Abstract
Background
The duration and effectiveness of immunity from infection with and vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are relevant to pandemic policy interventions, including the timing of vaccine boosters.
Methods
We investigated the duration and effectiveness of immunity in a prospective cohort of asymptomatic health care workers in the United Kingdom who underwent routine polymerase-chain-reaction (PCR) testing. Vaccine effectiveness (≤10 months after the first dose of vaccine) and infection-acquired immunity were assessed by comparing the time to PCR-confirmed infection in vaccinated persons with that in unvaccinated persons, stratified according to previous infection status. We used a Cox regression model with adjustment for previous SARS-CoV-2 infection status, vaccine type and dosing interval, demographic characteristics, and workplace exposure to SARS-CoV-2.
Results
Of 35,768 participants, 27% (9488) had a previous SARS-CoV-2 infection. Vaccine coverage was high: 97% of the participants had received two doses (78% had received BNT162b2 vaccine [Pfizer–BioNTech] with a long interval between doses, 9% BNT162b2 vaccine with a short interval between doses, and 8% ChAdOx1 nCoV-19 vaccine [AstraZeneca]). Between December 7, 2020, and September 21, 2021, a total of 2747 primary infections and 210 reinfections were observed. Among previously uninfected participants who received long-interval BNT162b2 vaccine, adjusted vaccine effectiveness decreased from 85% (95% confidence interval [CI], 72 to 92) 14 to 73 days after the second dose to 51% (95% CI, 22 to 69) at a median of 201 days (interquartile range, 197 to 205) after the second dose; this effectiveness did not differ significantly between the long-interval and short-interval BNT162b2 vaccine recipients. At 14 to 73 days after the second dose, adjusted vaccine effectiveness among ChAdOx1 nCoV-19 vaccine recipients was 58% (95% CI, 23 to 77) — considerably lower than that among BNT162b2 vaccine recipients. Infection-acquired immunity waned after 1 year in unvaccinated participants but remained consistently higher than 90% in those who were subsequently vaccinated, even in persons infected more than 18 months previously.
Conclusions
Two doses of BNT162b2 vaccine were associated with high short-term protection against SARS-CoV-2 infection; this protection waned considerably after 6 months. Infection-acquired immunity boosted with vaccination remained high more than 1 year after infection. (Funded by the U.K. Health Security Agency and others; ISRCTN Registry number, ISRCTN11041050.)
Real-world studies have shown the short-term effectiveness of vaccines with respect to symptomatic and asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the severity of coronavirus disease 2019 (Covid-19), and secondary transmission.1-4 The duration of this protection over longer periods remains uncertain and warrants ongoing study.
The population uptake of two doses of Covid-19 vaccines in the United Kingdom (in persons >12 years of age) as of February 2022 was 84.5%,5 and it has now been more than 6 months since the second dose was administered to prioritized groups (health care and social workers and clinically vulnerable persons). Given the sustained high levels of community infection5 and concerns about the potential waning of immunity,6-10 the government of the United Kingdom initiated a rollout of booster vaccination in prioritized groups in September 2021.11 Improved understanding and characterization of vaccine effectiveness at longer dose intervals and of potential variation in effectiveness according to demographic factors, vaccination schedules, and history of SARS-CoV-2 infection are urgently needed to inform vaccination strategies.
In the SARS-CoV-2 Immunity and Reinfection Evaluation (SIREN) study, which involved a large cohort of asymptomatic health care workers who underwent polymerase-chain-reaction (PCR) testing every 2 weeks, more than 30% of the participants were seropositive for SARS-CoV-2 at enrollment.4,12,13 In this analysis, we aimed to determine the level and durability of protection against SARS-CoV-2 infection in the study cohort from March 2020 through September 2021 by estimating vaccine effectiveness after two doses of Covid-19 vaccine, according to the type of vaccine and dosing interval, in participants without previous infection. We also evaluated immunity against reinfection conferred by previous infection plus Covid-19 vaccine.
Methods
Study Design and Oversight
The SIREN study is an ongoing, multicenter, prospective cohort study involving health care workers (≥18 years of age) in the United Kingdom. This study received approval from the Berkshire Research Ethics Committee, and the results were reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.15 All the authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Study Participants and Data
Participants underwent PCR testing for SARS-CoV-2, supplemented by widespread lateral-flow testing, every 2 weeks, as well as monthly antibody testing. Every 2 weeks, they also completed questionnaires that included questions about symptoms. This data collection has been described elsewhere.4
Vaccination data (the type of vaccine and dates of administration) were obtained through personal identifiers from each health administration, linked to a national vaccination register, and directly from the participants in questionnaires completed every 2 weeks. The dosing interval was categorized as “short” if the second dose was administered up to 6 weeks after the first dose and “long” if the second dose was administered 6 weeks or more after the first dose.14
Serum samples obtained from all the participants at baseline visits were collected centrally. These samples were tested at the U.K. Health Security Agency (formerly Public Health England) central testing laboratory at Porton Down with the use of the semiquantitative Elecsys Anti-SARS-CoV-2 nucleocapsid (N) protein assay and the fully quantitative Elecsys Anti-SARS-CoV-2 spike (S) protein assay (both manufactured by Roche Diagnostics).
Explanatory Variables and Exclusion Criteria
At the beginning of the analysis, the participants were assigned to one of two cohorts: participants with no history of SARS-CoV-2 infection (the previously uninfected cohort) and those who had ever received a PCR test result or an antibody test result consistent with previous SARS-CoV-2 infection (the previously infected cohort). Participants were excluded from this analysis if the cohort assignment could not be accurately completed or if the outcome could not be determined (e.g., if they did not undergo PCR testing during the follow-up period), if they had previous infection that occurred on or after the vaccination date, or if the date of onset of the primary infection, based on either a positive PCR test or Covid-19 symptoms, was not available. Participants were also excluded if they had received a Covid-19 vaccine other than the BNT162b2 vaccine (Pfizer–BioNTech) or the ChAdOx1 nCoV-19 vaccine (AstraZeneca) because of the small numbers of persons who had received other vaccines.
Primary Outcome
The primary outcome was a PCR-confirmed SARS-CoV-2 infection, irrespective of the participant’s symptom status. This outcome was defined as a primary infection in the previously uninfected cohort or a reinfection in the previously infected cohort (two PCR-positive samples ≥90 days apart or a new PCR-positive sample ≥28 days after an antibody-positive result consistent with previous infection).
Person-Time at Risk
Follow-up began on December 7, 2020 (the day before Covid-19 vaccination was introduced in the United Kingdom), and continued until September 21, 2021, a period that covered 10 calendar months. All the participants who were enrolled on or before December 7, 2020, were followed from that date onward. Participants who were enrolled after December 7, 2020, (i.e., those with delayed entry) were followed from the date of their enrollment. Unvaccinated participants who had a primary infection during follow-up were moved into the previously infected cohort 90 days after their PCR-positive date, at which point they were considered to be at risk for reinfection. For individual participants, the end of follow-up was the date of primary infection (in the previously uninfected cohort), the date of reinfection (in the previously infected cohort), or the date of the last PCR-negative test.
