Abstract
Background & Aims
The long-term immunogenicity of anti-SARS-CoV-2 vaccines in liver transplant (LT) recipients is unknown. We aimed to assess the long-term antibody response of the Pfizer-BioNTech® BNT162b2 vaccine in LT recipients compared to controls.
Methods
LT recipients underwent anti-SARS-CoV-2 anti-receptor-binding domain protein IgG (anti-RBD) and anti-nucleocapsid protein IgG antibody (anti-N) measurements at the first and 1, 4 and 6 months after the second vaccination dose.
Results
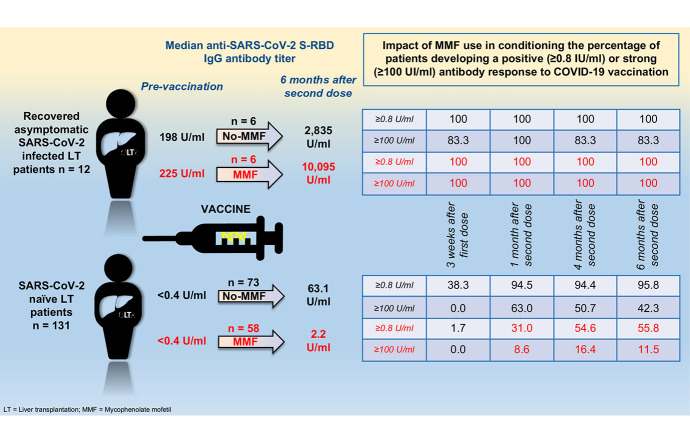
One hundred forty-three LT recipients and 58 controls were enrolled. At baseline, 131/143 (91.6%) LT recipients tested anti-N negative (COVID-19 naïve), and 12/143 (8.4%) tested positive (COVID-19 recovered) compared to negative controls. Among COVID-19 naïve, 22.1% were anti-RBD positives 1 month after the first vaccine dose, while 66.4%, 77%, and 78.8% were 1, 4 and 6 months following the second vaccine dose. In contrast, 100% of controls were positive at 4 months (p <0.001). The median anti-RBD titer 4 months after the second vaccine dose was significantly lower (32 U/ml) in COVID-19 naïve than in controls (852 U/ml, p <0.0001). A higher daily dose of mycophenolate mofetil (MMF) (p <0.001), higher frequency of ascites (p = 0.012), and lower serum leukocyte count (p = 0.016) were independent predictors of anti-RBD negativity at 6 months. All COVID-19 recovered patients tested positive for anti-RBD at each time point. The median antibody titer was similar in those taking MMF (9,400 U/ml, 11,925 U/ml, 13,305 U/ml, and 10,095 U/ml) or not taking MMF (13,950 U/ml, 9,575 U/ml, 3,500 U/ml, 2,835 U/ml, p = NS) 3 weeks after the first and 1, 4 and 6 months after the second vaccine dose, respectively.
Conclusions
In COVID-19-naïve LT recipients, the immunogenicity of anti-SARS-CoV-2 vaccination was significantly lower than that in controls. MMF was the main determinant of vaccination failure in SARS-CoV-2-naïve patients.
Lay summary
The immunogenicity of anti-SARS-CoV-2 vaccination in liver transplant recipients is currently unknown. Herein, we show that liver transplant recipients who have not previously had COVID-19 are less likely to mount effective antibody responses to vaccination than a control population. The main determinant of vaccination failure was the use of the immunosuppressive drug mycophenolate mofetil.
Keywords: mRNA vaccine, mycophenolate mofetil, liver transplantation
Graphical abstract
Introduction
The new coronavirus pathogen, SARS-CoV-2, has been identified as the cause of COVID-19.1 Preliminary reports indicated that in liver transplant (LT) recipients, the clinical outcome following COVID-19 was better compared to other solid organ transplant recipients2 and not per se worse compared to the general population.3 However, more recent reports indicate that mortality in LT recipients remains particularly elevated.4 , 5 Two anti-SARS-CoV-2 vaccines based on mRNA technology (Pfizer-BioNTech® BNT162b2 and Moderna® mRNA-1273)6 , 7 have been approved. After the administration of 2 doses of these vaccines in immunocompetent patients, nearly all of them developed neutralizing antibodies against SARS-CoV-2 s-receptor-binding domain (RBD) protein.8 The development of neutralizing antibodies seems to reduce the risk of symptomatic severe SARS-CoV-2-related disease in immunocompetent patients.9 However, in LT recipients, the short-term (up to 3 months) humoral immune response induced by SARS-CoV-2 mRNA vaccines seems to be lower than that in immunocompetent patients.[10], [11], [12] At present, no data are available regarding the rate and duration of the immune response after vaccination in the long term (up to 6 months) in this population. Despite this, all scientific societies recommend that LT patients should undergo 2 anti-SARS-CoV-2 doses with mRNA vaccines 3-6 months after LT, when immunosuppression can be reduced,[13], [14], [15] with the possibility of a third booster dose.16 The aim of this prospective study was to assess the safety and the long-term (up to 6 months) humoral immune response induced by 2 doses of the Pfizer-BioNTech® BNT162b2 vaccine in a cohort of LT recipients compared to healthy controls.
Materials and methods
Study protocol
The staff at the academic hospital in our Italian region launched the anti-SARS-CoV-2 vaccination program for all LT recipients, adopting 2 doses of the Pfizer-BioNTech® BNT162b2 vaccine, in March 2021. Both vaccine doses were administered directly in the hospital for all LT recipients who were in long-term follow-up at the hospital hepatology and liver transplantation unit. Patients fulfilling this condition along with their demographic and clinical characteristics were extracted from the electronic database. The exclusion criteria were aged <18 years at transplant, pregnancy, past known SARS-CoV-2 infection, and LT performed less than 3 months before vaccination. A group of physicians and nurses without known past SARS-CoV-2 infection who followed patients in the clinic served as controls. All patients and controls provided written informed consent to the vaccination protocol and to participate in this study, which was approved by the regional Ethics Committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Before vaccination, both patients and controls completed a detailed interview reporting the presence of signs and/or symptoms (i.e., fever, cough, anosmia, and diarrhea), suggesting recent or past SARS-CoV-2 exposure. In addition, controls were periodically tested for SARS-CoV-2 infection via real-time reverse transcription PCR (RT–PCR) on nasopharyngeal swabs. A vaccination self-reported side effects questionnaire was administered to participants within 30 days of receipt of the second vaccination dose.
