Abstract
Background and Purpose:
Vasospasm and delayed cerebral ischemia (DCI) contribute significantly to the morbidity/mortality associated with aneurysmal subarachnoid hemorrhage (SAH). While considerable research effort has focused on preventing or reversing vasospasm, SAH-induced brain injury occurs in response to a multitude of concomitantly acting pathophysiologic mechanisms. In this regard, the pleiotropic epigenetic responses to conditioning-based therapeutics may provide an ideal SAH therapeutic strategy. We previously documented the ability of hypoxic preconditioning (PC) to attenuate vasospasm and neurological deficits after SAH, in a manner that depends on the activity of endothelial nitric oxide synthase. The present study was undertaken to elucidate whether the NAD-dependent protein deacetylase sirtuin isoform SIRT1 is an upstream mediator of hypoxic PC-induced protection, and to assess the efficacy of the SIRT1-activating polyphenol Resveratrol as a pharmacologic preconditioning therapy.
Methods:
Wild-type C57BL/6J mice were utilized in the study and subjected to normoxia or hypoxic PC. Surgical procedures included induction of SAH via endovascular perforation or sham surgery. Multiple endpoints were assessed including cerebral vasospasm, neurobehavioral deficits, SIRT1 expression via quantitative real-time PCR for mRNA, and western blot for protein quantification. Pharmacological agents utilized in the study include EX-527 (SIRT1 inhibitor), and Resveratrol (SIRT1 activator).
Results:
Hypoxic PC leads to rapid and sustained increase in cerebral SIRT1 mRNA and protein expression. SIRT1 inhibition blocks the protective effects of hypoxic PC on vasospasm and neurological deficits. Resveratrol pretreatment dose-dependently abrogates vasospasm and attenuates neurological deficits following SAH – beneficial effects that were similarly blocked by pharmacologic inhibition of SIRT1.
Conclusion:
SIRT1 mediates hypoxic preconditioning-induced protection against neurovascular dysfunction after SAH. Resveratrol mimics this neurovascular protection, at least in part, via SIRT1. Activation of SIRT1 is a promising, novel, pleiotropic therapeutic strategy to combat DCI after SAH.
Keywords: Subarachnoid hemorrhage, Delayed cerebral ischemia, Vasospasm, Resveratrol, SIRT1, Sirtuin
INTRODUCTION
Delayed cerebral ischemia (DCI) is a major cause of poor outcome after aneurysmal subarachnoid hemorrhage (SAH). DCI is a multifactorial process caused by a combination of large artery vasospasm and microcirculatory deficits including autoregulatory dysfunction, microvessel thrombosis, and inflammation (Vergouwen et al., 2010). No effective therapeutic strategies are available to prevent or attenuate DCI and improve patient outcome. To address this issue, our efforts have focused on preconditioning (PC) – a powerful and pleiotropic phenomenon by which the brain’s inherent resistance to injury can be augmented by exposure to a sub-lethal stimulus. In an earlier proof-of-concept study, we demonstrated that activation of endogenous protection via hypoxic PC is beneficial in the setting of SAH (Vellimana et al., 2011). Specifically, we found that hypoxic PC nearly completely prevented vasospasm and markedly improved neurological outcome following experimental SAH, and that this protection was mediated by endothelial nitric oxide synthase (eNOS). However, the mechanism by which hypoxic PC upregulates eNOS to induce powerful neurovascular protection in the setting of SAH has not been elucidated. Moreover, hypoxia as a conditioning stimulus may have limited therapeutic potential, and the complexity of eNOS regulation also creates challenges for therapeutic targeting (Garcia and Sessa, 2019). To overcome these limitations, it is essential to identify the hypoxia-responsive, upstream regulatory molecule that is responsible for PC-induced neurovascular protection in SAH. If identified, this protein would represent a novel molecular target that could be therapeutically exploited to potentially improve patient outcome after aneurysmal SAH.
Sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide-dependent histone deacetylase, is a hypoxia-responsive cellular energy sensor with diffuse and abundant expression in neurons, glia, and the cerebral vasculature. SIRT1 has known mechanistic links to vascular function (Donato et al., 2011; Mattagajasingh et al., 2007; Tajbakhsh and Sokoya, 2012) including a critical regulatory impact on eNOS expression and activity (Donato et al., 2011; Mattagajasingh et al., 2007; Tajbakhsh and Sokoya, 2012). We therefore hypothesized that SIRT1 could be a potential mediator of the strong eNOS-mediated neurovascular protection against SAH afforded by hypoxic PC (Vellimana et al., 2018). That SIRT1 exerts protective effects in non-hemorrhagic models of neuronal injury and neurodegeneration (Della-Morte et al., 2009; Donmez and Outeiro, 2013; Liu et al., 2016; Pallas et al., 2008; Tang, 2009), and has been implicated in the pathophysiology of early brain injury (EBI) after SAH (Zhang et al., 2016; Zhou et al., 2014), lends further support to our hypothesis. Hence, we undertook the present study to examine SIRT1 as an upstream mediator of hypoxic PC in SAH, and to test whether a pharmacologic strategy to activate SIRT1 mimics the DCI protection afforded by hypoxic PC.
