Abstract
Background
The risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) decreases substantially among patients who have recovered from coronavirus disease 2019 (Covid-19). However, it is unknown how long protective immunity lasts. Current guidelines recommend vaccination of recovered patients even though data regarding vaccine effectiveness in such cases are still limited.
Methods
In this retrospective cohort study, we reviewed electronic medical records from a large health care organization in Israel to assess reinfection rates in patients who had recovered from SARS-CoV-2 infection before any vaccination against Covid-19. We compared reinfection rates among patients who had subsequently received the BNT162b2 vaccine (Pfizer–BioNTech) and those who had not been vaccinated between March 1 and November 26, 2021. We used a Cox proportional-hazards regression model with time-dependent covariates to estimate the association between vaccination and reinfection after adjustment for demographic factors and coexisting illnesses. Vaccine effectiveness was estimated as 1 minus the hazard ratio. In a secondary analysis, we evaluated the vaccine effectiveness of one dose as compared with two doses.
Results
A total of 149,032 patients who had recovered from SARS-CoV-2 infection met the eligibility criteria. Of these patients, 83,356 (56%) received subsequent vaccination during the 270-day study period. Reinfection occurred in 354 of the vaccinated patients (2.46 cases per 100,000 persons per day) and in 2168 of 65,676 unvaccinated patients (10.21 cases per 100,000 persons per day). Vaccine effectiveness was estimated at 82% (95% confidence interval [CI], 80 to 84) among patients who were 16 to 64 years of age and 60% (95% CI, 36 to 76) among those 65 years of age or older. No significant difference in vaccine effectiveness was found for one dose as compared with two doses.
Conclusions
Among patients who had recovered from Covid-19, the receipt of at least one dose of the BNT162b2 vaccine was associated with a significantly lower risk of recurrent infection.
Observational studies have shown that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) elicits an immune response and provides protection against recurrent infection.1-4 Nevertheless, the vaccination of patients who have recovered from coronavirus disease 2019 (Covid-19) has been recommended by the Centers for Disease Control and Prevention,5 the European Centre for Disease Prevention and Control,6 and the U.K. Health Security Agency7 since the degree and persistence of infection-acquired immunity over time have not yet been determined.8,9
Early biologic and epidemiologic evidence has suggested the effectiveness of Covid-19 vaccination in small cohorts of recovered patients in the short term.10-12 However, the effectiveness in large populations and for extended periods remains uncertain. In June 2021, the B.1.617.2 (delta) variant of SARS-CoV-2 became the dominant strain in Israel (Fig. S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org) and went on to cause a major resurgence of Covid-19 infection (Fig. S2). The surge of the delta variant provided an epidemiologic opportunity to assess whether the vaccination of patients who had recovered from Covid-19 would provide extra protection against recurrent infection. Thus, our study objective was to evaluate the effectiveness of the BNT162b2 messenger RNA (mRNA) Covid-19 vaccine (Pfizer–BioNTech) in preventing reinfection in patients who had recovered from Covid-19.
Methods
Study Design and Population
This observational, retrospective cohort study was based on routine data obtained from electronic medical records for members of Clalit Health Services, a large health care organization that covers approximately 52% of the Israeli population. The study included Clalit Health Services members between the ages of 16 and 110 years who had recovered from documented SARS-CoV-2 infection at least 100 days earlier and before the receipt of any vaccination against Covid-19. The primary infection date was defined as the timing of the first positive result on quantitative reverse-transcriptase–polymerase-chain-reaction (RT-qPCR) assay. Eligible patients had a primary infection that occurred between August 23, 2020 (190 days before study initiation) and May 31, 2021 (90 days after study initiation).
We compared rates of recurrent infection, as identified on ad hoc RT-qPCR testing, among patients who had received the BNT162b2 vaccine with rates among unvaccinated patients. A regression analysis was used to assess vaccine effectiveness.
