Abstract
Simple Summary
Liver resection for colorectal liver metastases (CRLM) represents the best curative option; however, few patients are candidates for surgery. Microwave ablation (MWA) can be a valid alternative in selected patients. This systematic review reports the oncological results of MWA for CRLM. The literature available on the Web was analyzed for reports concerning MWA for resectable CRLM, published before January 2021. Finally, 12 papers concerning MWA complications, recurrence-free (RF) cases, patients free from local recurrence (FFLR), and overall survival rates (OS) were selected. Global RF rates at 1, 3, and 5 years were 65.1%, 44.6%, and 34.3%, respectively. Global FFLR at 3, 6, and 12 months were 96.3%, 89.6%, and 83.7%, respectively. Global OS rates at 1, 3, and 5 years were 86.7%, 59.6%, and 44.8%, respectively. A better FFLR was achieved with an MWA surgical approach at 3, 6, and 12 months, with 97.1%, 92.7%, and 88.6%, respectively. Surgical MWA for CRLM smaller than 3 cm was a safe and valid option. MWA can be entered as part of the flowchart decision of CRLM curative treatment, especially for use in the parenchyma-sparing strategy and as a complement to surgery.
Abstract
(1) Background: colorectal liver metastases (CRLM) are the most common extra-lymphatic metastases in colorectal cancer; however, few patients are fit for curative surgery. Microwave ablation (MWA) showed promising outcomes in this cohort of patients. This systematic review and pooled analysis aimed to analyze the oncological results of MWA for CRLM. (2) Methods: Following PRISMA guidelines, PubMed, Scopus, EMBASE, Google Scholar, Science Direct, and the Wiley Online Library databases were searched for reports published before January 2021. We included papers assessing MWA, treating resectable CRLM with curative intention. We evaluated the reported MWA-related complications and oncological outcomes as being recurrence-free (RF), free from local recurrence (FFLR), and overall survival rates (OS). (3) Results: Twelve out of 4822 papers (395 patients) were finally included. Global RF rates at 1, 3, and 5 years were 65.1%, 44.6%, and 34.3%, respectively. Global FFLR rates at 3, 6, and 12 months were 96.3%, 89.6%, and 83.7%, respectively. Global OS at 1, 3, and 5 years were 86.7%, 59.6%, and 44.8%, respectively. A better FFLR was reached using the MWA surgical approach at 3, 6, and 12 months, with reported rates of 97.1%, 92.7%, and 88.6%, respectively. (4) Conclusions: Surgical MWA treatment for CRLM smaller than 3 cm is a safe and valid option. This approach can be safely included for selected patients in the curative intent approaches to treating CRLM.
Keywords: colorectal liver metastasis, microwave ablation, liver resection
1. Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, with 25–35% of patients presenting with or developing colorectal liver metastases (CRLM). Almost 20% of patients present synchronous CRLM and 10–15% of patients, metachronous CRLM [1,2]. Liver resection is the current gold standard for the curative-intent treatment of CRCLM, leading to a 5-year overall survival (OS) rate of 31–58% [3]. Unfortunately, only 15–25% of patients are suitable for oncological surgery, mainly due to patient factors (age, medical condition) and/or tumor factors (size, number, and localization) [4]. Modern chemotherapy (CHT) alone has improved the OS by 15–20 months [5]. To achieve better oncological results, local therapies, such as thermal ablation and external beam radiation (SBRT), with or without surgery have been developed. These techniques are aimed at optimizing the outcome by maximizing parenchymal preservation and minimizing the local recurrence [6,7]. In these settings, ablation techniques offer an advantage for surgery, treating deep lesions more easily [6,7]. The long-term reported results after radiofrequency ablation (RFA) for CRLM are promising; this technique represents the most commonly used modality, before microwave treatment and cryotherapy [8]. Recurrence rates from RFA are approximately three times lower than those for cryotherapy, but they are approximately three times higher compared to resection when used as a first-line treatment in patients with resectable disease [9,10,11]. RFA is safe and effective, both in percutaneous and surgical approaches, although its limitations include increased impedance as temperatures reach 100 degrees Celsius, a small zone of active heating, and decreased effectiveness with charring. The results of RFA in terms of outcome and safety have been extensively demonstrated [12,13,14]. RFA is less useful for the treatment