Statistical Analysis
In our Cox proportional-hazards model with delayed entry of some participants, the outcome was time to PCR-positive SARS-CoV-2 infection, stratified according to age group, geographic region, workplace setting, and frequency of exposure to persons with Covid-19. We chose stratification based on these categorical predictors because they were statistically significant when controlled for but did not satisfy the proportional-hazards assumption (Schoenfeld test, according to predictor and global fit). We also controlled for sex and race or ethnic group because we observed that these predictors were statistically significant, led to an increase in the likelihood value and Wald statistic, and satisfied the proportional-hazards assumptions.
The model accounted for calendar time, given the varying infection rate, through the baseline hazard, which could take any functional form. In this model, the hazard is assumed to be
Hi(t)=hoi(t)exp (β1x1 + … + βkxk),
with a time-varying baseline hazard hoi(t) for each stratum. We estimated the parameter β, report the hazard ratio HR=exp(β), and report vaccine effectiveness and protection from primary infection calculated as 1 minus the hazard ratio, along with Wald statistic confidence intervals. The estimates of the hazard ratios are independent of the baseline hazard, on which no assumption was made.
The analysis began on December 7, 2020, shortly before the second wave of SARS-CoV-2 infection peaked in the United Kingdom, and continued through the spring of 2021 and into the third wave (Fig. S3 in the Supplementary Appendix, available with the full text of this article at NEJM.org); thus, it was crucial to account for a varying hazard rate.
The main predictors — vaccination status and previous infection status — were categorical and varied according to time. We grouped these predictors according to the time since vaccination and divided the follow-up time into unvaccinated and postvaccination time intervals. We also grouped previous infection status into three categories: before primary infection, up to 12 months after the primary infection, and more than 12 months after the primary infection. We used robust variance estimates to guard against the potential for unmeasured confounders at the hospital organization (site) level.
We fitted the model first in the previously uninfected cohort, estimating vaccine effectiveness over time. Here, postvaccination intervals were categorized according to vaccine type and dosing interval, the latter to explore differences in protection in participants who received the second dose closer in time to their first dose. We then focused on all the recipients of the BNT162b2 vaccine, including those who were infected before vaccination, and fitted a model with interaction of the time since the primary infection and the time since vaccination. Recipients of the ChAdOx1 nCoV-19 vaccine and the categorization according to dosing interval for the BNT162b2 vaccine were excluded because of small numbers in the previously infected cohort. This allowed us to investigate vaccine effectiveness in previously infected persons. We report these estimates as well as estimates from an unadjusted model, without stratifying or controlling for any predictor other than the time since vaccination and infection. Goodness of fit was assessed with the use of the likelihood ratio test (against the null model) and Akaike information criterion values. The widths of the confidence intervals have not been adjusted for multiplicity and cannot be used to infer effects.
We performed sensitivity analyses to assess the extent of depletion-of-susceptibles bias and the effect of excluding participants in the previously infected cohort who did not have a reliable date of primary infection. All the sensitivity analyses provided results that were similar to those presented here, but the estimates were more uncertain (see Tables S6 through S11). All the analyses were conducted with the use of Stata software, version 15.1 (StataCorp). The results were independently replicated with the use of R software, version 4.1.1, survival package v.3.2-13 (R Foundation for Statistical Computing). Our annotated code is available at https://github.com/SIREN-study/SARS-CoV-2-Immunity.
Results
Study Population
A total of 44,546 participants were enrolled between June 18, 2020, and April 23, 2021, from 135 sites across the United Kingdom; 35,768 met the inclusion criteria for this analysis (Fig. S1). The characteristics of the participants are shown in Table 1; most participants were women (84%), and the median age was 46 years (interquartile range, 36 to 54). Table S2 shows a comparison of these characteristics with those of the national population.
Table 1. Demographic Characteristics of the Participants at Baseline and Vaccination Status as of September 21, 2021.*.
| Characteristic | Previously Uninfected Cohort (N=26,280) |
Previously Infected Cohort (N=9,488) |
Total (N=35,768) |
|---|---|---|---|
| number (percent) | |||
| Age group | |||
| <25 yr | 935 (3.6) | 362 (3.8) | 1,297 (3.6) |
| 25–34 yr | 5,023 (19.1) | 2,083 (22.0) | 7,106 (19.9) |
| 35–44 yr | 6,580 (25.0) | 2,268 (23.9) | 8,848 (24.7) |
| 45–54 yr | 8,007 (30.5) | 2,867 (30.2) | 10,874 (30.4) |
| 55–64 yr | 5,283 (20.1) | 1,802 (19.0) | 7,085 (19.8) |
| ≥65 yr | 452 (1.7) | 106 (1.1) | 558 (1.6) |
| Sex | |||
| Male | 4,051 (15.4) | 1,648 (17.4) | 5,699 (15.9) |
| Female | 22,190 (84.4) | 7,827 (82.5) | 30,017 (83.9) |
| Nonbinary, other, or prefer not to say | 39 (0.1) | 13 (0.1) | 52 (0.1) |
| Race or ethnic group† | |||
| White | 23,610 (89.8) | 8,024 (84.6) | 31,634 (88.4) |
| Asian | 1,581 (6.0) | 905 (9.5) | 2,486 (7.0) |
| Black | 381 (1.4) | 240 (2.5) | 621 (1.7) |
| Mixed race | 380 (1.4) | 155 (1.6) | 535 (1.5) |
| Other ethnic group | 278 (1.1) | 149 (1.6) | 427 (1.2) |
| Prefer not to say | 50 (0.2) | 15 (0.2) | 65 (0.2) |
| Medical conditions | |||
| None | 19,569 (74.5) | 7,101 (74.8) | 26,670 (74.6) |
| Immunosuppression | 623 (2.4) | 180 (1.9) | 803 (2.2) |
| Chronic respiratory condition | 3,306 (12.6) | 1,133 (11.9) | 4,439 (12.4) |
| Chronic nonrespiratory condition | 2,782 (10.6) | 1,074 (11.3) | 3,856 (10.8) |
| Occupation | |||
| Administrative or executive, office-based occupation | 4,280 (16.3) | 1,154 (12.2) | 5,434 (15.2) |