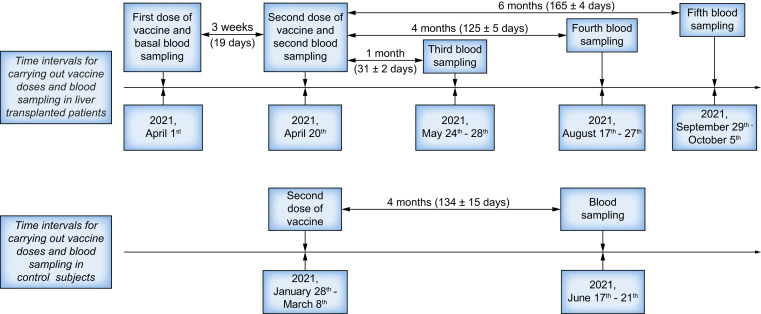
In all patients, a blood sample was collected at the following time points: at the first and the second vaccine doses (performed 19 days after the first) and at 1 (31±2 days), 4 (125±5 days), and 6 months (165±4 days) thereafter. One blood sample was collected 4 months (134±15 days) after the second vaccine dose in controls (Fig. 1 ). Anti-SARS-CoV-2-N protein IgM and IgG antibodies (iFlash® – Shenzhen Yhlo Biotech Co. Ltd) and anti-spike glycoprotein-specific immunoglobulin G receptor-binding domain (s-RBD) antibodies (Roche Elecsys®, F. Hoffmann-La Roche Ltd) were measured in blood samples collected at every time point in both LT patients and controls. In accordance with the manufacturer’s inserts, cut-off values used to identify positive patients were >10.0 kAU/L and ≥0.8 U/ml for the anti-SARS-CoV-2 N and s-RBD protein antibodies, respectively.
Fig. 1.
Timing schedule of the administration of the Pfizer-BioNTech® BNT162b2 vaccine doses and of the measurements of serum anti-SARS-CoV-2 antibodies in blood samples collected in liver-transplanted patients and controls.
Statistical analysis
Statistical analysis was performed by means of Stata 15.1 statistical software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Since normality testing of continuous variables failed in approximately half of the cases, a non-parametric rank-sum (Mann-Whitney) test was used, and data are presented as medians and IQRs. The comparison of categorical variables was carried out using the Pearson chi-square test, and data are presented as frequencies (%). Stepwise logistic regression analysis with a forward approach was used to select independent predictors for the development of a positive anti-SARS-CoV-2 vaccine-induced humoral response. All variables showing a p value ≤0.10 in the univariate analysis were included. Pseudo R2, the area under the ROC curve, and the percentage of correct classification are presented as quality estimations of the regression model. Multivariate linear regression analysis with a stepwise forward approach was used to discriminate the best fitting variables in predicting the antibody response after vaccination, considering antibody titer as a continuous variable. All variables significantly associated with antibody response post-vaccination in the univariate regression test were selected to run in the multivariate linear model.
Results
Patients
One hundred and sixty-four LT recipients were selected for enrollment in the study. Among them, 19 (11.6%) declined to participate in the vaccination program, and 2 did not receive the second vaccine dose: 1 died due to the progression of hepatocellular carcinoma recurrence, and the other was lost to follow-up. Thus, 143 LT recipients and 58 healthy controls were ultimately evaluated. LT recipients were more frequently male (71.7% vs. 32.9%, p <0.001) and older (67.7 vs. 47.6 years, p <0.001) than controls.
Prevalence of prevaccination anti-SARS-CoV-2-N protein antibodies in LT recipients and controls
None of the LT recipients nor controls reported in the interview as having a current or recovered SARS-CoV-2 infection. A positive anti-SARS-CoV-2-N antibody test, suggesting a history of asymptomatic previous SARS-CoV-2 infection, was detected only in the sample collected before vaccination in 12/143 (8.4%) LT recipients and in none of the controls. Table 1 reports the main demographic and clinical characteristics of patients with regard to the results of the anti-SARS-CoV-2-N protein antibody test. Antibody-positive patients presented a higher BMI (28.8 vs. 26.0 kg/m2, p = 0.010), were less frequently transplanted for alcohol-related liver disease (33.3% vs. 44.3%) and more frequently for hepatitis B (25% vs. 16%) (p = 0.036), and had a higher prevalence of diabetes mellitus (66.7% vs. 35.1%, p = 0.031) or recurrent post-transplant cirrhosis with esophageal varices (25% vs. 4.6%, p = 0.005) than antibody-negative patients. No significant differences between the 2 groups regarding either the immunosuppressive treatment schedule or the serum levels of immunosuppressive drugs were recorded. Considering this unexpected finding, these 2 groups of LT recipients were analyzed separately and classified as COVID-19-naïve (n = 131) and COVID-19-recovered (n = 12).
Table 1.
Baseline demographic and clinical characteristics of the studied population.
| COVID-19-naïve (n = 131) | COVID-19-recovered (n = 12) | p value | |
|---|---|---|---|
| Age at LT (years) | 57.9 (51.8-62.8) | 57.5 (52.5-59.8) | 0.453 |
| Male sex | 92 (70.2) | 10 (83.3) | 0.337 |
| BMI (kg/m2) | 26.0 (23.5-28.7) | 28.8 (27.2-30.8) | 0.010 |
| Months between LT and vaccination | 94 (49-189) | 157 (87-203) | 0.192 |
| Etiology: HCV, HBV, NASH, AH, AI, other (%) | 28, 21, 0, 58, 13, 11 (21.4, 16.0, 0.0, 44.3, 9.9, 8.4) | 2, 3, 1, 4, 1, 1 (16.7, 25.0, 8.3, 33.3, 8.3, 8.3) | 0.036 |
| HCC | 47 (35.9) | 2 (16.7) | 0.180 |
| DM | 46 (35.1) | 8 (66.7) | 0.031 |
| Dyslipidemia | 29 (22.1) | 4 (33.3) | 0.378 |
| Alcohol consumption >40 g/day | 9 (6.9) | 1 (8.3) | 0.849 |
| HTN | 58 (44.3) | 6 (50.0) | 0.703 |
