Abstract
Since the outbreak of the Coronavirus Disease 2019 (CoViD-19), the World Health Organization has recommended that, in absence of soap and water, alcohol-based hand sanitizer can be used to prevent the transmission of coronaviruses. Unfortunately, many media and anecdotal reports indicate that many alcohol-based hand sanitizers sold in South Africa are substandard and some contain potentially toxic ingredients. The study aimed to identify hand sanitizers used in the Johannesburg area during the CoViD-19 pandemic that do not contain the recommended alcohol concentration of at least 70% propanol or 60% ethanol, and contain traces of toxic ingredients. Hand sanitizers randomly collected from various traders around Johannesburg were analyzed using Agilent auto sampler coupled to a gas chromatograph utilizing flame ionisation detection. Of the 94 hand sanitizer samples collected, three preparations contained no alcohol, whereas the rest contained either ethanol, 2-propanol or 1-propanol or a combination of two alcohols. Of the alcohol-containing hand sanitizers, 37 (41%) contained less than 60% alcohol. Ethyl acetate, isobutanol and other non-recommended alcohols (methanol and 3-methyl-butanol) were also identified. Consumers are therefore warned that among the many brands of hand sanitizers found around Johannesburg, there are some substandard preparations and some that contain traces of toxic ingredients.
Subject terms: Biochemistry, Health care, Chemistry
Introduction
The gold standard for hand hygiene and prevention of the spread of non-airborne infectious diseases is regarded as washing with warm water and soap, because water and soap remove oils from hands that can harbour pathogens1. However, in the absence of water, hand sanitizers are recommended2,3. The transmission of respiratory pathogens spread by droplet or airborne routes is limited through respiratory hygiene/cough etiquette and physical space infection prevention measures4,5.
Since the outbreak of SARS-CoV-2, CoViD-19 (coronavirus), it is recommended by the World Health Organisation (WHO) that, in absence of water, the use of alcohol-based hand sanitizers can prevent the transmission of coronavirus6. Consequently, the demand for hand sanitizers has increased worldwide including South Africa, resulting in a surge in the trade of hand sanitizers and initially leading to shortages in their supply.
Hand sanitizer formulations exist in the form of liquids, gels and foams. Depending on the active ingredient used, hand sanitizers can be classified as one of two types: alcohol-based and alcohol-free. Alcohol-based hand sanitizers are recommended for general use, whereas the alcohol-free ones are not7,8. Hand sanitizers with less than the recommended alcohol content (60–95% alcohol) have been found not to work well for many types of pathogens, in that they may merely reduce their growth rate and hence reduce their numbers rather than kill them outright9,10.
Alcohol-based hand sanitizers are available in the form of rinses (liquid) and rubs (gel, foam and cream), and both are effective agents for reducing the number of viable pathogens, including coronavirus, on hands. Alcohol-based hand sanitizers may contain a variety of alcohols [e.g., isopropyl alcohol (isopropanol, 2-propanol), ethanol (ethyl alcohol), n-propanol (1-propanol)] or a combination of two of these, including other ingredients11–14.
For alcohol-based hand sanitizers, the US Centres for Disease Control and Prevention (CDC) recommends a concentration of 60–95% ethanol or 2-propanol mixed with distilled water15. Alcohol acts on microbes in the presence of water by making the organism cell membrane permeable leading to cytoplasm leakage, denaturing of proteins and eventually, cell lysis12. At higher concentrations (> 95%) alcohol is not effective since microbial denaturing of proteins only takes place in the presence of water16. Alcohols with four carbons and more are hence, not recommended to be used as hand sanitizers since they are less soluble in water2.
Ethanol has been shown to be effective against a variety of enveloped viruses, beginning at concentrations of 42.6%17. Addition of acids to ethanol can substantially improve the virucidal activity against most viruses17. For example, a formulation with low alcohol content and citric acid was found to inactivate all enveloped and non-enveloped viruses18. Several studies demonstrate that 2-propanol is considerably less effective compared to ethanol against viruses17. Some studies have also shown that ethanol gel formulations, unless they have been specially formulated and tested are less efficacious than ethanol solution formulations19, even though this has not yet been proven for SARS-CoV-2.
