Abstract
BACKGROUND:
The “trace call” results on Xpert® Ultra indicates extremely low TB levels and may be difficult to interpret. The prevalence of trace results among presumptive TB patients in high TB-HIV infection settings is unknown, as is the significance of divergent “trace call” result interpretations.
METHODS:
Presumptive TB patients attending a public health facility in Lusaka, Zambia, were prospectively enrolled. Participants underwent several TB investigations, including sputum smear microscopy, Ultra testing, and culture. The diagnostic accuracy of Ultra (culture-based reference) and the number of patients recommended for TB treatment was assessed according to several different interpretation criteria for “trace call” results.
RESULTS:
Among the 740 participants, 78 (10.5%) were Ultra-positive and an additional 37 (5.0%) had a “trace call” result. The prevalence of trace results did not differ according to HIV status (5.3% vs. 4.8%) or prior TB status (5.6% vs. 4.9%). Differing interpretations of trace results had modest effects on Ultra’s sensitivity (range 79.3–82.6%) and specificity (range 94.3–99.2%), but increased the number of patients recommended for treatment by up to 44.9%.
CONCLUSIONS:
Ultra trace results were common in this setting. The interpretation of trace results may substantially impact TB case yield.
Keywords: tuberculosis, diagnosis, HIV, CAD
Abstract
CONTEXTE :
La catégorie de résultats « traces » du test Xpert Ultra indique des taux de TB très faibles et peut être difficile à interpréter. La prévalence de résultats traces parmi des patients suspects de TB dans des zones à forte prévalence de TB-VIH est inconnue, tout comme la signification d’interprétations divergentes des résultats traces.
MÉTHODES :
Les patients suspects de TB consultant dans un centre de soins public de Lusaka, Zambie, ont été inclus de manière prospective. Les participants ont fait l’objet de plusieurs examens de détection de la TB, dont microscopie des frottis d’expectorations, test Xpert® Ultra et culture. La précision diagnostique du test Ultra (par rapport à la culture) et le nombre de patients recommandés pour traitement antituberculeux ont été évalués selon plusieurs critères d’interprétation des résultats traces.
RÉSULTATS :
Parmi les 740 participants, 78 (10,5%) étaient positifs au test Ultra et 37 autres participants (5.0%) avaient un résultat trace. La prévalence des résultats traces ne différait pas en fonction du statut VIH (5,3% vs. 4,8%) ou du statut tuberculeux antérieur (5,6% vs. 4,9%). Les interprétations divergentes des résultats traces avaient un effet modéré sur la sensibilité du test Ultra (écart 79,3–82,6%) et sur sa spécificité (écart 94,3–99,2%), mais elles augmentaient le nombre de patients à qui un traitement était recommandé de 44,9% maximum.
CONCLUSIONS :
Les résultats traces au test Ultra étaient fréquents. L’interprétation de ces résultats peut impacter considérablement la détection des cas de TB.
The Xpert® MTB/Rif Ultra (henceforth “Ultra”; Cepheid, Sunnyvale, CA, USA) assay was developed to address the challenges of diagnosing paucibacillary TB disease.1,2 A recent meta-analysis demonstrated an approximate 6% overall increase in sensitivity and 17% increase among culture-positive, smear-negative persons compared to Xpert® MTB/RIF (Cepheid) in general populations.3 This increase in sensitivity is at the expense of a small decrease in specificity (overall about 3%), which is largely among patients with a recent history of previous TB (e.g., treated in the last 5 years).2,4
Ultra’s increased sensitivity is largely due to the “trace call” result, a new result sub-category in addition to the previous “high, medium, low and very low” categories. It indicates the detection of minimal bacilli and presents a challenge for the clinical management of presumptive TB patients. Initial WHO guidance recommended that a “trace call” should be interpreted as a true-positive TB result (and treatment initiated) for persons living with HIV (PLHIV), children, and for extrapulmonary specimens.5 For HIV-negative presumptive TB patients with trace results, especially those previously treated for TB, caution was advised a because of Ultra’s ability to detect very low-level, non-viable and non-replicating bacilli.5 A second ‘tie-breaker’ sputum Ultra test was recommended in such circumstances but was rarely logistically feasible to perform in most high-burden settings, presenting clinicians with a dilemma as to whether anti-TB therapy should be initiated.
