Abstract
Capsaicin (CAP) is an ingredient of peppers that has biological activities at low doses but causes gastrointestinal (GI) discomfort at high doses. However, the GI effects of high doses of CAP and the evaluation criteria to determine this remain unknown. To elucidate the dose-related effects of CAP on GI health, CAP was administered to mice at 40, 60, and 80 mg/kg doses. The results showed that 40 mg/kg CAP did not negatively affect GI tissues, while 60 and 80 mg/kg CAP damaged GI tissues and caused significant inflammation in the jejunum, ileum, and colon. The levels of serum substance P (SP) and calcitonin gene-related peptide (CGRP) were CAP-dose-dependent, and short-chain fatty acids (SCFAs) content significantly increased in the 80 mg/kg group. Correlation analysis revealed that the underlying mechanisms might be related to the regulation of gut microbiota, especially Bifidobacterium, Lactobacillus, Faecalibacterium, and Butyricimonas. These results suggest that oral administration of 60 and 80 mg/kg CAP in mice causes intestinal inflammation and high levels of serum neuropeptides and cecal SCFAs, which may be related to alterations in gut microbiota.
Keywords: capsaicin, gastrointestinal, inflammation, neuropeptides, TRPV1, gut microbiota
1. Introduction
Capsaicin (CAP) is the main pungent ingredient of chili peppers [1], which are spices with a unique spicy flavor widely consumed in various diets. CAP has demonstrated broad potent biological characteristics, including antioxidant, anti-obesity, pain-alleviating, and anti-inflammation effects [2]. Nevertheless, recent studies have revealed that CAP shows bioactivities under low doses but tends to have side effects under high doses. When CAP is continuously consumed at high levels, people may feel some uncomfortable gastrointestinal (GI) symptoms—such as heartburn, diarrhea, pain, and other symptoms—in their daily life [3]. Therefore, the GI effects of CAP ingestion have received increasing attention.
Owing to its pungency, high-dose CAP may inhibit gastric acid production [4], damage the GI mucosa by inducing gastric inflammation [5,6], cause structural changes of the intestinal barrier [7,8,9], and further result in other GI symptoms [10,11,12]. Nevertheless, results from studies on CAP vary because of the dosage and duration of CAP and the species and characterization of research subjects, making it difficult to confirm the specific GI effects of CAP. Therefore, there are no uniform and clear experimental models and evaluation criteria to determine the effects of CAP ingestion on GI health.
Against this background, the present research aimed to elucidate the effects of CAP on GI health and to investigate the mechanisms of CAP-induced GI injury. Tissue inflammation, histopathological injury, and serum levels of GI neuropeptides in mice were detected to reflect the effects of different oral doses of CAP on GI health. Furthermore, a correlation analysis was performed to determine if CAP regulates short-chain fatty acids (SCFAs) and the composition of gut microbiota. This may correlate CAP ingestion with GI health and provide more insight into biological safety studies on CAP.
2. Materials and Methods
2.1. Animals and Experimental Design
Twenty-four SPF male C57BL/6J mice (18 ± 2 g, 6-week-old) were purchased from Charles River (Beijing, China) and reared at a temperature of 25 ± 2 °C under an artificial lighting period (12 h light/12 h dark) in a barrier facility of the Animal Center of Jiangnan University. After seven days’ adaptive feeding, all mice were randomly divided into four groups of six mice each.
The experimental protocol is presented in Table 1. Briefly, the control group was intragastrically administered 200 μL of vehicle (3% ethanol and 10% Tween 80 in 0.9% saline) daily [13,14], while three CAP groups were intragastrically administered 200 μL of different concentrations of CAP dissolved in vehicle (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). The intragastrically administrated CAP concentrations were 40, 60, and 80 mg/kg. During the seven-day trial, the bodyweight, food intake, and water intake were counted and recorded every day. After seven days, the mice were anesthetized and sacrificed. Blood samples were collected from the mice orbital plexus and then centrifuged at 3000× g for 30 min to get the serum. Fresh fecal and cecal content were collected, then the samples were immediately frozen at −80 °C. Tissues of the stomach, jejunum, ileum, and colon were collected, and a portion of each tissue was separated and fixed with 4% paraformaldehyde, the rest was frozen at −80 °C. All animal experiments used in this study were approved by the Ethics Committee of Jiangnan University, China (JN. no. 20200630c0640815(126)) and complied with the guidelines of the 2010/63/European Community.
Table 1.
Animal experimental design.
| Group | Daily Gavage Treatment | Volume | Sample Size |
|---|---|---|---|
| Control | Vehicle | 200 μL | n = 6 |
| 40 mg/kg | CAP at a concentration of 40 mg/kg bodyweight | 200 μL | n = 6 |
| 60 mg/kg | CAP at a concentration of 60 mg/kg bodyweight | 200 μL | n = 6 |
| 80 mg/kg | CAP at a concentration of 80 mg/kg bodyweight | 200 μL | n = 6 |
2.2. Histopathology and Measurement of Inflammatory Cytokine Levels in GI Tissues
Some tissues of the stomach, jejunum, ileum, and colon were fixed in 4% paraformaldehyde and stained with hematoxylin and eosin (H&E). Images of the paraffin sections were taken by scanning with a software named Pannoramic MIDI Digital Slide Scanner (3D-Histech Co., Ltd., Budapest, Hungary). The software CaseViewer 2.3 and Image J were used to measure the histopathological injury, and the valuation criteria are referred to the previous study [15].
Tissues, including the remaining stomach, jejunum, ileum, and colon, were homogenized with the phosphate buffer (containing 1% protease inhibitor cocktail) and centrifuged at 8000× g for 15 min. The concentrations of interleukin (IL)-10, TNF-α, and IL-1β were quantized using the ELISA kits (R&D Systems, Minneapolis, MN, USA).
2.3. Measurement of Serum Neuropeptide
The contents of substance P (SP) and calcitonin gene-related peptide (CGRP) in the serum of the mice were analyzed using commercial ELISA kits (Mlbio, Shanghai, China).
