Abstract
Background
Nirmatrelvir is an orally administered severe acute respiratory syndrome coronavirus 2 main protease (Mpro) inhibitor with potent pan–human-coronavirus activity in vitro.
Methods
We conducted a phase 2–3 double-blind, randomized, controlled trial in which symptomatic, unvaccinated, nonhospitalized adults at high risk for progression to severe coronavirus disease 2019 (Covid-19) were assigned in a 1:1 ratio to receive either 300 mg of nirmatrelvir plus 100 mg of ritonavir (a pharmacokinetic enhancer) or placebo every 12 hours for 5 days. Covid-19–related hospitalization or death from any cause through day 28, viral load, and safety were evaluated.
Results
A total of 2246 patients underwent randomization; 1120 patients received nirmatrelvir plus ritonavir (nirmatrelvir group) and 1126 received placebo (placebo group). In the planned interim analysis of patients treated within 3 days after symptom onset (modified intention-to treat population, comprising 774 of the 1361 patients in the full analysis population), the incidence of Covid-19–related hospitalization or death by day 28 was lower in the nirmatrelvir group than in the placebo group by 6.32 percentage points (95% confidence interval [CI], −9.04 to −3.59; P<0.001; relative risk reduction, 89.1%); the incidence was 0.77% (3 of 389 patients) in the nirmatrelvir group, with 0 deaths, as compared with 7.01% (27 of 385 patients) in the placebo group, with 7 deaths. Efficacy was maintained in the final analysis involving the 1379 patients in the modified intention-to-treat population, with a difference of −5.81 percentage points (95% CI, −7.78 to −3.84; P<0.001; relative risk reduction, 88.9%). All 13 deaths occurred in the placebo group. The viral load was lower with nirmaltrelvir plus ritonavir than with placebo at day 5 of treatment, with an adjusted mean difference of −0.868 log10 copies per milliliter when treatment was initiated within 3 days after the onset of symptoms. The incidence of adverse events that emerged during the treatment period was similar in the two groups (any adverse event, 22.6% with nirmatrelvir plus ritonavir vs. 23.9% with placebo; serious adverse events, 1.6% vs. 6.6%; and adverse events leading to discontinuation of the drugs or placebo, 2.1% vs. 4.2%). Dysgeusia (5.6% vs. 0.3%) and diarrhea (3.1% vs. 1.6%) occurred more frequently with nirmatrelvir plus ritonavir than with placebo.
Conclusions
Treatment of symptomatic Covid-19 with nirmatrelvir plus ritonavir resulted in a risk of progression to severe Covid-19 that was 89% lower than the risk with placebo, without evident safety concerns. (Supported by Pfizer; ClinicalTrials.gov number, NCT04960202.)
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and illness with the associated coronavirus disease 2019 (Covid-19) continue to threaten global health.1 Persons with particular characteristics such as older age, current smoking, or underlying clinical conditions such as cardiovascular disease, diabetes, obesity, and cancer are at high risk for severe Covid-19 and associated adverse outcomes.2-5 In a meta-analysis, patients with prespecified coexisting conditions were approximately twice as likely to have progression to severe Covid-19 and five times as likely to die from Covid-19 as patients without those conditions.4 Mortality among older adults may be greater than that among persons with prespecified coexisting conditions.2,3,6
A need exists for safe and effective oral Covid-19 treatments that can prevent the progression of infection to more severe disease, hospitalization, and death; shorten the time to clinical recovery; and reduce the transmission rate. Such treatments would help reduce current strains on health care systems, including overwhelmed hospital facilities and lack of beds in intensive care units. For nonhospitalized patients with mild-to-moderate Covid-19, treatment options include monoclonal antibodies, which are currently available under emergency use authorization by the Food and Drug Administration for patients at high risk for progression to severe Covid-19.7-10 Although monoclonal antibodies significantly reduce the risk of progression to severe Covid-19,11-13 limitations to their use include the need for administration and monitoring in a health care setting and the potential for reduced efficacy against emerging SARS-CoV-2 variants.10
Nirmatrelvir (PF-07321332) is an orally administered antiviral agent targeting the SARS-CoV-2 3-chymotrypsin–like cysteine protease enzyme (Mpro).14 Mpro is an attractive antiviral target because it is essential in the viral replication cycle (i.e., in processing viral polyproteins into functional units)15 and has a low likelihood of off-target activity, owing to the absence of recognized human analogues.16 Nirmatrelvir exhibited potent inhibition of Mpro activity and virus replication across a wide spectrum of coronaviruses in vitro; oral administration was associated with SARS-CoV-2 lung titers that were significantly lower than titers associated with placebo in a mouse model.14 Nirmatrelvir is metabolized mainly by CYP3A4.14 Coadministration of nirmatrelvir with a low dose (100 mg) of ritonavir, a CYP3A4 inhibitor, enhances nirmatrelvir pharmacokinetics.14,17 A first-in-human study in healthy participants (ClinicalTrials.gov number, NCT04756531) showed a clinically acceptable safety profile up to the highest dose and exposure evaluated (500 mg of nirmatrelvir plus 100 mg of ritonavir twice daily for 10 days). Simulations showed that twice-daily administration of 300 mg of nirmatrelvir plus 100 mg of ritonavir achieves and maintains plasma trough concentrations approximately five to six times the in vitro 90% effective concentration of nirmatrelvir (i.e., the concentration at which 90% inhibition of SARS-CoV-2 viral replication is observed) (data on file).