Recurrent infection was defined as a positive RT-qPCR test for SARS-CoV-2 at least 100 days after the primary infection. The 100-day cutoff for defining recovery was determined in order to account for prolonged PCR positivity and in accordance with the Israeli Ministry of Health guidelines that allow patients who have recovered from Covid-19 to be vaccinated at least 3 months after their primary documented infection.
The study start date was March 1, 2021, when the Israeli Ministry of Health approved vaccination of all patients who had recovered from Covid-19 at least 3 months earlier. The follow-up of patients whose primary infection had occurred 100 to 190 days before study initiation began on the study start date. The follow-up of patients who fit the inclusion criteria after the study start date began at 100 days after the primary infection. The study follow-up ended on November 26, 2021 (Fig. S3).
The exclusion criteria included reinfection before the study start date, a history of vaccination against Covid-19 before the primary infection, and vaccination before the 100-day recovery cutoff date. Since Clalit Health Services had only started to provide the mRNA-1273 (Moderna) vaccine to recovered patients in August 2021, mRNA-1273 recipients were also excluded.
The study population was divided into vaccinated and unvaccinated groups. Inclusion in the two groups was dynamic during the study period; participants who had received their first vaccine dose were counted in the unvaccinated group until 7 days after vaccination, after which they were transitioned to the vaccinated group. The 7-day time lag for the analysis of vaccine effectiveness is in concordance with the reported time for vaccine efficacy that was observed in randomized, controlled trials of the BNT162b2 vaccine with respect to the second and third doses.13,14 The time lag for vaccine effectiveness has also been validated in a previous large observational study regarding the BNT162b2 booster dose.15 A description of patients’ transition from the unvaccinated group to the vaccinated group is provided in Figure S4.
The study was approved by the Community Helsinki and Data Utilization committees of Clalit Health Services. The study was exempt from obtaining informed consent from the patients, owing to the retrospective design. No financial or in-kind support was provided for the conduct of the study.
Study Outcomes
The primary outcome was the rate of SARS-CoV-2 reinfection in the population that had recovered from Covid-19. In a secondary analysis, we assessed vaccine effectiveness among patients who had received one vaccine dose as compared with those who had received two doses.
Data Sources and Organization
In this analysis, we evaluated integrated patient-level data maintained by Clalit Health Services from two primary sources: the operational primary care database and the Covid-19 database. The operational database includes sociodemographic data and comprehensive clinical information, such as the history of community care visits and hospitalizations and the results of laboratory tests. The evaluation of coexisting chronic conditions was based on the registry of diagnoses of chronic diseases compiled from multiple data sources.
The Covid-19 database includes vaccination dates and the results of all ad hoc RT-qPCR tests performed for suspected infections and surveillance. The Covid-19 database is updated daily from the Covid-19 central repository of the Ministry of Health. The same databases were used in the primary studies that evaluated the effectiveness and safety of the BNT162b2 vaccine in a real-world setting.16,17 A description of the data repositories that were used in this study is provided in the Supplementary Appendix.
For each patient in the study, the following sociodemographic data were extracted: age, sex, population sector (general Jewish, ultra-Orthodox Jewish, or Arab), and the score for socioeconomic status (ranging from 1 [lowest] to 10 [highest]). Details regarding these data are provided in the Supplementary Appendix.
The following clinical data were extracted: vaccination dates, all RT-qPCR test results, and clinical risk factors for Covid-19 complications,18 including diabetes mellitus, chronic obstructive pulmonary disease, asthma, chronic kidney failure, lung cancer, hypertension, ischemic heart disease, chronic heart failure, obesity, and a history of stroke, transient ischemic attack, or smoking.
Statistical Analysis
We used descriptive statistics to characterize the study patients. Kaplan–Meier analysis with log-rank testing was performed for univariate analyses. We used a multivariate Cox proportional-hazards regression model with time-dependent covariates to estimate the association between all covariates and vaccination. A P value of less than 0.05 was considered to indicate statistical significance in all analyses.
