Abstract
Background
The B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified on November 25, 2021, in Gauteng province, South Africa. Data regarding the seroprevalence of SARS-CoV-2 IgG in Gauteng before the fourth wave of coronavirus disease 2019 (Covid-19), in which the omicron variant was dominant, are needed.
Methods
We conducted a seroepidemiologic survey from October 22 to December 9, 2021, in Gauteng to determine the seroprevalence of SARS-CoV-2 IgG. Households included in a previous seroepidemiologic survey (conducted from November 2020 to January 2021) were contacted; to account for changes in the survey population, there was a 10% increase in the households contacted, with the use of the same sampling framework. Dried-blood-spot samples were tested for IgG against SARS-CoV-2 spike protein and nucleocapsid protein with the use of quantitative assays. We also evaluated Covid-19 epidemiologic trends in Gauteng, including cases, hospitalizations, recorded deaths, and excess deaths from the start of the pandemic through January 12, 2022.
Results
Samples were obtained from 7010 participants, of whom 1319 (18.8%) had received a Covid-19 vaccine. The seroprevalence of SARS-CoV-2 IgG ranged from 56.2% (95% confidence interval [CI], 52.6 to 59.7) among children younger than 12 years of age to 79.7% (95% CI, 77.6 to 81.5) among adults older than 50 years of age. Vaccinated participants were more likely to be seropositive for SARS-CoV-2 than unvaccinated participants (93.1% vs. 68.4%). Epidemiologic data showed that the incidence of SARS-CoV-2 infection increased and subsequently declined more rapidly during the fourth wave than it had during the three previous waves. The incidence of infection was decoupled from the incidences of hospitalization, recorded death, and excess death during the fourth wave, as compared with the proportions seen during previous waves.
Conclusions
Widespread underlying SARS-CoV-2 seropositivity was observed in Gauteng before the omicron-dominant wave of Covid-19. Epidemiologic data showed a decoupling of hospitalizations and deaths from infections while omicron was circulating. (Funded by the Bill and Melinda Gates Foundation.)
The B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified on November 25, 2021, in Gauteng province, South Africa.1 The World Health Organization designated omicron as a variant of concern because of its predicted high transmissibility and its potential to evade immunity from neutralizing antibodies induced by vaccination or natural infection with wild-type virus.2 The omicron variant contains mutations that indicate that it could be more infectious, more transmissible, and possibly better able to evade innate immunity and neutralizing antibody activity than wild-type virus.3-5 In addition to having at least 32 mutations affecting the spike protein,6 the omicron variant harbors 3 mutations involving the membrane protein and 6 involving the nucleocapsid protein, whereas the antibody-evasive B.1.351 (beta) variant has only 7 spike-protein mutations and 1 nucleocapsid-protein mutation.7
The omicron variant outcompeted the B.1.617.2 (delta) variant in Gauteng and was responsible for 98.4% of new cases sequenced in South Africa in December 2021.8 This fourth wave of coronavirus disease 2019 (Covid-19) arose in the context of the rollout of Covid-19 vaccines, which began on May 17, 2021, in South Africa. We previously conducted a population-wide seroepidemiologic survey in Gauteng that was completed on January 22, 2021.9 We found that 19.1% of the population was seropositive for SARS-CoV-2, as assessed by the detection of IgG against the receptor-binding domain; the seroprevalence ranged from 5% to 43% across provincial subdistricts.9 After that survey was completed, South Africa faced a third wave of Covid-19, from approximately April 7 to November 1, that was largely due to the delta variant, which outcompeted the beta variant.10
We report the results of a follow-up seroepidemiologic survey in Gauteng that was completed on December 9, 2021, and thus provides seroprevalence data largely from before the fourth wave of Covid-19. Furthermore, we report data regarding Covid-19 epidemiologic trends in Gauteng, including cases, hospitalizations, recorded deaths, and excess deaths from the start of the pandemic through January 12, 2022.
Methods
Study Setting
Gauteng is divided into five health districts (Johannesburg, Ekurhuleni, Sedibeng, Tshwane, and West Rand) that comprise 26 subdistricts.11 Gauteng constitutes 1.5% of the landmass in South Africa but contains 26% of the population (15.5 of 59.6 million persons).11 The overall population density in Gauteng is 737 persons per square kilometer, with the value ranging from 3400 in Johannesburg, where 36.9% of the population lives, to 200 in West Rand, where 6.2% of the population lives (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Study Survey
This survey included the same households that were sampled during our previous survey, which was undertaken from November 4, 2020, to January 22, 2021.9 The previous survey was started 9 weeks after the onset of the second wave of Covid-19 in Gauteng, which was dominated by the beta variant. Details regarding the previous survey, including the sampling framework used, have been published9 and are summarized in the Supplementary Methods section of the Supplementary Appendix.
This survey was conducted from October 22 to December 9, 2021. To account for possible nonparticipation, out-migration, and death since the previous survey, there was a 10% increase in the households that were sampled; the additional households were sampled in the same clusters used previously. The survey was powered to evaluate seropositivity for SARS-CoV-2 at the district and subdistrict levels. Demographic and epidemiologic data were collected with the use of an electronic questionnaire.9 Details regarding the questionnaire are provided in the Supplementary Appendix.
The Human Research Ethics Committee at the University of the Witwatersrand granted a waiver for ethics approval of the survey, which was performed at the behest of the Gauteng Department of Health as part of public health surveillance. Nevertheless, all participants provided written informed consent; those who were approached to participate were free to decline participation. The authors designed the study, collected and analyzed the data, and vouch for the completeness and accuracy of the data and the fidelity of the study to the protocol. The authors wrote the manuscript; no one who is not an author contributed to the writing of the manuscript.