2.4. Determination of Levels of SCFAs in Cecal Content
The contents of SCFAS in the ceca were analyzed using gas chromatography–mass spectrometry (GC-MS) as previously described [16], including acetic, propionic, and butyric acid. The cecal contents were freeze-dried and approximately 50 mg of the samples were suspended with saturated NaCl for 30 min. SCFAs were then extracted by diethyl ether and then quantified by GC-MS (QP2010 Ultra system, Shimadzu Corporation, Kyoto, Japan).
2.5. 16S rRNA Gene Sequencing
Total DNA of fecal samples was extracted by a commercial kit (MP Biomedicals; Carlsbad, CA, USA). The 16S rRNA gene was PCR-amplified with primers (341F/806R) specific for the V3–V4 region. The PCR products were stained with 4% nucleic acid dye, electrophoresed on a 1.5% agarose gel (SBS Genetech Co., Ltd., Beijing, China), and used a QIAquick Gel Extraction Kit (Qiagen GmbH, Hilden, Germany) to purify. The purified DNA concentration was determined using Qubit 4 Fluorometer (Life Technologies Corporation, Carlsbad, CA, USA) and the amplicon libraries were pooled in equimolar amounts and sequenced by MiSeq (Illumina, Santiago, CA, USA) at Jiangnan University (Wuxi, China). The sequence data were mapped into operational taxonomic units (OTUs) with a similarity of 97%.
2.6. Statistical Analysis
Statistical analyses were conducted with Origin 2018 and SPSS v22.0. All data are indicated as the mean ± standard error (SEM), and one-way analysis of variance (ANOVA) according to LSD post hoc tests was used to compare multiple groups. p < 0.05 was considered as significant. Microbiota analysis was performed using QIIME 2 pipeline, Shannon indexes, and Chao1 index, while principal coordinates analysis (PCoA) was implemented using marker data profiling (https://www.microbiomeanalyst.ca/ (accessed on 7 August 2021)). Linear discriminant analysis (LDA) effect size (LEfSe) was implemented online (http://huttenhower.sph.harvard.edu/galaxy (accessed on 8 August 2021)). Correlation analysis was implemented using R version 3.6.3.
3. Results
3.1. Effects of CAP on Body Weight and Food and Water Intake
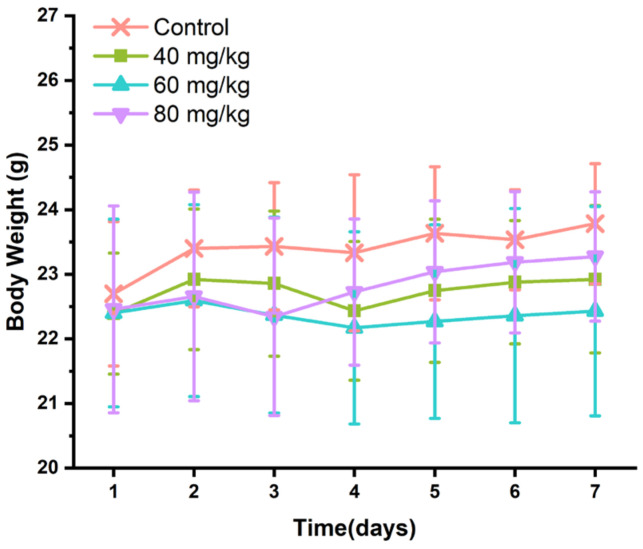
With the daily bodyweight of the mice measured, no significant weight change was observed in the 7-day treatment with CAP (p > 0.05). Interestingly, the average bodyweight of mice in different doses of CAP groups was lower than that in the control group, but not significant (Figure 1). Meanwhile, there were no significant differences in food intake and water intake (Supplementary Figure S1) between the experimental groups, but the mice treated with high-dose CAP (60 and 80 mg/kg) had a more erratic and dispersive water intake than the control group.
Figure 1.
Effects of CAP on body weight of mice.
3.2. Effects of CAP Administration on the Histopathology of GI Tissues
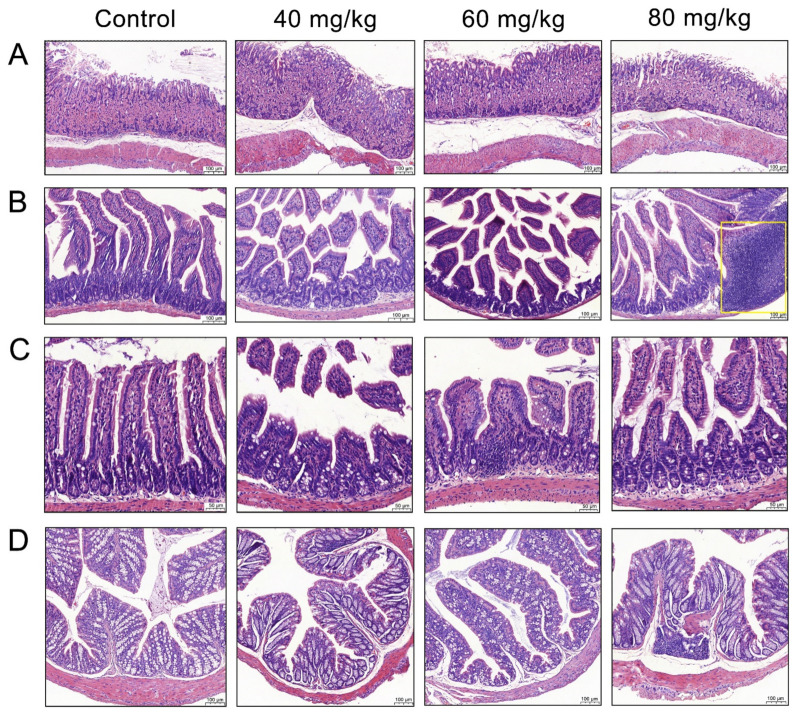
Owing to the pungent properties of CAP, the morphology of GI tissue was observed to determine whether CAP caused histopathological injury. H&E staining was used for the histopathological evaluation of the stomach, jejunum, ileum, and colon (Figure 2A).
Figure 2.
Morphology of the different gastrointestinal tissues. (A) Stomach, (B) jejunum, (C) ileum, and (D) colon.
