Abstract
BACKGROUND:
The level of antibiotic resistance of pathogens causing uncomplicated urinary tract infections (UTIs) is increasing. The 2017–2018 GLASS (Global Antimicrobial Resistance and Use Surveillance System) report indicated >70% resistance to ceftriaxone and ciprofloxacin in Escherichia coli in Pakistan.
METHODS:
A prospective study was conducted in the Médecins Sans Frontières (MSF) supported Timurgara District Hospital, Timurgara, Pakistan, from September 2017 to December 2018. Women aged 18–65 years presenting to the Emergency Department with symptoms of uncomplicated UTI (cystitis/pyelonephritis) were invited to participate. We conducted microbiological culture and sensitivity testing for samples with positive dipstick or nitrite test.
RESULTS:
Of the 200 patients who participated, 109 (54.5%) were diagnosed with pyelonephritis and 91 (45.5%) with cystitis. Forty-three samples (21.5%) were culture-positive: E. coli was isolated in 27 samples, Enterococcus spp. in 7 and Klebsiella pneumoniae in 6. Overall resistance to ciprofloxacin was observed in 51.8% of E. coli isolates, and ceftriaxone resistance in 66.7% of E. coli isolates and in 33.3% of K. pneumoniae. Resistance to fosfomycin was low (one E. coli isolate).
CONCLUSIONS:
This study found resistance to first- and second-line antibiotics for treating UTIs as per the MSF protocol. Heightened awareness and potential changes to local prescription practices are necessary to curb the spread of antimicrobial resistance pathogens causing UTIs.
Keywords: antibiotic resistance, Pakistan, urinary tract infection, Escherichia coli, developing country, emergency department
Abstract
OBJECTIF :
Le taux de résistance aux antibiotiques des pathogènes responsables d’infections urinaires non compliquées (UTI) est en hausse. Le rapport GLASS (Global Antimicrobial Resistance and Use Surveillance System) 2017–2018 a indiqué un taux de résistance >70% à la ceftriaxone et à la ciprofloxacine chez Escherichia coli (Pakistan).
MÉTHODES :
Une étude prospective a été réalisée dans l’hôpital du district de Timurgara géré par Médecins Sans Frontières (MSF), de septembre 2017 à décembre 2018. Les femmes de 18–65 ans consultant aux Urgences avec des symptômes d’UTI non compliquée (cystite/pyélonéphrite) ont été invitées à participer. Nous avons réalisé une culture microbiologique et un test de sensibilité pour les échantillons positifs à la bandelette urinaire et au test de détection des nitrites.
RÉSULTATS :
Deux cents patients ont participé, dont 109 (54,5%) avaient un diagnostic de pyélonéphrite et 91 (45,5%) un diagnostic de cystite. Quarante-trois échantillons (21,5%) étaient positifs à la culture ; E. coli a été isolé de 27 échantillons, Enterococcus spp. de sept échantillons et Klebsiella pneumoniae de six échantillons. Une résistance à la ciprofloxacine a été observée chez 51,8% des isolats de E. coli, et une résistance à la ceftriaxone chez 66,7% des isolats de E. coli et chez 33,3% des isolats de K. pneumoniae. La résistance à la fosfomycine était faible (un isolat de E. coli).
CONCLUSIONS :
Cette étude a rapporté une résistance aux antibiotiques de première et deuxième intention utilisés dans le traitement des UTI, conformément au protocole de MSF. Une sensibilisation accrue et un éventuel changement des pratiques locales de prescription sont nécessaires pour freiner la propagation des pathogènes responsables d’UTI résistants aux antimicrobiens.
Urinary tract infections (UTIs) are one of the most prevalent infectious disorders affecting people and the second most common reason for antibiotic prescriptions, after respiratory tract infections.1,2
The two main uncomplicated UTIs are acute uncomplicated cystitis (AUC) and acute uncomplicated pyelonephritis (AUP).2 AUC is an infection of the bladder and urethra that affects mainly women and girls from 2 years of age and pyelonephritis is an infection of the renal parenchyma. Escherichia coli is the most common causative pathogen of both diseases and accounts for approximately 85% of community-acquired UTIs and 50% of hospital-acquired UTIs.3–6 Other pathogens include Proteus mirabilis, Enterococcus spp, Klebsiella spp, and in young women, Staphylococcus saprophyticus.
