Abstract
Thoracic ultrasound is an appealing alternative to chest radiography for the diagnosis of TB. Based on research experience conducting thoracic ultrasound for adults and children in South Africa, three key considerations for potential scale-up were identified. First, thoracic ultrasound requires a comprehensive training programme for novice users; artificial intelligence may be used to simplify training and interpretation. Second, a robust ultrasound device is needed with good subpleural resolution and a probe suitable for children. Third, comprehensive scanning of the lungs is time-intensive, and shorter scanning protocols may be more feasible in clinical practice.
Keywords: tuberculosis, thoracic ultrasound, user experience
Abstract
L’échographie thoracique est une alternative attrayante à la radiographie pulmonaire pour le diagnostic de la TB. En prenant appui sur l’expérience acquise lors d’études ayant utilisé l’échographie thoracique chez l’adulte et l’enfant en Afrique du Sud, trois considérations clés pour une éventuelle utilisation accrue de cet outil ont été identifiées. Premièrement, tout nouvel utilisateur d’un échographe thoracique doit suivre un programme de formation exhaustif. L’intelligence artificielle pourrait être utilisée pour simplifier la formation et l’interprétation des résultats. Deuxièmement, un échographe de qualité est nécessaire, avec une bonne résolution sous-pleurale et une sonde adaptée à l’enfant. Troisièmement, une scannographie exhaustive des poumons est chronophage ; des protocoles de scannographie plus courts pourraient être plus faciles en pratique clinique.
TB is the leading cause of death due to a single infectious agent globally.1 Chest radiography is the first-line imaging modality for the diagnosis of TB,2 although there are often barriers to access in TB-endemic settings. Thoracic ultrasound detects peripheral lesions in patients with pulmonary TB such as consolidations and small subpleural consolidations (SPC);3–5 and although it may not be able to detect some centrally located features of TB, it could be an appealing alternative to chest radiography (CXR) for TB screening and diagnosis. Ultrasound is portable, safe, and has promising performance characteristics compared to CXR for diagnosis of other pulmonary pathologies such as pneumonia.6,7 Data on its diagnostic accuracy for TB are still limited.3–5 Furthermore, the recent availability of affordable, hand-held, chip-based devices has the potential to improve access to this rapidly evolving technology.
Based on our experience in performing thoracic ultrasounds for adult and paediatric study participants in Durban and Cape Town, South Africa, we describe challenges encountered and lessons learned, which may assist clinicians, researchers and device developers who are interested in the potential use of ultrasound for TB diagnosis.
Ethical approval was obtained for the adult study from the Biomedical Research Ethics Committee, University of KwaZulu-Natal, Durban, South Africa, and the London School of Hygiene & Tropical Medicine, London, UK; and from the Stellenbosch University Human Research Ethics Committee, Tygerberg, South Africa, for the paediatric study.
OPERATOR TRAINING
Ultrasounds in the adult study were performed by a senior board-certified radiologist (RJ) with substantial prior ultrasound experience, but not previously trained in lung ultrasound, and three pulmonologists (PM, MM, DK) with minimal prior ultrasound experience. In the paediatric study, ultrasounds were performed by a senior board-certified radiologist (RP) with substantial general, but not lung, ultrasound experience, a paediatrician (AR) with some prior ultrasound experience and a trained sonographer with no specific lung ultrasound experience. All study sonographers underwent similar lung ultrasound training. Our training programme consisted of a combination of hands-on bedside training, which focused on scan technique, as well as image review (which could be done virtually) centred on interpretation. Training was tailored to each sonographer’s level of experience, ranging from several hours for experienced sonographers to several days for more novice operators.
In general, users reported ease of use for image acquisition, but more challenges with the interpretation of findings, particularly for subtle pleural-based pathology. An operator’s ability to detect a lesion generally improved with their level of experience. We found that operator training was time-intensive, especially for novice operators and required on-site presence of a skilled trainer over an extended period of time for most sonographers, to ensure independent proficiency. Early quality assurance feedback was important to ensure consistency across operators, and clear codification of findings was required for the research process.