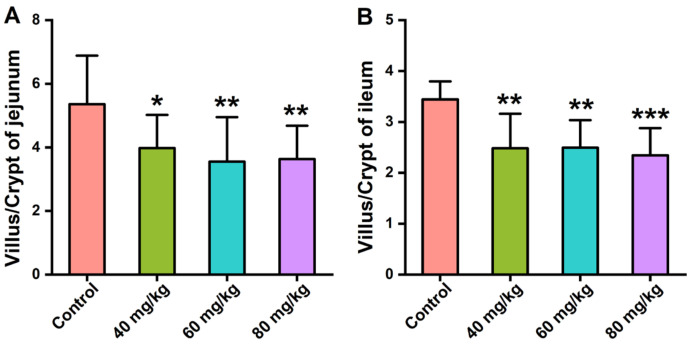
The gastric mucosa of the control group was intact, and their nuclei were clearly visible. The groups treated with different concentrations of CAP showed slight cell vacuolization, but there was no significant difference when compared with the control group. The jejunum of the control group mice had tidy villi with healthy crypt structures and intact mucous membranes, while the jejunal villi in the CAP-treated groups were irregularly arranged with local villus breakage and shedding (Figure 2B). The group treated with 80 mg/kg CAP showed inflammatory cell infiltration. We further evaluated the ratio of villus height to crypt depth (V/C) in the jejunum. Compared with the control group, treatment with 40, 60, and 80 mg/kg CAP reduced the V/C ratio in the jejunum by 25.7%, 33.7%, and 32.2%, respectively (Figure 3A). Similar results were observed in the histopathology of the ileum, where the ileal villi of the CAP-treated groups were shortened. Treatment with 40, 60, and 80 mg/kg CAP reduced the V/C ratio of the ileum by 27.9%, 27.6%, and 31.9%, respectively, compared with the control group (Figure 3B), accompanied by local villus breakage and crypt hyperplasia (Figure 2C).
Figure 3.
Effects of CAP on the villi/crypt of the intestine, specifically in the (A) jejunum and (B) ileum. p < 0.05, ** p < 0.01, and *** p < 0.001 vs. control.
The colon tissue of mice in the control group showed enriched goblet cells and healthy crypt structures with tidy villi, while the mice in the CAP-treated groups showed inflammatory cell infiltration, and loss of crypt and goblet cells (Figure 2D). Remarkably, mice treated with 60 mg/kg CAP showed a loss of mucus-producing goblet cells compared with mice in the control group, and inflammatory cell infiltration was evident in the 80 mg/kg CAP-treated mice. The statistical analyses of the colonic crypt height and goblet cell numbers were shown in Supplementary Figure S2.
3.3. Effects of CAP on the Levels of Inflammatory Cytokines in Gastrointestinal Tissues
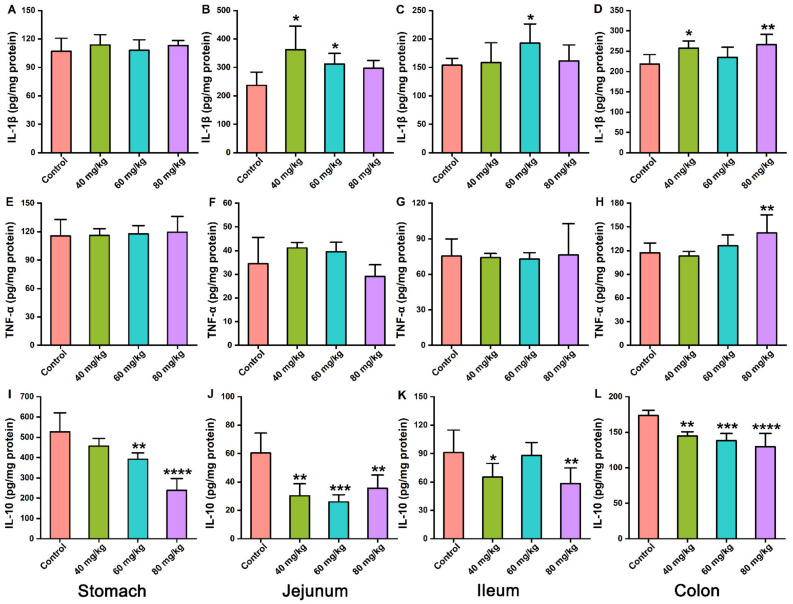
Levels of inflammatory cytokines in gastrointestinal tissues were measured to evaluate whether CAP induced gastrointestinal injury via an inflammatory effect. The results revealed that CAP administered orally at a dose of 40–80 mg/kg increased IL-1β levels in the jejunum and colon (Figure 4B,D) compared with the control group. The levels of TNF-α in the stomach, jejunum, ileum, and colon showed no significant differences among all CAP-treated groups, except in the colon of the 80 mg/kg CAP group (Figure 4E–H). However, all gastrointestinal tissues showed a decrease in IL-10 levels after CAP treatment (Figure 4I–L). Remarkably, the decrease seemed to be CAP-dose-dependent in colon tissues (Figure 4L). Consequently, oral administration of CAP influenced the levels of anti-inflammatory cytokines in the stomach and ileum, but did not induce severe inflammatory injury. However, CAP caused inflammatory injury in the jejunum and colon at high doses (80 mg/kg) by increasing IL-1β and TNF-α levels and decreasing IL-10 levels.
Figure 4.
Effects of CAP on inflammatory cytokine levels in the stomach, jejunum, ileum, and colon tissues. (A–D) IL-1β, (E–H) TNF-α, and (I–L) IL-10. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 vs. control.
3.4. Effects of CAP on the Neuropeptide Levels in Serum
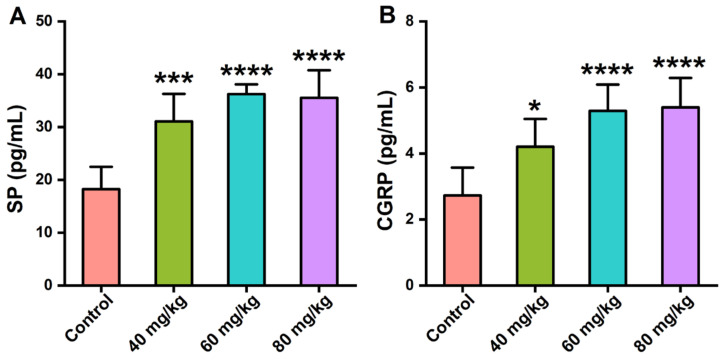
The serum levels of SP and CGRP were measured to reflect the effects of CAP on the secretion of gastrointestinal neuropeptides in mice. As shown in Figure 5, CAP orally administered at doses of 40, 60, and 80 mg/kg significantly increased the serum levels of SP and CGRP, which were CAP-dose-dependent.