Serologic Analysis
Dried-blood-spot samples were obtained from participants and tested for IgG against SARS-CoV-2 spike protein and nucleocapsid protein with the use of quantitative assays on the Luminex platform. Anti-nucleocapsid IgG was included to identify persons who were seropositive from natural infection rather than vaccination. Details regarding the serologic assays have been published12,13 and are summarized in the Supplementary Appendix.
Covid-19 Data Sources
Data regarding daily cases, hospitalizations, and recorded deaths were sourced from the South African National Institute for Communicable Diseases daily databases, including the DATCOV database, through January 12, 2022.14,15 Data regarding weekly excess deaths attributable to Covid-19 were defined by and sourced from the South African Medical Research Council through January 8, 2022.16 We analyzed these epidemiologic data for Gauteng and its five health districts, both overall and with stratification according to age group and sex when granular data were available.
Cases included asymptomatic and symptomatic infections with SARS-CoV-2 confirmed by either a nucleic acid amplification assay or a rapid antigen test. Hospitalizations included admissions for SARS-CoV-2 infection, as well as admissions for other illnesses in which SARS-CoV-2 infection was incidentally identified on routine screening at the time of admission. Definitions of recorded death and excess death attributable to Covid-19 are provided in the Supplementary Appendix.
Statistical Analysis
The sample-size justification and the methods for repeated random sampling of households that were used in our previous survey have been published9 and are summarized in the Supplementary Appendix, together with the methods for analyses of associations with seropositivity, which were performed with the use of generalized linear models with log link to estimate risk ratios. These were unadjusted, univariable analyses for each risk factor. Data regarding daily cases, hospitalizations, and recorded deaths and weekly excess deaths were converted to incidences with the use of population denominators from Statistics South Africa mid-2020 projections for South Africa and its provinces.11
Results
Participants
We obtained samples that were adequate for serostatus evaluation from 7010 of 7498 participants from 3047 households (Figure 1); 83% of the samples had been obtained by November 25, 2021, when the omicron variant was first identified (Fig. S1). Demographic and household characteristics, known underlying medical conditions and participant-reported human immunodeficiency virus status, and vaccination status of the survey participants are shown in Table 1. The degree to which the survey population was representative of the general population of Gauteng and of South Africa is described in Table S2. Vaccination in Gauteng according to district, age, and vaccine is summarized in Table S3. As of November 25, 2021, of the total population of 12,191,569 persons 12 years of age or older (who were eligible for vaccination), 4,386,646 (36.0%) had received at least one dose of BNT162b2 or Ad26.COV2.S, and 2,452,017 (20.1%) had received two doses. Of the 2,416,045 persons older than 50 years of age, 1,074,303 (44.5%) had received two doses of BNT162b2.
Figure 1. Survey Participants.
This survey (conducted from October 22 to December 9, 2021) included the same households that were sampled during our previous survey (conducted from November 4, 2020, to January 22, 2021).9 To account for possible nonparticipation, out-migration, and death since the previous survey, there was a 10% increase in the households that were sampled; the additional households were sampled in the same clusters used previously.
Table 1. Seroprevalence of IgG against SARS-CoV-2 Spike Protein or Nucleocapsid Protein in Gauteng, South Africa, from October 22 to December 9, 2021, and Risk Factors for Seropositivity.*.
| Variable | Overall | Seroprevalence | Association with Seropositivity† | |
|---|---|---|---|---|
| no. (%) | no. | % (95% CI) | risk ratio (95% CI) | |
| All participants‡ | 7010 (100) | 5123 | 73.1 (72.0–74.1) | — |
| Sex | ||||
| Male | 2941 (42.0) | 1998 | 67.9 (66.2–69.6) | Reference |
| Female | 4065 (58.0) | 3125 | 76.9 (75.5–78.1) | 1.13 (1.10–1.17) |
| Not reported | 4 (<0.1) | — | — | — |
| Age group§ | ||||
| <12 yr | 753 (10.7) | 423 | 56.2 (52.6–59.7) | Reference |
| 12 to 17 yr | 622 (8.9) | 459 | 73.8 (70.2–77.1) | 1.31 (1.21–1.42) |
| 18 to 50 yr | 4047 (57.7) | 2977 | 73.6 (72.2–74.9) | 1.30 (1.23–1.40) |
| >50 yr | 1588 (22.7) | 1264 | 79.7 (77.6–81.5) | 1.42 (1.32–1.52) |
| Vaccination status§ | ||||
| Unvaccinated | 5691 (81.2) | 3895 | 68.4 (67.2–69.6) | Reference |
| Vaccinated | 1319 (18.8) | 1228 | 93.1 (91.6–94.3) | 1.36 (1.33–1.39) |
| Vaccination status according to age group§ | ||||
| Unvaccinated | ||||
| <12 yr | 753 (10.7) | 423 | 55.8 (52.2–59.4) | 0.81 (0.76–0.86) |
| 12 to 17 yr | 603 (8.6) | 443 | 73.5 (69.8–76.8) | 1.06 (1.00–1.11) |
| 18 to 50 yr | 3356 (47.9) | 2334 | 69.5 (68.0–71.1) | Reference |
| >50 yr | 979 (14.0) | 695 | 71.0 (68.1–73.7) | 1.02 (0.97–1.07) |
| Vaccinated | ||||
| <12 yr | 0 | 0 | — | — |
| 12 to 17 yr | 19 (0.3) | 16 | 84.2 (60.8–94.8) | 1.21 (1.00–1.47) |
| 18 to 50 yr | 691 (9.9) | 643 | 93.1 (90.9–94.7) | 1.33 (1.30–1.38) |
| >50 yr | 609 (8.7) | 569 | 93.4 (91.2–95.1) | 1.34 (1.30–1.39) |
| Previous Covid-19 testing | ||||