There have been recent reports of an increasing level of antibiotic resistance in pathogens causing uncomplicated UTIs.2,7,8 The 2017–2018 WHO GLASS (Global Antimicrobial Resistance and Use Surveillance System) report indicates >70% resistance to ceftriaxone (CRO) and ciprofloxacin (CFX) in E. coli isolated from UTIs in Pakistan;7 a previous study on UTI pathogens in Karachi found that 49% of Gram-positive and 57% of Gram-negative bacteria were resistant to CFX.9
The aim of the present study was to identify the bacterial aetiology of uncomplicated UTIs and determine resistance patterns of the causative pathogens in a large district hospital in Pakistan.
METHODS
Study location
In August 2008, Médecins Sans Frontières Operational Centre Brussels (MSF OCB) began working in the Khyber Pakhtunkhwa Province, Pakistan, in response to the internally displaced people (IDP) crisis following military interventions. The Lower Dir District is one of the 35 districts in the province; located on the banks of the Panjkora River, Timurgara City is the district headquarter of the district. MSF has been collaborating with Pakistan’s Ministry of Health (MoH) since 2009 to support the Timurgara District Headquarters Hospital in Timurgara, which provides comprehensive emergency and obstetric care.
Study design, population and timing
This was a prospective observational study of women aged 18–65 years presenting with symptoms of uncomplicated UTIs (AUC and/or AUP) to the Emergency Department of the Timurgara District Hospital. Patients were excluded if they were pregnant, had had more than three episodes of UTI in the past 12 months, or were being treated for urinary tract anomalies or other complicating factors.4,10 In this study, the term “samples” refers to urine samples and “isolates” refers to the number of bacteria.
Patients who received a clinical diagnosis of un-complicated UTI (AUC; infection of the lower urinary tract or AUP; infection of the upper urinary tract) were invited to participate in the study. Patients were treated for AUC or AUP according to routine MSF clinical guidelines whether or not they agreed to participate.4 Participants were recruited from September 2017 to December 2018.
Case definitions and treatments
As per the MSF Guidelines,4 an acute uncomplicated UTI is classified as uncomplicated cystitis (AUC) or pyelonephritis (AUP), and defined where there are no
Pakistan; urinary tract infection; Escherichia coli; developing country; emergency department functional or anatomical anomalies in the urinary tract, no renal functional impairment, and no concomitant disease that would promote UTI.4,10 The clinical features of AUC include dysuria (burning pain on urination) and pollakiuria (passing of small quantities of urine more frequently than normal) with no fever (or mild fever) and no flank pain. The symptom of dysuria alone is insufficient to make a diagnosis.4 The clinical features of AUP include the signs of cystitis, a fever >38.5°C and flank pain (often unilateral) or abdominal tenderness.4 Treatment was decided according to MSF guidelines. More details on the treatment are available in the Supplementary Data.
Sample collection and laboratory testing
Urine samples were provided by patients for biochemical analysis using a dipstick (Analyticon Biotechnologies, Lichtenfels, Germany) as per routine clinical protocol.4 Samples with a result of ⩾25 leukocytes/μL and/or a positive nitrite test were sent to the Aga Khan Laboratory in Karachi, Pakistan, for microbiological culture and sensitivity testing. Samples were inoculated on cysteine lactose electrolyte deficient (CLED) media using 1 μl loop and incubated at 37°C in ambient air for 24 h. Culture plates were read and interpreted by the Senior Technologist. Single colony-forming units (CFU) were equivalent to 1,000 colonies, with colony counts of 104–105 and >105 considered as significant bacteriuria. The Kirby-Bauer method for disk diffusion and CLSI (Clinical and Laboratory Standards Institute) breakpoints were used to determine antibiotic sensitivity patterns.11
Ethical approval
This study was approved by the MSF Ethical Review Board, Paris, France (ID:1759) and the National Bioethics Committee Pakistan, Islamabad, Pakistan (ID: NBC-274). Patients received an informed consent form in two languages (Pashtun and English) and provided written informed consent.
RESULTS
Study population
Two hundred samples were collected over the 15-month enrolment period. The mean age of women participating in the study was 32.1 years; 45.5% were diagnosed with cystitis and 54.5% with pyelonephritis. Of the 200 urine samples collected in this study, 43 (21.5%) samples had detectable bacteriuria (“positive urine sample”). Of the 43 patients with a positive urine, 34 (76.7%) patients had experienced more than one UTI episode in the previous 12 months. Of these patients, seven had experienced three episodes of UTI in the past 12 months (21.2%). Seventeen patients (39%) with a positive urine had received at least one previous antibiotic treatment in the last 3 months (Table 1).
TABLE 1.
