Abstract
Cells display broad heterogeneity across multiple phenotypic features, including motility, morphology, and cell signaling. Live-cell imaging techniques are beginning to capture the importance and interdependence of these phenomena. However, existing image analysis pipelines often fail to capture the intricate changes that occur in small subpopulations, either due to poor segmentation protocols or cell tracking errors. Here we report a pipeline designed to image and track single-cell dynamic phenotypes in heterogeneous cell populations. We provide step-by-step instructions for three phenotypically different cell lines across two time scales as well as recommendations for adaptation to custom data sets. Our protocols include steps for quality control that can be used to filter out erroneous tracks and improve assessment of heterogeneity. We demonstrate possible phenotypic readouts including motility, nuclear receptor translocation, and mitosis.
Keywords: Live-cell imaging, Tracking, Heterogeneity, Phenotypes, Motility, Receptor translocation, Mitosis
1. Introduction
Time-lapse microscopy has revealed extensive heterogeneity in the dynamic behavior of living cells [1–4]. Variations in gene expression [5, 6], motility [7, 8], morphology [9–12], and responsiveness to drug treatment [13] are well documented and have been shown to correlate with the dynamics of critical signaling molecules. To deepen our understanding of the spatiotemporal dynamics of cell behavior, an array of both proprietary and open-source computational tools has been developed to track cells while collecting phenotypic information [14–25]. However, generating image analysis pipelines that are broadly available and easily adaptable to phenotypically diverse cell types has proven to be challenging.
Another persisting problem in time-lapse microscopy is the difficulty in determining whether outliers are technical artifacts (e.g., errors due to poor segmentation or tracking) or instead credible observations of a heterogeneous population. This problem has recently been addressed in a couple of ways: (1) by data filtering [4, 26] and (2) by correcting errors [25] based on a selected phenotypic readout.
To capture the complex changes occurring in single cells over time, there remains a need for a set of robust guidelines to rapidly fine-tune tracking tools to individual data sets. In this chapter, we provide detailed protocols to prepare, image, track, and analyze the dynamic phenotypes of individual cells. For image analysis, we used the open-source image analysis software CellProfiler. Our pipeline incorporates the quality control metric Tracking Aberration Measure (TrAM) to identify tracks with unrealistic jumps in multiple phenotypic features [4]. Our protocol can be adapted to a broad range of data sets. Here we demonstrate the flexibility of our pipeline by tracking three different cell lines across two time scales.
2. Materials
2.1. Cell Preparation
Tissue culture medium: RPMI 1640 (supplemented with L-glutamine, 10% fetal bovine serum, 1% penicillin/streptomycin) (see Note 1).
Imaging medium 1 (for motility and mitosis experiments): phenol red-free RPMI 1640 (supplemented with L-glutamine, 10% fetal bovine serum, and 1% penicillin/streptomycin) (see 2).
Imaging medium 2 (for AR translocation experiments): phenol red-free RPMI 1640 (supplemented with L-glutamine, 10% charcoal/dextran stripped fetal bovine serum, and 1% penicillin/streptomycin).
1× Phosphate buffered saline (PBS).
Trypsin 0.05%.
96-Well tissue culture-treated microplates with black walls and clear bottom (see Note 3).
Automated cell counter (see Note 4).
Nuclear markers: nuclear dyes for short-term time-lapse experiments and nuclear localization constructs for long-term time-lapse experiments (see Note 5).
If applicable: transfection reagent for transient expression of fluorescent proteins of interest (see Note 6).
If applicable: reagent for positive selection of cells stably expressing fluorescent proteins (see Note 7).
Androgen receptor ligand methyltrienolone (R1881) (see Note 8).
2.2. Image Acquisition
Imaging system with automated stage and multi-well mounting frame, environmental control, and ability to perform time-lapse imaging sequences (see Note 9).
Acquisition filters to collect fluorescent signals: DRAQ5 (620–640 nm excitation, 650–760 nm emission), dsRed (520–550 nm excitation, 560–630 nm emission), EGFP (460–490 nm excitation, 500–550 nm emission), and BF (transmission, 650–760 nm emission) (see Note 10).
2.3. Cell Tracking
Image analysis software with cell segmentation and tracking capabilities. Available readouts should include cell positions (XY), morphology features (nuclear area, nuclear roundness), and fluorescence intensity. Specific readouts required to apply data filtering steps and analyze dynamics are listed in the respective assay and in Table 1. To exemplify our workflow using an open-source application, we used CellProfiler (see Note 11).