| Never tested | 5956 (85.0) | 4271 | 71.7 (70.6–72.8) | Reference |
| Tested positive | 195 (2.8) | 172 | 88.2 (82.9–92.0) | 1.23 (1.17–1.30) |
| Tested negative | 859 (12.3) | 680 | 79.3 (76.3–81.8) | 1.10 (1.06–1.15) |
| Household members per room¶ | 1 (0.5–1.5) | — | — | 1.01 (1.00–1.02) |
| Occupation | ||||
| Unemployed | 4102 (58.5) | 3014 | 73.5 (72.1–74.8) | Reference |
| Production | 381 (5.4) | 279 | 73.2 (68.6–77.4) | 1.00 (0.94–1.06) |
| Education, public transportation, or retail | 661 (9.4) | 509 | 77.0 (73.6–80.1) | 1.05 (1.00–1.10) |
| Health care | 73 (1.0) | 63 | 86.3 (76.4–92.5) | 1.17 (1.07–1.29) |
| Office work or other | 353 (5.0) | 277 | 78.5 (73.9–82.4) | 1.06 (1.01–1.13) |
| Student | 1440 (20.5) | 981 | 68.1 (65.7–70.5) | 0.93 (0.89–0.96) |
| Smoking status‖ | ||||
| None | 4168 (59.5) | 3234 | 77.6 (76.3–78.8) | Reference |
| Daily | 1125 (16.1) | 748 | 66.5 (63.7–69.2) | 0.86 (0.82–0.90) |
| Once or twice a week | 244 (3.5) | 181 | 74.2 (68.3–79.3) | 0.96 (0.89–1.03) |
| Occasionally | 203 (2.9) | 157 | 77.3 (71.1–82.6) | 1.00 (0.92–1.08) |
| Coexisting conditions | ||||
| None | 4731 (67.5) | 3507 | 74.1 (72.9–75.4) | Reference |
| ≥1 | 2279 (32.5) | 1616 | 70.9 (69.0–72.7) | 0.96 (0.93–0.99) |
| HIV status | ||||
| Negative | 6460 (92.2) | 4727 | 73.2 (72.1–74.2) | Reference |
| Positive | 550 (7.8) | 396 | 72.0 (68.1–75.6) | 0.98 (0.93–1.04) |
| Dwelling type** | ||||
| Formal standalone house | 4700 (67.0) | 3488 | 74.2 (72.9–75.4) | Reference |
| Informal settlement | 1147 (16.4) | 761 | 66.3 (63.6–69.0) | 0.89 (0.86–0.93) |
| Block of flats or high-rise building | 423 (6.0) | 329 | 77.8 (73.6–81.5) | 1.05 (0.99–1.11) |
| Subsidized low-income housing | 666 (9.5) | 494 | 74.3 (70.8–77.4) | 1.00 (0.95–1.05) |
| Other | 74 (1.1) | 51 | 68.9 (57.5–78.4) | 0.93 (0.80–1.08) |
| District | ||||
| Johannesburg | 2468 (35.2) | 1880 | 76.2 (74.5–77.8) | Reference |
| Ekurhuleni | 1861 (26.5) | 1382 | 74.3 (72.2–76.2) | 0.97 (0.94–1.01) |
| Sedibeng | 564 (8.0) | 397 | 70.4 (66.5–74.0) | 0.92 (0.87–0.98) |
| Tshwane | 1464 (20.9) | 975 | 66.7 (54.2–69.0) | 0.87 (0.84–0.91) |
| West Rand | 653 (9.3) | 489 | 74.9 (71.4–78.1) | 0.98 (0.94–1.03) |
Covid-19 denotes coronavirus disease 2019, HIV human immunodeficiency virus, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Analyses of associations with seropositivity for SARS-CoV-2 were performed with the use of generalized linear models with log link to estimate risk ratios. These were unadjusted, univariable analyses for each risk factor. Confidence intervals were not adjusted for multiplicity and should not be used for inference.
Two participants had serologic test results that could not be linked to the main questionnaire and were excluded from the analyses.
Age group and vaccination status were not included in the regression model; instead, an interaction term between age group and vaccination status was introduced to account for the differences in seroprevalence according to vaccination status across age groups. Vaccination status was obtained from vaccination certificates for 1026 of the 1327 participants (77.3%) who reported being vaccinated.
The median and interquartile range are shown, rather than the number and percentage. The risk ratio shows the increase in the risk of seropositivity with one additional household member per room (i.e., for every additional household member, there is a 1.01 increase in risk).
Smoking status was assessed only in the 5740 participants who were 18 years of age or older.
Dwelling types were defined in accordance with the national census classification.
Seroprevalence
Among unvaccinated participants, the overall prevalence of anti-spike or anti-nucleocapsid IgG seropositivity was 68.4% (95% confidence interval [CI], 67.2 to 69.6), whereas the prevalence of anti-nucleocapsid IgG seropositivity was 39.7% (95% CI, 38.4 to 41.0), a finding that indicates a lack of sensitivity of anti-nucleocapsid IgG for the detection of previous infection. We thus focused on the overall prevalence of anti-spike or anti-nucleocapsid IgG seropositivity.
Among all participants, the overall seroprevalence was 73.1% (95% CI, 72.0 to 74.1) (Table 1). The seroprevalence was heterogeneous across provincial districts, ranging from 66.7% (95% CI, 54.2 to 69.0) in Tshwane, where the omicron variant was first identified, to 76.2% (95% CI, 74.5 to 77.8) in Johannesburg (Fig. S2). In addition, the seroprevalence was heterogeneous across subdistricts, ranging from 72.7% to 85.8% within Johannesburg and from 58.9% to 77.4% within Tshwane (Table S4).
Female participants were more likely to be seropositive than male participants (76.9% vs. 67.9%; risk ratio, 1.13; 95% CI, 1.10 to 1.17). The seroprevalence varied according to age group; it was lowest among children younger than 12 years of age (56.2%) and highest among adults older than 50 years of age (79.7%). Children 12 to 17 years of age were more likely to be seropositive than children younger than 12 years of age (73.8% vs. 56.2%; risk ratio, 1.31; 95% CI, 1.21 to 1.42). Participants who had received a Covid-19 vaccine were more likely to be seropositive than unvaccinated participants (93.1% vs. 68.4%; risk ratio, 1.36; 95% CI, 1.33 to 1.39). Among vaccinated participants, the seroprevalence was consistently high across age groups; among adults 18 to 50 years of age, those who were vaccinated had a higher seroprevalence than those who were unvaccinated.