Device selection and scanning technique
Both studies were proof-of-principle studies that sought to perform comprehensive ultrasound examinations with the best possible devices in order to assess, in principle, whether ultrasound could detect TB. Each began with a pilot phase, scanning all participants with both a standard ultrasound (GE Logiq P9, Boston, MA, USA for adults; Canon Aplio 400, Tokyo, Japan, for paediatrics) and a novel, hand-held, chip-based device (ButterflyIQ, Butterfly Network, Guilford, CT, USA). The pilot phase was designed to determine if the hand-held device performed well enough to be used alone for the rest of the study. Standard ultrasound was set to “abdominal” pre-set; tissue harmonics were turned off and depth was initially set to 11 cm in adults and 3.5 cm in paediatrics. The hand-held device was set to “lung” or “paediatric lung” pre-set according to patient age, which included pre-set depths of 8 cm and 4 cm, respectively.
The scanning technique chosen for this work was similar in both study populations and based on previously described techniques.3,4 It involved comprehensive, systematic interrogation of each intercostal space, from apices to diaphragm, in longitudinal and transverse planes for adults and transverse only for paediatrics. The chest was divided into a total of 18 regions (for each lung, upper, middle and lower regions anteriorly, laterally and posteriorly) per participant, and representative images were saved from each region for review by a masked expert.
STANDARD VS. NOVEL HAND-HELD ULTRASOUND
Standard ultrasound provided acceptable resolution in both adult and paediatric patients, and was taken as the reference standard for our informal comparison with the novel hand-held device during the pilot phase.
Adult experiences
According to our a priori-defined scanning protocol, the novel device provided suboptimal resolution for subtle, pleural-based pathology such as SPC and pleural line irregularities when used in adults at a standardised scanning depth of 11 cm (Figure 1). This was especially important because SPCs were considered important findings for TB based on previous studies.3–5 Reducing depth improved resolution at the pleural line but overlooked deeper structures in adults, making it impractical. Other pre-sets on the novel device were tried; however, these did not significantly improve resolution. The device was not able to consistently complete our lengthy scan protocol and would occasionally freeze before completing the examination. In addition, the process of labelling images on the novel device was time-consuming. Given these limitations of the novel device in adults, we chose to continue using the standard device as the primary imaging tool in adults and to use the novel device only for “spot” imaging.
FIGURE 1.
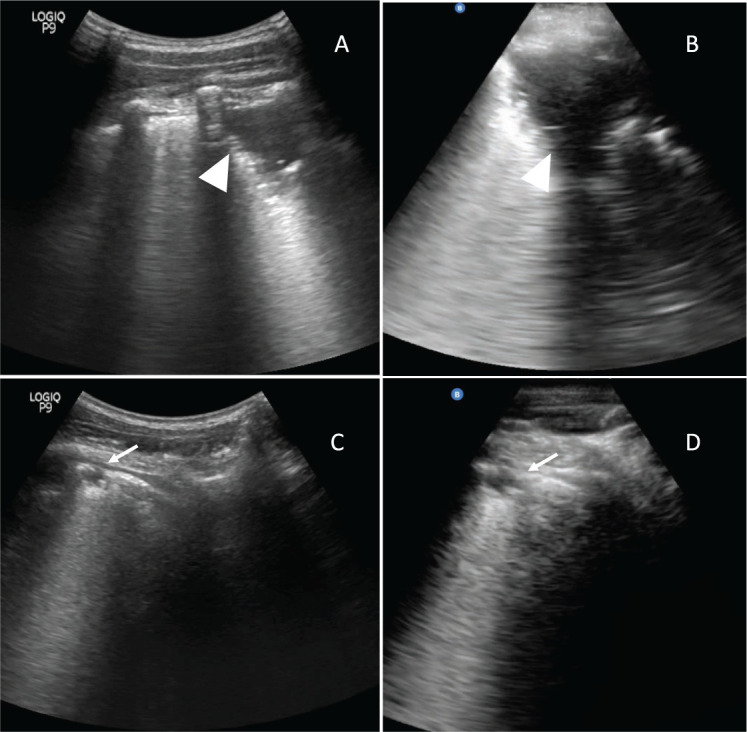
Comparison of standard and novel ultrasound devices in study participants. Large consolidation (arrowhead), characterised by subpleural echo poor or tissue-like region ⩾1 cm in depth or length, seen on standard device (Panel A) is adequately imaged on novel device (Panel B). More subtle, small subpleural consolidation (thin arrow), characterised by hypoechoic subpleural region less than 1 cm x 1 cm, with distinct borders and posterior trailing artifact, seen on standard device (Panel C) is sub-optimally imaged on novel device (Panel D).