| Presence of esophageal varices | 6 (4.6) | 3 (25.0) | 0.005 |
| Presence of ascites | 4 (3.1) | 1 (8.3) | 0.341 |
| IS treatment | |||
| Tacrolimus | 85 (64.9) | 8 (66.7) | 0.901 |
| Cyclosporine | 31 (23.3) | 4 (33.3) | 0.456 |
| MMF | 58 (44.3) | 6 (50.0) | 0.703 |
| Everolimus | 12 (9.2) | 0 (0.0) | 0.273 |
| Prednisone | 13 (9.9) | 1 (8.3) | 0.859 |
| Double-triple IS including MMF | 51 (38.9) | 6 (19.5) | 0.454 |
| MMF+T;+C;+E;+P;+T+P;+C+P (%) | 30, 16, 2, 1, 0, 2 (22.9, 12.2, 1.5, 0.8, 0.0, 1.5) | 4, 1, 0, 0, 1, 0 (33.3, 8.3, 0.0, 0.0, 8.3, 0.0) | |
| Double-triple IS excluding MMF | 14 (10.7) | 0 (0.0) | 0.233 |
| T+E, T+A, T+P, C+P, T+E+P (%) | 3, 1, 7, 1, 2 (2.3, 0.8, 5.3, 0.8, 1.5) | 0, 0, 0, 0, 0 | |
| Any double IS therapy | 61 (46.6) | 5 (41.7) | 0.745 |
| Any triple IS therapy | 4 (3.1) | 1 (8.3) | 0.341 |
| IS levels with respect to reference# | |||
| Below | 63 (48.1) | 7(58.3) | 0.497 |
| Above | 6 (4.6) | 0 (0.0) | 0.449 |
| Serum IS drug levels or daily dose# | |||
| Tacrolimus (ng/ml) | 3.05 ±0.82 | 4.12 ±0.62 | 0.581 |
| Cyclosporine (ng/ml) | 17.2 ±8.0 | 11.8 ±2.2 | 0.440 |
| MMF (g/day) | 0.73 ±0.08 | 0.88 ±0.27 | 0.602 |
| Everolimus (ng/ml) | 0.0 ±0.0 | 0.43 ±0.14 | 0.321 |
| Prednisone (mg/day) | 0.42 ±0.42 | 0.51 ±0.15 | 0.860 |
| Hemoglobin (g/dl) | 13.5 (12.1-14.8) | 12.5 (12.0-14.6) | 0.378 |
| Leukocytes (n/μl) | 5,640 (4,500-6,590) | 6,420 (4,825-7,610) | 0.214 |
| Neutrophils (n/μl) | 3,357 (2,725-4,175) | 3,687 (2,736-4,424) | 0.749 |
| Albumin (g/dl) | 4.23 (4.07-4.53) | 4.08 (3.75-4.29) | 0.067 |
| Total bilirubin (mg/dl) | 0.60 (0.42-0.90) | 0.67 (0.54-0.86) | 0.340 |
| eGFR (ml/min/1.73 m2) | 59.1 (45.9-75.6) | 55.2 (45.5-68.6) | 0.600 |
| AST (IU/ml) | 18 (15-24) | 24 (18-27) | 0.082 |
| ALT (IU/ml) | 16 (11-23) | 19 (15-28) | 0.206 |
| INR | 1.04 (0.98-1.13) | 1.02 (0.96-1.11) | 0.664 |
| 25-OH-Vitamin D (ng/ml) | 31 (26.0-35.0) | 33.3 (27.8-41.6) | 0.224 |
Patients were divided with regard to the presence (COVID-19-recovered) or absence (COVID-19-naïve) of prevaccination anti-SARS-CoV-2-N protein IgG/IgM antibodies. Categorical variables are presented as frequencies (%), and the Pearson chi-square test was used for statistical comparisons. Continuous variables are presented as medians (IQR), and immunosuppressive drug serum levels are presented as the means (±SE). The rank-sum test (Mann-Whitney) was used for statistical comparisons.
A, azathioprine; AH, alcoholic hepatitis; AI, autoimmune hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, cyclosporine; DM, diabetes mellitus; E, everolimus; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; HTN, arterial hypertension; INR, international normalized ratio; IS, immunosuppressive; LT, liver transplantation; NASH, non-alcoholic steatohepatitis, MMF, mycophenolate mofetil; P, prednisone; T, tacrolimus.
reference blood levels evaluated within 1 month before vaccination for each IS drug were calculated in accordance with Cillo et al.35
Anti-SARS-CoV-2 s-RBD antibody response after BNT162b2 vaccination in COVID-19 naïve patients and controls
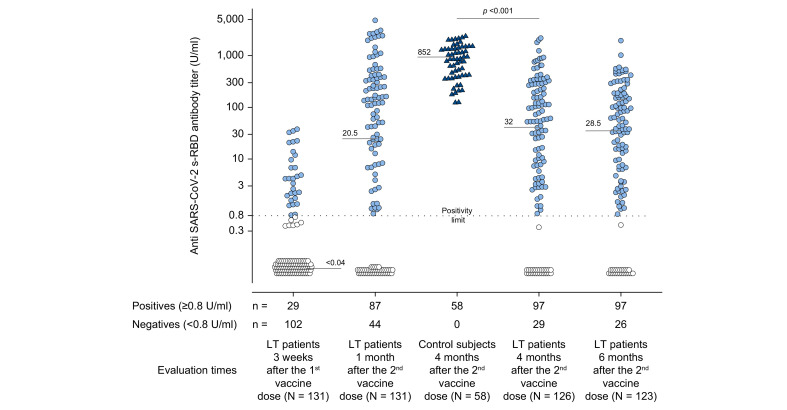
The median (IQR) time from LT to vaccination was 94 (49–189) months. At 4 and 6 months after the second vaccine dose, data were available in 126/131 (96.2%) and 123/131 (93.9%) LT recipients, respectively, since 5 and 3 patients were lost to follow-up at each time point. The number of LT recipients who tested positive for anti-s-RBD after each vaccination time point was as follows: 29/102 (22.1%) 3 weeks (19 days) after the first vaccine dose, 87/44 (66.4%) after 1 month (31±2 days), 97/29 (77%) after 4 months (125±5 days), and 97/26 (78.8%) after 6 months (165±4 days) compared to 58/58 (100%) of controls evaluated 4 months (134±15 days) following the second vaccine dose. Ten patients developed a late (between the first and fourth months) positive antibody response after vaccination, and none of the tested positive patients became antibody-negative within 6 months post-vaccination. The median anti-s-RBD antibody titers at 4 (32 U/ml) and 6 (28.5 U/ml) months remained stable in LT recipients, but were significantly lower (32 U/ml) than those in the controls (852 U/ml, p <0.0001) at 4 months (Fig. 2 ).
Fig. 2.
Anti-SARS-CoV-2 s-RBD antibody titers evaluated in COVID-19-naïve patients and controls.
In COVID-19-naïve patients, the antibody titers were evaluated 3 weeks (19 days) after the first dose of the Pfizer-BioNTech® BNT162b2 vaccine and after 1 month (31±2 days), 4 months (125±5 days), and 6 months (165±4 days) following the second vaccine dose. Four months (134±15 days) after the second vaccine dose, antibody titers were evaluated in controls. Positive responders to vaccination were defined as those having reached an antibody titer ≥0.8 U/ml (light blue circles for patients and dark blue triangles for controls) while antibody titer <0.8 U/ml identified patient non-responders (white circles). Medians of antibody titers are reported for each time point, and the statistical analysis was performed by means of a non-parametric rank-sum (Mann-Whitney) test.