Participants who had previously tested positive for SARS-CoV-2 infection were more likely to be seropositive than participants who had never been tested (88.2% vs. 71.7%; risk ratio, 1.23; 95% CI, 1.17 to 1.30). Participants living in an informal settlement had a lower seroprevalence than participants living in a standalone house (66.3% vs. 74.2%; risk ratio, 0.89; 95% CI, 0.86 to 0.93). Daily smoking was associated with a lower seroprevalence than was not smoking (66.5% vs. 77.6%; risk ratio, 0.86; 95% CI, 0.82 to 0.90).
Covid-19 Trends
Daily cases, weekly hospitalizations, daily recorded deaths, and weekly excess deaths attributable to Covid-19 in Gauteng are shown in Figure 2. Daily cases, hospitalizations, and recorded deaths are also shown with stratification according to age group (Figure 3) and according to sex (Fig. S3).
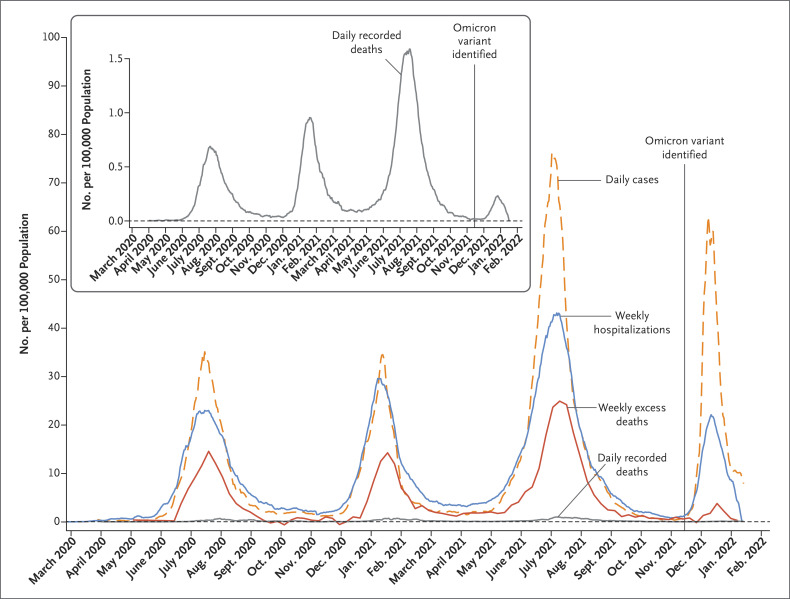
Figure 2. Cases, Hospitalizations, Recorded Deaths, and Excess Deaths Attributable to Covid-19 in Gauteng, South Africa, from the Start of the Pandemic through January 12, 2022.
Shown are incidences of daily cases, weekly hospitalizations, daily recorded deaths, and weekly excess deaths attributable to coronavirus disease 2019 (Covid-19). The inset shows the incidence of daily recorded deaths on an enlarged y axis. The horizontal dashed line indicates an incidence of zero. The data were sourced from the National Institute for Communicable Diseases daily databases through January 12, 2022, except for the data regarding weekly excess deaths attributable to Covid-19, which were defined by and sourced from the South African Medical Research Council through January 8, 2022.16 The B.1.1.529 (omicron) variant was first identified on November 25, 2021. Cases included asymptomatic and symptomatic infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) confirmed by either a nucleic acid amplification assay or a rapid antigen test. Changes in the frequency of testing limit direct comparisons of case numbers; in particular, the lower frequency of testing during the first wave, which was due to constraints in laboratory capacity and prioritization of testing for hospitalized persons, prevents the direct comparison of cases from the first wave with those from subsequent waves. Hospitalizations included admissions for SARS-CoV-2 infection, as well as admissions for other illnesses in which SARS-CoV-2 infection was incidentally identified on routine screening at the time of admission. The DATCOV system was developed during the first wave, with gradual onboarding of facilities; thus, hospitalizations from the first wave may be underestimated. Definitions of recorded death and excess death attributable to Covid-19 are provided in the Supplementary Appendix.
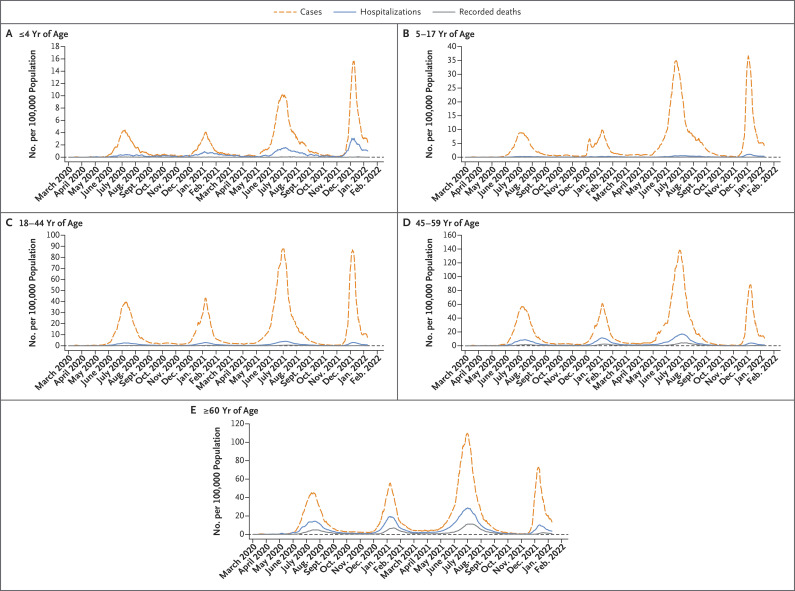
Figure 3. Covid-19 Cases, Hospitalizations, and Recorded Deaths in Gauteng, South Africa, According to Age Group.