of lesions near to large blood vessels because of the “heat-sink effect”, where the flowing blood carries heat away, bringing higher local recurrence rates. The principle of microwave ablation (MWA) is the tissue coagulation necrosis caused by the oscillation of polar molecules, with non-reliance on electrical conductivity; this procedure is less affected by the presence of blood vessels [15,16]. This technique also remains effective in temperatures above 100 degrees Celsius, allowing for the use of multiple antennae providing a potentially larger ablation zone, with a shorter procedure time [17,18]. In the past few years, MWA has established a larger place in the treatment of CRLM because of the previously cited reasons [19]. Interestingly, some authors suggested increasing the ablation zone by reducing the surrounding blood inflow during intraoperative MWA, by clamping the hepatic pedicle [20,21]. Microwave (MW) energy is more difficult to distribute than radiofrequency (RF) energy. MW energy is carried in wavelengths, which are more cumbersome than the small wires used to feed energy to RF electrodes and are prone to heating up when carrying a large amount of power. Consequently, MWA appears less feasible than RFA in the treatment of high-risk-located and subcapsular nodules. In addition, MWA is more expensive than RFA [22]. When comparing RFA and MWA, patients who underwent MWA had lower ablation-site recurrence rates (6% vs. 20%), and the 2-year control rate was significantly lower (7% vs. 18%) [23]. The existing literature on the outcomes following MWA for CRLM remains heterogeneous and sparse. This systematic review aims to report the results of the current state of evidence for CRLM treated by MWA, evaluating the oncological outcome in terms of overall survival, recurrence-free survival, and local recurrence-free survival, with all reporting enhanced by the benefits of pooled analysis.
2. Materials and Methods
The literature search methods, selection criteria, data collection, outcome measurements, and statistical analysis methods were selected according to the preferred items for systematic reviews and meta-analysis (PRISMA) statement [24]. No institutional review board or ethics committee approval was required.
2.1. Search Strategy
An online search of the PubMed, Scopus, EMBASE, Google Scholar, Science Direct, and the Wiley Online Library databases was performed to identify all studies on the treatment of CRLM through MWA ablation. Additional gray literature was searched using the POPLINE and SIGLE database to include doctoral dissertations, theses, and publications not found in the main databases. Additionally, we searched the Cochrane, mRCT, and clinicaltrials.gov databases to scan the ongoing or completed clinical trials on the matter.
When studies with overlapping cohorts were encountered, the report with a higher number of subjects or with the longest follow-up period was selected.
The systematic search of the literature was performed without publication-period restriction and was completed on 1 January 2021, including only those reports written in English.
A medical subject headings (MeSH) search was conducted for PubMed, Scopus, EMBASE, and Cochrane databases, applying the following terms: “((microwave) OR (MW) OR (MWA)) AND ((liver) OR (hepatic)) AND (metastases) AND ((colorectal) OR (colon) OR (colo-rectal))”.
To include the highest possible number of papers, the other databases were scanned using “Colorectal Liver Metastases Microwave” as a query.
References cited in the relevant papers were also examined to include all potentially noteworthy reports.
2.2. Selection Criteria
Randomized controlled trials (RCT) were studied, as well as observational studies with or without control arms that enrolled patients with CRLM treated via MWA.
Two reviewers (A.M. and F.P.) independently reviewed the results obtained by a literature search in a two-step method (Figure 1). After duplicates and citations were removed, the first step consisted of screening the titles and abstracts to determine their eligibility and relevance to the topic. During this phase, case reports, oral presentations, conferences, abstracts without a corresponding full text, reviews, meta-analyses, letters, editorials, books, non-English articles, reports describing study protocols, animal-model studies, and unrelated articles were excluded. In case of doubts or ambiguous abstracts, those studies were selected for full-text screening.
Figure 1.
Flowchart showing the selection process of the included studies. Abbreviations: colorectal liver metastases (CRLM), microwave ablation (MWA).