Figure 5.
Effects of CAP on the serum levels of gastrointestinal neuropeptides. (A) SP levels and (B) CGRP levels. * p < 0.05, *** p < 0.001, and **** p < 0.0001 vs. control.
3.5. Effects of CAP on the Levels of Cecal SCFAs
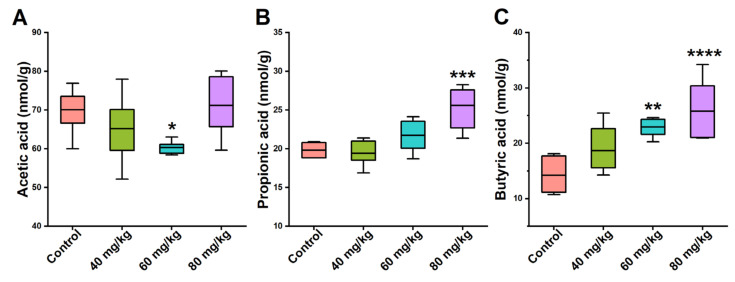
SCFAs are the main metabolites of intestinal microorganisms, mainly due to the breakdown of polysaccharides or dietary fiber. Therefore, the levels of SCFAs in cecal content—including acetic, propionic, and butyric acid—in the mice were quantified using GC-MS. Compared with the control group, the cecal level of acetic acid in the 60 mg/kg CAP-treated group was dramatically lower, while all the other groups showed no significant difference (Figure 6A). Orally administered CAP caused a dose-dependent increase in the cecal level of propionic acid. However, compared with the control group, only the 80 mg/kg dose showed a significant difference (Figure 6B). The trends in the levels of butyric acid in the 40, 60, and 80 mg/kg CAP-treated mice were similar to those of propionic acid. After 7-day treatment with 60 and 80 mg/kg CAP, butyric acid levels significantly increased (Figure 6C).
Figure 6.
Effects of CAP on the levels of cecal SCFAs. (A) Acetic acid, (B) propionic acid, and (C) butyric acid. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 vs. control.
3.6. CAP Modulates the Composition of Gut Microbiota
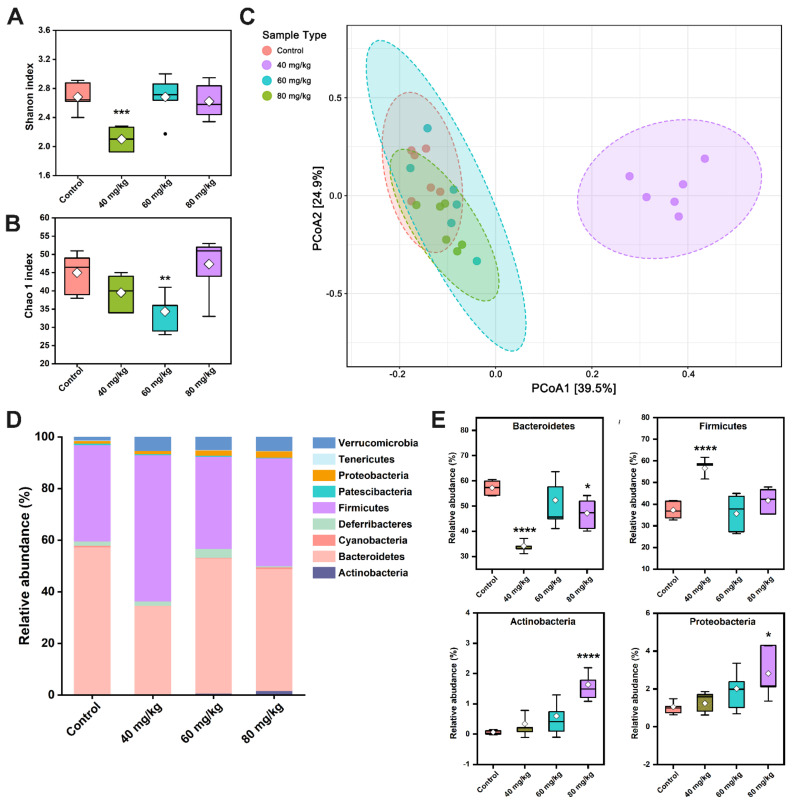
To assess if various doses of CAP regulate the gut microbiota, high-throughput sequencing was performed for analysis and comparison. The alpha diversity is reflected by the Shannon index and Chao1 index. Compared with the control group, the 40 mg/kg CAP-treated group showed dramatically decreased Shannon indices (p < 0.001) and a slightly decreased the Chao1 index, but there were no significant differences (Figure 7A). Meanwhile, 60 mg/kg CAP treatment significantly decreased the Chao1 index compared with the control, while the other groups showed no significant change (Figure 7B). Beta-diversity was measured using PCoA of the weighted UniFrac distance (Figure 7C), revealing distinct bacterial communities between the control and different doses of CAP-treated groups. The results reflected that the gut microbiota of mice in the 40 mg/kg CAP-treated group was significantly different from that in the control group. However, the 60 and 80 mg/kg CAP-treated mice showed no clear changes in intestinal microflora structure from the mice in the control group.
Figure 7.
Effect of CAP on the overall structure of gut microbiota. The (A) Shannon index, (B) Chao1 index, and (C) PCoA, with extended functionality for the labeling groups and with normal probability ellipsoids for different groups. (D) Distribution of bacterial taxa at the phylum level. (E) Comparison of the main phyla in the different groups: Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 vs. control.
At the phylum level, the dominant phyla in control mice were Bacteroidetes (57.24%) and Firmicutes (37.24%), followed by Deferribacteres (1.68%), Verrucomicrobia (1.41%), and Proteobacteria (1.06%) (Figure 7D). Compared with the control group, 40 mg/kg CAP treatment dramatically reduced the abundance of Bacteroidetes to 34.17% but increased the abundance of Firmicutes (56.64%) (p < 0.0001). Notably, CAP treatment at 80 mg/kg significantly reduced the relative abundance of Bacteroidetes, and increased the abundance of Actinobacteria and Proteobacteria (Figure 7E).