As previously indicated the global medical crisis as a result of the CoViD-19 pandemic has resulted in a great surge in the trade of hand sanitization products. This emergent situation is expected to continue for a considerable period of time until more efficient infection preventive measures become available, hence hand sanitizer demand will remain for an extended time. Unfortunately, many hand sanitizers in South Africa have not been verified to meet the regulators’ recommendations or that they are manufactured under the stipulated regulatory conditions20,21. In addition, the regulator [South African Bureau of Standards (SABS)] lacks verifiable information to ascertain the methods being used to prepare hand sanitizers at homes and to determine if these sanitizers are safe for use on human skin. As part of public awareness campaign and contribution to assist during the CoViD-19 pandemic, the project aimed to identify sanitizers available and used in the Johannesburg area that do not contain the recommended quality and alcohol content. In South Africa, alcohol-based hand sanitizers must comply with the standard SANS 490 as recommended by SABS20. The standard specifies that a minimum of 70% alcohol content is required if; alcohol, such as ethanol, isopropanol or n-propanol is the main ingredient; and that 60% alcohol content is required if there are other active ingredients. Solvents such as acetone (propanone), methanol, methylated spirits or other spirits are not allowed to be used.
Materials and methods
Collection of hand sanitizer samples
Ninety-four (94) samples of hand sanitizer sold in retail stores, spaza shops (informal convenience shop business in South Africa) and by street vendors, were randomly collected around Johannesburg during the period March to June 2020. The products were purchased ensuring not to buy repeat products/brands. Where two products of the same brand were included in the study it was so that one represents a gel and the other a liquid hand sanitizer. The hand sanitizer (HS) samples were labelled as HS1 to HS94 (Table 1).
Table 1.
A list of hand sanitizers whose alcohol content was assessed.
| Code | Trade name | Gel/liquid | % Alcohol (stated on container) |
|---|---|---|---|
| HS 1 | ADCO HYGIENE (HD) | Liquid | 70 |
| HS 2 | ADCO HYGIENE (WG) | Gel | 40 |
| HS 3 | Alcosan | Liquid | Not stated |
| HS 4 | Alcosan L (FG) | Aerosol | 40 |
| HS 5 | Ackermans | Gel | Not stated |
| HS 6 | BIOSOL | Gel | 70 |
| HS 7 | Century Chemicals | Gel | Not stated |
| HS 8 | Chaitoo Medical Supplies | Liquid | 70 |
| HS 9 | Classic Guard | Gel | 70 |
| HS 10 | Classic Guard | Liquid | 70 |
| HS 11 | Zamtha Hygiene | Gel | 70 |
| HS 12 | Clere | Gel | Not stated |
| HS 13 | Clere | Gel | Not stated |
| HS 14 | Clicks Expert | Gel | 70 |
| HS 15 | Cliks Helping Hand Trust | Gel | Not stated |
| HS 16 | Cosmo essentials | Gel | 63 |
| HS 17 | Cuticura | Gel | Not stated |
| HS 18 | Devlon | Liquid | 74 |
| HS 19 | DH | Gel | 70 |
| HS 20 | No Name | Gel | Not stated |
| HS 21 | dpachem | Liquid | 70 |
| HS 22 | ef-active | Gel | 72 |
| HS 23 | Garnier | Gel | 65 |
| HS 24 | Germ Bros | Liquid | 95 |
| HS 25 | Germ-xterminator | Gel | Not stated |
| HS 26 | GERMEX | Gel | Not stated |
| HS 27 | Lemon Verbena | Gel | Not stated |
| HS 28 | Handi Kleen | Gel | Not stated |
| HS 29 | HiDerm | Liquid | 70 |
| HS 30 | Hydralab | Gel | 70 |
| HS 31 | Identity | Gel | 65 |
| HS 32 | Identity | Gel | Not stated |
| HS 33 | i-Med | Aerosol | 70 |
| HS 34 | IMPO | Liquid | 70 |
| HS 35 | Izemo Services Group | Liquid | Not stated |
| HS 36 | Journey | Aerosol | 62 |
| HS 37 | Kmanufacturing | Liquid | 70 |
| HS 38 | Laboratoire Armille (Fyto) | Liquid | 70 |
| HS 39 | Lifebuoy | Gel | Not stated |
| HS 40 | Liquid Clinic | Liquid | 70 |