The EPIC-HR trial (Evaluation of Protease Inhibition for Covid-19 in High-Risk Patients) evaluated the safety and efficacy of nirmatrelvir plus ritonavir in nonhospitalized adults with mild-to-moderate Covid-19 at high risk for progression to severe disease.
Methods
Objectives, Patients, and Oversight
This phase 2–3, double-blind, randomized, placebo-controlled trial evaluated the efficacy, viral load, and safety associated with the use of nirmatrelvir plus ritonavir among nonhospitalized, symptomatic adults with Covid-19 who were at high risk for progression to severe disease. Eligible patients were required to be at least 18 years old; to have confirmed SARS-CoV-2 infection and symptom onset no more than 5 days before randomization, with at least one sign or symptom of Covid-19 on the day of randomization (see Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org); and to have at least one characteristic or coexisting condition associated with high risk of progression to severe Covid-19.2-4,6 The trial was approved by an ethics committee at each site, and all participants provided written informed consent.
Key exclusion criteria were previous confirmed SARS-CoV-2 infection or hospitalization for Covid-19, anticipated need for hospitalization within 48 hours after randomization, and prior receipt of convalescent Covid-19 plasma or SARS-CoV-2 vaccine. The Supplementary Appendix provides additional inclusion and exclusion criteria and information on prohibited prior or concomitant therapies, trial blinding, ethical conduct, and responsibilities of the sponsor. All the data were available to all the authors, who vouch for the accuracy and completeness of the data as well as the adherence of the trial to the protocol, which is available at NEJM.org and includes the statistical analysis plan.
Procedures
Eligible patients were randomly assigned in a 1:1 ratio, by means of an interactive response technology system, to receive either nirmatrelvir plus ritonavir or matched placebo every 12 hours for 5 days (10 doses total). Randomization was stratified by geographic region and by receipt or expected receipt (based on investigator opinion) of Covid-19 monoclonal antibodies. Nirmatrelvir and matching placebo were manufactured by Pfizer, ritonavir tablets were manufactured and tested by Hetero Labs, and blinding of the tablets was performed by Pfizer through over-encapsulation. The assessment schedule is outlined in Figure S1.
Efficacy
The primary objective of the trial was to assess the efficacy of nirmatrelvir plus ritonavir as compared with placebo by comparing the percentage of patients with Covid-19–related hospitalization or death from any cause through day 28 in the two groups. This comparison was performed in the modified intention-to-treat population, which included patients whose treatment began within 3 days after the onset of Covid-19 signs and symptoms and excluded patients who at randomization had received or were expected to receive monoclonal antibody treatment (see Table S2 for definitions of all analysis populations). A key secondary end point was the primary comparison analyzed similarly among patients whose treatment began within 5 days after the onset of Covid-19 signs and symptoms. A supplementary analysis was conducted to include patients who had received or were expected to receive monoclonal antibody treatment. Prespecified subgroup analyses of primary and secondary end points were conducted, and nominal 95% confidence intervals were provided to evaluate whether the treatment effect varied according to age, sex, race, body-mass index (BMI, the weight in kilograms divided by the square of the height in meters), baseline serology status and viral load, and number of baseline coexisting conditions and risk factors (see the Supplementary Appendix for details on the serology methods).
Viral Load
Detection and quantification of SARS-CoV-2 viral load in nasopharyngeal swabs by reverse-transcriptase–polymerase-chain-reaction assay was a secondary end point. Nasopharyngeal or nasal swabs were collected on day 1 (baseline) and days 3, 5, 10, and 14.
Safety
Safety end points included adverse events that emerged during or after the treatment period (starting on or before day 34), serious adverse events, and adverse events leading to discontinuation of the trial drug or placebo, as coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 24.0. Incidence data were provided for each treatment group within the safety analysis population, which included all patients who received at least one dose of drug or placebo. Investigators actively collected safety information through day 34.
Statistical Analysis
The trial planned to enroll approximately 3000 patients. Of these, 1717 were to be included in the primary analysis to ensure 90% power, accounting for an interim analysis, to detect a 50% difference in Covid-19–related hospitalization or death from any cause with nirmatrelvir plus ritonavir, as compared with placebo (anticipated event rate with placebo, 7.0%)11 in patients who had undergone randomization within 3 days after symptom onset and who received no monoclonal antibodies.
The primary analysis compared proportions of patients in the two groups who were hospitalized for Covid-19 or died from any cause through day 28, using the Kaplan–Meier method to account for all patients, including those prematurely withdrawn from the trial or lost to follow-up. A z-test was used for the comparison, with standard errors estimated from Greenwood’s formula. The end points were tested sequentially (i.e., first the primary end point, then the first key secondary end point, and finally other secondary end points) to ensure the overall alpha level of 0.05.
Changes from baseline to day 5 in log10-transformed viral load were compared between treatment groups with an analysis of covariance (ANCOVA) model adjusted for baseline viral load and serology status. Patients without detectable virus at baseline were excluded from the analysis. Viral loads below the limit of detection (2 log10 copies per milliliter) were imputed as 1.70 log10 copies per milliliter.
On the basis of a group sequential design utilizing a Lan–DeMets alpha-spending function with an O’Brien–Fleming stopping boundary,18,19 a prespecified interim analysis of the primary end point for efficacy, futility, and sample size re-estimation was performed by an external data monitoring committee once approximately 45% of patients in the primary analysis population had completed assessments through day 28. The prespecified significance level for early termination was 0.002 for efficacy and 0.9184 for futility. See the Supplementary Appendix for additional details regarding the statistical analyses, including handling of missing data.