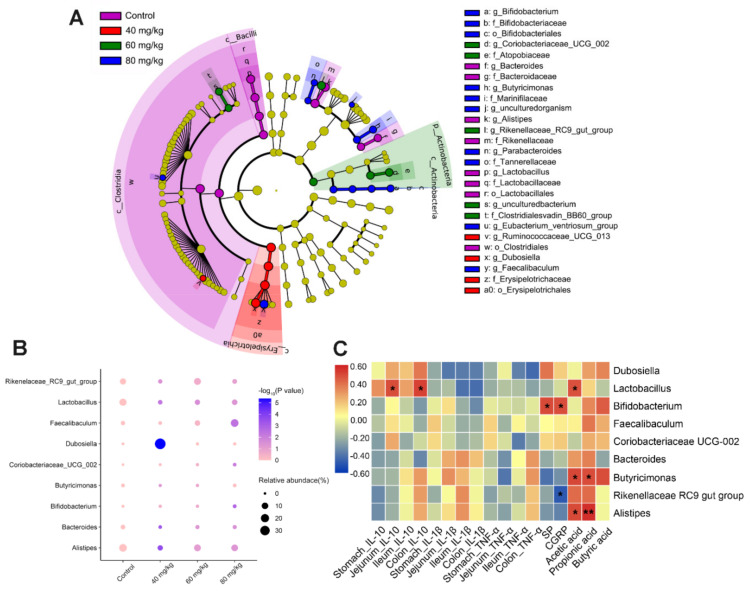
LEfSe was used to further identify the major bacterial biomarkers that revealed the dominant microorganisms in each group. Dominant bacterial markers of 8, 4, 5, and 10 taxa were found in the control, 40, 60, and 80 mg/kg CAP-treated groups, respectively (Figure 8A). The genera with the greatest differences included Butyricimonas, Lactobacillus, Faecalibaculum, Coriobacteriaceae_UCG_002, Bifidobacterium, Rikenellaceae_RC9_gutgroup, Bacteroides, Alistipes, and Dubosiella. The relative abundance of selected genera with significant differences was analyzed, showing that CAP treatment increased the proportion of Bifidobacterium and Faecalibaculum in a dose-dependent manner, but only showed a significant change in the 80 mg/kg CAP-treated group (Figure 8B). Moreover, the relative abundance of Lactobacillus and Alistipes was significantly reduced in the CAP-treated groups, especially in the group treated with 40 mg/kg CAP. Furthermore, compared with the control group, 40 mg/kg CAP treatment drastically elevated the proportion of Dubosiella, but reduced the abundance of Bacteroides, Butyricimonas, and Rikenellaceae_RC9_gut_group. Treatment with 80 mg/kg CAP resulted in an increased abundance of Coriobacteriaceae_UCG_002.
Figure 8.
Changes in gut microbiota after CAP treatment. (A) LEfSe analysis of gut microbiota is shown as a cladogram among all groups (LDA scores > 3.0). (B) Relative abundance of differential microorganisms at the genus level. p-values represent the discrepancy compared with the control group. Values are reported after one-way ANOVA followed by the LSD multiple-comparison test. (C) Heatmap of Spearman’s correlation between the relative abundance of gut microbiota at the genus level and the relevant indicators. * p < 0.05, and ** p < 0.01 vs. control.
3.7. Spearman’s Correlation Analysis between Gut Microbiota, Cytokines, Neuropeptides, and SCFAs
Spearman’s correlation analysis was performed among the nine main genera and related indicators to investigate the relationships between inflammatory cytokines, gastrointestinal neuropeptides, cecal SCFAs, and gut microbiota (Figure 8C). Lactobacillus correlated positively with IL-10 levels in the jejunum and colon (p < 0.05) but correlated negatively with pro-inflammatory cytokines (IL-1β and TNF-α). Bifidobacterium showed a positive relationship with serum SP and CGRP levels, whereas Rikenellaceae_RC9_gut_group showed a negative correlation with serum CGRP levels (p < 0.05). In addition, we found that Butyricimonas correlated positively with cecal SCFAs levels. Alistipes correlated positively with acetic acid and propionic acid levels, whereas Lactobacillus only correlated positively with acetic acid levels (p < 0.05).
4. Discussion
As the main ingredient of peppers, CAP is reported to have a variety of biological characteristics at low doses, such as anti-obesity, pain-relieving, antioxidant, and anti-inflammatory effects. However, when CAP is continuously consumed at high levels, people may experience GI discomforts, such as heartburn, diarrhea, pain, and other symptoms. Therefore, the GI effects of CAP ingestion have received increasing attention. Previous reports have revealed the average CAP consumption in humans, the estimated daily mean CAP intake was 30–150 mg per people, which was 8–37 mg/kg in mice, relatively [17,18]. Recent studies have revealed that oral administration at the dose of 20 mg/kg CAP for 14 days caused obvious inflammatory cell infiltration and cell vacuoles of gastric and jejunal mucosal tissues in rats [7]. Nevertheless, whether CAP affects other GI tissues remains unknown. There is still no clear experimental model and evaluation criteria for the effects of CAP ingestion on GI health. In this study, CAP was administered to mice at 40, 60, and 80 mg/kg to determine the gastrointestinal effects of normal and high CAP consumption.
The results showed that there was no significant damage to stomach tissue and that the damage to intestinal tissues was CAP-dose-related. In particular, compared with the control group, 60 and 80 mg/kg CAP treatment caused a decrease in the V/C ratio, local villus breakage, and shedding in the jejunum and ileum, similar to that shown in a previous report [19]. In addition, we found that oral administration of 80 mg/kg CAP caused inflammatory cell infiltration and significant mucus-producing goblet cell loss in colon tissues, which indicates that CAP induced the injury of GI tissues in a dose–response manner.