Factors influencing the anti-SARS-CoV-2 s-RBD IgG response after BNT162b2 vaccination in COVID-naïve patients
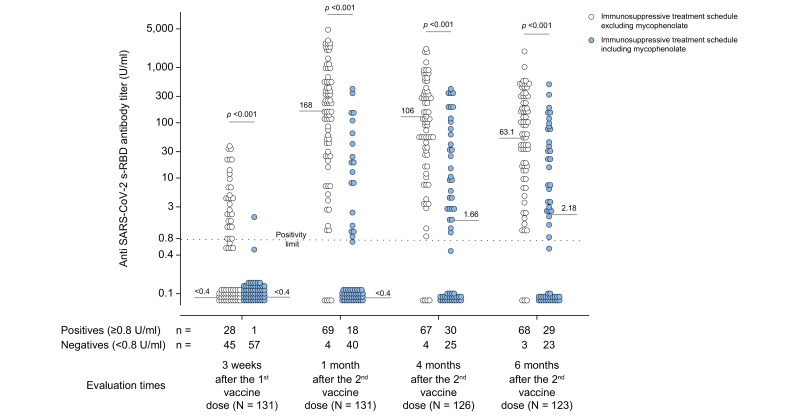
In the multivariate analysis, independent predictors of immune response failure (anti-SARS-CoV-2 s-RBD IgG antibody titer <0.8 U/ml) 3 weeks after the first dose of vaccination were alcohol consumption >40 g/day (p <0.001), taking a higher daily dose of mycophenolate mofetil (MMF) (p = 0.002), and having a lower estimated glomerular filtration rate (eGFR) (p = 0.016) (Table 2 ). In addition to taking a higher daily dose of MMF, immunosuppression employing >2 drugs, having lower serum leucocytes and being older at LT were selected as independent predictors of unsuccessful antibody response 1 and 4 months after the second vaccine dose, respectively (Table S1 and S2). A higher daily MMF dose assumption (p <0.001), a more frequent presence of ascites (p = 0.012) and having a lower number of leukocytes (p = 0.016) were selected as independent predictors of the negative antibody response 6 months after vaccination (Table 3 ). Moreover, patients treated with immunosuppressive schedules, including MMF, compared to those excluding MMF, presented significantly lower median antibody titers at each time point after vaccination (Fig. 3 ). The contribution of each immunosuppressive drug as well as any combination of immunosuppressive drugs in influencing the anti-SARS-CoV-2 s-RBD antibody titer, evaluated at each time point after vaccination, is presented in Table S3. In the stepwise multiple linear analysis, the daily dose of MMF and age at LT were selected as independent regressors of the entire span of anti-SARS-CoV-2 s-RBD antibody titers at every time point after vaccination. An eGFR and alcohol consumption >40 g/day were selected factors associated with the anti-SARS-CoV-2 s-RBD antibody titer at 3 weeks after the first vaccine dose and at 1 month after the second vaccine dose (Table S4).
Table 2.
Association of prevaccination demographic and clinical characteristics with antibody responses 3 weeks after the first dose of the Pfizer BTN162b2 vaccine.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Anti-s-RBD IgG negative (n = 102) | Anti-s-RBD IgG positive (n = 29) | p value | OR | 95% CI | p value | |
| Age at LT (years) | 59.5 (54.1-63.4) | 54.6 (48.3-59.1) | 0.008 | |||
| Male sex | 74 (72.6) | 18 (62.1) | 0.276 | |||
| BMI (kg/m2) | 25.5 (23.4-28.7) | 26.4 (23.8-29.3) | 0.407 | |||
| Months between LT and vaccination | 84.8 (36.7-189) | 153.9 (72.4-194.6) | 0.040 | |||
| Etiology: HCV, HBV, AH, AI, other (%) | 21, 18, 49, 7, 7 (20.6, 17.6, 48.0, 6.9, 6.9) | 7, 3, 9, 6, 4 (24.1, 10.3, 31.0, 20.7, 13.8) | 0.092 | |||
| HCC | 38 (37.3) | 9 (31.0) | 0.538 | |||
| DM | 37 (36.3) | 9 (31.0) | 0.602 | |||
| Dyslipidemia | 23 (22.6) | 6 (20.7) | 0.831 | |||
| Alcohol consumption >40 g/day | 9 (8.8) | 0 (0.0) | 0.097 | <0.001 | <0.001-<0.001 | <0.001 |
| HTN | 44 (43.1) | 14 (48.3) | 0.623 | |||
| Presence of esophageal varices | 5 (4.9) | 1 (3.5) | 0.741 | |||
| Presence of ascites | 3 (2.9) | 1 (3.5) | 0.889 | |||
| IS treatment | ||||||
| Tacrolimus | 63 (61.8) | 22 (75.9) | 0.160 | |||
| Cyclosporine | 26 (25.5) | 5 (17.2) | 0.356 | |||
| MMF | 57 (55.9) | 1 (17.1) | <0.001 | |||
| Everolimus | 10 (9.8) | 2 (4.9) | 0.632 | |||
| Prednisone | 11 (10.8) | 2 (6.9) | 0.537 | |||
| Double-triple IS including MMF | 50 (49.0) | 1 (3.5) | <0.001 | |||
| MMF+T; +C; +E; +P; +C+P (%) | 29, 16, 2, 1, 2 (28.4, 15.7, 2.0, 1.0, 2.0) | 1, 0, 0, 0, 0 (3.4, 0.0, 0.0, 0.0, 0.0) | ||||
| Double-triple IS excluding MMF | 12 (11.8) | 2 (6.9) | 0.454 | |||
| T+E, T+A, T+P, C+P, T+E+P (%) | 3, 1, 5, 1, 2 (2.9, 1.0, 4.9, 1.0, 2.0) | 0, 0, 2, 0, 0 (0.0, 0.0, 6.9, 0.0, 0.0) | ||||
| Any double IS therapy | 58 (56.9) | 3 (10.3) | <0.001 | |||
| Any triple IS therapy | 4 (3.9) | 0 (0.0) | 0.279 | |||
| Serum IS drug levels or daily dose# | ||||||
| Tacrolimus (ng/ml) | 4.26 ±0.46 | 3.63 ±0.46 | 0.678 | |||
| Cyclosporine (ng/ml) | 12.4 ±2.52 | 9.62 ±4.68 | 0.467 | |||
| Everolimus (ng/ml) | 0.45 ±0.16 | 0.34 ±0.25 | 0.523 | |||
| MMF (g/day) | 0.93 ±0.09 | 0.034 ±0.03 | <0.001 | 0.121 | 0.032-0.461 | 0.002 |
| Prednisone (mg/day) | 0.56 ±0.18 | 0.34 ±0.27 | 0.554 | |||
| IS levels with respect to reference# | ||||||
| Below | 48 (47.1) | 15 (51.7) | 0.657 | |||
| Above | 6 (5.9) | 0 (0.0) | 0.181 | |||
| Hemoglobin (g/dl) | 13.2 (12.1-14.8) | 14.0 (12.9-14.7) | 0.296 | |||
| Leukocytes (n/μl) | 5,780 (4,500-6,610) | 5,540 (4,680-6,330) | 0.727 | |||
| Neutrophils (n/μl) | 3,340 (2,770-4,180) | 3,420 (2,700-4,100) | 0.775 | |||
| Albumin (g/dl) | 4.24 (4.03-4.53) | 4.23 (4.10-4.50) | 0.857 | |||
| Bilirubin (mg/dl) | 0.59 (0.42-0.89) | 0.69 (0.43-1.01) | 0.351 | |||
| eGFR (ml/min/1.73 m2) | 58.0 (45.3-70.6) | 72.9 (50.7-84.2) | 0.009 | 1.031 | 1.005-1.058 | 0.016 |
| AST (U/ml) | 18 (14-23) | 21 (16-26) | 0.098 | |||
| ALT (U/ml) | 15 (11-23) | 19 (14-21) | 0.041 | |||
| INR | 1.04 (0.98-1.10) | 1.10 (1.00-1.20) | 0.083 | |||
| 25-OH-Vitamin D (ng/ml) | 31.0 (25.6-35.0) | 32.0 (26.7-36.2) | 0.437 | |||
Logistic model estimation parameters: pseudo R2 = 0.319; area under the ROC curve = 0.866; correct classification = 85.5%.