Patients’ data were censored 7 days after the receipt of a vaccine in the unvaccinated group and at the time of death from any cause or the end of follow-up in the two groups. Since the independent variable (vaccination status) varied over time, univariate and multivariate survival analyses were performed with time-dependent covariates according to the study design. The association between vaccination and reinfection was estimated by means of a multivariate Cox proportional-hazards regression model after adjustment for sociodemographic factors and coexisting illnesses. All the covariates were tested for interactions with the variable of interest (vaccination) and age group. Variables that met the testing criteria and were significantly associated with the outcome served as the inputs for multivariate regression analysis. The proportional-hazards assumption was tested for each variable by comparing survival curves and by performing Schoenfeld’s global test. Vaccine effectiveness was calculated as 1 minus the hazard ratio, as estimated by Cox proportional-hazards regression.
R statistical software, version 3.5.0 (R Foundation), was used for univariate and multivariate survival analyses with time-dependent covariates. SPSS software, version 26 (IBM), was used for all other statistical analyses.
Results
Patient Population
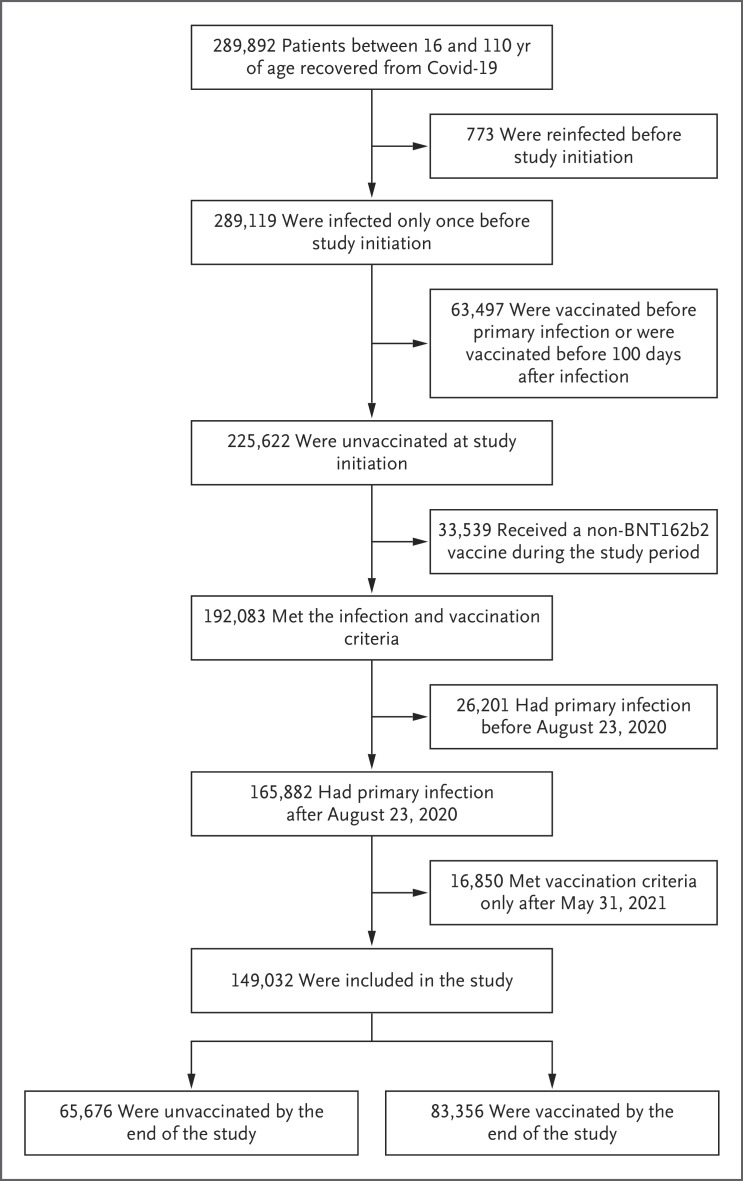
During the study period, 149,032 patients met the eligibility criteria. Of these patients, 83,356 (56%) received at least one dose of the BNT162b2 vaccine (Figure 1). The mean age of the study patients was 39.3 years. The most common coexisting conditions were obesity and current or former smoking (Table 1). The association between the patients’ characteristics and the rate of vaccine uptake is described in Table 2.