The inflammatory response is closely associated with GI injury. Studies have revealed that the main characteristics of CAP-induced GI inflammation in mice are elevated levels of inflammatory cytokines, especially IL-10, IL-1β, and TNF-α [7]. IL-10 is a key cytokine, which can reduce the release of inflammatory mediators and showed anti-inflammatory properties [20]. IL-1β and TNF-α are essential pro-inflammatory cytokines that cause mucosal inflammation and intestinal barrier damage [21,22]. Our results suggest that 80 mg/kg CAP treatment induces lower levels of IL-10 in all GI tissues and higher levels of IL-1β and TNF-α in the jejunum and colon. These results suggest that high-dose CAP may cause inflammatory injury to the jejunum and colon.
Treatment with CAP could activate the transient receptor potential channel of vanilloid subtype 1 and promote the release of gastrointestinal neuropeptides SP and CGRP, which could act on the central nervous system, regulate the immunoreaction, and are closely related to visceral pain in the GI tract [23]. Our results revealed that oral administration of 40, 60, and 80 mg/kg CAP significantly increased the serum levels of SP and CGRP, which seemed to be CAP-dose-dependent. Studies have shown that CGRP and SP antagonists significantly reduce the inflammatory cells infiltration in the colon of rats [24]. CGRP can induce the migration of T cell and the release of TNF-α by activating mast cells [25], and the blockage of the SP receptor can decrease the inflammation cells in mice with chronic colitis with T cell transfer [26]. Thus, we speculate that the enhanced levels of SP and CGRP in CAP-treated mice may further cause damage to the GI tract, inducing visceral pain.
It is reported that disturbances in the gut microbiota are associated with GI injury. Our results showed the alteration in gut microbiota and its SCFA metabolites after oral administration of different doses of CAP in mice. PCoA analysis revealed that the gut microbiota of 40 mg/kg CAP-treated mice was significantly different from that of the other groups, while the 60 and 80 mg/kg CAP treatments showed no clear changes. At the phylum level, only 40 mg/kg CAP treatment caused an increase in the relative abundance of Firmicutes and a decrease in the abundance of Bacteroidetes compared with the control group, which resulted an increase in Firmicutes/Bacteroidetes ratio. A previous report has revealed that CAP could improve glucose homeostasis in obese diabetic ob/ob mice by significantly increasing the Firmicutes/Bacteroidetes ratio [27]. In addition, 80 mg CAP treatment drastically elevated the relative abundances of Actinobacteria and Proteobacteria, similar to those discussed in previous results [28]. The presence of Proteobacteria is a mark of gut microbiota homeostasis imbalance, closely related to diarrhea symptoms and inflammation [29].
By identifying the major bacterial markers at the genus level, we found that the increase in the abundance of Actinobacteria was primarily attributed to the increased abundance of Bifidobacterium. The relative abundance of Bifidobacterium was dose-related to CAP, and the correlation analysis showed that the abundance of Bifidobacterium was also positively related to the serum levels of SP and CGRP. The local release of neuropeptides can alter the composition of the gut microbiota by regulating the immune response in the GI tract, whereas bacteria can also sense neuropeptides through membrane proteins [30,31]. Thus, we speculate that the dose-dependent increase in serum SP and CGRP in CAP-treated mice may be related to the promotion of the relative abundance of Bifidobacterium. Additionally, the 60 and 80 mg/kg CAP-treated groups significantly enriched the abundance of Faecalibacterium, similarly to previous results [32]. Faecalibacterium is considered a bioindicator of GI disorders and displayed a positive correlation with butyric acid/SCFA production [33,34]. This may be the reason for the significantly high level of cecal butyric acid in 60 and 80 mg/kg CAP-treated mice.
Moreover, the abundance of Lactobacillus, Bacteroides, and Alistipes was reduced in the CAP-treated groups. Lactobacillus belongs to the phylum Firmicutes, possessing the capacity to inhibit pathogens or relieve inflammation [35]. Our results showed that the abundance of Lactobacillus positively related to IL-10 levels in the jejunum and colon (p < 0.05), suggesting that CAP may influence the anti-inflammatory properties of GI tissues by decreasing the abundance of Lactobacillus in the intestinal microflora. The decreased abundance of Bacteroides after CAP treatment has been demonstrated in previous studies by [27,36]. Furthermore, Butyricimonas is known for its ability to produce butyric acid [37]. The abundance of Butyricimonas was decreased in the 40 and 60 mg/kg CAP groups, but significantly increased in the 80 mg/kg group, suggesting that high butyric acid levels in 80 mg/kg CAP-treated mice may be due to the promotion of Butyricimonas growth.
Notably, compared with the control group, 40 mg/kg CAP treatment drastically elevated the proportion of Dubosiella, and 80 mg/kg CAP treatment resulted in an increased abundance of Coriobacteriaceae_UCG_002. Dubosiella was reported to inhibit obesity in mice in previous studies [38], while Coriobacteriaceae_UCG_002 was beneficial to the host by producing the essential amino acids and fermenting dietary proteins. These surprising findings required more follow-up research [39].
5. Conclusions
In summary, mice were intragastrically administered with CAP at three doses to evaluate the effects of CAP on GI health. The results showed that administration of 40 mg/kg CAP did not have significant negative effects on the GI tract in mice, while 60 and 80 mg/kg CAP caused GI injury by damaging GI tissues and decreasing the levels of anti-inflammatory cytokines (IL-10). Inflammation and histopathological changes were significant in the jejunum, ileum, and colon, but only slight in the stomach. CAP increased serum SP and CGRP levels in a dose-dependent manner, which may induce an immune response and visceral pain. The levels of cecal SCFAs also significantly changed in the 80 mg/kg CAP-treated groups. These effects of CAP might be related to the regulation of gut microbiota, especially Bifidobacterium, Lactobacillus, Faecalibacterium, and Butyricimonas. Moreover, the underlying mechanism of the correlation between serum neuropeptides and specific gut microbiota needs to be studied, suggesting that probiotics, as members of the gut microbiota, may be an alternative in relieving CAP-induced GI injury. These data will reveal the effects of CAP on GI health, provide insight into the experimental model of CAP-induced GI injury, and enrich the correlation analysis between CAP ingestion and gut microbiota.