Patient characteristics of study participants, Timurgara District Hospital, Timurgara, Pakistan, September 2017–December 2018
| Overall (n = 200) n (%) | Negative urine samples (n = 157) n (%) | Positive urine samples (n = 43) n (%) | P value* | |
|---|---|---|---|---|
| Age, years, mean ± SD | 32.1 ± 11.1 | 31.6 ± 10.9 | 34.1 ± 11.7 | 0.2 |
| Hospitalisation | 9 (4.5%) | 7 (4.5%) | 2 (4.7%) | >0.9 |
| UTI episodes <12 months | 0.10 | |||
| 0 | 54 (27%) | 45 (29%) | 9 (21%) | |
| 1 | 50 (25%) | 33 (21%) | 17 (40%) | |
| 2 | 57 (28%) | 47 (30%) | 10 (23%) | |
| 3 | 39 (20%) | 32 (20%) | 7 (16%) | |
| Antibiotic use last 3 months | 0.4 | |||
| 0 | 128 (64%) | 102 (65%) | 26 (60%) | |
| 1 | 50 (25%) | 40 (25%) | 10 (23%) | |
| 2 | 18 (9.0%) | 13 (8.3%) | 5 (12%) | |
| 3 | 4 (2.0%) | 2 (1.3%) | 2 (4.7%) | |
| Diagnose | 0.3 | |||
| Cystitis | 91 (45.5%) | 68 (43) | 23 (53) | |
| Pyelonephritis | 109 (54.5%) | 89 (57%) | 20 (47%) |
*Wilcoxon rank-sum test, Fisher’s exact test or Pearson’s χ2 test.
SD = standard deviation; UTI = urinary tract infection.
Drug resistance and susceptibility
All study participants were prescribed antibiotics. Fosfomycin (101/200, 50%) and CFX (91/200, 45%) were the most commonly prescribed, whereas cefixime (6/200, 3%) and CRO (2/200, 1%) were given to a minority of study participants (Table 2). Among those with positive urine samples, 14% (6/43) had an isolate that was resistant to the antibiotic they received (Table 3); one-third of CFX recipients (33%, 4/12 prescriptions) and half of those prescribed CRO (50%, 1/2 prescriptions) had an isolate that was resistant to the antibiotic they received, although the latter antibiotic was very rarely prescribed (Table 2).
TABLE 2.
First-line antibiotics prescribed empirically in the Emergency Department and the antimicrobial susceptibility of patients’ positive isolates *
| Cefixime n | Ceftriaxone n (%) | Ciprofloxacin n (%) | Fosfomycin n (%) | Total n | |
|---|---|---|---|---|---|
| Number prescribed to all study participants (n = 200) | 6 | 2 | 91 | 101 | 200 |
| Number prescribed to those with a positive sample (n = 43) | 0 | 2 (100) | 12 (13.2) | 29 (28.7) | 43 |
| Antimicrobial susceptibility | |||||
| Resistant | 0 | 1 | 3 | 1 | 5 |
| Intermediate | 0 | 0 | 1 | 0 | 1 |
| Susceptible | 0 | 1 | 8 | 28 | 37 |
*Empirical treatments are prescribed according to MSF guidelines.
MSF = Médecins Sans Frontières.
TABLE 3.
E. coli and K. pneumoniae isolates that were resistant to first-line antibiotics in the MSF treatment protocol
| Number of first-line antibiotics with resistance | E. coli (n) | K. pneumoniae (n) |
|---|---|---|
| 0 | 7 | 3 |
| 1 | 8 | 3 |
| 2 | 10 | 1 |
| 3 | 1 | 0 |
| 4 | 1 | 0 |
MSF = Médecins Sans Frontières.
Microbial isolates and antimicrobial susceptibility
From the 43 positive samples we identified 44 isolates, which corresponded to six different pathogens, with one participant having E. coli and K. pneumoniae co-infection. Most organisms detected were Gram-negative (35/44, 79.5%). Overall, the most common organisms detected were E. coli (27/44, 61.4%), followed by K. pneumoniae and Enterococci spp. which were detected in seven participants each (7/44, 15.9%). The remaining pathogens, S. aureus, Pseudomonas aeruginosa and Beta Haemolytic Streptococcus Group B, were each isolated only once (1/44, 2.3%) (Supplementary Table S1).
Gram-negative bacteria, E. coli and K. pneumoniae, microorganisms that commonly cause UTIs, were moderately resistant to several antibiotics. More than 50% of E. coli isolates were not susceptible to nalidixic acid, cotrimoxazole and CRO. In contrast, more than half of the K. pneumoniae isolates were susceptible (Figure).
FIGURE.