| Nursing | 8,658 (32.9) | 3,526 (37.2) | 12,184 (34.1) |
| Health care assistant | 1,994 (7.6) | 907 (9.6) | 2,901 (8.1) |
| Doctor | 3,053 (11.6) | 1,195 (12.6) | 4,248 (11.9) |
| Midwife | 582 (2.2) | 195 (2.1) | 777 (2.2) |
| Physiotherapist, occupational therapist, or speech and language therapist | 996 (3.8) | 442 (4.7) | 1,438 (4.0) |
| Nonclinical support staff: maintenance staff, security guard, or hospital porter | 389 (1.5) | 141 (1.5) | 530 (1.5) |
| Pharmacist | 582 (2.2) | 155 (1.6) | 737 (2.1) |
| Health care scientist | 1,147 (4.4) | 243 (2.6) | 1,390 (3.9) |
| Medical, nursing, midwifery, or other student | 867 (3.3) | 333 (3.5) | 1,200 (3.4) |
| Other | 3,732 (14.2) | 1,197 (12.6) | 4,929 (13.8) |
| Occupational setting | |||
| Office | 5,481 (20.9) | 1,521 (16.0) | 7,002 (19.6) |
| Nonclinical setting | 1,064 (4.0) | 314 (3.3) | 1,378 (3.9) |
| Outpatient setting | 5,662 (21.5) | 1,679 (17.7) | 7,341 (20.5) |
| Maternity or labor ward | 361 (1.4) | 116 (1.2) | 477 (1.3) |
| Ambulance, emergency department, inpatient ward | 4,225 (16.1) | 2,231 (23.5) | 6,456 (18.0) |
| Intensive care | 1,273 (4.8) | 396 (4.2) | 1,669 (4.7) |
| Operating room | 657 (2.5) | 209 (2.2) | 866 (2.4) |
| Other | 7,557 (28.8) | 3,022 (31.9) | 10,579 (29.6) |
| Patient contact | |||
| No | 4,053 (15.4) | 1052 (11.1) | 5,105 (14.3) |
| Yes | 22,227 (84.6) | 8,436 (88.9) | 30,663 (85.7) |
| Frequency of contact with patient with Covid-19 | |||
| Every day | 5,585 (21.3) | 3,212 (33.9) | 8,797 (24.6) |
| Once per week | 4,340 (16.5) | 1,889 (19.9) | 6,229 (17.4) |
| Once per month | 2,368 (9.0) | 889 (9.4) | 3,257 (9.1) |
| Less than once per month | 3,697 (14.1) | 1,036 (10.9) | 4,733 (13.2) |
| Never | 10,290 (39.2) | 2,462 (25.9) | 12,752 (35.7) |
| Index of multiple deprivation‡ | |||
| 5 | 6,563 (25.0) | 2,308 (24.3) | 8,871 (24.8) |
| 4 | 5,982 (22.8) | 2,091 (22.0) | 8,073 (22.6) |
| 3 | 5,537 (21.1) | 1,978 (20.8) | 7,515 (21.0) |
| 2 | 4,408 (16.8) | 1,612 (17.0) | 6,020 (16.8) |
| 1 | 2,680 (10.2) | 1,178 (12.4) | 3,858 (10.8) |
| Not known | 1,110 (4.2) | 321 (3.4) | 1,431 (4.0) |
| Region | |||
| East Midlands | 1,963 (7.5) | 862 (9.1) | 2,825 (7.9) |
| East of England | 2,415 (9.2) | 948 (10.0) | 3,363 (9.4) |
| London | 2,432 (9.3) | 1,256 (13.2) | 3,688 (10.3) |
| Northeast | 453 (1.7) | 194 (2.0) | 647 (1.8) |
| Northwest | 2,174 (8.3) | 1,255 (13.2) | 3,429 (9.6) |
| Southeast | 2,568 (9.8) | 980 (10.3) | 3,548 (9.9) |
| Southwest | 4,503 (17.1) | 1,037 (10.9) | 5,540 (15.5) |
| West Midlands | 1,900 (7.2) | 817 (8.6) | 2,717 (7.6) |
| Yorkshire and Humber | 1,765 (6.7) | 879 (9.3) | 2,644 (7.4) |
| Scotland | 4,646 (17.7) | 803 (8.5) | 5,449 (15.2) |
| Northern Ireland | 888 (3.4) | 239 (2.5) | 1,127 (3.2) |
| Wales | 573 (2.2) | 218 (2.3) | 791 (2.2) |
| Vaccination status as of September 21, 2021 | |||
| Vaccinated | |||
| Second dose of BNT162b2 vaccine, long interval between doses | 20,843 (79.3) | 7,235 (76.3) | 28,078 (78.5) |
| Second dose of BNT162b2 vaccine, short interval between doses | 2,450 (9.3) | 609 (6.4) | 3,059 (8.6) |
| Second dose of ChAdOx1 nCoV-19 vaccine | 1,895 (7.2) | 908 (9.6) | 2,803 (7.8) |
| First dose of any vaccine | 630 (2.4) | 307 (3.2) | 937 (2.6) |
| Unvaccinated | 462 (1.8) | 429 (4.5) | 891 (2.5) |
Baseline was defined as the date of cohort assignment between December 2020 and April 2021. In the cohort of previously infected participants, 83% were seropositive (72% on U.K. Health Security Agency testing) and 17% were seronegative but had had a previous positive antibody or polymerase-chain-reaction (PCR) test. In this cohort of 9488 participants, 6815 (72%) had a primary infection in the period between February 2020 and May 2020, a total of 272 (3%) had a primary infection in the period between June and August 2020, and 2401 (25%) had a primary infection in the period between September 2020 and March 2021; the date of infection was either the date of the first positive PCR test or the date of onset of coronavirus disease 2019 (Covid-19) symptoms..
Race or ethnic group was reported by the participants.
The index of multiple deprivation, which is a measure of neighborhood relative deprivation calculated by the Office of National Statistics, was obtained through linkage with participant postal codes; the index ranges from 1 (most deprived) to 5 (least deprived).
At the beginning of the analysis, we assigned 26,280 participants to the previously uninfected cohort and 9488 to the previously infected cohort. The participants in the previously infected cohort were more likely than those in the previously uninfected cohort to be male, younger, from Black, Asian, or ethnic minority backgrounds, to work in clinical roles (e.g., to be doctors, nurses, or allied health professionals), and to report more frequent exposure to patients with Covid-19 (Table 1).
By the end of the analysis, 94.9% of the participants had received two doses of vaccine: 78.5% had received the BNT162b2 vaccine with a long interval between doses, 8.6% had received the BNT162b2 vaccine with a short interval between doses, and 7.8% had received the ChAdOx1 nCoV-19 vaccine (Table 1 and Fig. S2). We did not identify any major demographic differences among the participants according to vaccination schedule (Table S3).
Follow-up time varied according to participant, with a total of 7,482,388 participant person-days, of which 998,270 involved unvaccinated participants and 6,430,118 involved vaccinated participants (from the date of the first dose). A total of 62,291 PCR tests were performed during the “unvaccinated follow-up period,” which included follow-up time before vaccination in participants who were vaccinated during the analysis period and the total follow-up time in those who remained unvaccinated at the end of the analysis . A total of 427,951 PCR tests were performed during the period of the analysis in which participants were vaccinated (i.e., the “vaccinated follow-up period”). The average test interval was 16 days in the unvaccinated period and 15 days in the vaccinated period. In the previously uninfected cohort, 358,346 tests (average test interval, 14.8 days) were performed, and 131,896 tests were performed in the previously infected cohort (average test interval, 14.3 days).