Figure 1. Assessment for Eligibility.
The study included members of the Israeli Clalit Health Services who had recovered from documented coronavirus disease 2019 (Covid-19) at least 100 days earlier and who had not received any vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the time of infection. Eligible patients had a primary infection that occurred between August 23, 2020 (190 days before study initiation), and May 31, 2021 (90 days after study initiation).
Table 1. Characteristics of the Patients at Baseline.*.
| Characteristic | All Patients (N=149,032) |
|---|---|
| Age | |
| Mean ±SD — yr | 39.3±17.1 |
| Distribution — no. (%) | |
| 16–64 yr | 134,849 (90.5) |
| ≥65 yr | 14,183 (9.5) |
| Female sex — no. (%) | 81,162 (54.5) |
| Population sector — no. (%) | |
| General Jewish | 77,519 (52.0) |
| Ultra-Orthodox Jewish | 21,426 (14.4) |
| Arab | 50,087 (33.6) |
| Median score for socioeconomic status (IQR)† | 4 (2–6) |
| Clinical risk factors — no. (%) | |
| Diabetes | 14,727 (9.9) |
| Chronic obstructive pulmonary disease | 1,709 (1.1) |
| Asthma | 7,606 (5.1) |
| Chronic kidney failure | 2,332 (1.6) |
| Hypertension | 18,087 (12.1) |
| Ischemic heart disease | 5,720 (3.8) |
| Chronic heart failure | 1,868 (1.3) |
| Obesity | 36,938 (24.8) |
| Lung cancer | 155 (0.1) |
| History of stroke | 2,841 (1.9) |
| History of transient ischemic attack | 1,223 (0.8) |
| Current or former smoking | 34,290 (23.0) |
IQR denotes interquartile range.
The score for socioeconomic status ranges from 1 (lowest) to 10 (highest).
Table 2. Association between Characteristics of the Patients and Vaccine Uptake.*.
| Characteristic | Hazard Ratio (95% CI) | |
|---|---|---|
| Unadjusted Analysis | Adjusted Analysis | |
| Age group | ||
| 16–64 yr | Reference | Reference |
| ≥65 yr | 1.50 (1.47–1.53) | 1.35 (1.31–1.38) |
| Female sex | 0.97 (0.96–0.99) | 1.02 (1.00–1.03) |
| Population sector | ||
| General Jewish | Reference | Reference |
| Ultra-Orthodox Jewish | 0.57 (0.55–0.58) | 0.69 (0.68–0.71) |
| Arab | 1.18 (1.16–1.20) | 1.34 (1.31–1.36) |
| Score for socioeconomic status† | 1.08 (1.07–1.08) | 1.11 (1.11–1.12) |
| Clinical risk factors | ||
| Diabetes | 1.34 (1.31–1.37) | 1.08 (1.06–1.11) |
| Chronic obstructive pulmonary disease | 1.16 (1.09–1.23) | 0.95 (0.89–1.02) |
| Asthma | 1.02 (0.99–1.06) | 0.97 (0.94–1.00) |
| Chronic kidney failure | 1.18 (1.12–1.24) | 0.90 (0.85–0.95) |
| Hypertension | 1.41 (1.39–1.44) | 1.19 (1.16–1.22) |
| Ischemic heart disease | 1.36 (1.31–1.40) | 1.07 (1.03–1.11) |
| Chronic heart failure | 1.08 (1.01–1.14) | 0.77 (0.72–0.82) |
| Obesity | 1.17 (1.15–1.19) | 1.08 (1.06–1.10) |
| Lung cancer | 1.19 (0.97–1.46) | 0.95 (0.78–1.17) |
| History of stroke | 1.06 (1.01–1.11) | 0.77 (0.73–0.81) |
| History of transient ischemic attack | 1.27 (1.18–1.37) | 1.04 (0.96–1.12) |
| Current or former smoking | 0.98 (0.96–0.99) | 0.92 (0.90–0.94) |
The association between all covariates and vaccination uptake was estimated with the use of a multivariate Cox proportional-hazards regression model. The higher the hazard ratio, the greater the association between the listed characteristic and vaccine uptake. CI denotes confidence interval.