Paediatric experiences
In the paediatric study, the novel device provided adequate resolution for lung pathology compared to the standard ultrasound, including excellent pleural and subpleural detail. However, the novel device probe was too large to perform suprasternal notch scanning (Figure 2), which is the most important view to evaluate for potential mediastinal lymphadenopathy, a key radiological finding in paediatric TB. Despite this significant limitation, the paediatric study continued with the novel device as the primary imaging tool even after the pilot phase, as the pulmonary imaging was of acceptable quality. One possible explanation for the superior image quality in children compared to adults is that the shallower scanning depth used in paediatrics allowed for higher image resolution.
FIGURE 2.
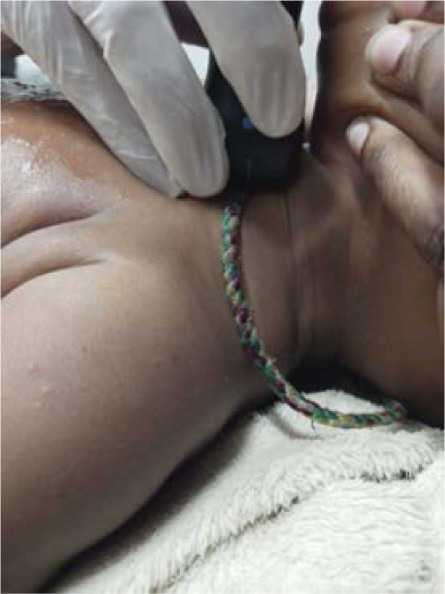
Example of suprasternal notch imaging in paediatric patient.
EXAMINATION TIME
A comprehensive scanning technique was used with a view to optimising sensitivity in these proof-of-principle studies. The median thoracic scan time was respectively 32 min (interquartile range [IQR] 27–40) and 20 (IQR 15–27) min in adult and paediatric patients, although there was considerable variation. This recorded time typically included all image labelling, archiving of images and simultaneous case report form completion by a second investigator according to a rigorous research protocol. Examination time is thus likely to be shorter in clinical practice. Longer scan times were noted for patients with more extensive pathology.
DISCUSSION
Our experiences reveal three main considerations related to the use of thoracic ultrasound in the TB diagnostic pathway: training, device characteristics and scanning technique.
First, thoracic ultrasound requires a comprehensive training programme for novice users. Effective training is an iterative process, and early feedback is essential to ensure there is consistency in regard to lesion identification and classification. This could be a significant barrier to broader implementation of thoracic ultrasound. There may be potential for artificial intelligence (AI) to assist with shortening the time needed to train operators in both image acquisition and interpretation. A finding such as a subtle SPC that flashes quickly across the screen may be more easily detected by a well-trained AI algorithm than by a novice sonographer with basic training. Thoracic ultrasound AI algorithms have already been developed to detect pneumonia8 and B-lines (sonographic signs of interstitial oedema), as well as to guide operators in cardiac imaging probe placement,9 and the field is rapidly evolving.
Second, the novel, chip-based, hand-held device may need software and hardware adaptations for adults to optimise resolution for pleural-based pathology, which may be particularly important in TB diagnosis. Additionally, in the specific context of TB diagnosis, a smaller probe footprint is needed to allow assessment for mediastinal lymphadenopathy in children.