Primary Outcome
The primary outcome was PCR-confirmed SARS-CoV-2 infection. Primary infections were noted in 2747 participants during follow-up, and reinfections were seen in 210, with cases peaking at the end of December 2020, declining by March and April 2021, and increasing in May 2021, a pattern that mirrored national trends (Fig. S3). At 14 days before or after the date of the positive PCR test, among the participants with primary infections, 1673 (61%) reported Covid-19–related symptoms, 368 (13%) reported other symptoms, 118 (4%) reported no symptoms, and 588 (21%) did not provide data on symptoms. In contrast, among the participants with reinfections, 71 (34%) reported Covid-19–related symptoms, 42 (20%) reported other symptoms, 45 (21%) reported no symptoms, and 52 (25%) did not provide data on symptoms. A total of 357 participants (13%) with primary infection reported a hospital visit for Covid-19–related symptoms, as compared with 18 (9%) of those with reinfection.
Vaccine Effectiveness against Primary Infection
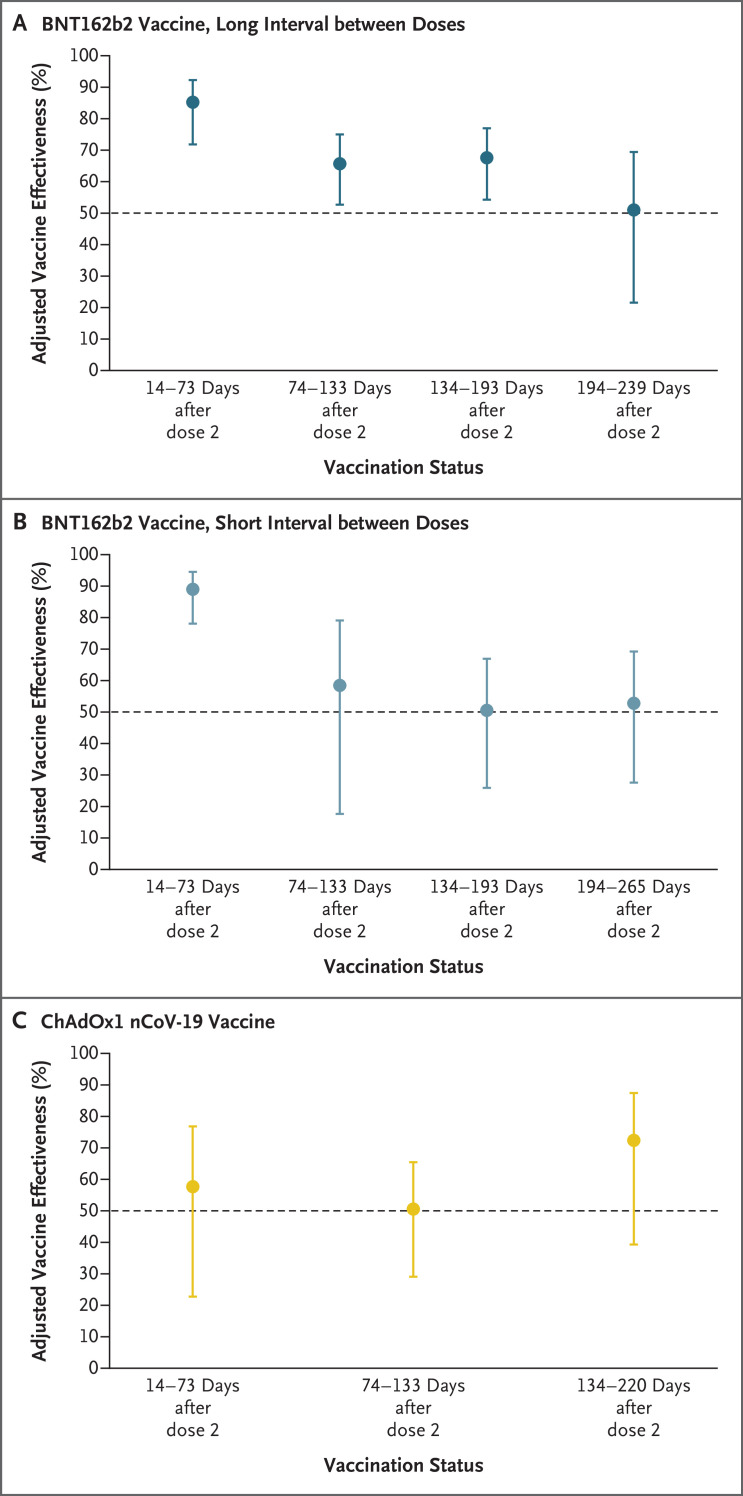
Among the participants without previous SARS-CoV-2 infection, two doses of BNT162b2 vaccine administered with a long interval between doses was associated with a decrease in the risk of infection of 85% (95% confidence interval [CI], 72 to 92) (i.e., the adjusted vaccine effectiveness in the first 2 months after the development of the full immune response, 14 to 73 days after the second dose) (Table 2 and S4 and Figure 1). Over time, the adjusted vaccine effectiveness declined but remained high, at 68% (95% CI, 54 to 77), 134 to 193 days after the second dose. At a median of 201 days (interquartile range, 197 to 205) after the second dose, we observed evidence of waning of protection, with an adjusted vaccine effectiveness of 51% (95% CI, 22 to 69).
Table 2. Incidence of SARS-CoV-2 Infection and Effectiveness of Covid-19 Vaccines against Symptomatic and Asymptomatic Infection in Participants without Previous SARS-CoV-2 Infection, December 7, 2020, through September 21, 2021.*.