A hazard ratio of more than 1.00 indicates an association between a higher score for socioeconomic status and vaccine uptake.
Study Outcomes
Testing of the interaction of the vaccination status with the other variables revealed a significant interaction with age group. Thus, we report results for the entire cohort and also separately for the two age groups.
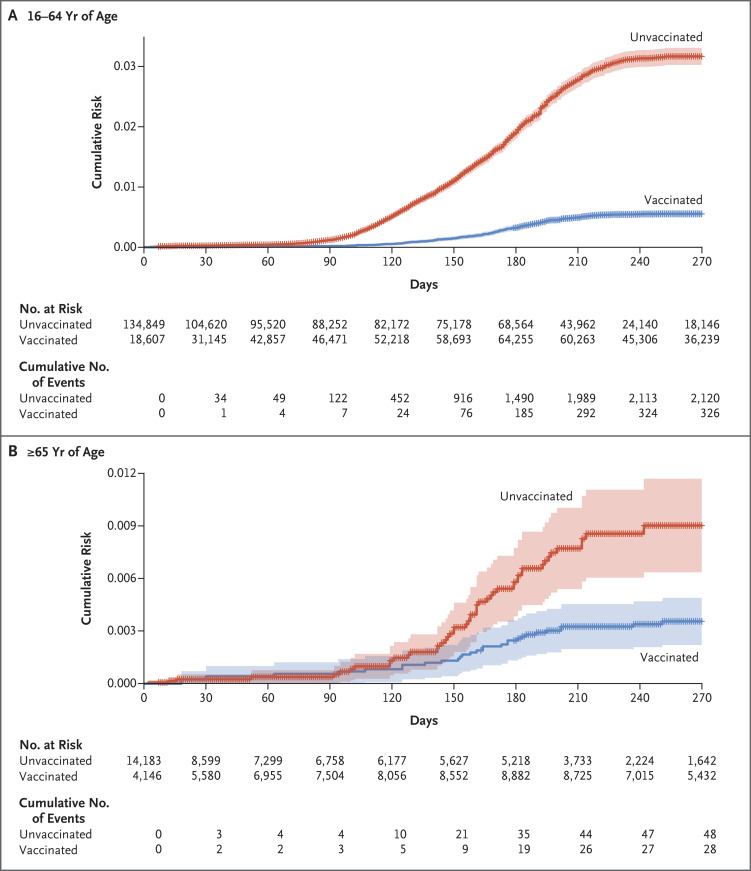
In the entire cohort, reinfection occurred in 354 of 83,356 vaccinated patients (2.46 cases per 100,000 persons per day) and in 2168 of 65,676 unvaccinated patients (10.21 cases per 100,000 persons per day). Among the patients between 16 and 64 years of age, reinfection occurred in 326 of 73,972 vaccinated patients (2.61 cases per 100,000 persons per day) and in 2120 of 60,877 unvaccinated patients (10.79 cases per 100,000 persons per day). Among the patients who were 65 years of age or older, reinfection occurred in 28 of 9384 vaccinated patients (1.46 cases per 100,000 persons per day) and in 48 of 4799 unvaccinated patients (3.02 cases per 100,000 persons per day).