Results
Patients
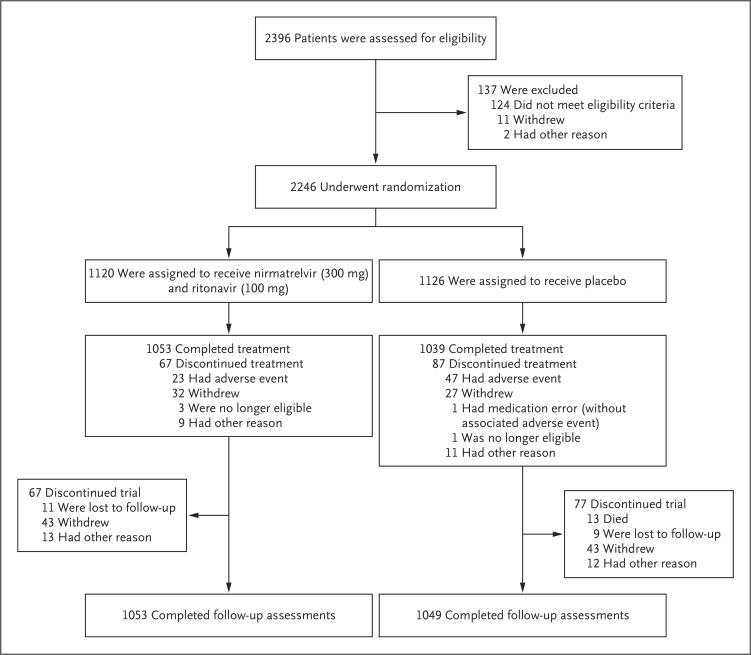
Between July 16 and December 9, 2021, a total of 2246 patients were enrolled at 343 sites worldwide; 1120 received nirmatrelvir plus ritonavir and 1126 received placebo (Figure 1). Of the 2246 patients, 2102 completed safety follow-up (day 34); no patients had completed long-term follow-up at the time of this analysis (i.e., through week 24).
Figure 1. Randomization, Treatment Assignments, and Follow-up.
Patients were recruited through December 9, 2021, from the United States (105 sites), Bulgaria (30 sites), South Africa (28 sites), Brazil (26 sites), India (19 sites), Mexico (18 sites), Ukraine (17 sites), Turkey (16 sites), Japan and Spain (10 sites each), Russia (9 sites), Argentina and Colombia (8 sites each), Poland and South Korea (7 sites each), Hungary (6 sites), Taiwan (5 sites), Malaysia and Czech Republic (4 sites each), and Thailand and Puerto Rico (3 sites each).
Patient characteristics were similar in the two groups (Table 1) and were largely representative of the expected patient population (Table S3). The median age was 46 years; 1148 patients (51.1%) were male, and 1607 (71.5%) and 315 (14.0%) were White and Asian, respectively. The most common prespecified characteristics and coexisting conditions associated with a risk of progression to severe Covid-19 at baseline were a BMI of 25 or above (1807 patients [80.5%]), current smoking (876 [39.0%]), and hypertension (739 [32.9%]); 1370 patients (61.0%) had two or more such characteristics or coexisting conditions. Most patients (2106 [93.8%]) had not received or were not expected to receive monoclonal antibodies for Covid-19 treatment at randomization, and 1489 (66.3%) received the first dose of the trial drug or placebo within 3 days after the onset of symptoms. Before receiving the trial drug or placebo, 4 patients had received monoclonal antibodies for Covid-19 treatment (3 in the nirmatrelvir group and 1 in the placebo group).
Table 1. Demographic and Clinical Characteristics of the Patients (Full Analysis Population).*.
| Characteristic | Nirmatrelvir plus Ritonavir (N=1120) |
Placebo (N=1126) |
Total (N=2246) |
|---|---|---|---|
| Median age (range) at randomization — yr | 45.00 (18.00–86.00) | 46.50 (18.00–88.00) | 46.00 (18.00–88.00) |
| Sex — no. of patients (%) | |||
| Male | 566 (50.5) | 582 (51.7) | 1148 (51.1) |
| Female | 554 (49.5) | 544 (48.3) | 1098 (48.9) |
| Race or ethnic group — no. of patients (%)† | |||
| White | 800 (71.4) | 807 (71.7) | 1607 (71.5) |
| Black | 60 (5.4) | 50 (4.4) | 110 (4.9) |
| Asian | 154 (13.8) | 161 (14.3) | 315 (14.0) |
| American Indian or Alaska Native | 96 (8.6) | 95 (8.4) | 191 (8.5) |
| Multiracial | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Not reported | 8 (0.7) | 9 (0.8) | 17 (0.8) |
| Other or unknown | 1 (0.1) | 2 (0.2) | 3 (0.1) |
| Time since first symptom | |||
| ≤3 days — no. of patients (%) | 754 (67.3) | 735 (65.3) | 1489 (66.3) |
| >3 days — no. of patients (%) | 366 (32.7) | 391 (34.7) | 757 (33.7) |
| Mean±SD — days | 2.93±1.12 | 2.99±1.09 | 2.96±1.10 |
| Median (range) — days | 3.00 (0.00–7.00) | 3.00 (0.00–9.00) | 3.00 (0.00–9.00) |
| Covid-19 monoclonal antibody treatment — no. of patients (%) | |||
| Received or expected to receive | 70 (6.2) | 70 (6.2) | 140 (6.2) |
| Not received or did not expect to receive | 1050 (93.8) | 1056 (93.8) | 2106 (93.8) |
| Serology status — no. of patients (%) | |||
| Negative | 518 (46.2) | 537 (47.7) | 1055 (47.0) |
| Positive | 581 (51.9) | 568 (50.4) | 1149 (51.2) |
| Median viral load (range) — log10 copies per milliliter | 5.41 (0.00–9.16) | 5.30 (0.00–9.15) | 5.35 (0.00–9.16) |
| Viral load ≥104 copies per milliliter — no. of patients (%) | 677 (60.4) | 676 (60.0) | 1353 (60.2) |
Shown are data for all patients who underwent randomization, regardless of whether the drug or placebo was administered.