| HS 41 | Liquid Clinic | Aerosol | 70 |
| HS 42 | Little Animals | Liquid | Not stated |
| HS 43 | LP | Gel | 71 |
| HS 44 | Mellow | Gel | 62 |
| HS 45 | Micro Safe | Gel | 70 |
| HS 46 | Milton | Gel | Not stated |
| HS 47 | Nature's Nourishment | Gel | 62 |
| HS 48 | No Germ | Liquid | 70 |
| HS 49 | No Name | Liquid | 70 |
| HS 50 | No Name | Liquid | Not stated |
| HS 51 | No Name | Liquid | 70 |
| HS 52 | No Name | Liquid | Not stated |
| HS 53 | No Name | Gel | Not stated |
| HS 54 | No Name | Gel | Not stated |
| HS 55 | No Name | Liquid | 70 |
| HS 56 | Noxaderm | Gel | 70 |
| HS 57 | Oh So Heavenly | Gel | Not stated |
| HS 58 | Omni Protect | Aerosol | 70 |
| HS 59 | Pakmed | Liquid | 70 |
| HS 60 | Pepper Tree | Gel | Not stated |
| HS 61 | Phepha Hand Cleansing Sanitizer | Liquid | 72 |
| HS 62 | ProCare | Gel | 70 |
| HS 63 | Puresse | Gel | Alcohol Free |
| HS 64 | Puridene | Liquid | 60 |
| HS 65 | Puridene | Liquid | 70 |
| HS 66 | Renew | Gel | 70 |
| HS 67 | Ryadsa Med (Lavender Blue) | Liquid | Not stated |
| HS 68 | SA Chemical Products (Hy-gene) | Liquid | Not stated |
| HS 69 | Safeguard | Gel | Not stated |
| HS 70 | Sani | Gel | 70 |
| HS 71 | SHARP | Liquid | 70 |
| HS 72 | Soft Chemical Laboratories | Aerosol | 70 |
| HS 73 | Triple K | Liquid | 75 |
| HS 74 | Unicad cleaning solutions (Eco Blast) | Aerosol | Not stated |
| HS 75 | Voi (pamper yourself) | Gel | Not stated |
| HS 76 | VP Herbal | Gel | 70 |
| HS 77 | Woolworths Food Hand Cleanser | Gel | Not stated |
| HS 78 | Woolworths Food Hand Cleanser (Pineapple) | Liquid | Not stated |
| HS 79 | Woolworths Food Hand Cleanser (spray cucumber) | Liquid | Not stated |
| HS 80 | Hand San Plus | Gel | 75 |
| HS 81 | Manly | Aerosol | 70 |
| HS 82 | Scarlet Hill (your hands biggest fan) | Gel | 70 |
| HS 83 | Life Trek | Liquid | 75 |
| HS 84 | No Name (Mr Price) | Liquid | Not stated |
| HS 85 | Paw Patrol | Gel | Not stated |
| HS 86 | Pride (Xtra Care) | Gel | Not stated |
| HS 87 | Sani Hand sanitiser (Blue) | Gel | Not stated |
| HS 88 | Bennetts Family Care | Aerosol | 80 |
| HS 89 | Clean | Gel | 70 |
| HS 90 | ProCare | Gel | Not stated |
| HS 91 | No Name | Liquid | Not stated |
| HS 92 | W.LAB | Gel | 70 |
| HS 93 | NIOH Sample | Liquid | Not stated |
| HS 94 | No Name | Liquid | Not stated |
Preparation of internal standard (2% acetaldehyde)
Two millilitres (2 ml) of acetaldehyde (Sigma-Aldrich, Germany) were added to a 100 ml volumetric flask. Deionized water was added to make up the volume to the mark.
Preparation of 2% stock standards
Two millilitres of each reagent (Sigma-Aldrich) were added to a 100 ml volumetric flask. Deionized water was added to make up the volume to the mark. A stock of each of the following reagents was prepared; methanol, ethanol, 1-propanol, 2-propanol, isobutanol, 3-methyl-butanol and ethyl acetate.
Preparation of calibration standards
A calibration standard was prepared in a range of 0.1–1.8% by diluting the stock solution with deionized water. The standards were each prepared in a 10 ml headspace vial, capped and mixed well on a vortex mixer. The standards were then immediately placed onto the headspace auto sampler tray for analysis.
Preparation of quality controls
A 2% quality control stock solution was prepared by adding 2 ml of alcohol (Sigma-Aldrich, Germany) to a 100 ml volumetric flask and filling up to the mark with deionized water. Three quality controls at low (QC 1, 0.2%), medium (QC 2, 1.0%) and high level concentration (QC 3, 1.6%) were prepared from the stock solution. Each QC was prepared in a 10 ml headspace vial, capped with septa and aluminium crimp cap and mixed well on a vortex mixer. All 3 QCs were prepared in duplicate and positioned on the auto sampler tray for analysis after calibration standards and after every 5 duplicate samples.