METHODS
Experimental animals
All experimental protocols were approved by the Animal Studies Committee at Washington University in St Louis. Three to four-month-old male C57BL/6J mice from Jackson Laboratories (Bar Harbor, Maine) were used for the mouse experiments.
Animals were assigned to different groups using block randomization. Animals used in the experiments bear no visible indication of intervention/treatment group and therefore group allocation was concealed. Interventions, assessments and data analyses were performed by researchers in a blinded manner per our laboratory protocol.
Hypoxic Preconditioning (PC)
Experimental mice were placed in a hypoxic chamber and exposed to air containing 8% O2/92% N2 for 4 hours, as previously described (Vellimana et al., 2011). Normoxic control mice were placed in chambers containing room air (Vellimana et al., 2011). All mice were given access to food and water ad libitum during the exposure period and returned to their home cages thereafter. PC was performed for 4 hours starting 24 hours prior to surgery.
Experimental SAH
Endovascular perforation SAH was performed as described (Han et al., 2012; Milner et al., 2015; Vellimana et al., 2011). Briefly, mice were anesthetized with isoflurane (4% induction, 1.5% maintenance), and a 5–0 nylon suture was introduced into the external carotid artery (ECA) and advanced through the internal carotid artery (ICA) until the ICA bifurcation. The suture was then advanced to induce SAH, then removed and the ECA ligated. Mice in the sham surgery groups underwent all of the above procedures except for suture-perforation. Body temperature of mice was maintained during surgery using a homeothermic heating pad and during recovery from anesthesia using an incubator.
Data from animals meeting any of the following exclusion criteria were removed from all statistical analysis per our standard laboratory protocol: 1) Mortality after SAH (<10% in this study), 2) Absence of subarachnoid blood on post-surgery day 2 in SAH groups 3) Presence of blood on post-surgery day 2 in Sham groups, 4) Neurological deficits suggestive of ischemic stroke (e.g. dense hemiparesis or hemisensory deficit) within 6 hours after surgery.
Drug Treatments
EX-527 [a selective SIRT1 inhibitor (Broussy et al., 2020; Zhao et al., 2013)] (10mg/kg), or vehicle (8% DMSO in β-Cyclodextrin:PBS), was administered to mice via intraperitoneal injection one hour prior to hypoxic PC or pharmacological treatment with Resveratrol. High dose Resveratrol (20 mg/kg) or vehicle (7.5% DMSO in corn oil) was administered to mice via intraperitoneal injection in two ways: 1) Single administration, in which high-dose Resveratrol or vehicle was given 1 hour prior to surgery; or 2) Multiple administration, in which high-dose Resveratrol or vehicle was given 1 hour prior to surgery and repeated every 12 hours until the morning of post-SAH day 2. Low-dose Resveratrol (10 mg/kg – high dose) or vehicle (7.5% DMSO in corn oil) was administered to mice via intraperitoneal injection 1 hour prior to surgery and repeated every 12 hours until the morning of post-SAH day 2.
Neurobehavioral Outcome Tests
Neurobehavioral outcome following SAH was examined daily using Neuroscore as described earlier (Han et al., 2012; Milner et al., 2015; Vellimana et al., 2011). Briefly, neurological function was graded based on a motor score (0–12) that evaluated spontaneous activity, symmetry of limb movements, climbing, and balance and coordination, along with a sensory score (4–12) that evaluated body proprioception and vibrissa, visual, and tactile responses.
Vasospasm Assessment
Vasospasm assessment was performed on post-SAH day 2 via cerebrovascular casting, as described (Han et al., 2012; Milner et al., 2015; Vellimana et al., 2011). Briefly, mice were anesthetized and transcardially perfused with PBS, 4% formalin and 3% gelatin-India ink solution. Brains were removed, SAH was graded as described, and blood vessels imaged under a microscope using a CCD camera. The narrowest diameter within the first 1000 μm of the middle cerebral artery (MCA) was measured to quantify vasospasm.
Quantitative, Real-Time PCR
Quantitative PCR was performed as we described previously (Reynolds et al., 2016). Briefly, brains were harvested after normoxia or at various time points after hypoxic PC. Tissue from the left cerebral hemisphere was lysed in Trizol (Life Technologies, Carlsbad, CA) and total RNA was extracted. High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA) was used to synthesize cDNA from the isolated RNA. Previously published sense and anti-sense oligonucleotide primers for SIRT1 were utilized (Morita et al., 2012). qPCR was performed on a 7500 Real-time PCR System (Applied Biosystems, Foster City, CA) utilizing SYBR Green PCR Master Mix reagents (Applied Biosystems, Foster City, CA). GAPDH expression was used as an internal control. The delta-delta calculation method was utilized to obtain fold change relative to controls (Livak and Schmittgen, 2001).