The Cox proportional-hazards regression model included only variables that met the criteria for the proportional-hazards assumption on the basis of Schoenfeld’s global test. Therefore, most sociodemographic and clinical factors were not incorporated into the model (Tables S1 and S2). The adjusted hazard ratio for reinfection in the vaccinated group, as compared with the unvaccinated group, was 0.18 (95% confidence interval [CI], 0.16 to 0.20) among patients between 16 and 64 years of age and 0.40 (95% CI, 0.24 to 0.64) among those who were 65 years of age or older (Table 3). Therefore, the vaccine effectiveness was estimated to be 82% (95% CI, 80 to 84) among patients between 16 and 64 years of age and 60% (95% CI, 36 to 76) among those who were 65 years of age or older. The cumulative risk curves are shown in Figure 2.
Table 3. Association between SARS-CoV-2 Reinfection and Demographic and Clinical Variables, According to Age Group.*.
| Variable | Hazard Ratio for Reinfection (95% CI) | |
|---|---|---|
| 16–64 Yr of Age (N=134,849) |
≥65 Yr of Age (N=14,183) |
|
| Vaccination | 0.18 (0.16–0.20) | 0.40 (0.24–0.64) |
| Female sex | 0.94 (0.87–1.02) | 0.85 (0.54–1.36) |
| Score for socioeconomic status | 1.09 (1.07–1.11) | 1.06 (0.94–1.19) |
| Ultra-Orthodox Jewish population | 1.19 (1.08–1.32) | 1.58 (0.82–3.05) |
| Clinical risk factor | ||
| Chronic obstructive pulmonary disease | 1.20 (0.66–2.16) | 2.34 (1.25–4.38) |
| Diabetes | 0.61 (0.48–0.77) | 1.24 (0.77–1.97) |
| Obesity | 0.87 (0.79–0.97) | 0.86 (0.54–1.38) |
The association between vaccination and reinfection was estimated by means of a multivariate Cox proportional-hazards regression model after adjustment for sociodemographic factors and coexisting illnesses. Variables that met the testing criteria and were significantly associated with the outcome served as the inputs for the multivariate regression analysis.
Figure 2. Cumulative Risk of Reinfection with SARS-CoV-2, According to Age and Subsequent Vaccination Status.
Shown is the cumulative risk of reinfection with SARS-CoV-2 among previously unvaccinated patients who had recovered from Covid-19 and who were between 16 and 64 years of age (Panel A) or were 65 years of age or older (Panel B). Shading indicates the 95% confidence interval, and hatch marks indicate data censoring.
Secondary Analysis
Of the 83,356 vaccinated patients, 67,560 (81.0%) received one vaccine dose, 15,251 (18.3%) received two doses, and 545 (0.7%) received three doses. The adjusted hazard ratio for reinfection among the patients who had received one vaccine dose as compared with those who had received two doses was 0.98 (95% CI, 0.64 to 1.50). The results of the Cox proportional-hazards regression model according to the number of vaccine doses are provided in Table S3.
Discussion
Our study showed that receipt of the BNT162b2 vaccine in patients who had recovered from Covid-19 was associated with substantially lower reinfection rates. These results are consistent with data from studies that have shown strong immunologic responses to vaccination in previously infected persons.11,19
Although vaccine effectiveness was lower among patients who were 65 years of age or older than among younger patients, the vaccine still offered substantial protection among older patients. However, among the unvaccinated patients, the reinfection rate among the older patients was much lower than that among the younger patients (3.02 cases per 100,000 persons per day vs. 10.79 cases per 100,000 persons per day). This observation may be explained if we assume that older patients who had already been infected with SARS-CoV-2 would have observed enhanced social distancing and other required precautions, especially during the surge of the delta variant, even if they had decided against vaccination. Therefore, the differences in reinfection rates between vaccinated and unvaccinated older patients were lower than those in the younger population.