Preparation of hand sanitizer samples
Preparation of liquid hand sanitizer samples
In a sterile polypropylene cup (urine container) was pipetted 350 µl sanitizer to which was added 25.65 ml deionized water. Then 900 µl of this solution was transferred to a 10 ml headspace vial to which 100 µl of internal standard was also added. The vial was capped and contents mixed thoroughly on a vortex mixer before analysis.
Preparation of gel hand sanitizer samples
In a sterile polypropylene cup on a weighing balance 10 g of deionized water was measured and 0.350 g of gel hand sanitizer was also added. The urine container was filled up with more deionized water until a mass of 25 g was reached. The cup was capped and shaken to mix contents well. Then 900 µl of this solution was pipetted into a 10 ml headspace vial to which was also added 100 µl of internal standard. The vial was capped and contents mixed on a vortexer before analysis.
Analysis of samples by headspace gas chromatography connected to a flame ionisation detector (HS-GC/FID)
Following sample preparation samples were immediately placed onto the Agilent G1888 headspace auto sampler tray (Agilent Technologies, USA) for analysis. The samples were analysed using a 6890N Agilent gas chromatograph (Agilent Technologies, USA) utilizing a flame ionisation detector. The column of choice was a SUPELCOWAX column (L = 30 m, ID = 0.25 mm and film thickness = 0.5 µl) purchased from Sigma-Aldrich.
Data acquisition and processing
Quantitation was performed using the Agilent OpenLab CDS ChemStation Edition C.01.05 integration software for GC Systems, accompanying the GC system22. A determination coefficient (r2) of more than 0.999 was obtained for the calibration curves. Method accuracy, precision and repeatability were assessed by calculating the standard deviation (SD) of replicate measurements, the standard error of the mean (SEM) and the coefficient of variation (CV %) (see Tables S2–S6, in Supplementary Material).
Data analysis
Results were analyzed using Microsoft Excel. Descriptive statistics using tables, mean and percentage was used to describe the data obtained.
Results
Ninety-four (94) samples of hand sanitizer sold in retail stores, spaza shops and by street vendors, were randomly collected around Johannesburg during the period March to June 2020. The samples consisted of fifty (50) gels and forty-four (44) liquids as presented in Fig. 1. Forty of the sanitizers (14 liquids and 26 gels) did not have their alcohol content stated on the container and only one sample was clearly indicated as alcohol-free (Table 1). This sample set represents most of the hand sanitizer brands available and/or sold in retail stores, spaza shops and by individuals, that are in use in different households and various workplaces around Johannesburg during the CoViD-19 pandemic.
Figure 1.
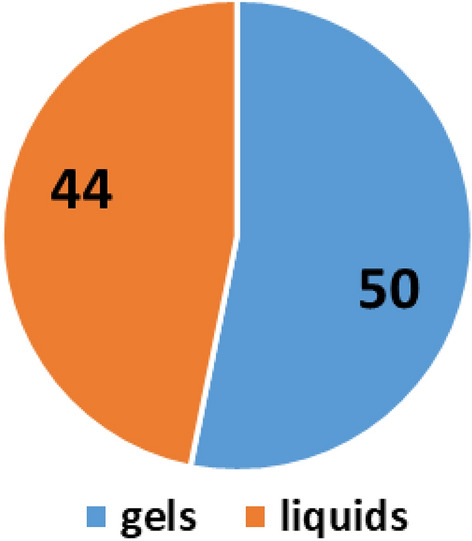
A pie chart of hand sanitizers collected around Johannesburg, comprising of gels and liquids.
Of the 94 hand sanitizer samples collected, three sanitizer preparations were found to contain no alcohol, whereas the rest contained either ethanol or 2-propanol or a combination of these two (Table S1). Only one hand sanitizer sample contained solely 1-propanol. By comparison, liquid formulations had on average less alcohol (56.38 ± 26.74%) than the gel formulations (66.14 ± 20.95%). Of the alcohol-containing sanitizers, 37 (41%) contained less than 60% alcohol. Toxic alcohol denaturants (ethyl acetate and isobutanol) and other non-recommended alcohols (methanol and 3-methyl-butanol) were also identified in 17% of these preparations (Fig. 2).