Association between prevaccination demographic and clinical characteristics of COVID-19-naïve liver transplanted patients (n = 131) with regards to the development of a positive (≥0.8 U/ml) or negative (<0.8 U/ml) anti-SARS-CoV-2 s-RBD antibody response, as assessed 3 weeks (19 days) after the first dose of the Pfizer BTN162b2 vaccine. Categorical parameters are presented as frequencies (%), and the Pearson chi-squared test was used for statistical comparisons. Continuous variables are presented as medians (IQR), and serum immunosuppressive drug levels are presented as the means (±SE). The rank-sum test (Mann-Whitney) was used for statistical comparisons. Stepwise regression with a forward approach was used to discriminate independent predictive variables to achieve a positive antibody response after vaccination in a multivariate logistic model analysis.
A, azathioprine; AH, alcoholic hepatitis; AI, autoimmune hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, cyclosporine; DM, diabetes mellitus; E, everolimus; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; HTN, arterial hypertension; INR, international normalized ratio; IS, immunosuppressive; LT, liver transplantation; NASH, non-alcoholic steatohepatitis, MMF, mycophenolate mofetil; P, prednisone; T, tacrolimus.
reference blood levels evaluated within 1 month before vaccination for each IS drug were calculated in accordance with Cillo et al.35
Table 3.
Association of prevaccination demographic and clinical characteristics with antibody responses 6 months after the second dose of the Pfizer BTN162b2 vaccine.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Anti-s-RBD IgG negative (n = 26) | Anti-s-RBD IgG positive (n = 97) | p value | OR | 95% CI | p value | |
| Age at LT (years) | 60.5 (57.7-65.8) | 57.4 (50.8-61.8) | 0.014 | |||
| Male sex | 16 (61.5) | 72 (74.21) | 0.203 | |||
| BMI (kg/m2) | 26.0 (23.5-228.7) | 25.5 (23.4-28.5) | 0.923 | |||
| Months between LT and vaccination | 54.1 (18.9-98.4) | 118 (59.1-189) | 0.006 | |||
| Etiology: HCV, HBV, AH, AI, other (%) | 6, 6, 8, 3, 3 (23.1, 23.1, 30.8, 11.5, 11.5) | 20, 15, 46, 8, 8 (20.6, 15.5, 47.4, 8.2, 8.2) | 0.636 | |||
| HCC | 9 (34.6) | 38 (39.2) | 0.671 | |||
| DM | 8 (30.8) | 34 (.5.1) | 0.683 | |||
| Dyslipidemia | 7 (26.9) | 20 (20.6) | 0.490 | |||
| Alcohol consumption >40 g/day | 3 (11.5) | 7 (7.2) | 0.474 | |||
| HTN | 11 (42.3) | 45 (46.4) | 0.710 | |||
| Presence of esophageal varices | 2 (7.7) | 4 (4.1) | 0.453 | |||
| Presence of ascites | 3 (11.5) | 1 (1.0) | 0.007 | 0.036 | 0.003-0.486 | 0.012 |
| IS treatment | ||||||
| Tacrolimus | 18 (69.2) | 63 (65.0) | 0.683 | |||
| Cyclosporine | 7 (26.9) | 21 (21.7) | 0.569 | |||
| MMF | 23 (88.5) | 29 (29.9) | <0.001 | |||
| Everolimus | 1 (3.9) | 11 (11.3) | 0.253 | |||
| Prednisone | 4 (15.4) | 8 (8.3) | 0.276 | |||
| Double-triple IS including MMF | 22 (84.6) | 24 (24.7) | <0.001 | |||
| MMF+T; +C; +E; +P; +C+P (%) | 16, 4, 0, 1, 1 (61.5, 15.4, 0, 3.8, 3.8) | 11, 10, 2, 0, 1 (11.3, 10.3, 2.1, 0, 1) | ||||
| Double-triple IS excluding MMF | 2 (7.7) | 11 (11.3) | 0.591 | |||
| T+E, T+A, T+P, C+P, T+E+P (%) | 0, 0, 1, 0, 1 (0, 0, 3.8, 0, 3.8) | 3, 1, 5, 1, 1 (3.1, 1, 5.2, 1, 1) | ||||
| Any double IS therapy | 22 (84.6) | 33 (34.0) | <0.001 | |||
| Any triple IS therapy | 2 (7.7) | 2 (2.1) | 0.151 | |||
| Serum IS drug levels or daily dose# | ||||||
| Tacrolimus (ng/ml) | 4.02 ±0.59 | 4.35 ±0.82 | 0.276 | |||
| Cyclosporine (ng/ml) | 5.30 ±2.34 | 11.8 ±2.72 | 0.561 | |||
| MMF (g/day) | 1.54 ±0.14 | 0.48 ±0.08 | <0.001 | 0.282 | 0.140-0.564 | <0.001 |
| Everolimus (ng/ml) | 0.18 ±0.18 | 0.57 ±0.18 | 0.277 | |||
| Prednisone (mg/day) | 0.0.67 ±0.36 | 0.46 ±0.17 | 0.303 | |||
| IS levels with respect to reference# | ||||||
| Below | 13 (50.0) | 46 (47.4) | 0.815 | |||
| Above | 0 (0.0) | 5 (5.2) | 0.237 | |||
| Hemoglobin (g/dl) | 12.7 (11.6-13.6) | 13.6 (12.8-15.0) | 0.007 | |||
| Leukocytes (n/μl) | 4,550 (3,700-5,630) | 5,980 (4,840-6,950) | 0.001 | 1.001 | 1.000-1.001 | 0.016 |
| Neutrophils (n/μl) | 2,850 (1,890-3,780) | 3,540 (2,910-4,310) | 0.016 | |||
| Albumin (g/dl) | 4.50 (4.07-4.61) | 4.24 (4.10-4.42) | 0.182 | |||
| Bilirubin (mg/dl) | 0.57 (0.37-0.73) | 0.63 (0.43-0.97) | 0.202 | |||
| eGFR (ml/min/1.73 m2) | 52.0 (45.3-70.6) | 63.1 (48.4-80.1) | 0.072 | |||
| AST (IU/L) | 15 (13-19) | 20 (16-25) | 0.001 | |||
| ALT (IU/L) | 11.5 (9-17) | 18 (12-27) | 0.001 | |||
| INR | 1.04 (0.96-1.14) | 1.04 (0.98-1.11) | 0.733 | |||
| 25-OH-Vitamin D (ng/ml) | 32.2 (28.6-35.0) | 31 (25.0-35.0) | 0.296 | |||
Logistic model estimation parameters: pseudo R2 = 0.393; area under the ROC curve = 0.905; correct classification = 88.6%.
Association between prevaccination demographic and clinical characteristics of COVID-19 naïve liver transplanted patients (n = 123) with regards to the development of a positive (≥0.8 U/ml) or negative (<0.8 U/ml) anti-SARS-CoV-2 s-RBD antibody response, as assessed 6 months (165±4 days) after the second dose of the Pfizer® BTN162b2 vaccine. Categorical parameters are presented as frequencies (%), and the Pearson chi-squared test was used for statistical comparisons. Continuous variables are presented as medians (IQR), for serum immunosuppressive drug levels, they are presented as the means (±SE), and the rank-sum test (Mann-Whitney) was used for statistical comparisons. Stepwise regression with a forward approach was used to discriminate independent predictive variables to achieve a positive antibody response after vaccination in a multivariate logistic model analysis.