Western Blot
Western blots were performed as we described previously (Han et al., 2012; Milner et al., 2015; Vellimana et al., 2011). Briefly, brains were harvested 48 h after sham or SAH surgery. Tissue from the hemisphere ipsilateral to endovascular perforation was lysed in a buffer containing 10mM HEPES, 5mM MgCl2, 1mM DTT, 2mM EDTA, 2mM EGTA, 1mM PMSF, 1% Triton X-100, 0.5mM sodium orthovanadate, 0.1uM okadaic acid, 25mM beta-glycerophosphate and protease inhibitor cocktail (Sigma, St. Louis, MO). The following primary antibodies were used: Rabbit anti-SIRT1 (Millipore, Burlington, MA)(Gueguen et al., 2014; Michan et al., 2014) and mouse anti-α-tubulin (Sigma, St. Louis, MO). α-tubulin was used as the loading control. Blots were subsequently incubated with anti-mouse or anti-rabbit horseradish peroxidase-conjugated IgG and visualized using an enhanced chemiluminescence kit (BioRad, Hercules, CA).
Statistical Analyses
Sample size estimation was performed a priori utilizing effect sizes based on our previous experience and/or pilot experiments for a power of 80% and significance level of 0.05. For assessments involving repeated measures on multiple days, all animals were assessed at each time point. Data are presented as the mean±SEM and were analyzed by one-way ANOVA followed by Tukey’s multiple comparison method unless otherwise indicated. For experiments involving two groups, Student’s t test was used. p<0.05 was considered as statistically significant.
RESULTS
SIRT1 expression is upregulated after hypoxic conditioning
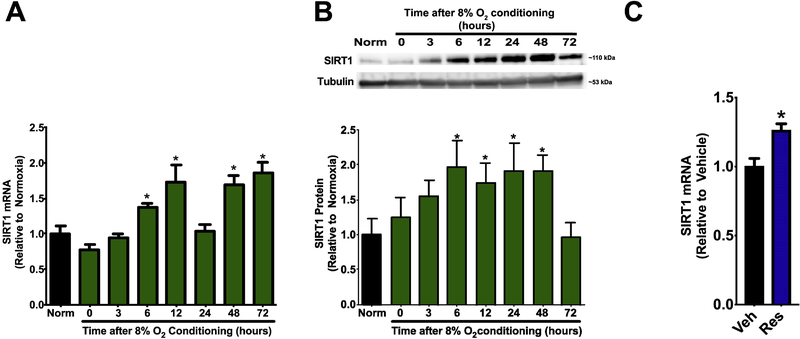
Significant increases in cerebral SIRT1 mRNA expression were measured 6, 12, 48 and 72 hours after hypoxic PC (p<0.05 vs. Normoxia) (Figure 1). In addition, significant increases in cerebral SIRT1 protein expression were observed 6, 12, 24, and 48 hours after hypoxic PC (p<0.05 vs. Normoxia) (Figure 1). This early upregulation of SIRT1 mRNA and protein expression after hypoxic PC suggests a potential role for SIRT1 as an initiator of hypoxic PC-induced protection.
Figure 1: Hypoxic conditioning and Resveratrol increase cerebral SIRT1 expression.
Wild type mice were subjected to normoxia (Norm) or hypoxic conditioning (8% oxygen for 4 hours). Brains were harvested at multiple time points. SIRT1 expression at the mRNA and protein level was assessed by qPCR (A) and Western blot (B), respectively; α-tubulin was used as a loading control. Data represent mean±SEM. *p<0.05 vs. Norm, by one-way ANOVA with Tukey multiple comparisons test. N=6 mice per group. (C) Mice were treated with Vehicle (Veh) or Resveratrol (Res, 20mg/kg, i.p.) and SIRT1 expression in the left cerebral hemisphere was assessed by qPCR 48 hours later. Data represent mean±SEM. *p<0.05 vs. Veh, by t-test. N=6 mice per group.
SIRT1 inhibition attenuates PC-induced neurovascular protection
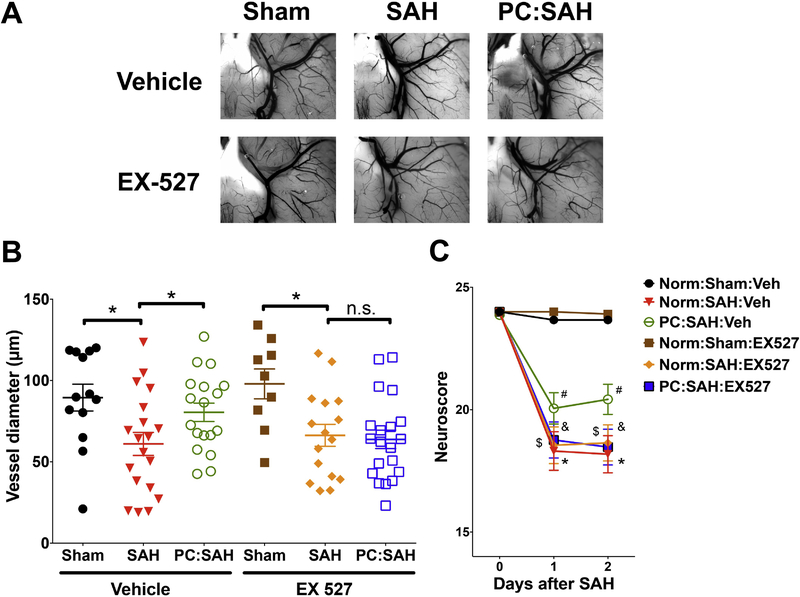
To determine if SIRT1 is necessary for hypoxic PC-induced protection, mice treated with vehicle or EX-527 (a selective SIRT1 inhibitor(Zhao et al., 2013) were subjected to normoxia or hypoxic PC followed by sham or SAH surgery. Consistent with our previous study (Vellimana et al., 2011), vehicle-treated mice with hypoxic PC were protected against both vasospasm and neurological deficits after SAH (Figure 2). In contrast, EX-527 administration blocked PC-induced protection against vasospasm and neurological deficits (Figure 2). These results suggest that SIRT1 is a critical mediator of PC-induced neurovascular protection following SAH.