Race or ethnic group was reported by the patient.
Efficacy
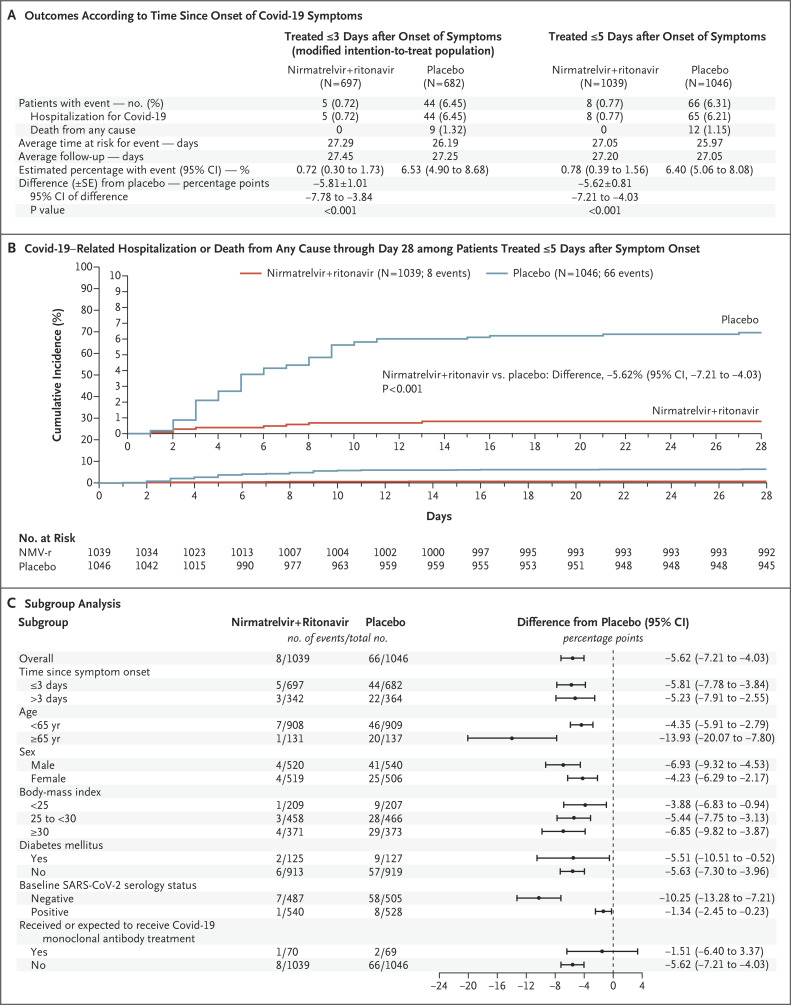
In the planned interim analysis of patients treated within 3 days after symptom onset (modified intention-to treat population, comprising 774 of the 1361 patients in the full analysis population), significantly fewer recipients of nirmatrelvir plus ritonavir had Covid-19–related hospitalization or death by day 28 (3 of 389 patients [0.77%]; 0 deaths) than placebo recipients (27 of 385 [7.01%]; 7 deaths), a difference of −6.32 percentage points (95% CI, –9.04 to –3.59; P<0.001). The relative risk reduction was 89.1%.
In the final analysis of patients who commenced treatment within 3 days after symptom onset and did not receive monoclonal antibodies (modified intention-to-treat population, comprising 1379 of the 2246 patients in the full analysis population), 5 of 697 patients (0.72%) in the nirmatrelvir group and 44 of 682 (6.45%) in the placebo group were hospitalized for Covid-19 or died from any cause through day 28 (Figure 2A). With use of the Kaplan–Meier method, the estimated event rates of Covid-19–related hospitalization or death from any cause at 28 days were 0.72% and 6.53% in the nirmatrelvir and placebo groups, respectively, corresponding to a difference of −5.81 percentage points (95% CI, –7.78 to –3.84; P<0.001) and an 88.9% relative risk reduction in Covid-19–related hospitalization or death from any cause. Nine deaths were reported in the placebo group and none in the nirmatrelvir group.
Figure 2. Efficacy of Nirmatrelvir plus Ritonavir (NMV-r) in Preventing Covid-19–Related Hospitalization or Death from Any Cause through Day 28.
Panel A shows efficacy results among patients who were treated within 3 days and within 5 days after symptom onset and who did not receive or were not expected to receive Covid-19 therapeutic monoclonal antibodies at randomization. The average time at risk for an event was computed as the time to the first event or as the time to the last day of participation or day 28, whichever was earlier. The average study follow-up was computed as the time to the last day of participation or day 28, whichever was earlier. Panel B shows the cumulative percentage of patients with Covid-19–related hospitalization or death from any cause through day 28 among patients treated within 5 days after symptom onset. The cumulative percentage was estimated for each treatment group with use of the Kaplan–Meier method. The inset shows the same data on an expanded y axis. Panel C shows subgroup analysis of the differences of the proportions of patients treated within 5 days after symptom onset who had Covid-19–related hospitalization or death from any cause through day 28, estimated for each treatment group with use of the Kaplan–Meier method. P values are based on normal approximation of the data. Study populations are described in Table S2.