In 2020, the WHO updated its guidance on the interpretation of a ‘trace call’ in the case of HIV-negative persons, recommending that it be considered a true-positive result in those without a prior TB episode or a recent history of TB treatment.6 However, the potential impact of this guidance change on the diagnosis and treatment of pulmonary TB has not been well-described. Furthermore, data on the overall prevalence of trace results in high TB and HIV burden settings are limited. Therefore, this study sought:1) to evaluate the prevalence of overall “trace call” results by HIV and previous TB status, 2) to characterize trace call-positive presumptive TB patients in detail, and 3) to determine how changes in interpreting trace results affect Ultra’s diagnostic accuracy and treatment recommendations for pulmonary TB.
STUDY DESIGN AND PROCEDURES
This was a prospective, cross-sectional study nested within a large implementation research project. It was conducted between July and December 2018 at a primary healthcare TB diagnostic center that serves an informal peri-urban settlement in Lusaka, Zambia.7 Consecutive adults aged 18 years who attended the health facility for TB screening were invited to participate in the study. Each consenting participant underwent a full clinical evaluation by a qualified medical practitioner, including TB symptom screening, HIV testing, point-of-care C-reactive protein (CRP), and digital chest X-ray (CXR) with computer-aided diagnosis (CAD4TB v1.5; Delft Imaging, Hertogenbosch, The Netherlands). CAD4TB is an artificial intelligence system that provides a score between 0 and 100, with higher scores indicating a greater likelihood that an individual has TB;8 the abnormality threshold was selected using a cut-off that achieved a minimum sensitivity of 90%, as is recommended for TB triage tests, while optimizing the area under the curve against a sputum-based culture reference standard. Two spot sputum samples were collected for microbiological TB testing, venous blood was collected for the determination of CD4 count from PLHIV and fingerstick blood was used to determine CRP levels.
Ethical approvals
The study was approved by the University of Zambia Biomedical Research Ethics Committee, Lusaka, Zambia (UNZABREC Ref:012-05-17). All participants provided written informed consent in their preferred language.
Laboratory testing
All samples, including sputum and blood samples, were transported to the Centre for Infectious Disease Research in Zambia (CIDRZ) Central Laboratory, Lusaka, Zambia, within 4–5 hours of collection. Each sputum sample was decontaminated using the N-acetyl-L-cysteine/sodium hydroxide method and each decontaminated sample was then split four-way to set up 1 MGIT culture (BACTEC™ MGIT™ 960 System; BD, Franklin Lakes, NJ, USA), 1 Löwenstein-Jensen solid culture, 1 concentrated Ziehl-Neelsen (ZN) smear and an Ultra assay. Mycobacterium tuberculosis in positive cultures was identified and confirmed using the MGIT™ TBc IDtest (BD). CRP levels were determined from fingerstick blood samples using the Actim CRP Rapid Test (Medix Biochemica, Espoo, Finland); CRP testing was done at the point-of-care by a study team member according to the manufacturer’s recommendations.
Data collection procedures
Demographic and clinical characteristics were collected on paper-based case record forms and later captured electronically using customized District Health Information Software 2 (DHIS2) forms. The data were stored in a PostgreSQL server database. Specified quality level (SQL) queries were written to evaluate for data inconsistencies and or discrepancies.