Third, the comprehensive scanning protocols of the lungs used in these research studies was time-intensive. Shorter scanning protocols may be more feasible in clinical practice. For example, a previously described six-zone, 12-view scan technique,10 which involves sliding from apices to diaphragm in anterior, lateral and posterior zones, could be adapted for TB by including additional views at the anterior and posterior apices, which are frequently affected by TB. Shorter scanning protocols should be evaluated in future studies to determine if acceptable sensitivity can be maintained with shorter scan times.
Strengths of this report include incorporation of experiences from both adult and paediatric populations, from a TB-endemic region, and across diverse ultrasound operators and devices. Limitations include heterogeneity in implementation practices between adults and paediatrics, and non-systematic assessment of operator experiences. Future studies of ultrasound for TB diagnosis should ideally include systematic evaluations of user experiences to guide best practices.
CONCLUSION
Thoracic ultrasound may have a potential role in screening and diagnosis of TB; if it proves to be a useful tool, implementation challenges will need to be addressed before it can be deployed effectively. Key issues to be addressed in the future include device design and selection, optimal scan protocol, the need for operator training, and the potential role for AI to simplify training and interpretation.
ACKNOWLEDGEMENTS
The authors wish to thank the people who consented to participate in this research; the team at Stellenbosch University (Tygerberg, South Africa); and the team at Africa Health Research Institute, including A Edwards, D Ramjit, D Gareta, N Dayi, N Ngcobo, Z Mhlane, F Karim and N Myeza.
In addition, we acknowledge support from the Spanish Ministry of Science and Innovation and State Research Agency through the “Centro de Excelencia Severo Ochoa 2019–2023” Programme (CEX2018-000806-S), and support from the Generalitat de Catalunya through the Centres de Recerca de Catalunya Programme. ELV is supported by a Spanish Paediatrics Association (AEP) fellowship and a Ramon Areces Foundation Fellowship.
This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation, Seattle, WA, USA (Grant Numbers OPP1212544 and OPP1212276). Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
Data Availability: This paper describes the experience of training users and performing thoracic ultrasound. The data supporting the findings of this study are available in the article
References
- 1.World Health Organization Global tuberculosis report, 2020. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 2.World Health Organization Systematic screening for active tuberculosis principles and recommendations. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 3.Fentress M, et al. Lung ultrasound findings compared with chest X-ray findings in known pulmonary tuberculosis patients: a cross-sectional study in Lima, Peru. Am J Trop Med Hyg. 2020;103(5):1827–1833. doi: 10.4269/ajtmh.20-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montuori M, et al. Lung ultrasonography in pulmonary tuberculosis: a pilot study on diagnostic accuracy in a high-risk population. Eur J Intern Med. 2019;66:29–34. doi: 10.1016/j.ejim.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Bigio J, et al. Diagnostic accuracy of point-of-care ultrasound for pulmonary tuberculosis: a systematic review. PLoS One. 2021 May 5;16:e0251236. doi: 10.1371/journal.pone.0251236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez MA, et al. Lung ultrasound for the diagnosis of pneumonia in adults: A systematic review and meta-analysis. Respir Res. 2014;15(1):50. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereda MA, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135(4):714–722. doi: 10.1542/peds.2014-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa M, et al. Automatic classification of pediatric pneumonia based on lung ultrasound pattern recognition. PLoS One. 2018;13(12):e0206410. doi: 10.1371/journal.pone.0206410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voelker R. Cardiac ultrasound uses artificial intelligence to produce images. JAMA. 2020;323(11):1034. doi: 10.1001/jama.2020.2547. [DOI] [PubMed] [Google Scholar]
- 10.Fentress M, et al. A Lung ultrasound scanning technique for children and adults in low-resource settings: preliminary experiences in sub-Saharan Africa. Am J Trop Med Hyg. 2021;105(5):1148–1151. doi: 10.4269/ajtmh.20-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]