After results of the primary analysis were found to be significant, the first key secondary analysis was performed among patients who commenced treatment within 5 days after symptom onset to evaluate hospitalization for Covid-19 or death from any cause. In the final analysis of this population, 8 of 1039 patients (0.77%) in the nirmatrelvir group and 66 of 1046 (6.31%) in the placebo group were hospitalized for Covid-19 or died from any cause through day 28 (P<0.001), corresponding to an 87.8% relative risk reduction (Figure 2A and 2B).
When 139 patients who received or were expected to receive monoclonal antibody treatment were included in the evaluation (6.25% of the total analysis population), hospitalizations due to Covid-19 or deaths from any cause were 0.81% and 6.10% in the nirmatrelvir and placebo groups, respectively (Table S4). Results from subgroup analyses were consistent, regardless of age, sex, race, BMI, baseline serology status, viral load, coexisting conditions, or number of coexisting conditions at baseline (Figure 2C and Fig. S2A through C).
Viral Load
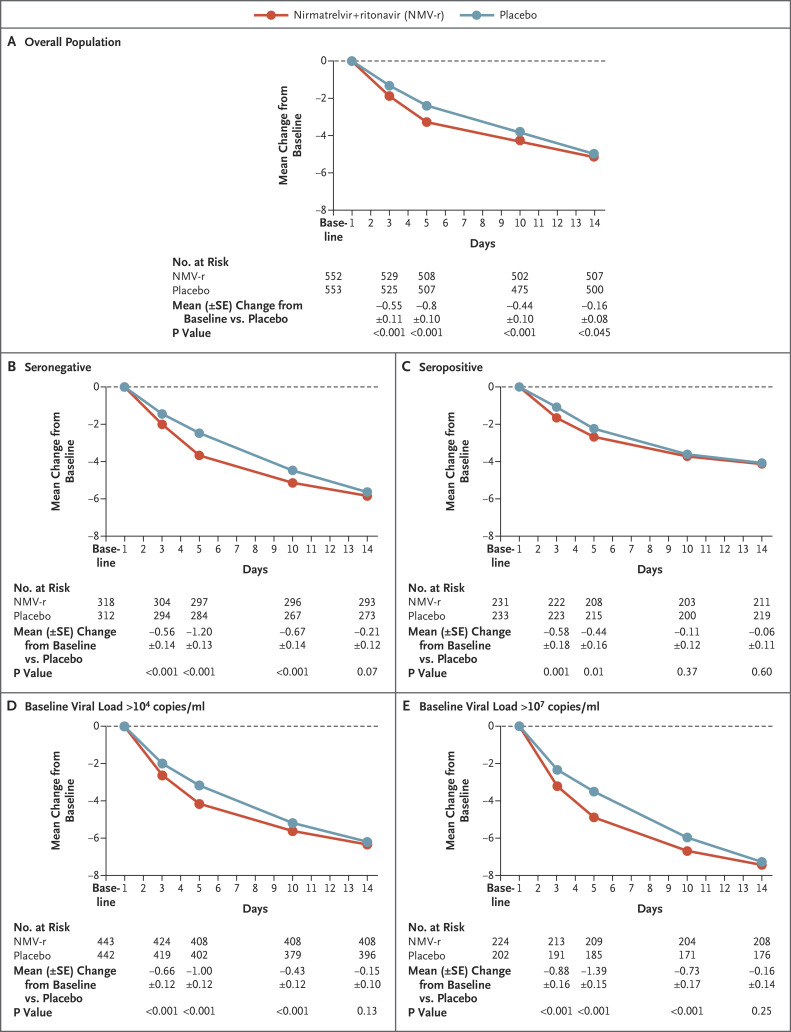
Data on SARS-CoV-2 viral load collected at baseline and day 5 were evaluated in 1574 patients (i.e., in 70% of the 2246 patients). After adjustment for baseline viral load, serology status, and geographic region, nirmatrelvir plus ritonavir reduced viral load at day 5 by an adjusted mean (±SE) of an additional 0.868±0.105 log10 copies per milliliter (95% CI, –1.074 to –0.6615; P<0.001) when treatment was initiated within 3 days after symptom onset, a decrease in viral load by a factor of 10 relative to placebo, and 0.695±0.085 log10 copies per milliliter (95% CI, –0.861 to –0.530; P<0.001) when treatment was initiated within 5 days after symptom onset (Figure 3A and Fig. S3A). When patients who received or were expected to receive monoclonal antibodies for Covid-19 treatment were included in the analysis, nirmatrelvir plus ritonavir showed a similar antiviral effect (nirmatrelvir plus ritonavir reduced viral load at day 5 by an additional 0.689±0.082 log10 copies per milliliter; 95% CI, –0.849 to –0.529 relative to placebo) (Fig. S4). Results from subgroup analyses were consistent with those in the overall population regardless of baseline viral load and serology status (Figure 3B through E and Fig. S3B through E). Preliminary analysis of 731 matched samples from day 1 and day 5 with available sequencing data suggests no significant associations between Mpro mutations and treatment failure.
Figure 3. Change from Baseline in Log10-Transformed Viral Load over Time (Modified Intention-to-Treat Population).