In the secondary analysis, we found that the receipt of more than one vaccine dose was not associated with greater effectiveness. However, it should be noted that only 19% of the vaccinated patients received more than one vaccine dose during the study period. Given the previous exposure to the virus, it seems that the primary vaccine dose in recovered patients provided a more robust and longer immunogenic response than the first dose alone in patients without previous Covid-19. These results are in concordance with the findings from a previous study conducted in Italy.11
Since March 2021, the Israeli Ministry of Health has recommended the administration of a single dose of vaccine in patients who have recovered from Covid-19, with the dose to be administered 3 months after recovery from the primary infection. However, not all patients who were eligible to receive this dose hurried to receive a postrecovery vaccine. Soon after Covid-19 vaccines had become available, Israel established an immunity passport policy, also known as the Green Pass, with the primary objective of allowing safe relaxation of Covid-19 restrictions.20 Initially, the Ministry of Health issued a Green Pass to all patients who had recovered from Covid-19 without any restriction. However, in October 2021, because of the surge in the delta variant, the Ministry of Health decided that patients who had not been vaccinated by 6 months after recovery would not be entitled to a Green Pass.21
In Israel, the receipt of a Covid-19 vaccine is a personal choice. Vaccine hesitancy after recovery from Covid-19 might have stemmed from personal safety concerns in patients who wanted to ensure that the vaccine was safe and beneficial for them. On the other hand, some patients who had a history of severe symptoms during their illness might have been willing to do anything that would avoid reinfection and therefore had a greater incentive to get the vaccine.
Our study has several strengths. First, the results are based on the integrated medical record system of Clalit Health Services, with detailed demographic, clinical, and laboratory testing data, including all dates and results of RT-qPCR testing, updated daily with information from the Ministry of Health data warehouse. Second, the large cohort is available for analysis with a relatively long-term follow-up. Third, the study period included the entire surge of the delta variant in Israel, during which the incidence of Covid-19 was one of the highest in the world.22 Thus, the number of reinfections was sufficient to show vaccine effectiveness.
Our study also has several limitations. As in any real-world observational study, the patients were not randomly assigned to receive or not to receive the vaccine. Much confounding is expected to arise from a lack of randomization because of substantial dissimilarities in the clinical backgrounds and sociodemographic characteristics of the two groups. This limitation is inherent in every real-world, population-based study of vaccine effectiveness, since the patients who received a vaccine may differ from those who did not.8,23 We attempted to overcome such bias by adjusting for variables known to affect rates of Covid-19 complications. However, measurement or correction may not have been performed adequately for unobserved or unmeasured sources of bias.
Another possible source of bias is the variation of exposure to SARS-CoV-2 during the study period. To minimize this potential bias, we entered patients in the study only until May 31, 2021, before the start of the surge in the delta variant. Therefore, we assumed that after adjustment for all covariates, the possible exposure variation had a similar effect in the vaccinated and unvaccinated groups.
A further limitation of this study is that reinfections were identified on the basis of a positive result on RT-qPCR assay, a procedure that would miss patients who were reinfected but were unaware of their infection or those who decided to avoid RT-qPCR testing, which would be more likely in mild cases. If we assume that infection was more likely to be asymptomatic or only mildly symptomatic in those who had been vaccinated, testing might have been less likely in this group. Thus, many records of infection may have been missed — a factor that could have substantially skewed reinfection rates in the vaccinated group and resulted in an overestimation of vaccine effectiveness. Therefore, we compared the overall testing rate in the two groups and found that testing was more frequent in the vaccinated group (Table S4).
