayer Vital GmbH, Bristol-Myers Squibb GmbH & Co., Doctrina Med AG, Meet The Experts Academy UG, Schoechl medical education GmbH, Mattsee, Stago GmbH, Mitsubishi Tanabe Pharma GmbH, and Novo Nordisk Pharma GmbH; has provided consulting services to Terumo; and has provided expert witness testimony relating to heparin-induced thrombocytopenia and non‐heparin-induced thrombocytopenia thrombocytopenic and coagulopathic disorders. All of these are outside the current work. A.S. declares no competing financial interests.
REFERENCES
- 1.Lee E-J, Beltrami-Moreira M, Al-Samkari H, et al. SARS-CoV-2 vaccination and ITP in de novo or pre-existing ITP patients. Blood. 2022;139(10):1564-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID research: a year of scientific milestones [published online ahead of print 5 May 2021]. Nature. doi: 10.1038/d41586-020-00502-w. [DOI] [PubMed] [Google Scholar]
- 3.Pishko AM, Bussel JB, Cines DB. COVID-19 vaccination and immune thrombocytopenia. Nat Med. 2021;27(7):1145-1146. [DOI] [PubMed] [Google Scholar]
- 4.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althaus K, Möller P, Uzun G, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106(8):2170-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18): 1680-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir K-L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.