Figure 2.
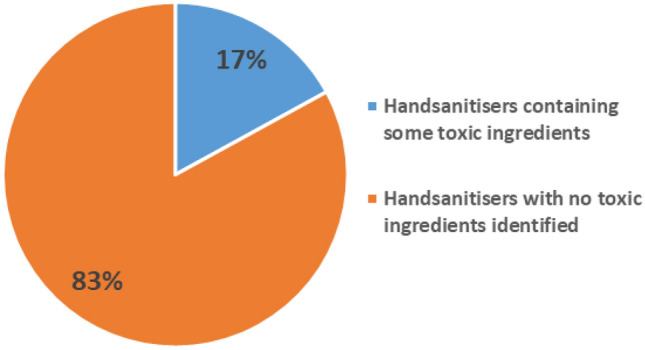
A pie chart showing a proportion of the analysed hand sanitizers found to contain toxic ingredients.
Results from this study indicate that there are about similar number of gel hand sanitizers in existence around Johannesburg as the liquid formulations (50 gels versus 44 liquids). However, there is no certainty that this is a true representation of the then available alcohol-based hand sanitizing products as no statistical techniques were employed to collect these hand sanitizer samples. By comparison, liquid formulations (56.38 ± 27%) had on average less alcohol than the gel formulations (66.14 ± 20.95%).
To assess the quality of the results, the following criteria were examined so as to confirm the accuracy and robustness of the analytical method23. All peaks including ethanol and isopropanol which eluted very close to each other, had baseline separation. Peaks were sharp with narrow baseline width. The results were repeatable and reproducible as shown in Tables S2 and S3, passing the repeatability and reproducibility tests with the difference between two results chosen randomly being less than 2.83 × SD.
The average and standard deviation of the retention times for all the compounds identified were calculated. Results showed acceptable performance within run and between runs. Data for the internal standard stability and reliability are provided in Table S4. Data were generated on different days and by two analysts. There was minimal drift in retention times for all three levels of quality control throughout all the analytical runs. Linearity for the responses was assessed by examining the correlation coefficients for the calibration for all analytical runs. It is evident as shown in Table S5 that there was strong positive association (linear response) between the concentrations and the signal responses for all the analytes. Furthermore, the deviations in linearity between the analytical runs were minimal with all correlations remaining at 99% positive linear association.
Two quality controls of each of the three levels were run immediately after calibration and thereafter after every 10 samples and at the end of the analytical run. Box and Whisker plots were used to identify and remove all outliers in the data sets. Results for the quality controls were acceptable as can be seen in Table S6. Average recoveries (Av Recovery %) were acceptable. As this was a non-standard in-house developed method, Guidelines for Standard Method Performance Requirements AOAC Official Methods of Analysis24 was used to estimate acceptable recoveries. The recoveries were deemed acceptable for this work.
Discussion
While more (56%) brands of hand sanitizer in this study contained the recommended concentration of alcohol, there were also many (44%) substandard and possibly subpotent preparations. Unfortunately, tests to determine if any of the analyzed hand sanitizers with lower alcohol content than is recommended had any of the virucidal activity enhancing ingredients, such as acids, were beyond the scope of this study. The study also found that only 30% (10 gels and 9 liquids) of the analyzed hand sanitizers contained ≥ 80% alcohol. Even though alcohol concentrations higher than 80% are known to be less potent against bacteria because proteins are not easily denatured in the absence of water, this bodes well for disinfection against SARS-CoV-2 as ethanol at ≥ 80% is highly effective against enveloped viruses19. Moreover, it was found that some hand sanitizers contained, in addition to the acceptable alcohols (ethanol, 1-propanol and 2-propanol), some toxic ingredients, such as ethyl acetate, 3-Methyl-1-butanol and methanol. This is worrying because even if a hand sanitizer contains enough alcohol as recommended or contains ingredients that enhance its virucidal activity in case of low alcohol content (< 60%), the presence of toxic ingredients renders the preparation harmful and unfit for human use. It is for this reason that it is recommended that all consumers (workplaces and the public in general) be aware of untrustworthy brands of hand sanitizer supplying substandard and possibly sub-potent sanitizer preparations or sanitizers with toxic ingredients.