Abbreviations
| ANOVA | Analysis of variance |
| CAP | Capsaicin |
| CGRP | Calcitonin gene-related peptide |
| GC-MS | Gas chromatograph-mass spectrometry |
| GI | Gastrointestinal |
| H&E | Hematoxylin and eosin |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis effect size |
| OTU | Operational taxonomic unit |
| PCoA | Principal coordinates analysis |
| SCFAs | Short-chain fatty acids |
| SEM | Standard error of the mean |
| SP | Substance P |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods11050686/s1, Figure S1. Effects of CAP on daily food intake and water intake of each mice: (A) Food intake, (B) Water intake; Figure S2. Assessment of colonic tissues: (A) Height of colonic crypts, (B) Goblet numbers per crypt.
Author Contributions
Conceptualization, Q.X. and B.M.; Methodology, Q.X. and X.T.; Software, Q.X. and S.C.; Validation, X.T.; Formal analysis, Q.X. and B.M.; Investigation, Q.X. and X.T.; Resources, Q.Z., J.Z. and H.Z.; Data curation, X.L. and S.C.; Writing—original draft preparation, Q.X.; Writing—review and editing, Q.X. and B.M.; Visualization, Q.X.; Supervision, H.Z. and W.C.; Project administration, X.L., J.Z. and W.C.; Funding acquisition, B.M., S.C. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant no. 31972086, 32172173) and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Experimental Animal Ethics Committee of Jiangnan University (JN. no. 20200630c0640815(126), 30 June 2020).
Data Availability Statement
All data presented in this study are available in the main body of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fujiwake H., Suzuki T., Oka S., Iwai K. Enzymatic formation of capsaicinoid from vanillylamine and iso-type fatty acids by cell-free extracts of Capsicum annuum var. annuum cv. Karayatsubusa. Agric. Biol. Chem. 1980;44:2907–2912. doi: 10.1271/bbb1961.44.2907. [DOI] [Google Scholar]
- 2.Srinivasan K. Biological Activities of Red Pepper (Capsicum annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016;56:1488–1500. doi: 10.1080/10408398.2013.772090. [DOI] [PubMed] [Google Scholar]
- 3.Hammer J., Vogelsang H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol. Motil. 2007;19:279–287. doi: 10.1111/j.1365-2982.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 4.Mozsik G., Vincze A., Szolcsanyi J. Four response stages of capsaicin-sensitive primary afferent neurons to capsaicin and its analog: Gastric acid secretion, gastric mucosal damage and protection. J. Gastroenterol. Hepatol. 2001;16:1093–1097. doi: 10.1046/j.1440-1746.2001.02598.x. [DOI] [PubMed] [Google Scholar]
- 5.Lu M., Cao Y., Ho C.-T., Huang Q. Development of Organogel-Derived Capsaicin Nanoemulsion with Improved Bioaccessibility and Reduced Gastric Mucosa Irritation. J. Agric. Food Chem. 2016;64:4735–4741. doi: 10.1021/acs.jafc.6b01095. [DOI] [PubMed] [Google Scholar]
- 6.Lu M., Cao Y., Ho C.-T., Huang Q. The enhanced anti-obesity effect and reduced gastric mucosa irritation of capsaicin-loaded nanoemulsions. Food Funct. 2017;8:1803–1809. doi: 10.1039/C7FO00173H. [DOI] [PubMed] [Google Scholar]
- 7.Han J., Zhang S., Liu X., Xiao C. Fabrication of capsaicin emulsions: Improving the stability of system and relieving the irritation to the gastrointestinal tract of rats. J. Sci. Food Agric. 2019;100:129–138. doi: 10.1002/jsfa.10002. [DOI] [PubMed] [Google Scholar]
- 8.Tsukura Y., Mori M., Hirotani Y., Ikeda K., Amano F., Kato R., Ijiri Y., Tanaka K. Effects of capsaicin on cellular damage and monolayer permeability in human intestinal Caco-2 cells. Biol. Pharm. Bull. 2007;30:1982–1986. doi: 10.1248/bpb.30.1982. [DOI] [PubMed] [Google Scholar]
- 9.Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita S., Furuse M., Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Drewes A.M., Schipper K.P., Dimcevski G., Petersen P., Gregersen H., Funch-Jensen P., Arendt-Nielsen L. Gut pain and hyperalgesia induced by capsaicin: A human experimental model. Pain. 2003;104:333–341. doi: 10.1016/S0304-3959(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 11.Van Avesaat M., Troost F.J., Westerterp-Plantenga M.S., Helyes Z., Le Roux C.W., Dekker J., Masclee A.A.M., Keszthelyi D. Capsaicin-induced satiety is associated with gastrointestinal distress but not with the release of satiety hormones. Am. J. Clin. Nutr. 2016;103:305–313. doi: 10.3945/ajcn.115.123414. [DOI] [PubMed] [Google Scholar]
- 12.Van Wanrooij S.J.M., Wouters M.M., Van Oudenhove L., Vanbrabant W., Mondelaers S., Kollmann P., Kreutz F., Schemann M., Boeckxstaens G.E. Sensitivity Testing in Irritable Bowel Syndrome With Rectal Capsaicin Stimulations: Role of TRPV1 Upregulation and Sensitization in Visceral Hypersensitivity? Am. J. Gastroenterol. 2014;109:99–109. doi: 10.1038/ajg.2013.371. [DOI] [PubMed] [Google Scholar]
- 13.Baboota R.K., Murtaza N., Jagtap S., Singh D.P., Karmase A., Kaur J., Bhutani K.K., Boparai R.K., Premkumar L.S., Kondepudi K.K., et al. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J. Nutr. Biochem. 2014;25:893–902. doi: 10.1016/j.jnutbio.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Tang C., Tang Y., Yin H., Liu X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr. Res. 2020;64:14. doi: 10.29219/fnr.v64.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieleman L.A., Palmen M., Akol H., Bloemena E., Pena A.S., Meuwissen S.G.M., van Rees E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao B., Li D., Ai C., Zhao J., Zhang H., Chen W. Lactulose Differently Modulates the Composition of Luminal and Mucosal Microbiota in C57BL/6J Mice. J. Agric. Food Chem. 2016;64:6240–6247. doi: 10.1021/acs.jafc.6b02305. [DOI] [PubMed] [Google Scholar]