TrAM functionality for CellProfiler: https://github.com/RudermanLab/TrAM_CellProfiler (see Note 11).
Open-source image processing program ImageJ to compile movies (avi files) using images exported from CellProfiler (tiff files).
Table 1.
Summary of time-lapse assays to measure cellular dynamics
| Steps of the workflow | Variable | Short-term dynamics: motility and protein translocation | Long-term dynamics: mitosis | ||
|---|---|---|---|---|---|
|
| |||||
| 1. Cell preparation | Cell line | HeLa | PC3 | Panc-1 | HeLa |
| Cell number/well | 5000 | 10,000 | 12,000 | 3000 | |
| Nuclear marker | DNA stain DRAQ5 | nuclear protein Nucleus-RFP | |||
| Other proteins of interest | GFP-tagged nuclear receptor AR | ||||
| 2. Image acquisition | Channels | DRAQ5 (620–640 nm excitation, 650–760 nm emission), EGFP (460–490 nm excitation, 500–550 nm emission), BF (transmission, 650–760 nm emission) | dsRed (520–550 nm excitation, 560–630 nm emission), BF (transmission, 650–760 nm emission) | ||
| # Conditions (wells), # technical replicates (fields) | 2, 4 | 2, 35 | |||
| Imaging increment, total time, number of time points | 1 min, 24 min, 25 | 30 min, 20 h, 41 | |||
| 3. Cell tracking | Border objects | discard | discard | ||
| Threshold | 1.0 | 1.1 | 1.0 | 1.0 | |
| Nuclear diameter (px) | 23–44 | 17–53 | 16–36 | 13–63 | |
| Nuclear area (px) | 400–3000 | 300–3000 | 200–3000 | 300–3000 | |
| Incomplete tracks | Minimum lifetime filter 24 | Minimum lifetime filter 40 | |||
| 4. Dynamic phenotype | TrAM filtering criteria | Motility: Nuclear area, nuclear roundness | Mitosis: XY positions, nuclear roundness | ||
| Nuclear translocation: XY positions | |||||
| Population stratification | Responders vs. non-responders: Total Math_NtoCRatio increase >0.147 | Mitotic cells: Step nuclear area increase (30 min) > 18.2% | |||
3. Methods
3.1. Preparation of Cells
Prepare tissue culture and imaging media as described in the materials section. Preheat all reagents in a 37 °C water bath.
Culture cells in a 10 cm dish, and maintain at 37 °C in a humidified incubator with 5% carbon dioxide. Passage at least twice before performing experiments.
To seed cells, aspirate tissue culture media and wash monolayer twice with 5 ml 1× PBS.
Aspirate PBS, add 3 ml trypsin, and incubate for 5 min at 37 °C or until cells have detached from the plate.
Add 7 ml imaging medium and transfer cell suspension to 15 ml canonical tube.
Centrifuge cells at 200 × g for 5 min at room temperature.
Aspirate supernatant and resuspend cell pellet in 2 ml imaging medium 1 or 2.
Count cells: mix 10 μl cell solution with 10 μl trypan blue, pipet mixture onto both sides of cell counting slide, and count cells.
Plate 3000–12,000 cells in 100 μl/well. For short-term time-lapse experiments, plate cells at 70% confluency. For long-term time-lapse experiments, where cell proliferation is expected, plate cells at 40% confluency to avoid overgrowth (see Note 12).
If applicable: if needed for protein translocation assay or long-term nuclear tracking in mitosis assay, perform transient transfection of fluorescently labeled proteins following manufacturer’s recommendations (see Note 13).
Incubate cells for 24 h at 37 °C.
If applicable: if nuclear dyes are used, replace overnight medium with 100 μl imaging medium 1 or 2, supplemented with nuclear dye at desired end concentration (see Note 14).
Transfer plate to microscope and begin time-lapse after 30 min incubation in the live-cell chamber (see Note 15).
3.2. Image Acquisition
Here it is key to balance temporal resolution (imaging increment) with total imaging time to avoid phototoxic imaging conditions. Below, we provide two protocols to exemplify different adjustments based on applications and needs.
Choose image acquisition filter combinations to detect nuclear stain and proteins of interest (e.g., fluorescently nuclear receptors) and collect bright-field images (see Table 1).