Figure 2: Pharmacological inhibition of SIRT1 attenuates hypoxic preconditioning (PC)-induced neurovascular protection.
Mice treated with EX-527 (SIRT1 inhibitor) or vehicle (Veh) were subjected to Normoxia (Norm) or hypoxic PC followed by sham or endovascular perforation SAH. Neurobehavioural assessments were performed at baseline prior to SAH (Day 0) and daily for 2 days via Neuroscore test. Large artery vasospasm was assessed in the same animals on post-SAH Day 2 via measurement of left middle cerebral artery (MCA) diameter. (A) Representative images of gelatin/India ink-casted vessels used for vasospasm assessment. (B) Left MCA vessel diameter measurements. Data represent mean±SEM. *p<0.05 and n.s. p>0.05 by one-way ANOVA with Tukey multiple comparisons test. (C) Neurobehavioral assessments. Data represent mean±SEM. *, $, & p<0.05 vs Norm:Sham:Veh and #p<0.05 vs Norm:SAH:Veh by Repeated Measures ANOVA with Newman-Keuls multiple comparisons test. N= 13, 19, 18, 9, 16, and 19 mice per group for Norm:Sham:Veh, Norm:SAH:Veh, PC:SAH:Veh, Norm:Sham:EX-527, Norm:SAH:EX527, PC:SAH:EX527, respectively. Mortality = 2 mice in Norm:SAH:EX527 group.
Resveratrol increases SIRT1 mRNA expression
Consistent with prior studies (Baur et al., 2006), mice treated with Resveratrol, an activator of SIRT1 (Bai et al., 2018; Bastianetto et al., 2015; Borra et al., 2005), demonstrated a nearly 30% increase in brain SIRT1 mRNA expression at 48 hours compared to vehicle-treated mice (p<0.05, Figure 1C).
Resveratrol protects against SAH-induced neurovascular dysfunction
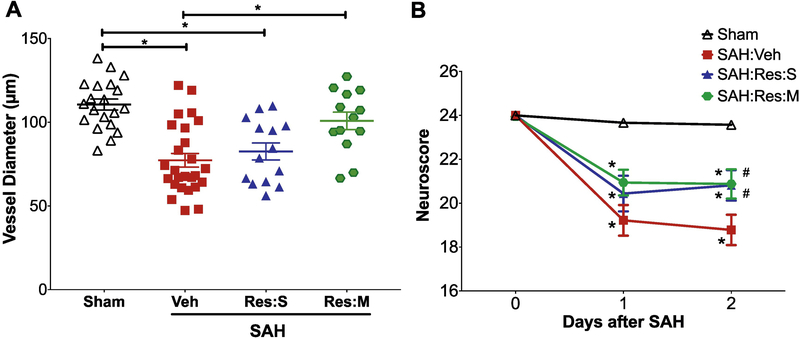
To determine if SIRT1 activation mimics hypoxic PC-induced protection against neurovascular dysfunction after SAH, mice were administrated vehicle or the SIRT1 activator Resveratrol followed by Sham or SAH surgery. We observed that a single pre-SAH administration of high dose (20mg/kg) Resveratrol (Res:S) induced a trend towards vasospasm protection and afforded significant protection against neurological deficits on post-SAH day 2 (Figure 3). Multiple administration of high dose (20mg/kg) Resveratrol (Res:M) induced a strong protection against vasospasm and neurological deficits (Figure 3). Taken together, these results indicate a dose-dependent, neurovascular protective effect of Resveratrol in the setting of SAH that may be mediated by SIRT1.
Figure 3: Resveratrol protects against SAH-induced neurovascular dysfunction.
Mice treated with vehicle (Veh), single dose Resveratrol 20mg/kg (Res:S), or multiple doses of Resveratrol 20mg/kg (Res:M) were subjected to sham surgery or endovascular perforation SAH. Large artery vasospasm was assessed on post-SAH day 2 via measurement of left middle cerebral artery (MCA) diameter. (A) Left MCA vessel diameter measurements. Data represent mean±SEM. *p<0.05 by one-way ANOVA with Tukey multiple comparisons test. (B) Neurobehavioral assessments were performed at baseline prior to SAH (Day 0) and daily for 2 days via Neuroscore test. Data represent mean±SEM. *p<0.05 vs Sham, and #p<0.05 vs SAH:Veh by Repeated Measures ANOVA with Newman-Keuls multiple comparisons test. N= 20, 25, 14 and 13 mice per group for Sham, SAH:Veh, SAH:Res:S and SAH:Res:M, respectively. Mortality = 1 each in SAH:Veh and SAH:Res:M groups.