Data analysis
The analysis was restricted to patients with valid culture, Ultra and smear microscopy results. CXRs were considered abnormal (and suggestive of active TB) if the CAD score was ⩾29; using our pre-defined criteria for selecting a threshold, this cut-off achieved a sensitivity and specificity of respectively 90% and 58%. Ultra was considered definitively positive for any “high”, “medium”, “low”, or “very low” M. tuberculosis detected result. Descriptive analyses were undertaken to provide an overview of participant characteristics. Patients with Ultra trace results were then stratified by HIV status and prior TB status. The diagnostic sensitivity, specificity of Ultra for TB and number of patients recommended for TB treatment were determined according to one of four Ultra “trace call” result interpretations: 1) all considered negative, 2) considered positive only among HIV-positive persons (original WHO guidance5), 3) considered positive among all HIV-positive persons and HIV-negative persons without prior TB or TB treatment in the last 5 years (updated WHO guidance6), 4) all considered positive. Data were analyzed using Stata Statistical Software v14 (Stata Corporation, College Station, TX, USA).
RESULTS
Of the 771 participants who consented to be part of the study, 763 submitted a sputum sample and 740 of these had valid results for culture, Ultra and smear microscopy and were included in the analysis (Figure). Participants had a median age of 38 years (inter-quartile range [IQR] 29.5–47.0), 58.2% were male, 43.1% were HIV-positive (median CD4 count: 285 cells/μL, IQR 165–490) and 19.3% reported having been previously treated for TB (Table 1). There were 73.4% who had a positive WHO symptom screen and 36.6% had an abnormal chest-X ray. The prevalence of TB isolated from sputum using mycobacterial culture was 12.4% (n = 92; 95%CI 10.1–15.0); 6.5% (n = 48; 95%CI 4.8–8.5) of participants had sputum smear-positive TB.
FIGURE.
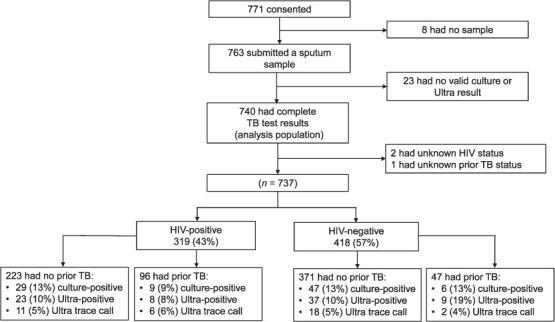
Flow diagram of participants recruited into the study.
TABLE 1.
Participant characteristics and TB diagnostic categories (n = 740)
| n (%) | |
|---|---|
| Age, years, median [IQR] | 38 [30–47] |
| Male sex | 431 (58.2) |
| HIV-positive | 319 (43.1) |
| CD4 count, cells/mm3, median [IQR]* | 285 [165–490] |
| Previous history of TB | 143 (19.3) |
| WHO Symptom screen-positive | 536 (73.4) |
| Chest X-ray abnormality | 271 (36.6) |
| Sputum TB investigations | |
| Smear-positive | 48 (6.5) |
| Culture-positive | 92 (12.4) |
| Xpert Ultra result | |
| Positive | 78 (10.5) |
| Trace positive | 37 (5.0) |
| Negative | 625 (84.5) |
*CD4 counts among HIV-positive participants.
IQR = interquartile range.
Prevalence of Ultra trace results
The prevalence of Ultra trace results was 5.0% (95%CI 3.5–6.8; n = 37); an additional 10.4% (n = 77; 95%CI 8.4–13.0) of participants had an Xpert (non-trace) positive result; therefore, trace results accounted for 32.2% (95%CI 23.8–41.5; n = 37/114) of all Ultra results in which TB was detected. There was no difference in the prevalence of Ultra trace results between HIV-positive (5.3%, 95% CI 3.1–8.4; n = 17/319) and HIV-negative (4.8%, 95%CI 2.9–7.3; n = 20/418) participants. The prevalence of Ultra trace results also did not differ between those with a history of previous TB (5.6%, 95%CI 2.4–10.7; n = 8/143) and those without previous TB (4.9%, 95%CI 3.3–6.9; n = 29/596).