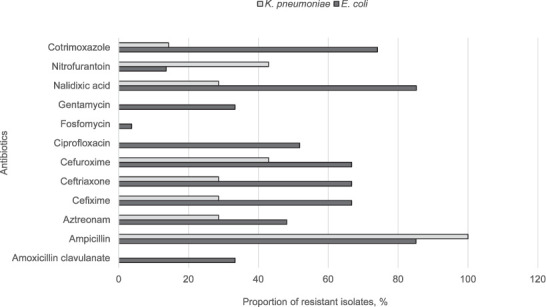
The proportion of non-susceptible isolates of Escherichia coli and Klebsiella pneumoniae isolates from study participants, Timurgara District Hospital, Timurgara, Pakistan, September 2017–December 2018. As K. pneumoniae are intrinsically resistant to ampicillin (due to chromosomal SHV-1), a 100% resistance rate was expected. For the results of the resistant isolates of Escherichia coli (n = 27), there were 5 results of nitrofuratoin missing. For all other antibiotics, the denominator was the same (K. pneumoniae, n = 7).
When examining the Gram-positive isolates, we found one isolate of Streptococcus agalactiae with no resistance to any antibiotics. Seven patients were positive for the Enterococci spp., of which 85.6% (6/7) of isolate were resistant to CFX. All the Enterococci spp. isolates were susceptible to ampicillin. One isolate of S. aureus – a methicillin-resistant S. aureus (MRSA) isolate – was detected and was tested against vancomycin (susceptible), amikacin (susceptible), gentamicin (resistant), levofloxacin (resistant), cotrimoxazole (resistant), tetracycline (susceptible), nitrofurantoin (susceptible) and oxacillin (resistant).
DISCUSSION
This study highlights the presence of resistance in urinary pathogens to first- and second-line antibiotics as per the MSF guidelines. It also indicates resistance to CFX and the presence of multidrug-resistant organisms (MDROs) in this population.
Low resistance rates to fosfomycin and nitrofurantoin are consistent with other reported results,1,12 and similar to some local reports.13–15 In contrast, 50% of isolates tested were resistant to CFX: with one-third of patients with a positive urine sample treated with CFX had a resistant pathogen. Similar levels of resistance to CFX have been reported in Pakistan.6 To note, CFX is readily available over the counter in an oral suspension throughout Pakistan.9 This high level of resistance to CFX indicates that the use of the antibiotic as a first-line treatment for UTI in Pakistan needs to be reconsidered. Guidelines recommend a single dose of 3 g fosfomycin as first-line definitive treatment for acute uncomplicated cystitis in non-pregnant females.4,14 In this study, the majority of participants did receive fosfomycin as empiric treatment; however, half of those who received fosfomycin were diagnosed with pyelonephritis. Due to its failure to achieve sufficient tissue concentrations in the kidneys, fosfomycin is not recommended as a treatment option for pyelonephritis.16
Specific extended spectrum β-lactamase (ESBL) testing was not performed by the laboratory; however, using zone of clearance interpretation for third-generation cephalosporins and monobactams, 29% of the K. pneumoniae and 65% of E. coli isolates may be ESBLs, as well as one MRSA isolate.17
The detection of ESBLs and MRSA in this study indicates that these organisms are circulating within the community. The probable high prevalence of ESBLs in this cohort casts doubt on the use of cephalosporins, and their presence should be taken into account when developing treatment guidelines for UTIs and other illnesses.
There are several limitations to this study. This was a single-site study with 200 samples from women; for a full review of empiric therapy of UTIs, a broader representation of the population with more samples should be included. There was also a potential for recall bias in the survey, as the history of UTIs and previous antibiotic prescriptions were self-reported. Also, the data indicated that MSF prescribing guidelines were not rigorously followed. The mis-treatment could be due to poor diagnostic quality and the inability to differentiate between cystitis and pyelonephritis, or a weak implementation of the guidelines. Furthermore, interpreting ESBLs based on clearance zone measurements rather than performing a specialized ESBL test limits ESBL detection within the community. A specific survey on MDROs in this population is needed.
The development and dissemination of a hospital antimicrobial stewardship program, including a local antibiogram profile, could be used as part of a strategy to improve prescribing patterns among physicians.4,11 Antimicrobial stewardship programs can take the lead in advocating for the use of antibiotics that remain active against the most prevalent UTI causative agents while simultaneously having the narrowest antibacterial range and the least adverse effect on the gut microbiota.18 It should also be noted that microbiological analysis of urine for UTIs is not a routine clinical process in this setting. The resistance to empirical treatments indicates the need for access to microbiological analysis on a broader scale.