Shown are 7-day moving averages of the incidences of daily cases, hospitalizations, and recorded deaths among participants 4 years of age or younger (Panel A), 5 to 17 years of age (Panel B), 18 to 44 years of age (Panel C), 45 to 59 years of age (Panel D), and 60 years of age or older (Panel E). The horizontal dashed line indicates an incidence of zero. Because the incidences differ across age groups, different y-axis scales are used for each age group to provide clarity and aid in the visual interpretation of the trends in each group.
During the fourth wave of Covid-19, in which the omicron variant was dominant, the daily case incidence increased more rapidly and also appeared to be decreasing more quickly than it had during the three previous waves (Figure 2). The time from the onset to the peak of the wave was 1 month in the fourth wave, as compared with 2 months in the third wave. As of January 12, 2022, the case incidence had not yet fully returned to the level before the onset of the fourth wave, but the wave was nearing its end, on the basis of the trajectory shown in Figure 2. At that time, there were almost no recorded or excess deaths attributable to Covid-19 per 100,000 population.
The number of documented Covid-19 cases in the fourth wave (226,932) was higher than that in the second wave (182,564) and lower than that in the third wave (511,638), whereas the incidences of hospitalization, recorded death, and excess death attributable to Covid-19 in the fourth wave were consistently lower than the incidences in earlier waves (Table 2). In addition, the peak incidences of hospitalization, recorded death, and excess death in the fourth wave were lower than the peak incidences in previous waves. The fourth wave contributed 11.2%, 3.9%, and 3.3% of overall hospitalizations, recorded deaths, and excess deaths due to Covid-19, respectively, whereas the third wave, in which the delta variant was dominant, contributed 43.6%, 49.3%, and 52.7%. Similar trends were observed across all districts (Fig. S4). Although there is a lag in the reporting of weekly excess deaths, the incidence in the fourth wave as of January 8, 2022 (12 per 100,000 population), was lower than the incidence in the third wave (197 per 100,000 population). As of January 12, 2022, incidences were on an ongoing downward trajectory, with a 7-day moving average of 7.28 cases, 0.96 hospitalizations, and 0.11 recorded deaths per 100,000 population — a decrease by a factor of 9.3, 3.3, and 2.4 from the peak incidence of 67.56 cases, 3.18 hospitalizations, and 0.26 recorded deaths per 100,000 population, respectively. The incidences were nearing prewave levels (as of October 25, 2021) of 0.46 cases, 0.15 hospitalizations, and 0.04 recorded deaths per 100,000 population.
Table 2. Cumulative Reported Cases, Hospitalizations, Recorded Deaths, and Excess Deaths Attributable to Covid-19 in Gauteng, South Africa, According to Covid-19 Wave.*.
| Outcome | Wave 1, Wild-Type | Wave 2, Beta | Wave 3, Delta | Wave 4, Omicron | Total |
|---|---|---|---|---|---|
| Cases† | |||||
| Period of wave | March 7– Nov. 13, 2020 | Nov. 14, 2020– March 30, 2021 | March 31– Oct. 25, 2021 | Oct. 26, 2021– Jan. 12, 2022 | March 7, 2020– Jan. 12, 2022 |
| No. | 232,130 | 182,564 | 511,638 | 226,932 | 1,153,264 |
| No. per 100,000 population | 1498 | 1178 | 3301 | 1464 | 7440 |
| Percent of total | 20.1 | 15.8 | 44.4 | 19.7 | 100 |
| Hospitalizations‡ | |||||
| Period of wave | March 7– Nov. 6, 2020 | Nov. 7, 2020– April 6, 2021 | April 7– Oct. 31, 2021 | Nov. 1, 2021– Jan. 12, 2022 | March 7, 2020– Jan. 12, 2022 |
| No. | 33,315 | 30,685 | 61,642 | 15,789 | 141,431 |
| No. per 100,000 population | 215 | 198 | 398 | 102 | 912 |
| Percent of total | 23.6 | 21.7 | 43.6 | 11.2 | 100 |
| Recorded deaths§ | |||||
| Period of wave | March 31– Dec. 14, 2020 | Dec. 15, 2020– May 2, 2021 | May 3– Nov. 19, 2021 | Nov. 20, 2021– Jan. 12, 2022 | March 31, 2020– Jan. 12, 2022 |
| No. | 6443 | 7084 | 14,256 | 1116 | 28,899 |
| No. per 100,000 population | 42 | 46 | 92 | 7 | 186 |
| Percent of total | 22.3 | 24.5 | 49.3 | 3.9 | 100 |
| Excess deaths§ | |||||
| Period of wave | May 9– Dec. 19, 2020 | Dec. 20, 2020– March 26, 2021 | March 27– Nov. 25, 2021 | Nov. 26, 2021– Jan. 8, 2022 | May 9, 2020– Jan. 8, 2022 |
| No. | 13,476 | 11,970 | 30,546 | 1,927 | 57,919 |
| No. per 100,000 population | 87 | 77 | 197 | 12 | 374 |
| Percent of total | 23.3 | 20.7 | 52.7 | 3.3 | 100 |
Data are shown for the first wave of Covid-19, dominated by wild-type virus; the second wave, dominated by the B.1.351 (beta) variant; the third wave, dominated by the B.1.617.2 (delta) variant; and the fourth wave, dominated by the B.1.1.529 (omicron) variant. The data were sourced from the National Institute for Communicable Diseases daily databases through January 12, 2022, except for the data regarding weekly excess deaths attributable to Covid-19, which were defined by and sourced from the South African Medical Research Council through January 8, 2022.16 As compared with the reporting of cases, there was a lag in the reporting of hospitalizations, recorded deaths, and excess deaths; thus, each of these outcomes has a different period for each wave. As of January 12, 2022, the case incidence had not yet fully returned to the level before the onset of the fourth wave; numbers, incidences, and proportions of cases, hospitalizations, and deaths were anticipated to continue to increase somewhat. However, the subsequent increases were limited, with the incidence of excess death attributable to Covid-19 having declined to 0 per 100,000 population by January 15, 2022.