Characteristics of Ultra trace call-positive participants
Detailed characteristics of the 37 participants with an Ultra trace results are shown in Table 2. The majority (75.7%) had at least one TB symptom, 56.8% had an abnormal digital CXR, 73.0% had an elevated CRP level (⩾10 mg/mL); only three participants with a trace-positive result were culture-positive (Table 2). More than half (54.0%, n = 20/37) of all Ultra trace results were among HIV-negative participants and the large majority (78.4%, n = 29/37) did not report a prior history of TB treatment. To note, 9 (24.3%) participants with an Ultra trace result did not report any symptoms of TB (2 HIV-positive and 7 HIV-negative), of which 55.6% had an abnormal CXR and 44.4% had an elevated CRP level (⩾10 mg/mL); none were culture-positive. While numbers were small, compared to HIV-negative participants, HIV-positive participants appeared to be more symptomatic and have more elevated CRP levels (Table 2). Of the 18 HIV-negative participants without previous TB and who had a trace result, 11 (61.1%) had a positive WHO symptom screen, 11 (61.1%) had an elevated CRP level (⩾10 mg/mL), 8 (44.4%) had an abnormal CXR, and 2 (11.1%) were culture-positive.
TABLE 2.
Clinical characteristics and TB investigations among participants with an Xpert Ultra “trace call” result
| All trace read results (n = 37) | HIV-positive | HIV-negative | |||
|---|---|---|---|---|---|
|
| |||||
| No prior TB (n = 11) | Prior TB (n = 6) | No prior TB (n = 18) | Prior TB (n = 2)* | ||
| Age, years, median [IQR] | 35 [26–46) | 40 [32–47] | 40.5 [31–47] | 28.5 [24–42] | 44.5 [40–49] |
| Male sex | 24 (64.0) | 7 (63.6) | 5 (83.3) | 11 (61.1) | 1 (50.0) |
| CD4 count, cells/mm3, median [IQR] | 325 [191–403] | 325 [191–403] | 287 [173–489] | — | — |
| TB symptoms | |||||
| Cough | 25 (67.6) | 9(81.0) | 5 (83.3) | 9 (50.0) | 2 (100) |
| Fever | 6 (16.2) | 3(27.3) | 1 (16.7) | 2 (11.1) | 0 |
| Night sweats | 10 (27.0) | 2 (18.2) | 3 (50.0) | 5 (27.8) | 0 |
| Weight loss | 11 (29.7) | 4 (36.4) | 4 (66.7) | 3 (16.7) | 0 |
| Chest pain | 24 (64.9) | 7 (63.6) | 4 (66.7) | 11 (61.1) | 2 (100) |
| Shortness of breath | 14 (37.8) | 5 (45.5) | 3 (50.0) | 6 (33.3) | 0 |
| Number of WHO TB symptoms† | |||||
| 0‡ | 9 (24.3) | 2 (18.2) | 7 (38.9) | ||
| 1 | 12 (32.4) | 9 (81.8) | 6 (100) | 6 (33.3) | 2 (100) |
| 2 | 5 (13.5) | 0 | 0 | 0 | 0 |
| 3 | 9 (24.3) | 0 | 0 | 4 (22.2) | 0 |
| 4 | 2 (5.4) | 0 | 0 | 1 (5.6) | 0 |
| dCXR | |||||
| Abnormal | 21 (56.8) | 6 (54.6 | 5 (83.3 | 8 (44.4) | 2 (100) |
| Normal | 13 (35.1)* | 4 (36.6) | 1 (16.8) | 8 (44.4) | |
| C-reactive protein, mg/mL | |||||
| <10 | 10 (27.0) | 1 (9.1) | 1 (16.7) | 7 (38.9) | 1 (50.0) |
| 10–40 | 7 (18.9) | 2 (18.2) | 1 (16.7) | 4 (22.2) | 0 |
| 40–80 | 5 (13.5) | 2 (18.2) | 1 (16.7) | 2 (11.1) | 0 |
| >80 | 15 (40.5) | 6 (54.5) | 3 (50.0) | 5 (27.8) | 1 (50.0) |
| Smear results | |||||
| Positive | 0 | 0 | 0 | 0 | 0 |
| Negative | 37 (100) | 11 (100) | 6 (100) | 18 (100) | 2 (100) |
| Culture results | |||||
| Positive | 3 (8.1) | 1 (9.1) | 0 | 2 (11.1) | 0 |
| Negative | 34 (91.9) | 10 (90.9) | 6 (100) | 16 (88.9) | 2 (100) |
*Participants with prior TB treated in 2016 (2 years before study entry) and 2014 (4 years before study entry). Such patients would not be recommended under current WHO guidelines6 to receive anti-TB therapy as they had been treated for TB in the previous 5 years.