| Vaccination Status and Time since Vaccination | Participants | Days of Follow-up | Participants with Primary Infection | Crude Incidence Rate |
Vaccine Effectiveness (95% CI) |
Adjusted Vaccine Effectiveness (95% CI) |
|---|---|---|---|---|---|---|
| no. | no. | no. | no. of infections/ 10,000 person-days at risk |
% | % | |
| Unvaccinated | 18,094 | 649,643 | 1,038 | 15.98 | — | — |
| Vaccinated with first dose | ||||||
| BNT162b2 vaccine | ||||||
| 21–27 days | 15,549 | 102,894 | 52 | 5.05 | 0.59 (0.44 to 0.71) | 0.59 (0.42 to 0.71) |
| 28–41 days | 15,247 | 201,531 | 60 | 2.98 | 0.64 (0.47 to 0.76) | 0.66 (0.52 to 0.76) |
| 42–55 days | 15,691 | 207,857 | 29 | 1.4 | 0.71 (0.56 to 0.81) | 0.70 (0.54 to 0.81) |
| 56–280 days | 16,376 | 341,183 | 53 | 1.55 | 0.67 (0.53 to 0.77) | 0.63 (0.46 to 0.75) |
| ChAdOx1 nCoV-19 vaccine | ||||||
| 21–27 days | 1,471 | 10,204 | 2 | 1.96 | 0.63 (−0.61 to 0.92) | 0.63 (−0.80 to 0.92) |
| 28–41 days | 1,495 | 20,496 | 1 | 0.49 | 0.87 (0.13 to 0.98) | 0.85 (0.16 to 0.97) |
| 42–55 days | 1,494 | 20,445 | 3 | 1.47 | 0.42 (−0.66 to 0.80) | 0.32 (−0.87 to 0.75) |
| 56–249 days | 1,470 | 38,308 | 10 | 2.61 | 0.24 (−0.56 to 0.63) | 0.09 (−0.87 to 0.55) |
| Vaccinated with second dose | ||||||
| BNT162b2 vaccine, long interval between doses | ||||||
| 14–73 days | 18,562 | 1,063,102 | 16 | 0.15 | 0.85 (0.71 to 0.93) | 0.85 (0.72 to 0.92) |
| 74–133 days | 17,332 | 950,734 | 264 | 2.78 | 0.70 (0.60 to 0.78) | 0.66 (0.53 to 0.75) |
| 134–193 days | 13,539 | 528,245 | 479 | 9.07 | 0.73 (0.64 to 0.79) | 0.68 (0.54 to 0.77) |
| 194–239 days | 2,261 | 20,774 | 81 | 38.99 | 0.46 (0.19 to 0.64) | 0.51 (0.22 to 0.69) |
| BNT162b2 vaccine, short interval between doses | ||||||
| 14–73 days | 2,259 | 118,505 | 10 | 0.84 | 0.85 (0.70 to 0.92) | 0.89 (0.78 to 0.94) |
| 74–133 days | 2,238 | 130,389 | 6 | 0.46 | 0.62 (0.19 to 0.82) | 0.58 (0.18 to 0.79) |
| 134–193 days | 2,122 | 118,192 | 47 | 3.98 | 0.58 (0.39 to 0.70) | 0.50 (0.26 to 0.67) |
| 194–265 days | 1,706 | 69,352 | 87 | 12.54 | 0.62 (0.45 to 0.74) | 0.53 (0.28 to 0.69) |
| ChAdOx1 nCoV-19 vaccine | ||||||
| 14–73 days | 1,414 | 79,806 | 15 | 1.88 | 0.52 (0.15 to 0.73) | 0.58 (0.23 to 0.77) |
| 74–133 days | 1,213 | 59,593 | 51 | 8.56 | 0.54 (0.32 to 0.68) | 0.50 (0.29 to 0.65) |
| 134–220 days | 715 | 16,936 | 26 | 15.35 | 0.67 (0.40 to 0.82) | 0.72 (0.39 to 0.87) |
Vaccine effectiveness was defined as 1 minus the hazard ratio. The crude incidence rate was not adjusted for the variable baseline hazard. The unadjusted vaccine effectiveness model was adjusted for the time since vaccination (combined with the dosing interval and type of vaccine) and baseline hazard only. The adjusted vaccine effectiveness model was adjusted for the baseline hazard time since vaccination (combined with the dosing interval and type of vaccine) and constant predictors (sex and race or ethnic group) and stratified across workplace setting, frequency of contact with patients with Covid-19, geographic area of the participant’s workplace, and age. In order to provide an estimate of absolute protection, we defined the reference group as the unvaccinated participants in the previously uninfected cohort. Additional details are provided in Table S3. CI denotes confidence interval, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Figure 1. Adjusted Vaccine Effectiveness over Time in Previously Uninfected Participants, According to Vaccine Type and Dosing Interval.
Shown is the adjusted vaccine effectiveness of two doses of coronavirus disease 2019 (Covid-19) BNT162b2 vaccine with a long interval between doses (Panel A), BNT162b2 vaccine with a short interval between doses (Panel B), and ChAdOx1 nCoV-19 vaccine with short dose intervals and long dose intervals combined (Panel C) in participants without previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Data are for the period from December 7, 2020, through September 21, 2021. 𝙸 bars indicate 95% confidence intervals.
A similar trend was observed in the participants who received a second dose of BNT162b2 vaccine with a short interval between doses, with high protection at 14 to 73 days (adjusted vaccine effectiveness, 89%; 95% CI, 78 to 94) that decreased to 53% (95% CI, 28 to 69) at a median of 238 days (interquartile range, 220 to 249) after the second dose. We found no significant difference between the BNT162b2 vaccine participants who had a long interval and those who had a short interval between doses with respect to protection after the second dose, with a hazard ratio for infection of 1.34 (95% CI, 0.58 to 3.10) at 14 to 73 days with the use of the short interval as the reference group.
The adjusted effectiveness of two doses of the ChAdOx1 nCoV-19 vaccine was 58% (95% CI, 23 to 77) 14 to 73 days after the second dose. The effectiveness did not differ considerably with longer periods of time after the second dose, with overlapping confidence intervals of vaccine effectiveness reflecting the small number of participants with data used to calculate this estimate (Table 2 and Figure 1). At 14 to 73 days after the second dose, the BNT162b2 vaccine with a short interval between doses was 74% more effective (95% CI, 36 to 89) and the BNT162b2 vaccine with a long interval between doses was 65% more effective (95% CI, 21 to 85) than the ChAdOx1 nCoV-19 vaccine. The Wald chi-square test of the model was 371.46 (31 degrees of freedom), with an Akaike information criterion of 15,367.