Cases included asymptomatic and symptomatic SARS-CoV-2 infections confirmed by either a nucleic acid amplification assay or a rapid antigen test. Changes in the frequency of testing limit direct comparisons of case numbers; in particular, the lower frequency of testing during the first wave, which was due to constraints in laboratory capacity and prioritization of testing for hospitalized persons, prevents the direct comparison of cases from the first wave with those from subsequent waves.
Hospitalizations included admissions for SARS-CoV-2 infection, as well as admissions for other illnesses in which SARS-CoV-2 infection was incidentally identified on routine screening at the time of admission. The DATCOV system was developed during the first wave, with gradual onboarding of facilities; thus, hospitalizations from the first wave may be underestimated.
Definitions of recorded death and excess death attributable to Covid-19 are provided in the Supplementary Appendix.
During the fourth wave, decreased incidences of hospitalization and recorded death were evident across all age groups older than 17 years and among both men and women. The incidences of hospitalization and recorded death among children 17 years of age or younger, which have consistently been markedly lower than the incidences in older age groups, were similar to the incidences during earlier waves, except for a lower mortality among children 5 to 17 years of age during the fourth wave than during the third (delta-dominant) wave (Figure 3 and Tables S5, S6, and S7).
Discussion
In Gauteng, the resurgence of Covid-19 that was dominated by the omicron variant evolved at a time when Covid-19 vaccine coverage was 36.0% among persons 12 years of age or older, with only 20.1% having received at least two doses of a Covid-19 vaccine as part of the national vaccine rollout program. Nevertheless, the results of our survey showed widespread underlying SARS-CoV-2 seropositivity across the province (73.1%), including a prevalence at the subdistrict level of up to 85.8%, before the onset of the omicron-dominant wave. This high seroprevalence was primarily induced by previous SARS-CoV-2 infection, as evidenced by the 68.4% seroprevalence among participants who had not received a Covid-19 vaccine. The methods used for selecting the random sample of households in the survey, with a distribution proportionate to subdistrict population sizes, ensured that the sample was representative of the general population of Gauteng.
In this context, we observed a dramatic decoupling of hospitalizations and deaths from infections during the fourth wave of Covid-19, as compared with the proportions seen during the three previous waves. The biologic basis for this decoupling could be the extensive cell-mediated immunity in the population that was induced by previous natural infection and vaccination. At least one vaccine dose had been administered to 61.2% of adults older than 50 years of age (1,479,288 of 2,416,045), who had accounted for 81.0% of all deaths (22,269 of 27,500) due to Covid-19 in Gauteng through the end of the third wave.17 Although we did not evaluate cell-mediated immunity, other studies have shown that natural infection induces a diverse polyepitopic cell-mediated immune response that targets the spike protein, nucleocapsid protein, and membrane protein.18 Consequently, cell-mediated immunity is likely to be more durable than neutralizing antibody–mediated immunity in the context of small mutations,19 particularly those mainly affecting the spike protein, such as those in the omicron variant. Furthermore, natural infection induces robust memory T-cell responses, including long-lived cytotoxic (CD8+) T cells, which have a half-life of 125 to 255 days.20
We think that the evolution of cell-mediated immunity from previous natural infection and vaccination has resulted in the decoupling of the high case incidence seen with the omicron variant from the incidence of severe disease (hospitalizations and deaths). This decoupling has occurred despite evidence that the omicron variant evades neutralizing antibody activity induced by spike-protein–based vaccines and by previous infection with other variants that did not harbor the same full set of putatively antibody-evasive mutations. Our hypothesis is supported by two recent preprint publications, which indicated that most of the T-cell response induced by vaccination or natural infection cross-recognizes the omicron variant, thereby probably contributing to protection against severe disease.21,22 An alternative or additional mechanism by which protection against severe disease may be conferred, despite the reduced neutralizing antibody activity against the omicron variant, is through Fc-mediated effector functions of non-neutralizing antibodies that induce antibody-mediated cellular phagocytosis, complement deposition, and natural killer–cell activation.19,23 In addition, the omicron variant may be less potent in causing serious illness.
We saw a high incidence of Covid-19 cases due to the omicron variant despite the high seroprevalence of humoral immune responses, a finding consistent with the antibody-evasive nature of the omicron variant. Reports have indicated that the omicron variant is more capable of evading neutralizing antibody activity than even the beta variant.7,24-26 Neutralizing antibody activity against the omicron variant after two doses of BNT162b2 or AZD1222 (also known as ChAdOx1 nCoV-19) was shown to be substantially lower than vaccine-induced neutralizing antibody activity against wild-type virus.27,28 Nevertheless, the majority of persons with hybrid immunity from natural infection and BNT162b2 or AZD1222 vaccination have measurable neutralizing antibody activity against the omicron variant, albeit a lower level than that against the wild-type virus.24 In this context, a high incidence of breakthrough cases and reinfections with the omicron variant was to be expected in South Africa, where the majority of persons had immunity from natural infection, which induces a lower magnitude of anti-spike neutralizing and binding antibody responses than vaccination.25 Furthermore, as part of its vaccine rollout at the time of the evolution of the fourth wave, South Africa was providing only a single dose of Ad26.COV2.S, which induces lower titers of neutralizing and blocking antibodies than two doses of BNT162b225; the third (booster) dose of BNT162b2 had not been introduced in South Africa at that time.