†3 participants did not have a valid dCXR result available.
‡Of the 9 participants with a negative WHO symptom screen, 4 were presumptive TB patients on the basis of current chest pain and 5 were presumptive TB patients on the basis of an abnormal dCXR.
IQR = interquartile range; dCXR = digital chest X-ray.
The impact of Ultra trace call interpretation on diagnostic accuracy and treatment recommendations
Using current international guidance to interpret trace results, Ultra’s sensitivity and specificity were respectively 82.6% and 94.3%. This represented a small increase in sensitivity compared to all “trace call” results being considered negative (+3.3%) or being considered positive only for HIV-positive persons (+1.8%) (Table 3). Interpreting trace results according to current guidance would result in small decreases in specificity (range 2.5–4.9%) using a sputum culture-based reference standard (Table 3). Implementing current recommendations for interpreting Ultra trace results would have resulted in an additional 35 (+44.9%) and 18 (+18.9%) individuals being recommended for TB treatment, respectively, compared to if all trace results were considered negative or positive only for HIV-positive persons (Table 3).
TABLE 3.
The effect of Xpert Ultra trace call result interpretation on Xpert Ultra diagnostic accuracy for TB and TB treatment recommendations
| Sensitivity | Specificity | Number recommended for TB therapy based on Ultra result (yield) | |||
|---|---|---|---|---|---|
|
|
|
||||
| n/N | % (95% CI) | n/N | % (95% CI) | ||
| Ultra Trace call result considered negative (all considered negative) | |||||
| All | 73/92 | 79.3 (69.6–87.1) | 643/648 | 99.2 (98.2–99.7) | 78 |
| Smear-positive | 45/46 | 97.8 (88.5–99.9) | 1/2 | 50.0 (1.26–98.7) | 46 |
| Smear-negative | 28/46 | 60.9 (45.4–74.9) | 641/645 | 99.4 (98.4–99.8) | 32 |
| Ultra Trace call result considered positive (according to original WHO guidance)* | |||||
| All | 74/92 | 80.8 (70.9–88.0) | 627/648 | 96.8 (95.1–98.0) | 95 |
| Smear-positive | 45/46 | 97.8 (86.0–99.5) | 1/2 | 50.0 (1.3–98.7) | 46 |
| Smear-negative | 29/46 | 63.0 (47.5–76.8) | 625/645 | 96.9 (95.3–98.1) | 49 |
| Ultra Trace call result considered positive (according to current WHO guidance)† | |||||
| All | 76/92 | 82.6 (73.3–89.7) | 611/648 | 94.3 (92.2–95.9) | 113 |
| Smear-positive | 45/46 | 97.8 (88.5–99.9) | 1/2 | 50.0 (1.3–98.7) | 46 |
| Smear-negative | 31/46 | 67.4 (52.0–80.5) | 609/645 | 94.5 (92.4–96.1) | 67 |
| Ultra Trace call result considered positive (all considered positive) | |||||
| All | 76/92 | 82.6 (73.3–89.7) | 608/647 | 94.0 (91.9–95.7) | 115 |
| Smear-positive | 45/46 | 97.9 (88.5–99.9) | 1/2 | 50.0 (1.3–98.7) | 46 |
| Smear-negative | 31/46 | 67.4 (52.0–80.5) | 607/645 | 94.1 (92.0–95.8) | 69 |
*Trace call result considered a true positive result for HIV-positive persons and a false-positive result for HIV-negative persons.