Durability of Protection after Primary Infection
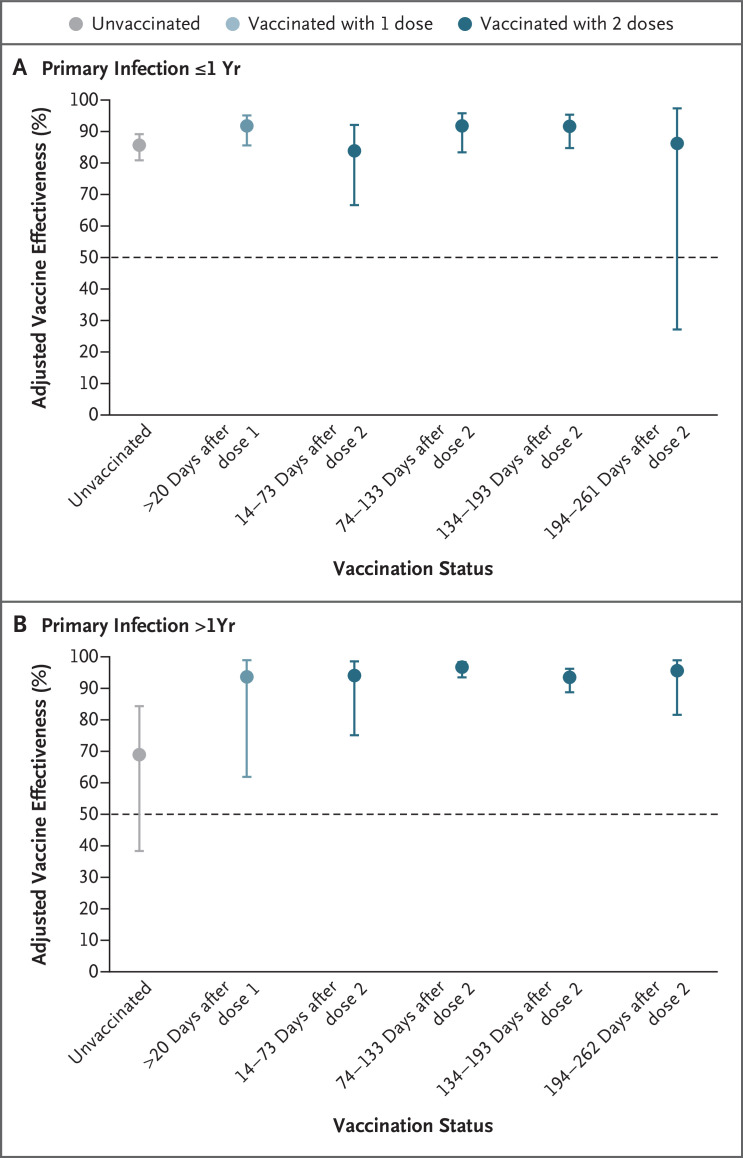
A total of 6169 participants in the previously infected cohort were followed in the unvaccinated follow-up period and up to 1 year after a primary infection. These participants were predominantly infected in the spring of 2020 and were followed in the period before emergence of the delta (B.1.617.2) variant. The risk of reinfection among these participants was 86% (95% CI, 81 to 89) lower than the risk of primary infection among the unvaccinated participants in the previously uninfected cohort (Table 3 and Figure 2). There was evidence of considerable waning of protection more than 1 year after infection, with a reduction to 69% (95% CI, 38 to 84); protection during the first year after infection was 54% (95% CI, 3 to 78) higher than that after more than 1 year.
Table 3. Incidence of SARS-CoV-2 Reinfection and Effectiveness of the BNT162b2 Vaccine against Symptomatic and Asymptomatic Reinfection among Participants with Previous SARS-CoV-2 Infection, December 7, 2020, through September 21, 2021.*.
| Infection and Vaccination Status and Time since Vaccination | Participants | Days of Follow-up |
Participants with Reinfection | Crude Incidence Rate | Vaccine Effectiveness (95% CI) |
Adjusted Vaccine Effectiveness (95% CI) |
|---|---|---|---|---|---|---|
| no. | no. | no. | no. of reinfections/ 10,000 person-days at risk |
% | % | |
| Follow-up ≤1 yr after primary infection | ||||||
| Unvaccinated | 6,169 | 258,088 | 58 | 2.25 | 0.82 (0.76 to 0.87) | 0.86 (0.81 to 0.89) |
| Vaccinated with first dose, 21–271 days | 7,381 | 303,281 | 13 | 0.43 | 0.91 (0.84 to 0.95) | 0.92 (0.86 to 0.95) |
| Vaccinated with second dose | ||||||
| 14–73 days | 5,075 | 201,580 | 8 | 0.40 | 0.81 (0.60 to 0.91) | 0.84 (0.67 to 0.92) |
| 74–133 days | 2,480 | 119,013 | 12 | 1.01 | 0.90 (0.82 to 0.95) | 0.92 (0.83 to 0.96) |
| 134–193 days | 1,533 | 51,893 | 13 | 2.51 | 0.91 (0.85 to 0.95) | 0.92 (0.85 to 0.95) |
| 194–261 days | 192 | 3,346 | 3 | 8.97 | 0.75 (−0.19 to 0.95) | 0.86 (0.27 to 0.97) |
| Follow-up >1 yr after primary infection | ||||||
| Unvaccinated | 486 | 50,041 | 12 | 2.40 | 0.71 (0.42 to 0.85) | 0.69 (0.38 to 0.84) |
| Vaccinated with first dose, 21–274 days | 1,642 | 38,422 | 2 | 0.52 | 0.90 (0.60 to 0.97) | 0.94 (0.62 to 0.99) |
| Vaccinated with second dose | ||||||
| 14–73 days | 4,852 | 234,484 | 2 | 0.09 | 0.93 (0.72 to 0.98) | 0.94 (0.75 to 0.99) |
| 74–133 days | 4,970 | 261,549 | 9 | 0.34 | 0.96 (0.92 to 0.98) | 0.97 (0.93 to 0.98) |
| 134–193 days | 3,772 | 137,473 | 18 | 1.31 | 0.95 (0.91 to 0.97) | 0.93 (0.89 to 0.96) |
| 194–262 days | 654 | 15,808 | 2 | 1.27 | 0.96 (0.84 to 0.99) | 0.95 (0.82 to 0.99) |
The crude incidence rate was not adjusted for the variable baseline hazard. In order to provide an estimate of absolute protection, we defined the reference group as the unvaccinated participants in the previously uninfected cohort. Vaccine effectiveness in the unvaccinated group refers to protection against reinfection. Infection rates in the unvaccinated cohort with previous infection were compared with those in the unvaccinated cohort without previous infection. In the assessment of unadjusted absolute protection against reinfection, the model was adjusted for combinations of time since vaccination with BNT162b2 vaccine and since primary infection and the baseline hazard only. In the assessment of adjusted absolute protection against reinfection, the model was adjusted for the baseline hazard, combinations of time since vaccination with BNT162b2 vaccine and since primary infection, and constant predictors (sex and race or ethnic group) and was stratified across workplace setting, frequency of contact with patients with Covid-19, geographic area of the participant’s workplace, and age. Additional details are provided in Table S4.
Figure 2. Protection against Reinfection with SARS-CoV-2 up to 18 Months after the Primary Infection.
Data are for the period from December 7, 2020, through September 21, 2021, for both the BNT162b2 and ChAdOx1 nCoV-19 vaccines and with all dosing intervals. 𝙸 bars indicate 95% confidence intervals.
Durability of Protection Conferred by Infection and Vaccination
In the previously infected cohort, with unvaccinated participants in the previously uninfected cohort as the reference group (Table 3 and Figure 2), a beneficial boosting of infection-acquired immunity was apparent, with combined protection of more than 90% after vaccination (after both the first and second doses). Waning of protection was not observed more than 1 year after infection or more than 6 months after vaccination. The Wald chi-square of the model was 789.68 (30 degrees of freedom), with an Akaike information criterion of 14,841.