This clinical evidence of the antibody-evasive nature of the omicron variant is corroborated by early studies that showed limited vaccine effectiveness against omicron at 25 weeks after two doses of AZD1222 or BNT162b2.29 However, vaccine effectiveness was substantially increased at 2 weeks after a booster dose of BNT162b2,29 which results in much higher neutralizing antibody titers than two doses of the vaccine30 and thus may partly mitigate the relative antibody-evasiveness of the omicron variant. In addition, in South Africa, vaccine effectiveness against hospitalization was 70% with the omicron variant, as compared with 93% with the delta variant.31 These data, together with the very limited neutralizing antibody activity against the omicron variant after two doses of AZD1222 or BNT162b2, further corroborate the evidence that protection against severe Covid-19 due to the omicron variant is probably mediated by much lower neutralizing antibody titers than those required to protect against SARS-CoV-2 infection or mild Covid-1925 or is provided by cell-mediated immunity or the Fc-effector functions of non-neutralizing antibodies (or a combination of these mechanisms).19,23
The antibody-evasive nature of the omicron variant is analogous to the antibody-evasiveness of the beta variant in recipients of AZD1222, the AstraZeneca chimpanzee adenovirus–based vaccine. AZD1222 was shown to have no effectiveness against mild-to-moderate Covid-19 due to the beta variant.32 However, vaccine effectiveness against hospitalization or death due to the beta or P.1 (gamma) variant was 80% in a report from Canada.33 Although AZD1222 induced nominal neutralizing antibody activity against the beta variant, only 11 of the 87 spike-protein epitopes targeted by T-cell immune responses induced by AZD1222 were affected by mutations in the beta variant.32 The dissociation between the lack of AZD1222-induced neutralizing antibody activity and the protection against severe disease involving the lower respiratory tract was also observed in a challenge study with AZD1222 against the beta variant in a Syrian golden hamster model.34
Evidence of the high transmissibility of the omicron variant is corroborated by the rapid rise in reported Covid-19 cases in Gauteng during the fourth wave. Indeed, the increase in the case incidence during the fourth wave occurred faster than that during any previous wave, a finding that indicates that the omicron variant is more transmissible than even the delta variant, which had an estimated reproductive number (R0) of 5 to 6.35
Our study has some limitations. First, we used publicly available data regarding Covid-19 morbidity and mortality that were collated in surveillance systems and could have changed over time, which could affect comparisons across the four waves. The DATCOV database does not distinguish between patients hospitalized for SARS-CoV-2 infection and patients hospitalized for other illnesses who incidentally had a positive test for SARS-CoV-2 on routine screening. Nevertheless, data from these systems are unlikely to have changed since the third wave. Second, changes in the frequency of testing over time limit head-to-head comparisons of case numbers across waves, although the criteria for testing have been similar since the start of the second wave. Finally, the fourth wave had not fully subsided at the time of this analysis. The numbers, incidences, and proportions of total cumulative cases, hospitalizations, and deaths attributable to this wave — in particular, the data for hospitalizations and deaths, because there is a lag in the reporting of these data — were anticipated to continue to increase somewhat. However, the subsequent increases were limited, with the incidence of excess death attributable to Covid-19 having declined to 0 per 100,000 population by January 15, 2022.
Our hypothesis that cell-mediated immunity primarily due to natural infection, with or without Covid-19 vaccination, has resulted in the decoupling of cases from severe disease remains to be investigated. In particular, the extent to which the polyepitopic T-cell response induced by vaccination against the spike protein — as well as the even more diverse polyepitopic T-cell response stimulated by natural infection, with or without vaccination — remains cross-reactive against the omicron variant warrants further investigation.21,22 Another possible contributing factor to the decoupling of cases from severe disease with the omicron variant, as compared with the proportions seen with previous variants, is that the omicron variant may be more adept at infecting the upper airways and less adept at infecting the lower airways, which could result in reduced virulence.36 The difference in the prevalence of immunity across waves limits our ability to draw any conclusions regarding the relative roles of reduced virulence and higher prevalence of underlying cell-mediated immunity in contributing to the decoupling of cases from severe disease observed with the omicron variant in our study.
We think that the decoupling of the incidence of Covid-19 cases from the incidences of hospitalization and death during the omicron-dominant wave in South Africa heralds a turning point in the Covid-19 pandemic, if the primary goal is protection against severe disease and death rather than prevention of infection. The 70% vaccine effectiveness against severe disease with BNT162b2 in South Africa31 might well be due to the hybrid cell-mediated immunity induced by vaccination and natural infection. Whether the same protection against severe Covid-19 due to the omicron variant will be seen in countries in which immunity is mainly from vaccination remains to be determined.
Acknowledgments
We thank the epidemiology team (Andronica Moipone Shonhiwa, Genevie Ntshoe, Joy Ebonwu, Lactatia Motsuku, Liliwe Shuping, Mazvita Muchengeti, Jackie Kleynhans, Gillian Hunt, Victor Odhiambo Olago, Husna Ismail, Nevashan Govender, Ann Mathews, Vivien Essel, Veerle Msimang, Tendesayi Kufa-Chakezha, Nkengafac Villyen Motaze, Natalie Mayet, Tebogo Mmaborwa Matjokotja, Mzimasi Neti, Tracy Arendse, Teresa Lamola, Itumeleng Matiea, Darren Muganhiri, Babongile Ndlovu, Khuliso Ravhuhali, Emelda Ramutshila, Salaminah Mhlanga, Akhona Mzoneli, Nimesh Naran, Trisha Whitbread, Mpho Moeti, Chidozie Iwu, Eva Mathatha, Fhatuwani Gavhi, Masingita Makamu, Matimba Makhubele, Simbulele Mdleleni, Bracha Chiger, Jackie Kleynhans, and Michelle Groome) and the information technology team (Tsumbedzo Mukange, Trevor Bell, Lincoln Darwin, Fazil McKenna, Ndivhuwo Munava, Muzammil Raza Bano, and Themba Ngobeni) at the National Institute for Communicable Diseases Notifiable Medical Conditions Surveillance System; Steve Hill, Ph.D., of Ashfield MedComms (an Ashfield Health company, part of UDG Healthcare) for medical writing and editing support for an earlier version of the manuscript, which was funded by AstraZeneca in accordance with Good Publication Practice guidelines; and the Gauteng Department of Health for providing the vaccine coverage data.