The tendency for unscrupulous manufacturers in South Africa is to mislead the public by labelling their products as “SABS Approved” yet not carrying the SABS Mark Scheme number. The SABS provides on its website the information that must be available on every container of approved hand sanitizer sold in South Africa21. Unknowingly, using a hand sanitizer with no virucidal activity against SARS-CoV-2 may give one a false sense of security, while those using sanitizers containing toxic ingredients are likely to suffer from the associated risks. For example, exposure to methanol through both ingestion and transdermal absorption, if left untreated, can be extremely dangerous, leading to significant disability and death25. Even if toxic substances are just traces, the typical frequent use of hand sanitizer products throughout the day can result in very high total exposure with consequent adverse health effects.
The US FDA is therefore continually adding certain hand sanitizers found to contain toxic ingredients to import alerts, to stop these products from legally entering the U.S. market26, while the South African Bureau of Standards (SABS) has also warned consumers about some unscrupulous manufacturers that are making false claims that their products are SABS-approved27.
Conclusion
Just like several other countries around the world, South Africa (SA) has relaxed legislation to make it easier for local businesses to rapidly produce alcohol-based hand sanitizers to meet the great surge in demand for hand sanitization products during the SARS-CoV-2 outbreak. However, those producing hand sanitizers are still advised to follow both the WHO and SABS guidelines, and avoid using poor alcohol quality which is likely to contain toxic substances. The SA public is also advised to remain alert to media reports that continually keep surfacing about hand sanitizer brands in violation of the SABS guidelines21, by producing sanitizer preparations that are subpotent or contain toxic substances.
It is also worth noting that the presence or addition of other pharmaceutical ingredients (e.g., chlorhexidine, triclosan, iodine/iodophores and benzalkonium chloride) may assist in instances where the alcohol-based hand sanitizers may fall short against certain bacteria and viruses11,12. However, though the presence of these other ingredients may impart additional antiviral and antimicrobial properties to the alcohol-based hand sanitizers, they may as well possibly exhibit some toxicity to humans. For example, although iodine is effective against most viruses and bacteria, it is also believed to cause skin irritation and discolouring, thus its presence may be harmful to humans12,18.
Supplementary Information
Acknowledgements
The authors would like to thank the National Institute for Occupational Health (NIOH), a Division of the National Health Laboratory Service (NHLS), for supporting this research. Also, greatly acknowledged are the NIOH Analytical Services team members (Angela Mawela, Lesiba Sethosa, Jane Mulaudzi and Sesitjie Moremi) for assisting in the collection of the hand sanitizer samples.
Author contributions
P.M. wrote the manuscript text. B.S., T.M. and B.D. performed data collection and analysis. P.P. assisted with analysis and results interpretation. B.K. reviewed and edited the manuscript, and supervised the team. All authors reviewed the final manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-08117-z.
References
- 1.Cohen SR, Ligda KO. Infectious Diseases. Basic Clinical Anesthesia. Springer Nature Switzerland AG; 2004. p. 647. [Google Scholar]
- 2.Bloomfield SF, Aiello AE, Cookson B, O'Boyle C, Larson EL. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am. J. Infect. Control. 2007;35(10):S27–S64. doi: 10.1016/j.ajic.2007.07.001. [DOI] [Google Scholar]
- 3.Thomson E, Bullied A. Production of ethanol-based hand sanitizer in breweries during the COVID-19 crisis. Tech. Q. 2020;57(1):47–52. [Google Scholar]
- 4.Chavis S, Ganesh N. Respiratory hygiene and cough etiquette. Infect. Control Dent. Off. 2019;18:91–103. doi: 10.1007/978-3-030-30085-2_7. [DOI] [Google Scholar]
- 5.World Health Organization. Transmission of SARS-CoV-2: Implications for infection prevention precautions: Scientific brief, 09 July 2020 (World Health Organization, 2020).
- 6.World Health Organization. Recommendations to Member States to improve hand hygiene practices to help prevent the transmission of the COVID-19 virus: Interim guidance, 1 April 2020 (No. WHO/2019-nCov/Hand_Hygiene_Stations/2020.1) (World Health Organization, 2020).
- 7.Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin. Microbiol. Rev. 2004;17:863–893. doi: 10.1128/CMR.17.4.863-893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd ECD, Michaels BS, Holah J, Smith D, Greig JD, Bartleson CA. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 10. Alcohol-based antiseptics for hand disinfection and a comparison of their effectiveness with soaps. J. Food Prot. 2010;73:2128–2140. doi: 10.4315/0362-028X-73.11.2128. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO guidelines on hand hygiene in health care (advanced draft): Global safety challenge 2005–2006: Clean care is safer care (World Health Organization, 2006).