Select wells (conditions) and imaging fields (technical replicates) to be imaged (see Note 16).
Select imaging increment and number of time points to achieve desired total imaging time. Details for two assays are listed below and can be found in Table 1.
Applicable to short-term time-lapse imaging to measure cell motility and nuclear translocation: generate sequence to image every minute for 24 min, resulting in 25 time points (see Note 17).
Applicable to long-term time-lapse imaging to track cell division: generate sequence to image cells every 30 min for 20 h, resulting in 41 time points.
Start time-lapse experiment.
3.3. Cell Tracking
The following section provides step-by-step instructions to build a CellProfiler pipeline to track cells. We list specific parameters for three different cell lines across two time scales and provide guidelines to customize the pipeline as needed. Detailed information on each step and alternative settings can be found by clicking on the respective question marks in CellProfiler.
3.3.1. Data Import
Download and install the open-source software CellProfiler [20]. Accept default options and add-ons.
Download and install TrAM for CellProfiler at https://github.com/RudermanLab/TrAM_CellProfiler.
Import data into CellProfiler using the four default import modules (Images, Metadata, NamesAndTypes, and Groups). The included CellProfiler pipelines contain a regular expression in the Metadata module that parses out information from the image file names (row, column, field, channel, time point). These metadata are used to group the images by well and organize into time series (see Note 18).
To analyze images from different imaging channels, assign names to each channel. This can be done under NamesAndTypes. We assigned images acquired in channel 2 Nuclear_Marker and images acquired in channel 4 Nuclear_ Receptor. Adjust these names accordingly.
For batch analysis of multiple image sequences, group images by the desired metadata item, e.g., well or field. Verify under grouping list that the resulting image count represents the total number of imaging time points.
3.3.2. Identify Cells
Detect and segment nuclei using images of nuclear fluorescence marker by selecting the corresponding channel.
Select IdentifyPrimaryObjects module.
Select Nuclear_Marker (name that identifies images acquired in nuclear marker channel from NamesAndTypes), and name the primary objects to be identified: Nuclei.
Set min/max to define object diameter range in pixel units: to include cells of all sizes, start with broad range of 10–60 pixels (8–30 μm) and adjust once you reach point 13 of the protocol (see Note 19).
Discard objects outside the diameter range and discard objects touching the border of the image.
Apply adaptive two-class thresholding strategy using the Otsu method [27], and keep the threshold smoothing scale at the default value 1.3488.
Set threshold correction factor to 1.0 with lower and upper bounds set to 0.0 to 1.0.
Size adaptive window to 50.
Use Shape to distinguish clumped objects and draw dividing lines between clumped objects.
Automatically calculate size of smoothing filter for declumping and minimum allowed distance between local maxima.
Speed up by using lower-resolution image to find local maxima.
Fill holes in identified objects after both thresholding and declumping.
Handling of objects if excessive number of objects is identified: Continue.
Select MeasureObjectSizeShape module to measure features of the Nuclei.
Within the FilterObjects module, select to filter Nuclei and name the output objects FilteredNuclei. Filter Measurements by setting limits to AreaShape, using the measurement Area. Set a min/max for nuclear area to 300–3000 pixels to exclude objects that are too small (debris) and too large (e.g., clusters not well segmented) (see Note 19).
3.3.3. Fine-Tune Nuclear Segmentation
We recommend incorporating the following steps to fine-tune nuclear segmentation to custom data sets.
Fine-tune threshold: start Test Mode and click Step. Inspect output image NucleiOutlines to verify preliminary cell segmentation. The threshold correction factor can be empirically determined based on the identified objects, e.g., if objects are missed, reduce the threshold correction factor. If areas of the background are erroneously identified as objects, increase this number (Fig. 1a). Test thresholds on images from various time points (click Next Image Set and Step within Test Mode), to account for any photobleaching.
Fine-tune nuclear area: within the Test Mode, inspect output image NucleiOutlines to verify preliminary cell segmentation. Click Step twice to obtain image of FilteredNuclei. If needed, go back to FilterObjects module, and fine-tune nuclear area thresholds (Fig. 1b).