A, azathioprine; AH, alcoholic hepatitis; AI, autoimmune hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, cyclosporine; DM, diabetes mellitus; E, everolimus; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; HTN, arterial hypertension; INR, international normalized ratio; IS, immunosuppressive; LT, liver transplantation; NASH, non-alcoholic steatohepatitis, MMF, mycophenolate mofetil; P, prednisone; T, tacrolimus.
reference blood levels evaluated within 1 month before vaccination for each IS drug were calculated in accordance with Cillo et al.35
Fig. 3.
Anti-SARS-CoV-2 s-RBD antibody titers evaluated in COVID-19-naïve patients who did or did not receive mycophenolate mofetil.
Antibody titers were measured 3 weeks (19 days) after the first dose of Pfizer-BioNTech® BNT162b2 vaccine, and after 1 month (31±2 days), 4 months (125±5 days), and 6 months (165±4 days) following the second vaccine dose with regards to the inclusion (light blue circles) or the exclusion (white circles) of mycophenolate mofetil monotherapy or in combination with other immunosuppressive drugs. Positive responders to vaccination were defined as those having reached an antibody titer ≥0.8 U/ml. Medians of antibody titers are reported for each time point, and the statistical analysis was performed by means of a non-parametric rank-sum (Mann-Whitney) test.
To identify patients who developed a strong antibody response after the full course of vaccination, the anti-SARS-CoV-2 s-RBD IgG antibody cut-off titer was selected at 100 U/ml. This was derived from the observation that adoptive transfer of purified polyclonal IgG from convalescent macaques robustly protected naïve recipient rhesus macaques against challenge with SARS-CoV-2 when the antibody titer was at least 100 U/ml.17 Considering this cut-off level, the number of patients who tested positive for anti-SARS-CoV-2 s-RBD IgG antibodies at 1, 4 and 6 months after the second vaccine dose was 51 (38.9%), 45 (35.7%) and 36 (29.3%), respectively. In the multivariate analysis, independent predictors of the achievement of a strong antibody response (>100 U/ml) were a younger age at LT (p = 0.0013), alcohol consumption <40 g/day (p <0.001) and taking a lower daily dose of MMF (p <0.001) at 1 month and no alcohol consumption at 4 months after the second vaccine dose (Table S5 and S6, respectively). A lower daily dose of MMF (p = 0.006) in addition to a younger age at LT (p = 0.012), alcohol consumption <40 g/day (p <0.001) and higher hemoglobin serum levels (p = 0.047) were selected as independent predictors of a strong antibody response 6 months after vaccination (Table S7).
Anti-SARS-CoV-2 s-RBD antibody response after BNT162b2 vaccination in LT COVID-19-recovered patients
All 12 recovered COVID-19 patients presented a positive anti-SARS-CoV-2 s-RBD antibody response before vaccination. Furthermore, all increased their antibody titers following vaccination, which were significantly higher than those reported in COVID-19-naïve patients (Fig. 4 ). Interestingly, the median antibody titers developed 3 weeks after the first vaccine dose were significantly higher than those present before vaccination but not significantly different from those developed 1 month after the second vaccine dose. As reported for COVID-19 naïve patients, the mean antibody titers remained stable 4 and 6 months after vaccination, and none of the patients became antibody-negative. Unlike COVID-19-naïve patients, in COVID-19-recovered patients, the positive antibody response rate was the same in those receiving MMF (n = 6) as in those who did not (n = 6), and the median anti-SARS-CoV-2 s-RBD antibody titer was evaluated both at baseline and at each time point after vaccination (Fig. 5 ).
Fig. 4.
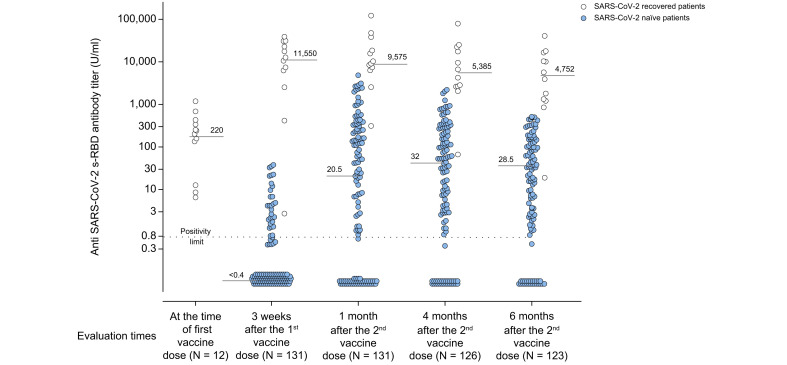
Anti-SARS-CoV-2 s-RBD antibody titers in COVID-19-recovered and in COVID-19-naïve patients.
Anti-SARS-CoV-2 s-RBD antibody titers in COVID-19-recovered (light blue circles) and in COVID-19-naïve (white circles) patients evaluated before and after 3 weeks (19 days) of the first dose of Pfizer-BioNTech® BNT162b2 vaccine, as well as after 1 month (31±2 days), 4 months (125±5 days), and 6 months (165±4 days) following the second vaccine dose. Positive responders to vaccination were defined as those having reached an antibody titer ≥0.8 U/ml. Medians of antibody titers are reported for each time point.
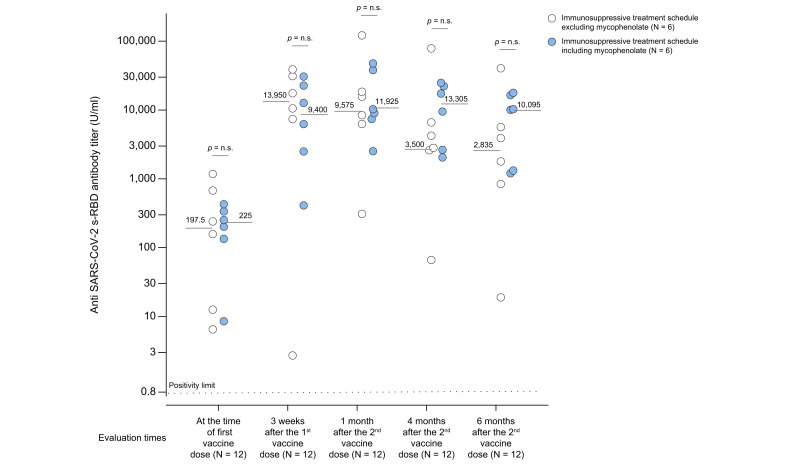
Fig. 5.
Anti-SARS-CoV-2 s-RBD antibody titers evaluated in COVID-19-recovered patients who did or did not receive mycophenolate mofetil.
Anti-SARS-CoV-2 s-RBD antibody titers evaluated in COVID-19-recovered (n = 12) patients before and after 3 weeks (19 days) of the first dose of Pfizer-BioNTech® BNT162b2 vaccine, as well as after 1 month (31±2 days), 4 months (125±5 days), and 6 months (165±4 days) following the second vaccine dose. Patients were divided with regard to adopting immunosuppressive treatment, including (light blue circles) or excluding (white circles) mycophenolate mofetil. Positive responders to vaccination were defined as those having reached an antibody titer ≥0.8 U/ml. Medians of antibody titers are reported for each time point, and the statistical analysis was performed by means of a non-parametric rank-sum (Mann-Whitney) test.