Panel A shows the adjusted mean change in viral load from baseline among all the patients who received at least one dose of the drug or placebo, had at least one visit between day 1 and day 28, did not receive or were not expected at baseline to receive Covid-19 therapeutic monoclonal antibody treatment, and were treated within 3 days after the onset of Covid-19 (modified intention-to-treat population). Panel B shows findings for the subgroup of patients whose baseline SARS-CoV-2 serology status was negative, and Panel C shows findings for the subgroup of patients whose baseline SARS-CoV-2 serology status was positive. Panel D shows findings among patients whose baseline viral load was more than 104 copies per milliliter, and Panel E shows findings among patients whose baseline viral load was more than 107 copies per milliliter. Patients were excluded from the analysis if the viral load was not detected or if data on baseline viral load were missing. Results obtained with unvalidated swabs were also excluded. Results were obtained with the use of a mixed-effects repeated-measures analysis of covariance model. Treatment, visit, and visit-by-treatment interactions were fixed effects in the analysis. Geographic region, baseline SARS-CoV-2 serology status, baseline viral load, and nasopharyngeal sample site were covariates, and participant was a random effect.
Safety
The incidence of adverse events that emerged during or after the treatment period was similar among recipients of nirmatrelvir plus ritonavir (22.6%) and recipients of placebo (23.9%) (Table 2). The most frequently reported such events (affecting at least 1% of patients) — both events considered by the investigator to be related to the assigned drug or placebo and those not considered to be related — among recipients of nirmatrelvir plus ritonavir were dysgeusia (5.6%, as compared with 0.3% of placebo recipients), diarrhea (3.1% vs. 1.6%), fibrin D-dimer increase (1.9% vs. 2.8%), alanine aminotransferase increase (1.5% vs. 2.4%), headache (1.4% vs. 1.3%), creatinine renal clearance decrease (1.4% vs. 1.6%), nausea (1.4% vs. 1.7%), and vomiting (1.1% vs. 0.8%); these adverse events were nonserious, were mostly grade 1 or 2, and resolved (Table S5).
Table 2. Summary of Adverse Events, Serious Adverse Events, and Adverse Events Leading to Discontinuation through Day 34 (Safety Analysis Population).*.
| Adverse Event Category | Nirmatrelvir plus Ritonavir (N=1109) |
Placebo (N=1115) |
|---|---|---|
| Events that emerged during treatment period | ||
| No. of adverse events | 476 | 525 |
| Patients with adverse events — no. (%) | ||
| Any adverse event | 251 (22.6) | 266 (23.9) |
| Serious adverse event | 18 (1.6) | 74 (6.6) |
| Maximum grade 3 or 4 adverse event | 45 (4.1) | 93 (8.3) |
| Maximum grade 5 adverse event | 0 | 13† (1.2) |
| Discontinued drug or placebo because of adverse event | 23 (2.1) | 47 (4.2) |
| Had dose reduction or temporary discontinuation owing to adverse event | 4 (0.4) | 4 (0.4) |
| Events considered to be related to drug or placebo | ||
| No. of adverse events | 123 | 52 |
| Patients with adverse events — no. (%) | ||
| Any adverse event | 86 (7.8) | 42 (3.8) |
| Serious adverse event | 1 (<0.1) | 0 |
| Maximum grade 3 or 4 adverse event | 5 (0.5) | 5 (0.4) |
| Maximum grade 5 adverse event | 0 | 0 |
| Discontinued drug or placebo because of adverse event | 9 (0.8) | 7 (0.6) |
| Had dose reduction or temporary discontinuation owing to adverse event | 2 (0.2) | 3 (0.3) |
Shown are data for all patients who received at least one dose of drug or placebo.
All reported deaths were related to Covid-19; causes of death included Covid-19 pneumonia (8 patients), Covid-19 (3 patients), pneumonitis (1 patient), and acute respiratory failure (1 patient).
Adverse events considered by the site investigator to be related to the trial drug or placebo were more common among recipients of nirmatrelvir plus ritonavir (7.8%) than among placebo recipients (3.8%). This difference was largely attributed to dysgeusia (4.5% vs. 0.2%) and diarrhea (1.3% vs. 0.2%), which were the only treatment-related adverse events reported in at least 1% of recipients of nirmatrelvir plus ritonavir; the majority of such events were resolved and were grade 1 or 2, with the exception of one case of grade 3 dysgeusia. Percentages were lower and similar across groups for related grade 3 events (nirmatrelvir plus ritonavir, 0.5%; placebo, 0.4%) and grade 4 events (nirmatrelvir plus ritonavir, 0; placebo, <0.1%).