SIRT1 mediates Resveratrol-induced protection against neurovascular dysfunction
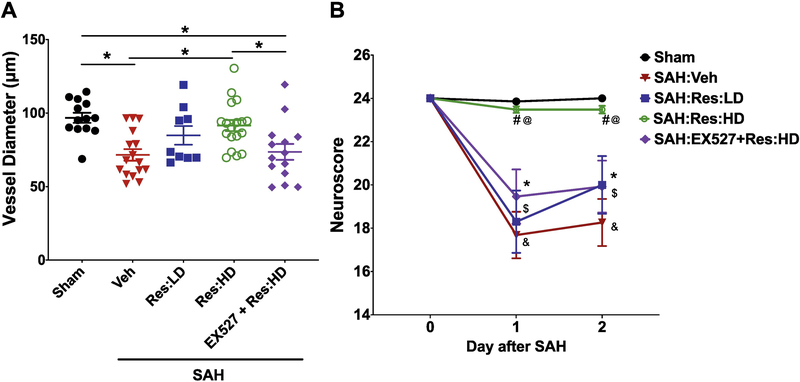
To determine if the protection afforded by Resveratrol is SIRT1 mediated, separate cohorts of mice were administered vehicle, low-dose (10mg/kg) Resveratrol (Res:LD), high-dose (20mg/kg) Resveratrol (Res:HD), or EX-527 plus high-dose (20mg/kg) Resveratrol (EX-527+Res:HD), followed by Sham or SAH surgery. Drug administration was continued twice daily including the morning of post-SAH day 2. While multiple administration of low-dose Resveratrol exhibited a trend towards protecting against vasospasm and neurological deficits, multiple administration of high-dose Resveratrol robustly and significantly abrogated SAH-induced vasospasm and neurological deficits (Figure 4), similar to findings in our prior experiment with multiple administration of high dose Resveratrol (Figure 3, Res:M group). Importantly, this protective effect was lost in mice that received EX-527 prior to Resveratrol treatment (Figure 4, EX527+Res:HD group). These results indicate that SIRT1, at least in part, mediates the protective effects of Resveratrol against SAH-induced vasospasm and neurological deficits.
Figure 4: Resveratrol-induced protection against post-SAH neurovascular dysfunction is SIRT1 mediated.
Mice treated with vehicle (Veh), low-dose Resveratrol (Res:LD, 10mg/kg), high-dose Resveratrol (Res:HD, 20mg/kg), or EX-527 + high-dose Resveratrol (EX-527+Res:HD) were subjected to sham surgery or endovascular perforation SAH. Drug treatment was continued twice daily until morning of post-SAH day 2. (A) Large artery vasospasm was assessed on post-SAH day 2 via measurement of left middle cerebral artery (MCA) diameter. Data represent mean±SEM, *p<0.05 by one-way ANOVA with Tukey multiple comparisons test. (B) Neurobehavioral testing was performed at baseline prior to SAH (Day 0) and daily for 2 days via Neuroscore test. Data represent mean±SEM, *,$,&p<0.05 vs Sham, #p<0.05 vs. SAH:Veh, and @p<0.05 vs. SAH:EX527+Res:HD by Two-way ANOVA with Tukey’s multiple comparisons test. N= 13, 16, 9, 17 and 14 mice per group for Sham, SAH:Veh, SAH:Res:LD, SAH:Res:HD and SAH:EX527+Res:HD, respectively. Mortality = 2 mice in SAH:Veh and 1 mouse in SAH:Res:HD group.
DISCUSSION
Our study advances multiple novel and important findings. First, we observed a time-dependent and sustained upregulation of SIRT1 expression in the brain, at both the mRNA and protein level, after hypoxic PC in vivo. Second, using a pharmacologic approach, we provide mechanistic evidence that SIRT1 is a critical mediator of hypoxic PC-induced protection against vasospasm and neurological dysfunction after SAH. Third, we showed that pretreatment with Resveratrol, an activator of SIRT1, induces a robust, dose-dependent protection against vasospasm and neurological dysfunction after SAH, and that this neurovascular protective effect is dependent on SIRT1. Overall, the vascular and neuroprotective effect of SIRT1 activation seen in our study provides additional foundational support for expanding the potential of SIRT1-based therapeutics beyond ischemic stroke (Khoury et al., 2018; Xu et al., 2018) to encompass hemorrhagic stroke as well.