An additional limitation is that we did not assess data on the severity of infection or on hospitalization or death in the reinfected patients since those outcomes were outside the scope of the study. However, in a recent study involving a large national cohort in Qatar, the risk that reinfection would result in hospitalization or death was 90% lower than the risk associated with primary infection.24
Finally, our findings were limited to the BNT162b2 vaccine. Although a recently published study provided evidence that the mRNA-1273 vaccine is slightly more effective than the BNT162b2 vaccine in participants who had received two vaccine doses,25,26 we cannot deduce whether this observation is relevant in averting reinfection with respect to patients who have recovered from Covid-19. Despite these limitations, we believe that our results may provide meaningful answers to a crucial question regarding vaccination policy with respect to patients after recovery from Covid-19.
Our study showed that among patients who had recovered from Covid-19, the receipt of one dose of the BNT162b2 vaccine was associated with an 82% lower risk of recurrent SARS-CoV-2 infection among those between 16 and 64 years of age and a 60% lower risk among those 65 years of age or older. No substantial difference was found in reinfection risk for two doses of vaccine as compared with one dose. The evidence that was gathered in this study during a surge of the delta variant in Israel supports a public health policy of vaccinating patients who have recovered from Covid-19, particularly in places where the delta variant is still of concern.
Supplementary Appendix
Disclosure Forms
This article was published on February 16, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet 2021;397:1204-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine 2021;35:100861-100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021;397:1459-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale J, Mumoli N, Clerici P, et al. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med 2021;181:1407-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html).
- 6.European Centre for Disease Prevention and Control. Questions and answers on COVID-19: vaccines (https://www.ecdc.europa.eu/en/covid-19/questions-answers/questions-and-answers-vaccines).
- 7.UK Health Security Agency. COVID-19 vaccination programme: information for healthcare practitioners. February 2, 2022. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042558/COVID-19-vaccine-information-for-healthcare-practitioners-21Dec21.pdf).
- 8.Rubin EJ, Baden LR, Morrissey S. Audio interview: what’s gone right in our battle against Covid-19. N Engl J Med 2021;385(22):e95-e95. [DOI] [PubMed] [Google Scholar]
- 9.Kojima N, Klausner JD. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect Dis 2022;22:12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamatatos L, Czartoski J, Wan YH, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021. March 25 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzoni A, Di Lauria N, Maggi L, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest 2021;131(12):e149150-e149150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination — Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep 2021;70:1081-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez JL. Efficacy & safety of BNT162b2 booster — C4591031 2 month interim analysis. Atlanta: Centers for Disease Control and Prevention, November 19, 2021. (https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-19/02-COVID-Perez-508.pdf). [Google Scholar]
- 15.Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med 2021;385:2413-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One 2021;16(3):e0247461-e0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA 2021;326:1930-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waitzberg R, Triki N, Alroy-Preis S, Lotan T, Shiran L, Ash N. The Israeli experience with the “Green Pass” policy highlights issues to be considered by policymakers in other countries. Int J Environ Res Public Health 2021;18:11212-11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israeli Ministry of Health. Green Pass: how to get the Green Pass, what places must comply with Green Pass restrictions, and current restrictions (https://corona.health.gov.il/en/directives/green-pass-info/).
- 22.Our World in Data. Daily new confirmed COVID-19 cases per million people. September 22, 2021. (https://ourworldindata.org/covid-cases?country=IND~USA~GBR~CAN~DEU~FRA~ISR~AFG~OWID_WRL#daily-confirmed-cases-per-million-people).
- 23.Rubin EJ, Baden LR, Morrissey S. Audio interview: waning immunity against SARS-CoV-2. N Engl J Med 2021;385(24):e99-e99. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Raddad LJ, Chemaitelly H, Bertollini R; National Study Group for COVID-19 Epidemiology. Severity of SARS-CoV-2 reinfections as compared with primary infections. N Engl J Med 2021;385:2487-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med 2022;386:105-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin EJ, Longo DL. Covid-19 mRNA vaccines — six of one, half a dozen of the other. N Engl J Med 2022;386:183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.