Fine-tune nuclear diameter: within Test Mode, click Step to obtain output table from the next module MeasureObjectSizeShape of FilteredNuclei. Calculate adjusted values: median MinFeretDiameter—STD rounded down and median MaxFeretDiameter + STD rounded up. Repeat for two to three time points throughout the time course and use the broadest obtained range to adjust nuclear diameter range. For long time-lapse experiments, broaden the adjusted range further by a factor of 1.5 to ensure tracking of nuclei during all stages of mitosis: multiply the maximum calculated value, and divide minimum calculated value by 1.5. Finally, inspect new output image NucleiOutlines, and compare with the previous version to verify optimized segmentation (Fig. 1c). Exit Test Mode and return to protocol overview. For short time-lapse experiments, we used values 23–44 for HeLa cells, 17–53 for PC3 cells, and 16–36 for small Panc-1 cells. To track mitotic events in long time-lapse experiments, we used 13–63 for HeLa cells (see Note 19).
Fig. 1.
Step-by-step fine-tuning of nuclear segmentation. Example Test Mode images of PC3 cells to adjust (a) threshold, (b) nuclear area, and (c) nuclear diameter to custom data sets. (a) Outlines in yellow represent border objects; outlines in magenta represent objects outside of nuclear diameter range. Green outlines are retained nuclei. (b) Boxes demonstrate objects rejected due to below threshold nuclear area. (c) Morphology table contains feature values to adjust nuclear diameter. Outlines in magenta represent objects outside of diameter range. Green outlines are retained nuclei
3.3.4. Track Cells
The following steps connect sequential cell measurements to track cells over time.
Select TrackObjects module and choose the Overlap tracking method.
Select the objects to track: FilteredNuclei.
Set maximum pixel distance to consider matches to 10 (see Note 20).
Filter incomplete tracks, i.e., cells without tracking data at every time point. Within the TrackObjects module, filter objects by lifetime and set the minimum lifetime filter to the total number of acquired time points - 1: set to 24 for short and 40 for long time-lapse experiments.
Select display option: Color and Number and save color-coded image.
Name the output image: TrackedCells.
3.3.5. Additional Modules to Measure Nuclear Translocation of Receptor Proteins
First, complete the steps listed above to obtain tracked cells, and then, before running the pipeline, include the additional steps listed below.
Select IdentifySecondaryObjects module.
Select input image Nuclear_Marker and input objects FilteredNuclei. Name the primary objects to be identified: Cells.
Select the Distance N method to expand primary objects by pixels and generate cell masks that extend beyond the nuclear membrane.
Select IdentifyTertiaryObjects module. Select Cells as larger identified objects and FilteredNuclei as smaller identified objects.
Name the tertiary objects PerinuclearCytoplasm.
Shrink smaller object prior to subtraction.
Retain outlines of the tertiary objects.
Name the outline image PerinulcearCytoplasmOutlines (see Note 21).
Select MeasureObjectIntensity module to use Nuclear_Receptor images to measure objects FilteredNuclei and PerinuclearCytoplasm.
Measure nuclear to cytoplasmic fluorescence intensity ratio: within the CalculateMath module, select the Divide operation and name the output measurement NtoCRatio.
To define the numerator measurement, select Object, FilteredNuclei, category Intensity, measurement MeanIntensity, and image Nuclear_Receptor.
To define the denominator measurement, select Object, PerinuclearCytoplasm, category Intensity, measurement MeanIntensity, and image Nuclear_Receptor.
3.3.6. Additional Steps to Measure Track Quality
This section consists of steps to compute the Tracking Aberration Measure (TrAM) for each cell to determine the credibility of its tracking. A detailed description of the TrAM method, benchmarking, and validation including examples of the effect on single-cell and population-based data can be found in our paper [4].