†Trace call result considered a true-positive result for HIV-positive persons and HIV-negative persons not previously treated for TB and a false-positive result for HIV-negative persons with a prior history of TB.
CI = confidence interval.
DISCUSSION
In this study, we carefully evaluated the prevalence of Ultra trace results among presumptive TB patients in a high TB-HIV burden setting. We found that an important minority (5%) of all presumptive TB patients had an Ultra trace result and that notably, the prevalence of trace results did not differ according to HIV status or whether or not a patient had a previous TB history. Furthermore, we found that while differential interpretation of trace results had modest effects on Ultra’s overall diagnostic accuracy, there are potentially large impacts on the overall number and relative yield of TB diagnoses and the number of presumptive TB patients who are recommended for TB treatment. As Ultra trace results present a challenge for clinical management of presumptive TB patients in high-burden settings due to the risk of overdiagnosis – particularly in previous treated individuals – our results have important implications.
Ultra trace results were common, accounting for nearly one-third (32%) of all Ultra-positive results in our study. This finding is somewhat higher than previous studies, which have reported a range between 3% and 30%.1,2,9–14 It was notable that only about 22% (n = 8/37) of presumptive TB patients with a “trace call” result in our study did not report a prior history of TB treatment. While this measure may be subject to some reporting bias (e.g., due to TB stigma), this is a substantially lower proportion than most previous studies; however, data on this are limited. For example, two studies from South Africa and a multi-country study in the United States found that 46% (n = 6/13), 35% (n = 15/43), 100% (n = 21/21), 58% (n = 11/19) had a prior TB history;2,11 only another small study from South Africa found that 20% (n = 1/5) of patients with a “trace call” result had a previous TB history.
WHO guidance regarding Ultra “trace calls” now includes strong consideration for HIV-negative adults without a prior or recent TB history (last 5 years) to be considered true-positives.6 This builds upon original recommendations that suggested that trace call results be considered positive in HIV-positive persons, children and extrapulmonary specimens.5 We found that 49% of all trace results were among HIV-negative adults without a previous or recent TB history. Such patients tended to have at least one classic TB symptom (61%), abnormal chest radiography (44%), and elevated CRP levels (61%); two were culture-positive. While Ultra trace results may represent transient colonization among persons without prior TB, our results suggest that a majority of such patients likely had either early or paucibacillary disease. As symptoms-based screening failed to detect nearly 40% of presumptive TB patients with Ultra trace results, additional screening and triage tools such as digital CXRs with automated AI reading and/or point-of-care CRP assays may help detect more TB cases and identify them sooner. Implementation of the updated WHO guidance6 on interpretation of Ultra trace calls from prior guidance would have resulted in a nearly 20% increase (18 additional cases) in the number of patients with TB diagnosed and recommended for treatment, while not significantly reducing diagnostic specificity. Collectively our results provide support for updated WHO guidance that recommend Ultra trace results among HIV-negative persons be considered positive, as prior guidance may be associated with substantial underdiagnoses of TB.6 However, there remains an important need for further research to guide optimized management of presumptive TB patients with “trace call” Ultra results.
Study strengths were the inclusion of many well-characterized, presumptive TB patients enrolled from within routine programmatic settings. Despite a relatively high prevalence of Ultra trace results, and one of the largest reports of Ultra trace results to date,3 the absolute number was relatively small, which limited subgroup analyses by HIV and previous TB status. Repeat sputum culture testing was not available to further help differentiate true-from false-positive results among those with a Ultra trace result. Furthermore, systematic extrapulmonary sampling was not used, possibly resulting in diagnostic misclassification, including underestimation of Ultra specificity when incorporating “trace call” results. Finally, as these results are drawn from a single, busy ambulatory public health setting in Zambia, and the point estimates of the prevalence of “trace call” results may not be generalizable to other settings in the region.