Discussion
A total of 18 months after the emergence of SARS-CoV-2 and 10 months after the rapid deployment of Covid-19 vaccines, we assessed the durability of protection against SARS-CoV-2 infection conferred by both infection-acquired and vaccine-acquired immunity. Most of our cohort of 26,280 previously uninfected health care workers received two doses of BNT162b2 vaccine, which was administered with a long interval between doses; this regimen was associated with a considerably reduced risk of infection over the first 6 months that peaked in the first 2 months, with an adjusted vaccine effectiveness between 72% and 92%. However, we found evidence of considerable waning of immunity, with protection declining to between 22% and 69% after 6 months. We found no significant differences in the risk of infection when the BNT162b2 vaccine was administered with a short or long interval between doses, although we found considerably lower protection after two doses of the ChAdOx1 nCoV-19 vaccine than after two doses of the BNT162b2 vaccine. The period of waning of protection coincided with the period when the delta variant was the predominant circulating strain; this may account for the more pronounced waning of protection in our cohort, given the reported reduced vaccine effectiveness against the delta variant.16
Among unvaccinated participants, the risk of infection was between 81% and 89% lower up to a year after infection among those who were previously infected than among those who were previously uninfected, but we found evidence of waning of protection more than 1 year after infection. Vaccination after previous infection appeared to boost and extend immunity, and we found no indication of waning of this immunity even more than 1 year after primary infection. Protection against symptomatic infection in the cohort of participants who were vaccinated after previous infection was similar to that reported after a three-course vaccination (two doses and a booster dose).17
Our finding of reduced protection from infection in previously uninfected participants after 6 months following the receipt of two doses of vaccine strengthens the accruing evidence base. Our design overcomes several biases of recent studies, including underestimation of the proportion of participants with previous infection.18 Previous studies have typically investigated symptomatic infection and used test-negative case–control or retrospective cohort designs and national testing surveillance data.6,8,10 These real-world studies have shown consistently lower protection and more pronounced waning than a recent clinical trial of BNT162b2 vaccine that showed an efficacy of 83.7% (95% CI, 74.7 to 89.9) against symptomatic infection 4 to 6 months after the second dose19; this reduced protection was probably related to the reduced vaccine effectiveness reported against the delta variant.16 The considerably lower protection observed after ChAdOx1 nCoV-19 vaccination than after BNT162b2 vaccination in the current study has also been reported in other recent studies.6,19 Several studies have shown lower antibody titers after vaccination with ChAdOx1 nCoV-19 than after vaccination with BNT162b220,21; a shorter interval to a reduction in titers below a putative protective antibody threshold from this lower baseline has been proposed as a causal mechanism for the lower vaccine effectiveness.19 We found no significant difference between the BNT162b2 vaccine administered with a short interval between doses and that administered with a long interval between doses with respect to protection against infection after two doses, despite the findings of other studies showing considerably higher antibody, B-cell, and T-cell responses in participants who had long-interval regimens than in those who had short-interval regimens14,22,23 and the findings of one observational study showing higher vaccine effectiveness against symptomatic infection associated with long-interval regimens.14 It is plausible that the threshold for the prevention of all SARS-CoV-2 infections may be higher than that for the prevention of symptomatic infection.
Recent studies have shown that vaccination confers more durable protection against severe outcomes of hospitalization and death than against symptomatic and asymptomatic infection.6,24 Although we have estimated vaccine effectiveness against all infections, including asymptomatic infections that have limited clinical significance, a reduction in vaccine effectiveness against infection will increase transmission to and the risk of infection among high-risk persons, some of whom may have progression to severe disease. Given the relatively young and healthy profile of our cohort and the rarity of severe disease observed in this study, we are unable to assess protection against severe outcomes.
Because of the limited length of follow-up, it remains unclear how long immune protection will last after previous infection; however, some studies have suggested that protection could last for up to 61 months, and others have shown protection ranging from 5 to 12 months.20,25-28 We found that protection conferred by primary infection remained high at up to 1 year but then began to wane. Most follow-up investigations of unvaccinated, previously infected participants occurred before the delta variant wave, with most of this cohort infected in the spring of 2020 and vaccinated by the end of January 2021. Our ability to study infection-acquired immunity in unvaccinated persons at longer intervals was limited given the very small number of participants in our cohort who remained unvaccinated. It is possible that the sustained infection-acquired protection in our cohort was affected by repeated low-dose occupational exposure to Covid-1929 and that it is therefore less generalizable to populations with lower exposure. It is also possible that sustained protection results from a broader diversity of T-cell immunity against different SARS-CoV-2 spike protein epitopes that emerges after infection, enhancing protection against variants and inducing long-lasting memory T-cell populations.26,30,31 Although our finding of greater protection associated with infection-acquired immunity than with vaccine-acquired immunity has been reported by other authors,32,33 others have reported that both types of immunity are equivalent34,35 or that vaccine-acquired immunity is superior.36 Although infection-acquired immunity is associated with a high level of protection, it wanes after 1 year in unvaccinated persons. In keeping with previous studies, we found an additional benefit of vaccination in previously infected participants,33,37,38 and our finding of high levels of protection associated with immunity from infection plus vaccination has also been observed previously.39 Until thresholds for protective antibody titers against SARS-CoV-2 infection are established, it will be challenging to accurately estimate how much vaccine-induced immunity is required to prevent reinfection at an individual level.
The key strengths of our study include the size of the cohort of participants who underwent frequent testing, independent of disease status, with an average PCR test interval of 16.6 days in the unvaccinated follow-up period and 14.5 days in the vaccinated follow-up period, supplemented by the widespread use of lateral-flow testing, which means we can be confident that most infections were detected. We were able to simultaneously investigate vaccination and previous infection status in this well-defined cohort and to adjust for important confounders, including workplace exposures. The most important limitation of our study is the relatively small number of participants who contributed follow-up data on key vaccination exposures; these participants included those who were unvaccinated, those who received the ChAdOx1 nCoV-19 vaccine, and those who received the BNT162b2 vaccine with a short interval between doses. This small number of participants particularly affected the precision of our estimates and our ability to assess potential waning after two doses of the ChAdOx1 nCoV-19 vaccine. The strengths of our study design and the speed of vaccine deployment considerably limited the effect of depletion-of-susceptibles bias (which particularly affects studies on waning of immunity from vaccination).18 Although the effect of this bias was not apparent in our sensitivity analysis (see the Supplementary Appendix), some residual confounding may remain.
BNT162b2 vaccine administered with a short or long interval between two doses was associated with a considerably reduced risk of SARS-CoV-2 infection (asymptomatic and symptomatic) in the short term, but this protection waned after 6 months, during a period when the delta variant predominated. Protection associated with two doses of ChAdOx1 nCoV-19 vaccine was considerably lower than that associated with the BNT162b2 vaccine overall. The highest and most durable protection was observed in participants who received one or two doses of vaccine after a primary infection. Strategic use of booster doses of vaccine to avert waning of protection (particularly in double-vaccinated, previously uninfected persons) may reduce infection and transmission in the ongoing response to Covid-19.