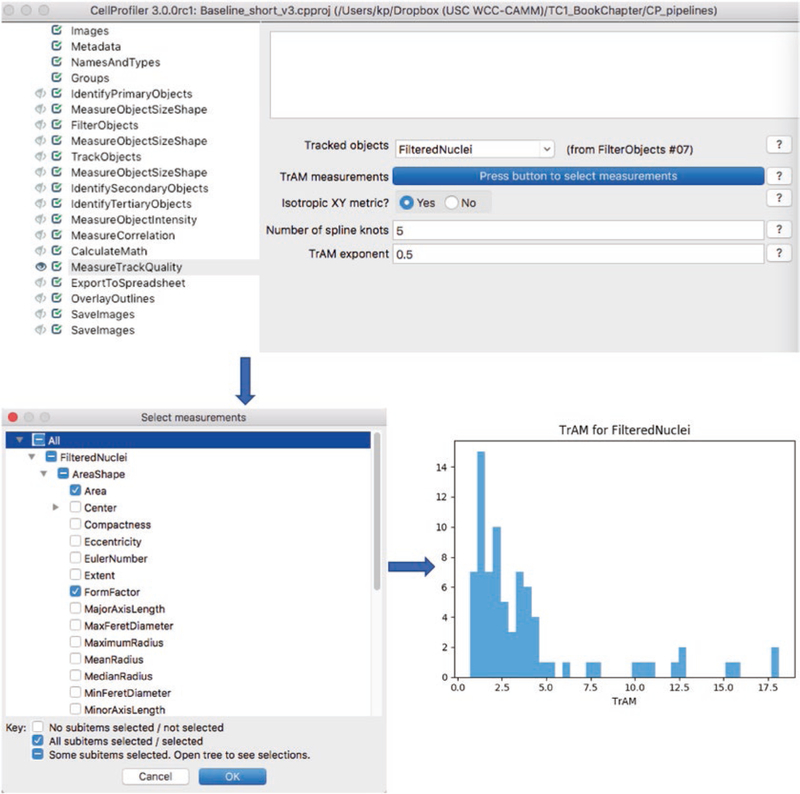
Select the MeasureTrackQuality module (Fig. 2).
Select the tracked FilteredNuclei. Press button to select measurements and compute TrAM, as listed in Table 1: for motility, use nuclear area (Area) and roundness (FormFactor); to measure receptor translocation, use X and Y coordinates (Center X, Y); and for long-term tracking across multiple generations, use X and Y coordinates (Center X, Y) and nuclear roundness (FormFactor) (see Note 22).
Select number of spline knots: 5 for 25 time point and 8 for 41 time point experiments.
TrAM values are computed and reported as MeasureTrackQuality_TrAM in the data spreadsheets that are exported for downstream processing (see steps below).
Fig. 2.
Computation of the track quality metric TrAM. Top: snapshot of TrackQC module and associated settings. Middle: press button to select measurements opens a new window with check boxes to select features for computation of TrAM. Bottom: TrAM histogram visualizes distribution across filtered nuclei. Low TrAM values represent higher-quality tracks
3.3.7. Export Data, Images, and Movies
The following steps are incorporated in the pipeline to specify movies that are useful for data quality control, pipeline verification and to export data for downstream analysis.
Export data: within the ExportToSpreadsheet module, select the comma delimiter to separate columns and select output file location and a prefix to generated file names. Export all measurement types.
Generate overlay images: select RescaleIntensity module and apply to Nuclear_Marker images. Choose specific values to be reset to the full intensity range using min/max for each image and save out ScaledNuclei. Select OverlayOutlines module and name the output image NucOverlay. Overlay ScaledNuclei images with segmentation outlines of FilteredNuclei or, if present, PerinuclearCytoplasm.
Export cell segmentation images and compile movie: select SaveImages module to export images of NucOverlay. Use sequential numbers and the prefix TrackedSegmentation to save tiff image at every cycle. Compile movie from tiff files, e.g., in ImageJ.
Export tracking images and compile movie: select SaveImages module to export images of TrackedCells. Use sequential numbers and the prefix TrackedCells to save tiff file at every cycle. Compile movie from tiff files, e.g., in ImageJ.
Once the protocol is set up, data sets are ready to be analyzed. To reduce run time, keep the eye in the MeasureTrackQuality modules open while closing all others and click Analyze Images (see Note 23).
3.4. Analysis of Dynamic Phenotypes
The following section describes steps downstream of CellProfiler to validate the data and analyze various phenotypic readouts.
Verify data quality by evaluating generated output movies of tracking and segmentation.
Evaluate tracking using TrAM histogram: for high-quality data, expect most cells to have low TrAM values (<5) and a steep slope. Broader peaks including higher TrAM values indicate lower tracking quality (Fig. 2).
Optional filtering step based on TrAM (see Note 24). To filter out tracks with a high probability of poor segmentation, set a TrAM threshold (x-axis) where the cell number (y-axis) slope flattens out (Fig. 3). In our experience, poor segmentation tracks always had TrAM values above 5 and therefore can be used as a low-stringency cutoff (see Note 25).
Optional step to verify TrAM cutoff: the spreadsheet FilteredNuclei contains a TrackObjects_Label and a MeasureTrackQuality_TrAM value for each cell. Compare tracking and segmentation movies to verify that errors have TrAM values above the chosen threshold, and good tracks are retained in the data set (Fig. 3).