CONCLUSION
Xpert Ultra trace call results, which indicate a minimal TB burden, are commonly encountered in high TB-HIV burden settings and present new clinical challenges in interpretation, especially among previously treated patients. The interpretation of Ultra trace results may have minimal effects on diagnostic accuracy, but a significant impact on the number of TB patients diagnosed and treated for TB. Where available, adjunct investigations such as CXRs and CRP levels may help to guide optimized management of presumptive TB patients with Ultra trace call results; however, further research is needed.
ACKNOWLEDGMENTS
The authors thank CIDRZ Staff (J Bwalya for his role in data entry, K Siame and P Chisenga for their role in participant enrollment and data collection); and Zambian Ministry of Health staff (B Banda, M Tembo, N Nkumbwizya, B Nchimunya, C Mumba, A Chinkondya, M Chansa, and W Njeleka for working closely with the project staff).
Support from the Stop TB Partnership’s TB REACH Initiative with funding from the Government of Canada made this work possible. The funders played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest: none declared.
References
- 1.Berhanu RH, et al. Performance of Xpert MTB/RIF, Xpert Ultra, and Abbott RealTime MTB for diagnosis of pulmonary tuberculosis in a high-HIV-burden setting. J Clin Microbiol. 2018;56(12):e00560–18. doi: 10.1128/JCM.00560-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorman SE, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(1):76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zifodya JS, et al. Cochrane Database Syst Rev. 2. 2021. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis; p. CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organisation WHO Meeting Report of a Technical Expert-Consultation: Non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 5.Stop TB Partnership Technical information note Xpert® MTB/RIF and Ultra. Geneva, Switzerland: Stop TB Partnership; 2019. http://www.stoptb.org/assets/documents/gdf/drugsupply/Xpert_info_note.pdf . [Google Scholar]
- 6.World Health Organization, WHO Guidelines Approved by the Guidelines Review Committee WHO consolidated guidelines on tuberculosis: Module 3: diagnosis – rapid diagnostics for tuberculosis detection. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 7.Kagujje M, et al. Active TB case finding in a high burden setting; comparison of community and facility-based strategies in Lusaka, Zambia. PLoS One. 2020;15(9):e0237931. doi: 10.1371/journal.pone.0237931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Geneva, Switzerland: WHO; 2021. WHO consolidated guidelines on tuberculosis. Module 2: screening: systematic screening for tuberculosis disease. Web Annex C: GRADE evidence to decision tables. https://apps.who.int/iris/bitstream/handle/10665/340243/9789240022713-eng.pdf . [PubMed] [Google Scholar]
- 9.Chang K, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012;64(6):580–588. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Chakravorty S, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio. 2017;8(4) doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra H, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF for diagnosis of tuberculosis in an HIV-endemic setting with a high burden of previous tuberculosis: a two-cohort diagnostic accuracy study. Lancet Respir Med. 2020;8(4):368–382. doi: 10.1016/S2213-2600(19)30370-4. [DOI] [PubMed] [Google Scholar]
- 12.Opota O, et al. Added value of Xpert MTB/RIF Ultra for diagnosis of pulmonary tuberculosis in a low-prevalence setting. J Clin Microbiol. 2019;57(2):e01717–18. doi: 10.1128/JCM.01717-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira GR, et al. Evaluation of Xpert MTB/RIF Ultra performance for pulmonary tuberculosis (TB) diagnosis in a city with high TB incidence in Brazil. Respir Med. 2020;162:105876. doi: 10.1016/j.rmed.2020.105876. [DOI] [PubMed] [Google Scholar]
- 14.Piersimoni C, et al. Comparative evaluation of Xpert MTB/RIF and the new Xpert MTB/RIF ultra with respiratory and extra-pulmonary specimens for tuberculosis case detection in a low incidence setting. J Clin Tuberc Other Mycobact Dis. 2019;15:100094. doi: 10.1016/j.jctube.2019.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]