Our in vivo findings that SIRT1 mRNA and protein expression is upregulated as early as 6 hours after hypoxic PC and that the upregulation of SIRT1 protein expression lasted until 48 hours after the conditioning stimulus are consistent with previously published in vitro studies that demonstrated SIRT1 activation by hypoxia in cultured mammalian cells (Joo et al., 2015; Lim et al., 2010). Our results with hypoxic PC are also consistent with previously published ex vivo and in vivo studies wherein preconditioning with brief ischemia led to SIRT1 activation in the brain and heart. Specifically, Raval et al. reported an increase SIRT1 activity in hippocampal slice cultures after oxygen glucose deprivation (Raval et al., 2006). In a subsequent study, they noted a similar increase in SIRT1 activity in the rat hippocampus after in vivo ischemic PC (Della-Morte et al., 2009). Similar activation of SIRT1 has been observed in models of cardiac ischemic PC (Nadtochiy et al., 2010). The molecular mechanisms by which hypoxia and brief ischemia result in SIRT1 activation include a complex metabolic interplay of oxygen- and redox-responsive signal transduction systems and will require further elucidation in tissue- and cell-specific studies (Wang et al., 2019). Nevertheless, the consistency of our hypoxic PC results and those of prior studies using brief ischemic conditioning implicate SIRT1 as playing a central role in conditioning-induced protection in the brain against multiple insults.
We also documented that pharmacologic inhibition of SIRT1 with the indole analogue EX527 (Zhao et al., 2013) abrogates hypoxic PC-induced protection against vasospasm and neurological dysfunction after SAH. While prior studies have not examined SIRT1 in the context of SAH-induced vasospasm and DCI, which was the focus of the present investigation, two recent publications have examined the role of SIRT1 in a less common form of secondary brain injury after SAH known as early brain injury (EBI). Zhou et al. demonstrated in rats that SIRT1 inhibition with Sirtinol exacerbated several elements of EBI, including cerebral edema and blood-brain-barrier breakdown, and worsened neurological function after experimental SAH (Zhou et al., 2014). Zhang et al. also reported that SIRT1 inhibition with Sirtinol exacerbated EBI, and that SIRT1 activation with Activator 3 attenuated EBI, after experimental SAH in rats (Zhang et al., 2016). These prior results regarding SAH-induced EBI suggest that an upregulation of SIRT1 may be an endogenous, self-protective response of the brain to SAH-induced injury. Coupled with our present findings regarding the role of SIRT1 in hypoxic PC-induced reductions in DCI metrics following SAH, experimental support for the contention that SIRT1 activation may represent a novel therapeutic strategy for patients with aneurysmal SAH is substantially strengthened.
To that end, several findings in our study demonstrate that Resveratrol may be one pharmacologic approach to enhance SIRT1 activation after SAH, and thereby provide neurovascular protection in this disease. First, we demonstrated a dose-dependent reduction in SAH-induced vasospasm and neurological deficits with Resveratrol pretreatment. This protective effect against DCI complements the findings of Li et al. who observed Resveratrol induced protection against EBI in a rat SAH model (Li and Han, 2018). The protective effect of Resveratrol in SAH also parallels the neuroprotective effect seen with a Resveratrol conditioning in middle cerebral artery occlusion model of ischemic stroke and asphyxial cardiac arrest (Della-Morte et al., 2009; Narayanan et al., 2015). Second, we showed that Resveratrol increases SIRT1 mRNA levels in brain, consistent with its role as a transcriptional activator of SIRT (Bai et al., 2018; Bastianetto et al., 2015; Borra et al., 2005; Li and Han, 2018). And third, the premise that a substantial part of Resveratrol’s therapeutic effect results from SIRT1 activation and not other SIRT1-independent actions is supported by our finding that the SIRT1 inhibitor EX527 blocked Resveratrol’s ability to protect against DCI after SAH. This latter finding is consistent with the loss of Resveratrol-mediated protective effects against EBI when rats with SAH were treated with the SIRT1 inhibitor Sirtinol (Qian et al., 2017).
Further studies are warranted to identify the mechanisms by which physiologic (hypoxic PC) and pharmacologic (Resveratrol) activation of SIRT1 results in attenuation of SAH-induced vasospasm and neurological dysfunction. Results from preclinical studies of cerebral SIRT1 suggest widespread epigenetics-based regulatory roles in maintaining genomic integrity, deacetylating histones and signaling proteins, affecting redox balance and reducing oxidative stress, and promoting cell survival (Ajami et al., 2017). While prior studies examining conditioning-induced SIRT1 activation in ischemic stroke suggest attenuation of oxidative stress(Koronowski et al., 2015; Xue et al., 2016) and inhibition of neuronal apoptosis as mechanisms for the observed neuroprotection,(Xue et al., 2016; Yan et al., 2013) the SIRT1-dependent vasospasm protection seen in our study indicates an additional direct vascular conditioning effect of SIRT1 activation. SIRT1 and Resveratrol have been demonstrated to activate eNOS (Donato et al., 2011; Mattagajasingh et al., 2007; Tajbakhsh and Sokoya, 2012), which was found to be critical in our prior study for the protection afforded by hypoxic PC against SAH-induced vasospasm and DCI metrics.(Vellimana et al., 2011) This relationship suggests that eNOS is likely a key downstream contributor to SIRT1-dependent vascular protection afforded by hypoxic PC against SAH-induced neurovascular dysfunction. Of course, other vascular and neuroprotective mechanisms of SIRT1 activation (Ajami et al., 2017; Sarubbo et al., 2018) also likely contribute to the beneficial phenotypic outcomes we measured herein.