Patients who received nirmatrelvir plus ritonavir reported fewer grade 3 or 4 adverse events than placebo recipients (4.1% vs. 8.3%), fewer serious adverse events (1.6% vs. 6.6%), and fewer adverse events leading to discontinuation of the drug or placebo (2.1% vs. 4.2%) (Table 2). The most frequently reported serious adverse events (those occurring in at least 2 patients) among recipients of nirmatrelvir plus ritonavir were Covid-19 pneumonia (6 patients [0.5%], as compared with 37 [3.3%] in the placebo group), Covid-19 (2 patients [0.2%], as compared with 8 [0.7%]), and decreased renal creatinine clearance (2 patients [0.2%], as compared with 3 [0.3%]); none were considered by the investigator to be related to nirmatrelvir or placebo (Table S6). Through day 34, no serious adverse events resulted in death among recipients of nirmatrelvir plus ritonavir; there were 13 deaths among placebo recipients, and all the deaths were Covid-19–related (Covid-19 pneumonia, 8 patients; Covid-19, 3 patients; pneumonitis, 1 patient; and acute respiratory failure, 1 patient). Adverse events that led to discontinuation of the trial drug or placebo in more than one patient in either treatment group (listed in order of frequency across treatment groups) were Covid-19 pneumonia, nausea, decreased renal creatinine clearance, vomiting, Covid-19, decreased glomerular filtration rate, pneumonia, pneumonitis, decreased white-cell count, and dysgeusia. Among recipients of nirmatrelvir plus ritonavir who discontinued the drug owing to an adverse event, events were mostly mild-to-moderate (grade 1 or 2) and were resolved or resolving at the time of this analysis. Twelve patients had an adverse event that was life-threatening (grade 4) (2 recipients of nirmatrelvir plus ritonavir and 10 placebo recipients). Few events (≤0.8%) leading to discontinuation of drug or placebo in either treatment group were considered by the investigator to be related to the trial drug or placebo.
Discussion
Results from this phase 2–3 trial in unvaccinated persons demonstrate the efficacy of oral administration of nirmatrelvir (300 mg) with ritonavir (100 mg) every 12 hours for 5 days. This regimen, commencing within 3 days after the onset of Covid-19 symptoms, was found to be efficacious at the planned interim analysis, with an 89.1% relative risk reduction in Covid-19–related hospitalization or death from any cause by day 28 among nonhospitalized adults at high risk for progression to severe disease. At the full analysis, relative risk reductions of 88.9% and 87.8% were observed among patients commencing treatment within 3 days and within 5 days after symptom onset, respectively, with 0 deaths occurring in the group that received nirmatrelvir plus ritonavir and 13 deaths occurring in the placebo group. This efficacy was supported by subgroup analyses of the primary end point; patients treated with nirmatrelvir plus ritonavir either had no Covid-19–related hospitalization or death from any cause or had a risk that was significantly lower than that with placebo, regardless of age, sex, race, BMI, baseline serology status, viral load, coexisting conditions, or number of coexisting conditions at baseline. Treatment with nirmatrelvir plus ritonavir was also associated with an additional reduction in SARS-CoV-2 viral load at day 5, by a factor of 10, as compared with placebo.
Nirmatrelvir plus ritonavir targets an essential protein that is conserved across coronaviruses.15,20,21 Given the well-conserved nature of the Mpro active site, inhibitors of Mpro may be more likely to retain activity against future variants.20,21
Fewer serious adverse events and adverse events leading to treatment discontinuation occurred with nirmatrelvir plus ritonavir than with placebo. The most frequent adverse events occurring more often in recipients of nirmatrelvir plus ritonavir were dysgeusia, diarrhea, and vomiting.
The concomitant use of nirmatrelvir plus ritonavir and certain drugs may result in potentially important drug interactions. Such interactions need to be managed through dose reduction of the concomitant medication, use of an alternative concomitant medication, increased monitoring for adverse events or concomitant medication drug levels, temporary discontinuation of concomitant medications, or avoidance of coadministration. Drug interactions with low-dose ritonavir (100 mg) given over a short duration of 5 days for treatment of Covid-19 are likely to be of lesser clinical consequence than long-term use of low-dose or standard-dose (600 mg) ritonavir for patients with human immunodeficiency virus. Nirmatrelvir plus ritonavir is contraindicated with use of certain drugs because of the risk of serious adverse events.
The trial has several strengths and limitations. Patients from diverse regions were included, enabling broad geographic generalizability. Although only persons at high risk for progression to severe Covid-19 were included, the corresponding demographic and clinical characteristics are relatively common: cardiovascular disease, obesity, and diabetes have been estimated at 7 to 14.9% worldwide prevalence in recent years,22-24 and approximately 12% of the world population in 2017 was 60 years of age or older.25 Of importance, this trial was restricted to unvaccinated persons, although a separate, ongoing trial of nirmatrelvir plus ritonavir (EPIC-Standard Risk [SR]; NCT05011513) includes vaccinated, high-risk persons.
Molnupiravir is an orally administered antiviral Covid-19 treatment that currently has emergency use authorization in the United States for use in the high-risk, nonhospitalized population,26 achieving a 30% reduction in Covid-19–related hospitalization or death through 29 days after randomization.27,28 Covid-19 therapeutic monoclonal antibodies are associated with relative risk reductions of approximately 70 to 85% against mild-to-moderate Covid-19 in outpatient settings11-13; however, monoclonal antibodies require intravenous or subcutaneous administration in a health care setting, and they may be less effective against emerging variants harboring mutations in the monoclonal antibody–targeted SARS-CoV-2 spike protein.7-9,29,30 Remdesivir, an agent administered intravenously that is approved for use in patients hospitalized for Covid-19, has shown an 87% reduction in the risk of progression to severe disease in the outpatient setting.31
Our data show that treatment with nirmatrelvir plus ritonavir early in Covid-19 illness can decrease progression to severe disease and quickly reduce SARS-CoV-2 viral load.