- 17.Kwon Y. Estimation of Dietary Capsaicinoid Exposure in Korea and Assessment of Its Health Effects. Nutrients. 2021;13:2461. doi: 10.3390/nu13072461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nair A., Morsy M.A., Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018;79:373–382. doi: 10.1002/ddr.21461. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y., Zhu Y., Wan J., Yang X., Firempong C.K., Yu J., Xu X. Enhanced oral bioavailability, reduced irritation and increased hypolipidemic activity of self-assembled capsaicin prodrug nanoparticles. J. Funct. Foods. 2018;44:137–145. doi: 10.1016/j.jff.2018.03.006. [DOI] [Google Scholar]
- 20.Evrard B., Coudeyras S., Dosgilbert A., Charbonnel N., Alame J., Tridon A., Forestier C. Dose-Dependent Immunomodulation of Human Dendritic Cells by the Probiotic Lactobacillus rhamnosus Lcr35. PLoS ONE. 2011;6:e18735. doi: 10.1371/journal.pone.0018735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horiuchi T., Mitoma H., Harashima S.-I., Tsukamoto H., Shimoda T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology. 2010;49:1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smirnova M.G., Kiselev S.L., Gnuchev N.V., Birchall J.P., Pearson J.P. Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur. Cytokine Netw. 2002;13:161–172. [PubMed] [Google Scholar]
- 23.Xiang Q., Guo W., Tang X., Cui S., Zhang F., Liu X., Zhao J., Zhang H., Mao B., Chen W. Capsaicin—The spicy ingredient of chili peppers: A review of the gastrointestinal effects and mechanisms. Trends Food Sci. Technol. 2021;116:755–765. doi: 10.1016/j.tifs.2021.08.034. [DOI] [Google Scholar]
- 24.Hoffmann P., Mazurkiewicz J., Holtmann G., Gerken G., Eysselein V.E., Goebell H. Capsaicin-sensitive nerve fibres induce epithelial cell proliferation, inflammatory cell immigration and transforming growth factor-alpha expression in the rat colonic mucosa in vivo. Scand. J. Gastroenterol. 2002;37:414–422. doi: 10.1080/003655202317316042. [DOI] [PubMed] [Google Scholar]
- 25.Engel M.A., Becker C., Reeh P.W., Neurath M.F. Role of Sensory Neurons in Colitis: Increasing Evidence for a Neuroimmune Link in the Gut. Inflamm. Bowel Dis. 2011;17:1030–1033. doi: 10.1002/ibd.21422. [DOI] [PubMed] [Google Scholar]
- 26.Gad M., Pedersen A.E., Kristensen N.N., de Felipe Fernandez C., Claesson M.H. Blockage of the Neurokinin 1 Receptor and Capsaicin-Induced Ablation of the Enteric Afferent Nerves Protect SCID Mice Against T-Cell-Induced Chronic Colitis. Inflamm. Bowel Dis. 2009;15:1174–1182. doi: 10.1002/ibd.20902. [DOI] [PubMed] [Google Scholar]
- 27.Song J., Ren H., Gao Y., Lee C., Li S.F., Zhang F., Li L., Chen H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front. Physiol. 2017;8:12. doi: 10.3389/fphys.2017.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang T., Song J., Wang H., Zhang Y., Xin J., Suo H. Qingke beta-glucan synergizes with a beta-glucan-utilizing Lactobacillus strain to relieve capsaicin-induced gastrointestinal injury in mice. Int. J. Biol. Macromol. 2021;174:289–299. doi: 10.1016/j.ijbiomac.2021.01.164. [DOI] [PubMed] [Google Scholar]
- 29.Reeves A.E., Theriot C.M., Bergin I.L., Huffnagle G.B., Schloss P.D., Young V.B. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes. 2011;2:145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzer P. Neuropeptides, Microbiota, and Behavior. In: Cryan J.F., Clarke G., editors. Gut Microbiome and Behavior. Volume 131. Elsevier Academic Press Inc.; San Diego, CA, USA: 2016. pp. 67–89. [DOI] [PubMed] [Google Scholar]
- 31.Sperandio V., Torres A.G., Jarvis B., Nataro J.P., Kaper J.B. Bacteria-host communication: The language of hormones. Proc. Natl. Acad. Sci. USA. 2003;100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang C., Zhang Y., Zhu X., Liu K., Wang X., Chen M., Wang J., Chen H., Hui S., Huang L., et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016;101:4681–4689. doi: 10.1210/jc.2016-2786. [DOI] [PubMed] [Google Scholar]
- 33.Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 34.Miquel S., Martin R., Rossi O., Bermudez-Humaran L.G., Chatel J.M., Sokol H., Thomas M., Wells J.M., Langella P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Wang W., Liu D., Guo Y. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS ONE. 2017;12:e0188634. doi: 10.1371/journal.pone.0188634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F., Huang X., Chen Y., Zhang D., Chen D., Chen L., Lin J. Study on the Effect of Capsaicin on the Intestinal Flora through High-Throughput Sequencing. ACS Omega. 2020;5:1246–1253. doi: 10.1021/acsomega.9b03798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakamoto M., Takagaki A., Matsumoto K., Kato Y., Goto K., Benno Y. Butyricimonas synergistica gen. nov., sp. nov and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family ‘Porphyromonadaceae’ isolated from rat faeces. Int. J. Syst. Evol. Microbiol. 2009;59:1748–1753. doi: 10.1099/ijs.0.007674-0. [DOI] [PubMed] [Google Scholar]
- 38.Guo X., Cao X., Fang X., Guo A., Li E. Inhibitory effects of fermented Ougan (Citrus reticulata cv. Suavissima) juice on high-fat diet-induced obesity associated with white adipose tissue browning and gut microbiota modulation in mice. Food Funct. 2021;12:9300–9314. doi: 10.1039/D0FO03423A. [DOI] [PubMed] [Google Scholar]
- 39.Xie Y., Zhou G., Wang C., Xu X., Li C. Specific Microbiota Dynamically Regulate the Bidirectional Gut-Brain Axis Communications in Mice Fed Meat Protein Diets. J. Agric. Food Chem. 2019;67:1003–1017. doi: 10.1021/acs.jafc.8b05654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study are available in the main body of the manuscript.