Patients and controls reported side effects of anti-SARS-CoV-2 vaccination
No severe side effects of vaccination were reported by either patients or controls. The more frequent reported side effect was modest pain in the vaccination injection site. This was reported in 23/58 (39.7%) controls and in 61/143 (42.6%) patients (p = 0.695). Considering both vaccination doses, the frequency of systemic symptoms, such as fever, asthenia or myalgia, was reported in 12/58 (20.6%) controls and in 7/143 (4.9%) patients (p <0.001). During routine post-vaccination patient follow-up, no significant liver test abnormalities or clinical alterations were recorded.
Discussion
The long-term antibody response to the full course of BNT162b2 vaccination was recorded in 78.8% of COVID-naïve patients compared to 100% of the controls. Moreover, the peak of responder patients was reached 4 months after the second vaccine dose and remained stable up to 6 months. Recent reports indicated that the rate of antibody response to anti-SARS-CoV-2 vaccination in LT patients ranged from 45.5% to 82%,11 , 12 , 16 , [18], [19], [20], [21], [22] which is comparable to what we observed. However, all these studies evaluated the early (up to 3 months) immune response to vaccination. Our study presents, for the first time to our knowledge, data regarding the persistence of the antibody response to vaccination in the long term (up to 6 months). Our findings could be considered unexpected, since the effect of immunosuppressive therapies could reduce antibody response duration over time more rapidly compared to immunocompetent patients. In a recent report conducted in healthcare professionals, 6 months after a full course of BNT162b2 vaccine, antibody titer decline was observed in approximately 89.6% of cases, and approximately 45% of them became seronegative.23 However, the peak response in these patients was reached 1 month after the second vaccine dose, which was earlier than what we observed in our series. One possible explanation could be that the vaccine-induced antibody response in immunosuppressed patients might be delayed and therefore detected to last longer. Whether this kinetic antibody response might be used to plan the vaccine booster dose would require appropriate clinical studies. In any case, to maintain the immunological response to vaccination for a long time, and in the hope of increasing the number of patient responders, it is desirable that booster doses are carried out in this category of patients. This strategy appears to be further justified by the recent emergence of the Omicron viral variant, whose clinical impact on LT recipients is not yet known.
No sex differences in the rate of long-term vaccine antibody response were detected. This agrees with previous reports11 , 18 , 22 but is in contrast to what was reported in LT recipients by Herrera et al.,19 who documented a significantly lower response rate in females. However, this difference may be due to the different vaccine types (mRNA1273) adopted in these patients compared to BNT162b2 adopted in our patients.
A detrimental effect on the long-term antibody response to vaccination was exerted by increasing the daily dose of MMF rather than adopting double or triple immunosuppressive drug combinations. This confirms data reported both in liver and in other solid organ transplants.24 Rabinowich et al. 18 showed that the use of MMF, in addition to a triple immunosuppression regimen, higher doses of steroids and lower eGFR were selected as negative predictors of vaccination response. In our series, only 13/131 (9.9%) patients were taking prednisone at a dosage >5 mg compared to 24/80 (30%) of those reported in the aforementioned study. Thus, the impact of steroids may be influenced by the different number of treated patients between the 2 studies. Furthermore, only 4 patients adopting triple immunosuppression were enrolled in our study. The influence of eGFR in conditioning the antibody vaccine response has seldom been evaluated in studies.11 , 12 , 19 , 22 In our series, a lower eGFR was selected as a negative predictor of vaccine response only 3 weeks after the first dose and not thereafter, which may be considered in agreement with what has been demonstrated in other series.22 In addition to the use of MMF, the presence of severe graft dysfunction leading to ascites formation and a lower serum leukocyte count were associated with a poor antibody response. Patients with advanced liver disease frequently show a suboptimal response to the anti-SARS-CoV-2 vaccine,12 which is also observable when employing other types of vaccines, such as that for hepatitis B.25 The detrimental combination of immunosuppressive treatment and the presence of graft cirrhosis after liver transplantation may justify our results. This may also explain the negative impact of a lower leukocyte count, which could be considered a surrogate marker of drug-induced immunosuppression and severe portal hypertension.26
The strong vaccination response was evaluated adopting an antibody cut-off value of 100 U/ml.17 The percentage of responder patients 6 months after vaccination decreased from 78.8% if evaluated by an antibody cut-off of 0.8 U/ml to 29.3%. Interestingly, alcohol consumption >40 g/day had a negative impact on a strong vaccination response, in addition to previously reported negative predictors. This finding agrees with studies indicating that alcohol consumption decreases the humoral response to some vaccines, such as those against streptococcal pneumonia.27 Since alcohol relapse after LT is described in up to 5% of recipients,28 the effectiveness of anti-SARS-CoV-2 vaccination in this category of patients may be further reduced.
In COVID-19-recovered patients in our series, higher BMI, diabetes mellitus, and recurrent cirrhosis with portal hypertension were significantly more frequent than in COVID-19-naïve patients. In contrast, no significant differences were recorded regarding which immunosuppression treatment was adopted, particularly regarding the use of MMF. This may be expected, since the presence of metabolic comorbidities has been associated with a worse clinical outcome for COVID-19 in LT recipients but not as a factor leading to increased susceptibility to infection.29 , 30 Similar consideration may be made with regard to the use of MMF, since it has been associated only with a more severe form of COVID-19,31 whereas the use of tacrolimus has been associated with a more benign course.32 Although in solid organ transplant recipients, a robust antibody and T cell response can be elicited regardless of COVID-19 severity,33 , 34 to our knowledge, no data are available on the impact of MMF use on developing natural immunity after SARS-CoV-2 infection in LT. The novelty of our findings, although derived from a small number of patients, is that among the COVID-19-recovered patients (50% treated with MMF), prevaccination anti-s-RBD protein antibody titers were detectable in all of them, independent of the use of MMF. Moreover, after the first vaccine dose, the antibody titer increased significantly and was comparable to that obtained 1 month after the second vaccine dose and remained positive up to 6 months, regardless of the use of MMF. This finding, if confirmed in a larger series, seems to support the observation that every immunosuppressive regimen adopted after LT has no meaningful impact on the ability to mount natural antibody responses after SARS-CoV-2 infection.34
Regarding vaccine safety in our study, no severe adverse events were reported in either patients or controls, nor were any liver biochemical abnormalities found during routine post-vaccination patient follow-up. These observations agree with previous studies performed in solid organ transplant recipients.10 , 18 , 19
Our study has some limitations. First, we did not evaluate the cellular immune response to vaccination. The correlation between humoral and cellular immune responses to anti-SARS-CoV-2 vaccination remains unclear. In a recent report evaluating 138 LT recipients, there was no evidence of a spike-specific T cell response in the majority of those without any detectable antibody response,22 suggesting that, in some cases, humoral and cellular immune responses could overlap. Second, we did not adopt systematic surveillance of patients to assess the efficacy of vaccination in preventing SARS-CoV-2 infection. Although this was not the aim of our study, we did not observe symptomatic SARS-CoV-2 infections in vaccinated patients during the follow-up period. Third, we enrolled patients with a long interval between transplant and vaccination; thus, our results may not be comparable to those obtainable when vaccination has been performed close to transplant.