We acknowledge several limitations of our study. The relationship between vasospasm and neurological outcome, and our contention that SIRT1 improves neurological function secondary to attenuation of vasospasm would be strengthened by additional measurements of cerebrovascular function such as cerebral blood flow and by including additional behavioral measures of neurological outcome. We also did not measure SIRT1 protein levels under all of the experimental conditions we studied, but only documented its increase in response to hypoxic PC and Resveratrol. Finally, we only used male mice for our experiments; the ability of SIRT1 activation by hypoxic PC or Resveratrol to benefit SAH-induced vasospasm and related neurological deficits should also be demonstrated in females, given the evidence that some regulatory actions of SIRT1 may exhibit sexual dimorphism (Lei et al., 2019).
In conclusion, SIRT1 activation represents a promising therapeutic strategy to combat vasospasm and DCI and improve neurological outcome after aneurysmal SAH. However, additional pre-clinical studies are needed prior to translation of SIRT1 activation into early phase clinical trials. Such studies would include examination of the effectiveness of a post-SAH SIRT1 activation treatment paradigm, assessment of the effect of SIRT1 activation on non-vasospasm components of DCI including autoregulatory dysfunction and the formation of microvessel thrombi, comparative effectiveness studies examining different classes of SIRT1 activators and NAD biosynthesis intermediates, elucidation of the optimal post-SAH therapeutic window, and cross-species validation of our findings.
HIGHLIGHTS.
Hypoxic preconditioning protects against deficits after subarachnoid hemorrhage
Hypoxic preconditioning induces robust upregulation of SIRT1
SIRT1 mediates hypoxic preconditioning induced protection in subarachnoid hemorrhage
Resveratrol mimics preconditioning induced protection in subarachnoid hemorrhage
ACKNOWLEDGMENTS
The authors thank Ernesto Gonzales for performing subarachnoid hemorrhage surgery.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health grants R01 NS091603 awarded to G.J.Z., R25 NS090978 to G.J.Z. and A.K.V., American Heart Association Postdoctoral Fellowship Grant (11POST7650038) awarded to A.K.V., and Neurosurgery Research and Education Foundation Research Fellowship Grant awarded to A.K.V.
Footnotes
DISCLOSURES
None
REFERENCES
- Ajami M, Pazoki-Toroudi H, Amani H, Nabavi SF, Braidy N, Vacca RA, Atanasov AG, Mocan A, Nabavi SM, 2017. Therapeutic role of sirtuins in neurodegenerative disease and their modulation by polyphenols. Neurosci Biobehav Rev 73, 39–47. [DOI] [PubMed] [Google Scholar]
- Bai X, Yao L, Ma X, Xu X, 2018. Small Molecules as SIRT Modulators. Mini Rev Med Chem 18, 1151–1157. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Menard C, Quirion R, 2015. Neuroprotective action of resveratrol. Biochim Biophys Acta 1852, 1195–1201. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA, 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM, 2005. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280, 17187–17195. [DOI] [PubMed] [Google Scholar]
- Broussy S, Laaroussi H, Vidal M, 2020. Biochemical mechanism and biological effects of the inhibition of silent information regulator 1 (SIRT1) by EX-527 (SEN0014196 or selisistat). J Enzyme Inhib Med Chem 35, 1124–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA, 2009. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159, 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR, 2011. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589, 4545–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez G, Outeiro TF, 2013. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5, 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Sessa WC, 2019. Endothelial NOS: perspective and recent developments. Br J Pharmacol 176, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen C, Palmier B, Plotkine M, Marchand-Leroux C, Besson VC, 2014. Neurological and histological consequences induced by in vivo cerebral oxidative stress: evidence for beneficial effects of SRT1720, a sirtuin 1 activator, and sirtuin 1-mediated neuroprotective effects of poly(ADP-ribose) polymerase inhibition. PLoS One 9, e87367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han BH, Vellimana AK, Zhou ML, Milner E, Zipfel GJ, 2012. Phosphodiesterase 5 inhibition attenuates cerebral vasospasm and improves functional recovery after experimental subarachnoid hemorrhage. Neurosurgery 70, 178–186; discussion 186–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HY, Yun M, Jeong J, Park ER, Shin HJ, Woo SR, Jung JK, Kim YM, Park JJ, Kim J, Lee KH, 2015. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1alpha (HIF-1alpha) via direct interactions during hypoxia. Biochem Biophys Res Commun 462, 294–300. [DOI] [PubMed] [Google Scholar]
- Khoury N, Koronowski KB, Young JI, Perez-Pinzon MA, 2018. The NAD(+)-Dependent Family of Sirtuins in Cerebral Ischemia and Preconditioning. Antioxid Redox Signal 28, 691–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronowski KB, Dave KR, Saul I, Camarena V, Thompson JW, Neumann JT, Young JI, Perez-Pinzon MA, 2015. Resveratrol Preconditioning Induces a Novel Extended Window of Ischemic Tolerance in the Mouse Brain. Stroke 46, 2293–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Wang J, Wang D, Li C, Liu B, Fang X, You J, Guo M, Lu XY, 2019. SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression-related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefrontal cortex. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Han X, 2018. Resveratrol alleviates early brain injury following subarachnoid hemorrhage: possible involvement of the AMPK/SIRT1/autophagy signaling pathway. Biol Chem 399, 1339–1350. [DOI] [PubMed] [Google Scholar]
- Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW, 2010. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell 38, 864–878. [DOI] [PubMed] [Google Scholar]
- Liu S, Sun J, Li Y, 2016. The Neuroprotective Effects of Resveratrol Preconditioning in Transient Global Cerebral Ischemia-Reperfusion in Mice. Turk Neurosurg 26, 550–555. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K, 2007. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A 104, 14855–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Juan AM, Hurst CG, Cui Z, Evans LP, Hatton CJ, Pei DT, Ju M, Sinclair DA, Smith LE, Chen J, 2014. Sirtuin1 over-expression does not impact retinal vascular and neuronal degeneration in a mouse model of oxygen-induced retinopathy. PLoS One 9, e85031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner E, Johnson AW, Nelson JW, Harries MD, Gidday JM, Han BH, Zipfel GJ, 2015. HIF-1alpha Mediates Isoflurane-Induced Vascular Protection in Subarachnoid Hemorrhage. Ann Clin Transl Neurol 2, 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Wada-Hiraike O, Yano T, Shirane A, Hirano M, Hiraike H, Koyama S, Oishi H, Yoshino O, Miyamoto Y, Sone K, Oda K, Nakagawa S, Tsutsui K, Taketani Y, 2012. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: an implicative role of SIRT1 in the ovary. Reprod Biol Endocrinol 10, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS, 2010. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan SV, Dave KR, Saul I, Perez-Pinzon MA, 2015. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke 46, 1626–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, Pizarro JG, Gutierrez-Cuesta J, Crespo-Biel N, Alvira D, Tajes M, Yeste-Velasco M, Folch J, Canudas AM, Sureda FX, Ferrer I, Camins A, 2008. Modulation of SIRT1 expression in different neurodegenerative models and human pathologies. Neuroscience 154, 1388–1397. [DOI] [PubMed] [Google Scholar]
- Qian C, Jin J, Chen J, Li J, Yu X, Mo H, Chen G, 2017. SIRT1 activation by resveratrol reduces brain edema and neuronal apoptosis in an experimental rat subarachnoid hemorrhage model. Mol Med Rep 16, 9627–9635. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA, 2006. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab 26, 1141–1147. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Singh I, Azad TD, Holmes BB, Verghese PB, Dietrich HH, Diamond M, Bu G, Han BH, Zipfel GJ, 2016. Heparan sulfate proteoglycans mediate Abeta-induced oxidative stress and hypercontractility in cultured vascular smooth muscle cells. Mol Neurodegener 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo F, Esteban S, Miralles A, Moranta D, 2018. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr Neuropharmacol 16, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh N, Sokoya EM, 2012. Regulation of cerebral vascular function by sirtuin 1. Microcirculation 19, 336–342. [DOI] [PubMed] [Google Scholar]
- Tang BL, 2009. Sirt1’s complex roles in neuroprotection. Cell Mol Neurobiol 29, 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellimana AK, Diwan D, Clarke J, Gidday JM, Zipfel GJ, 2018. SIRT1 Activation: A Potential Strategy for Harnessing Endogenous Protection Against Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Neurosurgery 65, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellimana AK, Milner E, Azad TD, Harries MD, Zhou ML, Gidday JM, Han BH, Zipfel GJ, 2011. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke 42, 776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB, 2010. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41, 2391–2395. [DOI] [PubMed] [Google Scholar]
- Wang Y, He J, Liao M, Hu M, Li W, Ouyang H, Wang X, Ye T, Zhang Y, Ouyang L, 2019. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur J Med Chem 161, 48–77. [DOI] [PubMed] [Google Scholar]
- Xu J, Jackson CW, Khoury N, Escobar I, Perez-Pinzon MA, 2018. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front Endocrinol (Lausanne) 9, 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Huang JW, Ding PY, Zang HG, Kou ZJ, Li T, Fan J, Peng ZW, Yan WJ, 2016. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav Brain Res 309, 1–8. [DOI] [PubMed] [Google Scholar]
- Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C, Xiong L, 2013. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. J Cereb Blood Flow Metab 33, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XS, Wu Q, Wu LY, Ye ZN, Jiang TW, Li W, Zhuang Z, Zhou ML, Zhang X, Hang CH, 2016. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis 7, e2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Allison D, Condon B, Zhang F, Gheyi T, Zhang A, Ashok S, Russell M, MacEwan I, Qian Y, Jamison JA, Luz JG, 2013. The 2.5 A crystal structure of the SIRT1 catalytic domain bound to nicotinamide adenine dinucleotide (NAD+) and an indole (EX527 analogue) reveals a novel mechanism of histone deacetylase inhibition. J Med Chem 56, 963–969. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Zhang X, Zhang XS, Zhuang Z, Li W, Sun Q, Li T, Wang CX, Zhu L, Shi JX, Zhou ML, 2014. SIRT1 inhibition by sirtinol aggravates brain edema after experimental subarachnoid hemorrhage. J Neurosci Res 92, 714–722. [DOI] [PubMed] [Google Scholar]