Acknowledgments
We thank all the patients who volunteered for this trial, the study site personnel for their contributions, and the members of the C4671005 external data monitoring committee for their dedication and their diligent review of the data. We also thank the following colleagues at Pfizer: Charlotte Allerton, Mohanish Anand, Annaliesa Anderson, Jeremias Antinew, Daniel Arenson, Edmund Arthur, Ayman Ayoub, Karen Baker, Elizabeth Bateman, Arthur Bergman, Mary Boylon-Bost, Kevin Brown, Carol Buck, Marjorie Buonanno, Paul Butler, Victoria Butler, Regina Calderon, Anna Maria Calella, Rhonda Cardin, Kristopher Chrisman, Ellen Chung Yi, Carol Connell, Tamara Costopoulos, Darren Cowan, Donna Cox, Amit Dagar, Bernita Davies, Gabriela Davila, Caridad Del Castillo-Castaneda, Gretchen Dean, Mikael Dolsten, Elizabeth Dushin, Martin Eck, Susannah Fitzgerald, Michael T. Gaffney, Ramakanth GSH, Eileen Mary Girten, Marie-Pierre Hellio Le Graverand-Gastineau, Jim Huang, Ileana Ionita, Farhad Kazazi, David Keller, Kelly Kincaid, Charles Knirsch, Stephen Langman, Catherine Lee, Joep Lelieveld, Cathy Q. Li, Katherine Liau, Carlos Linn, Marianne Lorenzen, Rod MacKenzie, Kannan Natarajan, Demetris Neophytou Zambas, Seleen Ong, Russ Orrico, Dafydd Owen, Seema Pai, Shyam Parvatini, Claire Penick, Jay Purdy, Amanda Radola, Kathleen Reilly Thompson, Amy Robey, Liz Rodgers, Glenda Rojas, Eve Rowe, Jean Satish, Cara Sclafani, Namita Singh, Ravi Shankar Singh, Phylinda Chan, Kawleen Singh Oberoi, Karen Singletary, Lisa Skeens, Patricia Smith, Holly Soares, Karan Somasundar, Anna Spolnik, Craig Stevenson, Kathleen Szymczak, Margaret Tawadrous, Manjit Toor, Arianna Trevisiol, Sarah Tweedy, Sophie Van De Leest, Jia Yi Liang, and all the colleagues not named here who contributed to the success of this trial. We thank Judith Kandel, Ph.D., Sheena Hunt, Ph.D., and Tricia Newell, Ph.D. of ICON, who wrote the first draft of the manuscript under direction from the authors, with funding from Pfizer.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on February 16, 2022, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Pfizer.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Center for Systems Science and Engineering. COVID-19 Dashboard. Johns Hopkins University (https://coronavirus.jhu.edu/map.html).
- 2.Docherty AB, Harrison EM, Green CA, et al. Features of 20133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985-m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021;72(9):e206-e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakur B, Dubey P, Benitez J, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep 2021;11:8562-8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 2020;81(2):e16-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-1242. [DOI] [PubMed] [Google Scholar]
- 7.O’Shaughnessy JA. Re: emergency use authorization 091. Silver Spring, MD: Food and Drug Administration. January 24, 2021. (https://www.fda.gov/media/145610/download). [Google Scholar]
- 8.O’Shaughnessy JA. Re: emergency use authorization 094. Silver Spring, MD: Food and Drug Administration. January 24, 2021. (https://www.fda.gov/media/145801/download). [Google Scholar]
- 9.O’Shaughnessy JA. Re: emergency use authorization 100. Silver Spring, MD: Food and Drug Administration. December 16, 2021. (https://www.fda.gov/media/149532/download). [Google Scholar]
- 10.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines (https://www.covid19treatmentguidelines.nih.gov/). [PubMed]
- 11.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med 2021;385:1382-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021;385:1941-1950. [DOI] [PubMed] [Google Scholar]
- 13.Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med 2021;385(23):e81-e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021;374:1586-1593. [DOI] [PubMed] [Google Scholar]
- 15.Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J 2014;281:4085-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003;300:1763-1767. [DOI] [PubMed] [Google Scholar]
- 17.Sevrioukova IF, Poulos TL. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U S A 2010;107:18422-18427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan KKG, DeMets D. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659-663. [Google Scholar]
- 19.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549-556. [PubMed] [Google Scholar]
- 20.Yang H, Xie W, Xue X, et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol 2005;3(10):e324-e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning L, Liu L, Li W, et al. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: case report. Am J Transplant 2020;20:1864-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Diabetes. November 10, 2021. (https://www.who.int/news-room/fact-sheets/detail/diabetes).
- 25.United Nations. World Population Ageing. 2017. (https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf).
- 26.Food and Drug Administration. FDA to hold advisory committee meeting to discuss Merck and Ridgeback’s EUA application for COVID-19 oral treatment. October 14, 2021. (https://www.fda.gov/news-events/press-announcements/fda-hold-advisory-committee-meeting-discuss-merck-and-ridgebacks-eua-application-covid-19-oral).
- 27.Merck. Merck and Ridgeback’s investigational oral antiviral molnupiravir reduced the risk of hospitalization or death by approximately 50 percent compared to placebo for patients with mild or moderate COVID-19 in positive interim analysis of phase 3 study. October 1, 2021. (https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/).
- 28.Merck. Merck and Ridgeback Biotherapeutics provide update on results from MOVe-OUT study of molnupiravir, an investigational oral antiviral medicine, in at risk adults with mild-to-moderate COVID-19. November 26, 2021. (https://www.merck.com/news/merck-and-ridgeback-biotherapeutics-provide-update-on-results-from-move-out-study-of-molnupiravir-an-investigational-oral-antiviral-medicine-in-at-risk-adults-with-mild-to-moderate-covid-19/).
- 29.Mahase E. Covid-19: what new variants are emerging and how are they being investigated? BMJ 2021;372:n158-n158. [DOI] [PubMed] [Google Scholar]
- 30.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021;19:409-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2021;386:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.