Analysis of dynamic nuclear translocation: plot Math_NtoCRatio across time to see changes in subcellular distribution. Optional step to stratify responders vs. non-responders in nuclear translocation assay: set threshold to total Math_NtoCRatio >0.147 (Fig. 3) (see Note 27).
Analysis of mitosis: MeasureTrackQuality_Is_Parent: marks cells that divide (1) vs. cells that don’t divide (0) during the time-lapse experiment. MeasureTrackQuality_Labels: TrAM value that is assigned to a trajectory. Parent cells can have multiple values, determined by the respective track of their daughter cells. MeasureTrackQuality_Split_Trajectory: marks daughter cells (1) that are born during the time-lapse experiment. Calculate hourly proliferation rate:
Fig. 3.
Overview of TrAM filtering to measure heterogeneity. (a) TrAM histogram to determine cutoff (red line). (b) Verification of TrAM cutoff. Cell not rejected due to low TrAM value (TrAM = 3.1) vs. cell filtered out due to high TrAM value (TrAM = 7.1). Nuclear segmentation images are shown after 3 min and 6 min imaging. (c) Speed distribution of PC3 cells before and after TrAM filtering
Optional step to stratify mitotic vs. non-mitotic cells: set threshold to step nuclear area increase (30 min) >18.2%.
Acknowledgments
We thank Beth Cimini and Anne Carpenter for their support in building the CellProfiler pipeline. We thank Thomas Do and Dane Lemons for help plating cells and generating cell tracking movies. We thank Naim Matasci, Torin Gerhart, and Colleen Garvey for technical discussions throughout the project. We would like to express our deepest gratitude to our philanthropic supporters: the Stephenson family, Emmet, Toni, and Tessa, for their donation of the Operetta HCS platform.
Footnotes
Media and supplements can be replaced as needed to culture other cell types.
Media and supplements can be replaced as needed to image other cell types. To increase signal-to-noise ratios, we suggest using phenol red-free medium with reduced levels of riboflavin (0.05 mg/l vs. 0.2 mg/l).
Other multi-well formats, e.g., 384-well plates, can be used to save on reagents, although the smaller wells are more difficult if system perturbations are conducted manually, i.e., without a liquid handler. To increase the signal-to-noise ratio, we chose specialized imaging plates with a thin plastic bottom. Other plates can be used as well, including glass-bottom multi-well plates, if they are tissue culture treated and black.
Other methods of cell counting, i.e., hemocytometer, may be used.
We used 2.5 μM DRAQ5 nuclear dye for short-term time-lapse experiments. Other nuclear stains can be used, e.g., siR-DNA or Hoechst. Be aware of possible drug interactions when using Hoechst. We used Nucleus-RFP nuclear localization constructs for long-term time-lapse experiments; other constructs can be used as well, e.g., H2B-RFP. The advantage of nuclear constructs is the avoidance of cytotoxic effects caused by nuclear dyes. However, transfection rates and levels of expression can vary widely, making cell detection and segmentation less efficient.
Transfection reagent and optimal conditions for your cell line should be determined empirically. We used Promega FuGENE to express GFP-tagged androgen receptors (AR) in all three cell lines. Similarly, we used 80 PPC (particles per cell) of Nucleus-RFP Bac-Mem to track nuclei over several hours.
We used 200 ng/ml G418 sulfate solution to select for cells stably expressing GFP-AR construct containing the neomycin resistance gene. Care should be taken that the appropriate reagent is chosen for the respective construct.
If working with R1881 or any other ligands or drugs, it is important to be aware of potential light sensitivity. R1881 is rapidly destroyed upon exposure to UV light. In these cases, we suggest either avoiding dyes/proteins that emit light in the blue spectrum or using compounds that are light stable, e.g., Cl-4AS-1.
For all our assays, we used the PerkinElmer Operetta high-content imaging system.
Not all channels are needed for each assay; necessary are filter combinations that image nuclear stain and, if applicable to the assay, protein of interest. We suggest acquiring bright-field images in parallel, as they can provide useful information for quality control, e.g., detection of cell debris and assessment of cell health.
Previously, we used proprietary software applications Harmony 2 (PerkinElmer) and Imaris version 8.3.1 (Bitplane). Protocols for these applications including access to TrAM outside of CellProfiler (Github, https://github.com/RudermanLab/tram) can be found in our previously published paper [4].