In conclusion, in COVID-19-naïve LT patients, the anti-SARS-CoV-2 vaccination antibody response rate, although significantly lower than that in controls, was maintained for at least 6 months. The increasing daily dose of MMF remains the main determinant of vaccination failure. This implies that modifying the daily dose of MMF in the immediate pre- and post-vaccination period may be hypothesized in COVID-19-naïve non-responders to increase the immunogenicity of booster vaccine doses. In contrast, LT recipients who recovered from COVID-19 had a full long-term antibody response to vaccination that was detectable after the first vaccine dose regardless of the use of MMF.
Abbreviations
LT, liver transplantation; MMF, mycophenolate mofetil; RBD, receptor-binding protein.
Financial support
The project did not receive financial support.
Authors’ contributions
PT, RP and LG had the idea for the study, and PT wrote the paper. LV collected the data and prepared the database. EFa and CF performed the statistical analysis and contributed to writing the paper. SC, AC, AS, and FC collected blood samples and performed all laboratory tests. DB, EFo, and EFu performed the clinical evaluation of the patients. RP and LR coordinated the vaccination processes and the logistics of blood sampling.
Data availability statement
De-identified data available on request.
Conflicts of interest
The authors declare no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
The authors thank Cristina Minissale, Federica Sandri, Marcello Ferro, Deborah Di Giusto, Annalisa Sostero for their logistic support and all patients and their families for permitting the realization of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.02.015.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trapani S., Masiero L., Puoti F., Rota M.C., Del Manso M., Lombardini L., et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study. Am J Transpl. 2021;21:2509–2521. doi: 10.1111/ajt.16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becchetti C., Gschwend S.G., Dufour J.F., Banz V. COVID-19 in liver transplant recipients: a systematic review. J Clin Med. 2021;10(17):4015. doi: 10.3390/jcm10174015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becchetti C., Zambelli M.F., Pasulo L., Donato M.F., Invernizzi F., Detry O., et al. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832–1840. doi: 10.1136/gutjnl-2020-321923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belli L.S., Duvoux C., Cortesi P.A., Facchetti R., Iacob S., Perricone G., et al. COVID-19 in liver transplant candidates: pretransplant and post-transplant outcomes - an ELITA/ELTR multicentre cohort study. Gut. 2021;70:1914–1924. doi: 10.1136/gutjnl-2021-324879. [DOI] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kageyama T., Ikeda K., Tanaka S., Taniguchi T., Igari H., Onouchi Y., et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021;27(12):1861.e1–1861.e5. doi: 10.1016/j.cmi.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 10.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss A.T., Hallett A.M., Boyarsky B.J., Ou M.T., Werbel W.A., Avery R.K., et al. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl. 2021;27(12):1852–1856. doi: 10.1002/lt.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75(6):1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fix O.K., Blumberg E.A., Chang K.M., Chu J., Chung R.T., Goacher E.K., et al. AASLD expert panel consensus statement: vaccines to prevent COVID-19 infection in patients with liver disease. Hepatology. 2021;74(2):1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo F.P., Piano S., Bruno R., Burra P., Puoti M., Masarone M., et al. Italian association for the study of the liver position statement on SARS-CoV2 vaccination. Dig Liver Dis. 2021;53:677–681. doi: 10.1016/j.dld.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toniutto P., Aghemo A., Grossi P., Burra P. Permanent Transplant Commission of the Italian Association for the Study of the L. Clinical update on the efficacy of anti-SARS-CoV-2 mRNA vaccines in patients on the waiting list for liver transplantation and in liver transplant recipients. Dig Liver Dis. 2021;53(10):1232–1234. doi: 10.1016/j.dld.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera S., Colmenero J., Pascal M., Escobedo M., Castel M.A., Sole-Gonzalez E., et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transpl. 2021;21(12):3971–3979. doi: 10.1111/ajt.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarino M., Cossiga V., Esposito I., Alessandro F., Morisco F. Effectiveness of SARS-Cov-2 vaccination in liver transplanted patients: the debate is open! J Hepatol. 2021;76(1):237–239. doi: 10.1016/j.jhep.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashidi-Alavijeh J., Frey A., Passenberg M., Korth J., Zmudzinski J., Anastasiou O.E., et al. Humoral response to SARS-Cov-2 vaccination in liver transplant recipients-A single-center experience. Vaccines (Basel) 2021;9(7):738. doi: 10.3390/vaccines9070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruether D.F., Schaub G.M., Duengelhoef P.M., Haag F., Brehm T.T., Fathi A., et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2021;20(1):162–172. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayart J.L., Douxfils J., Gillot C., David C., Mullier F., Elsen M., et al. Waning of IgG, total and neutralizing antibodies 6 Months post-vaccination with BNT162b2 in healthcare workers. Vaccines (Basel) 2021;9(10):1092. doi: 10.3390/vaccines9101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall V.G., Ferreira V.H., Ierullo M., Ku T., Marinelli T., Majchrzak-Kita B., et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transpl. 2021;21(12):3980–3989. doi: 10.1111/ajt.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Tajes S., Pocurull A., Lens S., Marino Z., Olivas I., Soy G., et al. Efficacy of an accelerated double-dose hepatitis B vaccine regimen in patients with cirrhosis. J Viral Hepat. 2021;28:1019–1024. doi: 10.1111/jvh.13509. [DOI] [PubMed] [Google Scholar]
- 26.Yongxiang W., Zongfang L., Guowei L., Zongzheng J., Xi C., Tao W. Effects of splenomegaly and splenic macrophage activity in hypersplenism due to cirrhosis. Am J Med. 2002;113:428–431. doi: 10.1016/s0002-9343(02)01210-x. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann P., Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084–18. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singal A.K., Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA. 2021;326:165–176. doi: 10.1001/jama.2021.7683. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colmenero J., Rodriguez-Peralvarez M., Salcedo M., Arias-Milla A., Munoz-Serrano A., Graus J., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belli L.S., Fondevila C., Cortesi P.A., Conti S., Karam V., Adam R., et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology. 2021;160(4):1151–1163. doi: 10.1053/j.gastro.2020.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartzell S., Bin S., Benedetti C., Haverly M., Gallon L., Zaza G., et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transpl. 2020;20:3149–3161. doi: 10.1111/ajt.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez-Ruiz M., Olea B., Almendro-Vazquez P., Gimenez E., Marcacuzco A., San Juan R., et al. T cell-mediated response to SARS-CoV-2 in liver transplant recipients with prior COVID-19. Am J Transpl. 2021;21:2785–2794. doi: 10.1111/ajt.16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cillo U., De Carlis L., Del Gaudio M., De Simone P., Fagiuoli S., Lupo F., et al. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: consensus recommendations from an Italian Working Group. Hepatol Int. 2020;14:930–943. doi: 10.1007/s12072-020-10091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data available on request.