Choosing an appropriate antimicrobial for the management of UTIs should take into consideration sex, clinical presentation, resistance prevalence in the local community, and the presence of anatomic or functional abnormalities.18 In this study, 14% of patients with bacteriuria were treated with an antibiotic to which the bacterium was resistant, which meant that medication would likely fail and that more therapy would be required. With the increase of microbial resistance, empiric therapy recommendations that do not take into account local resistance data could lead to poor treatment outcomes. For uncomplicated UTIs, first-line agents include nitrofurantoin (in patients with normal renal function) and fosfomycin (cystitis only), along with CFX and cefixime.4 However, nitrofurantoin was not available at the clinic for prescription at the time of this study.
CONCLUSION
This study highlights the presence of resistance in urinary pathogens to first- and second-line antibiotics for the treatment of UTIs as per MSF guidelines. It also indicates a high prevalence of resistance to CFX and the presence of MDROs in this population. In order to inform local prescription recommendations, a larger population-wide survey and continued surveillance should be implemented.
ACKNOWLEDGEMENTS
The authors would like to thank the nurses of Timurgara District Hospital for their assistance throughout the project: A M Khan, M Parvez, Mujeeb, A Yssel, N Ahmad and N Ur Rehman; G Benedetti for assisting with the drafting of the protocol and E Repetto for reviewing the manuscript. Conflicts of interest: none declared.
Data availability statement: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Mazzariol A, Bazaj A, Cornaglia G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: a review. J Chemother. 2017;29:2–9. doi: 10.1080/1120009X.2017.1380395. [DOI] [PubMed] [Google Scholar]
- 2.Bishop MC. Uncomplicated urinary tract infection. EAU Update Series. 2004;2:143–150. [Google Scholar]
- 3.Tandogdu Z, Wagenlehner FME. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. 2016;29:73. doi: 10.1097/QCO.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 4.Médecins Sans Frontières Clinical guidelines: diagnosis and treatment manual. Geneva, Switzerland: MSF; 2018. [Google Scholar]
- 5.Hooton TM, Gupta K. Acute simple cystitis in women. UptoDate Mar 19, 2019 https://www.uptodate.com/contents/acute-complicated-uri-nary-tract-infection-including-pyelonephritis-in-adults Accessed August 2019.
- 6.Sabir S, et al. Isolation and antibiotic susceptibility of E. coli from urinary tract infections in a tertiary care hospital. Pak J Med Sci. 2014;30:389–392. [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Global Antimicrobial Resistance Surveillance System (GLASS) report, early implementation 2017–2018. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 8.World Health Organization Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva, Switzerland: WHO; 2012. http://apps.who.int/iris/bitstream/10665/44863/1/9789241503501_eng.pdf . [Google Scholar]
- 9.Abdullah FE, et al. Increasing ciprofloxacin resistance of isolates from infected urines of a cross-section of patients in Karachi. BMC Res Notes. 2012;5:696. doi: 10.1186/1756-0500-5-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagenlehner FME, et al. Uncomplicated urinary tract infections. Dtsch Arztebl Int. 2011;108:415–423. doi: 10.3238/arztebl.2011.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing. 29th ed. Wayne, PA, USA: CLSI; 2019. [Google Scholar]
- 12.Silver LL. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med. 2017;7:a025262. doi: 10.1101/cshperspect.a025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohail M, et al. Characteristics and antibiotic resistance of urinary tract pathogens isolated from Punjab, Pakistan. Jundishapur J Microbiol. 2015;8:e19272. doi: 10.5812/jjm.19272v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain A, Sohail M, Abbas Z. Prevalence of Enterococcus faecalis mediated UTI and its current antimicrobial susceptibility pattern in Lahore, Pakistan. J Pak Med Assoc. 2016;66:1232–1236. [PubMed] [Google Scholar]
- 15.Jafri SA, et al. Antibiotic resistance of E. coli isolates from urine samples of Urinary Tract Infection (UTI) patients in Pakistan. Bioinformation. 2014;10:419–422. doi: 10.6026/97320630010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence Pyelonephritis (acute): antimicrobial prescribing. London, UK: NICE; 2018. https://www.nice.org.uk/guidance/ng111 . [Google Scholar]
- 17.Centers for Disease Control and Prevention Laboratory detection of extended-spectrum β-lactamases (ESBLs) Atlanta, GA, USA: CDC; 2010. https://www.cdc.gov/hai/settings/lab/lab_esbl.html Accessed March 2019. [Google Scholar]
- 18.Rosa R, et al. Antimicrobial resistance in urinary tract infections at a large urban ED: Factors contributing to empiric treatment failure. Am J Emerg Med. 2017;35:397–401. doi: 10.1016/j.ajem.2016.11.021. [DOI] [PubMed] [Google Scholar]