Exact cell numbers to be plated to achieve the desired confluency depends on cell area in monolayer. For short experiments, we plated 10,000 PC3 cells, 5000 HeLa cells (cells are larger and flatter), and 12,000 Panc-1 cells (smaller cells). For long experiments, we plated 3000 HeLa cells to avoid confluency. Confluency makes tracking very difficult and alters proliferation and migration properties. Ideally, cells should be within exponential phase of growth throughout the experiment.
In both cases, we performed reverse transfections while plating cells for experiments the next day. Alternatively, traditional transfections can be performed 1 day after cell seeding. In this case, one must consider the extra day cells are plated until the experiment is performed when determining optimal cell seeding number.
We used 2.5 μM DRAQ5 to stain the nuclei.
A 30 min incubation step in the microscope ensures efficient nuclear staining and increased stabilization of environmental settings in the live-cell chamber. Thermal instability can lead to a plate shift and biased results.
A number of wells and fields that can be read within desired increment time will vary depending on the assay. Examples are listed in Table 1.
Adjustment of total assay time may be necessary to adapt to slower kinetics of other nuclear receptors.
We imported images generated by the PerkinElmer Operetta. These files use a naming convention to denote where and when an image was collected from a plate. An example filename might be “r05c09f03p01-ch3sk26fk1fl1.tiff” where the letters r, c, f, p, ch, and sk denote row, column, field, plane, channel, and time point, respectively. Our CellProfiler pipelines use a regular expression to parse these values out of the filename. In this case, a regular expression defines a pattern that is used to extract values into variables. From the example above, the correct parsing would be row = 5, column = 9, field = 3, plane = 1, channel = 3, and time point = 26. Since the metadata extraction relies on naming convention, data collected on different imaging systems will not just “plug and play” with our analysis routines.
These values are based on 0.5 μm/px images. Adjust pixel values according to the magnification.
When working with 0.5 μm/px images and imaging every min, this protocol assumes cells migrate at a maximum speed of 5 μm/min. If needed, adjust pixel values to image size and imaging increment.
Appropriate segmentation of nuclei and surrounding perinuclear cytoplasm can be verified in Test Mode.
To compute TrAM, we suggest choosing measurements that are nonessential for the respective assay to minimize bias, e.g., we specifically avoided using Center X, Y for motility or nuclear area for mitosis.
Closing the eyes in image analysis modules will prevent pop-ups and reduce run time. The open eye in the MeasureTrackQuality module is necessary to obtain TrAM histogram.
TrAM filtering is especially useful if the goal of the assay is to capture the full range of cellular behavior, e.g., to detect small subpopulations of cells within a much larger population. These steps can also affect averages across populations and thus increase overall data accuracy. A full description of the method can be found in our methods paper [4].
Here we establish thresholds based on a by-eye estimation of the TrAM distribution curve. Alternatively, one can establish a data-specific ground truth to statistically determine thresholds that maximize balanced accuracy, i.e., sum of sensitivity and specificity. Details and protocols for this approach can be found in our paper [4].
Formula assumes 0.5 μm/px images. Adjust pixel values to different image size.
The value was determined using ROC approach. See publication for details [4].