- 10.Centers for Disease Control (CDC). Show me the science—When and how to use hand sanitizer in community settings (2019). https://www.cdc.gov/handwashing/show-me-the-science-hand-sanitizer.html. Accessed 29 March 2021.
- 11.Jing JL, Pei Yi T, Bose RJ, McCarthy JR, Tharmalingam N, Madheswaran T. Hand sanitizers: A review on formulation aspects, adverse effects, and regulations. Int. J. Environ. Res. Public Health. 2020;17(9):3326. doi: 10.3390/ijerph17093326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golin AP, Choi D, Ghahary A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control. 2020;48(9):1062–1067. doi: 10.1016/j.ajic.2020.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold, N. A., Mirza, T. M. & Avva. U. Alcohol Sanitizer. In StatPearls [Internet] (StatPearls Publishing, 2020). https://www.ncbi.nlm.nih.gov/books/NBK513254/. Accessed 29 March 2021. [PubMed]
- 14.World Health Organization. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. Annex G, Use of disinfectants: Alcohol and bleach (2014). https://www.ncbi.nlm.nih.gov/books/NBK214356/. Accessed 28 May 2020. [PubMed]
- 15.Centers for Disease Control and Prevention (CDC) Guidelines for hand hygiene in healthcare settings. MMWR. 2002;51(16):1–44. [Google Scholar]
- 16.DeMattei, C., Holman, D., Rossman, P. K., Auchtung, T. A. & Working Bugs LLC. Method for the formulation of hand sanitizer. U.S. Patent 9456602, 04 Oct 2016 (2016).
- 17.Kampf G. Efficacy of ethanol against viruses in hand disinfection. J. Hosp. Infect. 2018;98(4):331–338. doi: 10.1016/j.jhin.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ionidis G, Hübscher J, Jack T, Becker B, Bischoff B, Todt D, Hodasa V, Brill FH, Steinmann E, Steinmann J. Development and virucidal activity of a novel alcohol-based hand disinfectant supplemented with urea and citric acid. BMC Infect. Dis. 2016;16(1):1. doi: 10.1186/s12879-016-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochwoto M, Muita L, Talaam K, Wanjala C, Ogeto F, Wachira F, Osman S, Kimotho J, Ndegwa L. Anti-bacterial efficacy of alcoholic hand rubs in the Kenyan market, 2015. Antimicrob. Resist. Infect. Control. 2017;6:17. doi: 10.1186/s13756-017-0174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.South African Bureau of Standards. Alcohol-based hand sanitizer and handrub Amdt 2. South African National Standard. SANS 490:2020 Edition 1.2, ISBN 978-0-626-39219-2 (South African Bureau of Standards, 2020).
- 21.South African Bureau of Standards. SABS Mark Approved manufacturers and brands: HAND SANITISERS—SANS 490 | SANS 1853 (2020). https://www.sabs.co.za/COVID19-SABS-Mark/index.asp. Accessed 03 Oct 2021.
- 22.Agilent. OpenLab CDS ChemStation (Edition C.01.05) [Computer program] (Agilent Technologies, 2014). http://www.agilent.com/chem/openlabcds
- 23.Cool P, Ockendon M. Stats Book. WordPress; 2015. [Google Scholar]
- 24.Paez, V., Barrett, W. B., Deng, X., Diaz-Amigo, C. et al. AOAC SMPR® 2016.002. J. AOAC Int.99(4), 1122–1124 (2016). [DOI] [PubMed]
- 25.Ashurst, J. V., Nappe, T. M. Methanol toxicity. In StatPearls [Internet] (StatPearls Publishing, 2020). https://www.ncbi.nlm.nih.gov/books/NBK482121/. Accessed 29 March 2021.
- 26.FDA updates. FDA updates on hand sanitizers consumers should not use: [7/31/2020] FDA continues to find issues with certain hand sanitizer products (2020). https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-hand-sanitizers-consumers-should-not-use#60237d57f3846. Accessed 10 Feb 2021.
- 27.Media Xpose. Ensure claims that sanitizers have been sabs approved are valid. Media Xpose, Apr 21, 2020 (2020). https://mediaxpose.co.za/2020/04/21/ensure-claims-that-sanitizers-have-been-sabs-approved-are-valid/. Accessed 04 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.