Supplementary Appendix
Disclosure Forms
This article was published on February 23, 2022, at NEJM.org.
Data are available at www.wits-vida.org; requests for data sharing should be directed to Dr. Madhi at shabir.madhi@wits.ac.za.
Footnotes
Supported by the Bill and Melinda Gates Foundation (INV-023514). DATCOV, a national surveillance system, is funded by the National Institute for Communicable Diseases and the South African Government.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021;600:21-21. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. November 26, 2021. (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern).
- 3.European Centre for Disease Prevention and Control. Implications of the emergence and spread of the SARS-CoV-2 B.1.1. 529 variant of concern (Omicron) for the EU/EEA. November 26, 2021. (https://www.ecdc.europa.eu/sites/default/files/documents/Implications-emergence-spread-SARS-CoV-2%20B.1.1.529-variant-concern-Omicron-for-the-EU-EEA-Nov2021.pdf).
- 4.UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 29. November 26, 2021. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1036501/Technical_Briefing_29_published_26_November_2021.pdf).
- 5.Chen J, Wang R, Gilby NB, Wei GW. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model 2022;62:412-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaway E, Ledford H. How bad is Omicron? What scientists know so far. Nature 2021;600:197-199. [DOI] [PubMed] [Google Scholar]
- 7.Cele S, Gazy I, Jackson L, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature 2021;593:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.South African National Institute for Communicable Diseases. Network for genomic surveillance in South Africa SARS-CoV-2 sequencing update. December 31, 2021. (https://www.nicd.ac.za/wp-content/uploads/2022/01/Update-of-SA-sequencing-data-from-GISAID-30-Dec-2021_dash.pdf).
- 9.Mutevedzi PC, Kawonga M, Kwatra G, et al. Estimated SARS-CoV-2 infection rate and fatality risk in Gauteng Province, South Africa: a population-based seroepidemiological survey. Int J Epidemiol 2021. October 30 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.South African National Institute for Communicable Diseases. SARS-CoV-2 genomic surveillance update June 2021. July 1, 2021. (https://www.nicd.ac.za/wp-content/uploads/2021/07/Sequencing-update-1July-2021_V14.pdf).
- 11.Statistics South Africa. Statistical release P0302: mid-year population estimates 2020. July 31, 2021. (http://www.statssa.gov.za/publications/P0302/P03022020.pdf).
- 12.Kwatra G, Nunes M, Dhar N, et al. Correlation of dried blood spots and plasma for quantification of Immunoglobulin (IgG) against receptor binding domain and full length spike protein of SARS-CoV-2. J Virol Methods 2022;300:114394-114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021;8(9):e568-e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Communicable Diseases. Daily hospital surveillance (DATCOV) report (https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/daily-hospital-surveillance-datcov-report/).
- 15.Jassat W, Mudara C, Ozougwu L, et al. Difference in mortality among individuals admitted to hospital with COVID-19 during the first and second waves in South Africa: a cohort study. Lancet Glob Health 2021;9(9):e1216-e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradshaw D, Laubscher R, Dorrington R, Groenewald P, Moultrie T. Report on weekly deaths in South Africa 26 Dec 2021 — 1 Jan 2022 (week 52). South African Medical Research Council, January 4, 2022. (https://www.samrc.ac.za/sites/default/files/files/2022-01-05/weekly1Jan2022.pdf).
- 17.National Institute for Communicable Diseases. Weekly hospital surveillance (DATCOV) update (https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/weekly-hospital-surveillance-datcov-update/).
- 18.Milne G, Hames T, Scotton C, et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med 2021;9:1450-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alter G, Yu J, Liu J, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021;596:268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371:eabf4063-eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keeton R, Tincho MB, Ngomti A, et al. SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. December 28, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.26.21268380v1). preprint.
- 22.Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological memory able to cross-recognize variants from Alpha to Omicron. December 28, 2021. (https://www.biorxiv.org/content/10.1101/2021.12.28.474333v1). preprint. [DOI] [PMC free article] [PubMed]
- 23.Gorman MJ, Patel N, Guebre-Xabier M, et al. Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M vaccination. Cell Rep Med 2021;2:100405-100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. December 11, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.08.21267491v1). preprint.
- 25.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022;375:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dejnirattisai W, Huo J, Zhou D, et al. Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. December 22, 2021. (https://www.biorxiv.org/content/10.1101/2021.12.03.471045v2). preprint. [DOI] [PMC free article] [PubMed]
- 27.Dejnirattisai W, Shaw RH, Supasa P, et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022;399:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cele S, Jackson L, Khan K, et al. SARS-CoV-2 omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. December 17, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.08.21267417v3). preprint.
- 29.Andrews N, Stowe J, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. December 14, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.14.21267615v1). preprint.
- 30.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021;398:2258-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N Engl J Med 2021;386:494-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasreen S, Chung H, He S, et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada. July 16, 2021. (https://www.medrxiv.org/content/10.1101/2021.06.28.21259420v2). preprint.
- 34.Fischer RJ, van Doremalen N, Adney DR, et al. ChAdOx1 nCoV-19 (AZD1222) protects Syrian hamsters against SARS-CoV-2 B.1.351 and B.1.1.7. Nat Commun 2021;12:5868-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med 2021;28:taab124-taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond M, Halfmann P, Maemura T, et al. The SARS-CoV-2 B.1.1.529 omicron virus causes attenuated infection and disease in mice and hamsters. December 29, 2021. (https://www.researchsquare.com/article/rs-1211792/v1). preprint.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.