References
- 1.Purvis JE, Lahav G (2013) Encoding and decoding cellular information through signaling dynamics. Cell 152:945–956. 10.1016/j.cell.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschuler SJ, L FW (2010) Cellular heterogeneity: do differences make a difference? Cell 141:559–563. 10.1016/j.cell.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snijder B, Pelkmans L (2011) Origins of regulated cell-to-cell variability. Nat Rev Mol Cell Biol 12:119–125. 10.1038/nrm3044 [DOI] [PubMed] [Google Scholar]
- 4.Patsch K, Chiu C-L, Engeln M et al. (2016) Single cell dynamic phenotyping. Sci Rep 6:34785. 10.1038/srep34785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aw Yong KM, Zeng Y, Vindivich D et al. (2014) Morphological effects on expression of growth differentiation factor 15 (GDF15), a marker of metastasis. J Cell Physiol 229:362–373. 10.1002/jcp.24458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purvis JE, Karhohs KW, Mock C et al. (2012) p53 dynamics control cell fate. Science 336:1440–1444. 10.1126/science.1218351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmieri B, Bresler Y, Wirtz D, Grant M (2015) Multiple scale model for cell migration in monolayers: elastic mismatch between cells enhances motility. Sci Rep 5:11745. 10.1038/srep11745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M-H, W P-H, Staunton JR et al. (2012) Mismatch in mechanical and adhesive properties induces pulsating cancer cell migration in epithelial monolayer. Biophys J 102:2731–2741. 10.1016/j.bpj.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CS, Mrksich M, Huang S et al. (1997) Geometric control of cell life and death. Science 276:1425–1428. 10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- 10.Rangamani P, Lipshtat A, Azeloglu EU et al. (2013) Decoding information in cell shape. Cell 154:1356–1369. 10.1016/j.cell.2013.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakal C, Aach J, Church G, Perrimon N (2007) Quantitative morphological signatures define local signaling networks regulating cell morphology. Science 316:1753–1756 [DOI] [PubMed] [Google Scholar]
- 12.Sero JE, Sailem HZ, Ardy RC et al. (2015) Cell shape and the microenvironment regulate nuclear translocation of NF-κB in breast epithelial and tumor cells. Mol Syst Biol 11:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slack MD, Martinez ED, LF W, Altschuler SJ (2008) Characterizing heterogeneous cellular responses to perturbations. Proc Natl Acad Sci U S A 105:19306–19311. 10.1073/pnas.0807038105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Held M, Schmitz MHA, Fischer B et al. (2010) CellCognition: time-resolved phenotype annotation in high-throughput live cell imaging. Nat Methods 7:747–754. 10.1038/nmeth.1486 [DOI] [PubMed] [Google Scholar]
- 15.Georgescu W, Wikswo JP, Quaranta V (2012) CellAnimation: an open source MATLAB framework for microscopy assays. Bioinformatics 28:138–139. 10.1093/bioinformatics/btr633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huth J, Buchholz M, Kraus JM et al. (2011) TimeLapseAnalyzer: multi-target analysis for live-cell imaging and time-lapse microscopy. Comput Methods Prog Biomed 104:227–234. 10.1016/j.cmpb.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 17.Huth J, Buchholz M, Kraus JM et al. (2010) Significantly improved precision of cell migration analysis in time-lapse video microscopy through use of a fully automated tracking system. BMC Cell Biol 11:24. 10.1186/1471-2121-11-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Q, Busetto AG, Fededa JP et al. (2012) Unsupervised modeling of cell morphology dynamics for time-lapse microscopy. Nat Methods 9:711–713. 10.1038/nmeth.2046 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez G, Fusco L, Benmansour F, et al. (2013) Automated quantification of morphodynamics for high-throughput live cell time-lapse datasets. In: 2013 IEEE 10th Int. Symp biomed imaging. IEEE, pp 664–667. [Google Scholar]
- 20.Carpenter AE, Jones TR, Lamprecht MR et al. (2006) CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7:R100. 10.1186/gb-2006-7-10-r100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyson DR, Garbett SP, Frick PL, Quaranta V (2012) Fractional proliferation: a method to deconvolve cell population dynamics from single-cell data. Nat Methods 9:923–928. 10.1038/nmeth.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzyubachyk O, van Cappellen WA, Essers J et al. (2010) Advanced level-set-based cell tracking in time-lapse fluorescence microscopy. IEEE Trans Med Imaging 29:852–867. 10.1109/TMI.2009.2038693 [DOI] [PubMed] [Google Scholar]
- 23.Dzyubachyk O, Essers J, van Cappellen WA et al. (2010) Automated analysis of time-lapse fluorescence microscopy images: from live cell images to intracellular foci. Bioinforma 26:2424–2430 [DOI] [PubMed] [Google Scholar]
- 24.Harder N, Mora-Bermudez F, Godinez WJ et al. (2009) Automatic analysis of dividing cells in live cell movies to detect mitotic delays and correlate phenotypes in time. Genome Res 19:2113–2124. 10.1101/gr.092494.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadanandan SK, Baltekin O, Magnusson KEG et al. (2016) Segmentation and track-analysis in time-lapse imaging of bacteria. IEEE J Select Topics Signal Process 10:174–184. 10.1109/JSTSP.2015.2491304 [DOI] [Google Scholar]
- 26.Masuzzo P, Huyck L, Simiczyjew A et al. (2017) An end-to-end software solution for the analysis of high-throughput single-cell migration data. Sci Rep 7:42383. 10.1038/srep42383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sezgin M, Sankur B (2004) Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging 13:146–168 [Google Scholar]