Acknowledgments
We thank all the participants for their ongoing contributions and commitment to this study; all the research teams for their hard work and support at all 135 sites and for making the study possible; colleagues at the U.K. Health Security Agency Sero-epidemiology Unit for their support in biobanking and processing the high volumes of serum samples; colleagues at the U.K. Health Security Agency Porton Down for organizing and performing all the centralized serologic testing, including testing of 35,000 baseline samples, with particular thanks to Caoimhe Kelly, Anaya Ellis, Gabrielle Harker, Olivia Carr, Aaron Lloyd, Hannah Selman, Matthew Royds, and Georgia Hemingway; and Shaun Seman (Medical Research Council Biostatistics Unit, University of Cambridge) for his advice on handling the depletion-of-susceptibles analysis.
Protocol
Supplementary Appendix
Disclosure Forms
This article was published on February 16, 2022, at NEJM.org.
Footnotes
Supported by the U.K. Health Security Agency, the U.K. Department of Health and Social Care (with contributions from the governments in Northern Ireland, Wales, and Scotland), the National Institute for Health Research, and grants from the Medical Research Council.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org
References
- 1.UK Health Security Agency. COVID-19 — SARS-CoV-2. In: The green book. 2021. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/984310/Greenbook_chapter_14a_7May2021.pdf).
- 2.Department of Health and Social Care. Optimising the COVID-19 vaccination programme for maximum short-term impact. 2021. (https://www.gov.uk/government/publications/prioritising-the-first-covid-19-vaccine-dose-jcvi-statement/optimising-the-covid-19-vaccination-programme-for-maximum-short-term-impact).
- 3.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088-n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021;397:1725-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UK Health Security Agency. Coronavirus (COVID-19) in the UK: vaccinations in United Kingdom. 2021. (https://coronavirus.data.gov.uk/details/vaccinations).
- 6.Andrews N, Tessier E, Stowe J, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. October 6, 2021. (https://www.medrxiv.org/content/10.1101/2021.09.15.21263583v2). preprint.
- 7.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 2021;398:1407-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021;385(24):e83-e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385(24):e84-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021;385(24):e85-e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Health Security Agency. COVID-19 vaccination: booster dose resources. 2021. (https://www.gov.uk/government/publications/covid-19-vaccination-booster-dose-resources/covid-19-vaccination-a-guide-to-booster-vaccination).
- 12.Wallace S, Hall V, Charlett A, et al. SIREN protocol: impact of detectable anti-SARS-CoV-2 on the subsequent incidence of COVID-19 in 100,000 healthcare workers: do antibody positive healthcare workers have less reinfection than antibody negative healthcare workers? December 18, 2020. (https://www.medrxiv.org/content/10.1101/2020.12.15.20247981v1). preprint.
- 13.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet 2021;397:1459-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amirthalingam G, Bernal JL, Andrews NJ, et al. Higher serological responses and increased vaccine effectiveness demonstrate the value of extended vaccine schedules in combatting COVID-19 in England. July 28, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.26.21261140v1). preprint.
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-1457. [DOI] [PubMed] [Google Scholar]
- 16.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews N, Stowe J, Kirsebom F, Gower C, Ramsay M, Bernal JL. Effectiveness of BNT162b2 (Comirnaty, Pfizer-BioNTech) COVID-19 booster vaccine against Covid-19 related symptoms in England: test negative case-control study. November 15, 2021. (https://www.medrxiv.org/content/10.1101/2021.11.15.21266341v1). preprint.
- 18.Alderete JF, Newton E, Dennis C, Neale KA. The vagina of women infected with Trichomonas vaginalis has numerous proteinases and antibody to trichomonad proteinases. Genitourin Med 1991;67:469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldridge RW, Yavlinsky A, Nguyen V, et al. Waning of SARS-CoV-2 antibodies targeting the spike protein in individuals post second dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and risk of breakthrough infections: analysis of the Virus Watch community cohort. November 9, 2021. (https://www.medrxiv.org/content/10.1101/2021.11.05.21265968v1). preprint.
- 20.Wei J, Pouwels KB, Stoesser N, et al. SARS-CoV-2 anti-spike IgG antibody responses after second dose of ChAdOx1 or BNT162b2 and correlates of protection in the UK general population. January 14, 2022. (https://www.medrxiv.org/content/10.1101/2021.09.13.21263487v3). preprint.
- 21.Parry H, Bruton R, Stephens C, et al. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun Ageing 2021;18:34-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021;184(23):5699.e11-5714.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry H, Bruton R, Stephens C, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. May 17, 2021. (https://www.medrxiv.org/content/10.1101/2021.05.15.21257017v1). preprint. [DOI] [PMC free article] [PubMed]
- 24.Tenforde MW, Self WH, Naioti EA, et al. Sustained effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 associated hospitalizations among adults — United States, March–July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1156-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend JP, Hassler HB, Wang Z, et al. The durability of immunity against reinfection by SARS-CoV-2: a comparative evolutionary study. Lancet Microbe 2021;2(12):e666-e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milne G, Hames T, Scotton C, et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med 2021;9:1450-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021;595:426-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet 2021;397:1204-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swadling L, Diniz MO, Schmidt NM, et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022;601:110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojima N, Klausner JD. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect Dis 2022;22:12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jagannathan P, Wang TT. Immunity after SARS-CoV-2 infections. Nat Immunol 2021;22:539-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satwik R, Satwik A, Katoch S, Saluja S. ChAdOx1 nCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections. Eur J Intern Med 2021;93:112-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. August 25, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.24.21262415v1). preprint.
- 34.Lumley SF, Rodger G, Constantinides B, et al. An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Clin Infect Dis 2021. July 3 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shenai MB, Rahme R, Noorchashm H. Equivalency of protection from natural immunity in COVID-19 recovered versus fully vaccinated persons: a systematic review and pooled analysis. September 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.09.12.21263461v1). preprint. [DOI] [PMC free article] [PubMed]
- 36.Bozio CH, Grannis SJ, Naleway AL, et al. Laboratory-confirmed COVID-19 among adults hospitalized with COVID-19-like illness with infection-induced or mRNA vaccine-induced SARS-CoV-2 immunity — nine states, January–September 2021. MMWR Morb Mortal Wkly Rep 2021;70:1539-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination — Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep 2021;70:1081-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murugesan M, Mathews P, Paul H, Karthik R, Mammen JJ, Rupali P. Protective effect conferred by prior infection and vaccination on COVID-19 in a healthcare worker cohort in South India. August 31, 2021. (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3914633). preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA 2021;326:1930-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.