During the second step, a full-text screening of articles meeting the specific inclusion criteria was made by the same reviewers. Studies were excluded when they did not include MWA as a treatment, had a sample of fewer than 10 patients, used MWA as a palliative treatment, did not report a sufficient follow-up, combined MWA and other treatments on the same subjects, or were unrelated, as well as those studies in which the patients affected by CRLM and/or treated with MWA could not be correctly isolated from those with other liver metastases and/or were treated with other procedures (i.e., RFA). We excluded all reports with a sufficient follow-up time that failed to report our outcomes of interest. Any disagreement was jointly resolved by the authors and reviewed by two expert surgeons (R.K., T.P.).
The exclusion and inclusion criteria are summarized in Table 1.
Table 1.
Inclusion and exclusion criteria. Abbreviations: colorectal liver metastases (CRLM), microwave ablation (MWA), chemotherapy (CHT), radiofrequency ablation (RFA).
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| MWA only | Combined procedures (i.e., RFA) |
| MWA + resections of other liver portions | MWA and subsequent resection of the same target |
| CRLM (+ mixed studies with identifiable results for CRLM only) | No adenocarcinoma CRLM |
| No lesions’ dimensional limits | Palliative treatment |
| Patients aged above 18 years old | Pregnant women |
| Sample > 10 Patients | Case reports and sample < 10 patients |
| Regardless neoadjuvant CT | |
| Recurrences | |
| Treated surgically and percutaneously |
2.3. Data Extraction and Outcomes of Interest
Once the articles meeting the specific inclusion criteria were identified, a series of datasets were extracted independently by the two reviewers. These included: study type and design, patients’ characteristics, the total number of MWA performed, mean/median lesion dimensions, the number of lesions per patient, synchronous/metachronous lesions, treatment at recurrence, operation time, length of hospital stay, complications, follow-up time, and the items defining the inclusion criteria (see below). All the selected articles evaluated the postoperative complications using the Clavien–Dindo Score (CDs) [25]. Additional data, such as neoadjuvant and adjuvant treatments, specific postoperative complications, and types of MWA device and needle tip were registered as well.
Subsequently, and upon existing data, we stratified for lesions according to diameter (<30 mm vs. >30 mm), for percutaneous/surgical treatment procedures, and for the type of surgical approach (open, vs. laparoscopic, robotic) in the latter group.
The following primary outcome was extracted: rates of patients free from local recurrence (FFLR, at 3, 6, and 12 months at least). The following secondary outcomes were extracted: recurrence-free rates (RF, at 3, 6, and 12 months, at least) and overall survival rates (OS, at 3, 6, and 12 months, at least). We defined the overall recurrence rate as the appearance of new lesions during postoperative follow-up, irrespective of their location. Local recurrence rate was defined as a postoperative relapse occurring specifically on the ablated liver lesions.
Additional outcomes included the evaluation of recurrence-pattern patients with hepatic and extra-hepatic progression.
2.4. Quality Assessment
The quality of all included studies was assessed independently by A.M. and F.P. using the Newcastle-Ottawa scale (NOS) of quality assessment [26]. Any disagreements were resolved in consensus.
2.5. Statistical Analysis
Data of interest were collected as presented in the original manuscripts or were calculated from the reported raw data whenever possible. Quantitative data were presented descriptively as mean and standard deviations (SD). When continuous data were presented as medians and range, the method developed by Hozo et al. was used [27]. When continuous data were presented as medians and interquartile range, we used the method developed by Wan et al. [28]. Categorical variables were summarized as frequencies and percentages with 95% confidence intervals (CIs).
We extracted the recurrence and survival outcomes from the Kaplan–Meier curves and/or explicit data, when available. Subgroup survival analysis on patients with lesions of <30 mm, treated percutaneously or surgically, was performed as well as outcomes after MWA under laparoscopic versus open procedures. Finally, grouped analysis incorporated papers providing data on the local recurrence rates.
3. Results
3.1. Literature Analysis
The literature search identified 4822 records. After excluding duplicates and citations, a pool of 3578 papers was screened. The first step of the literature analysis consisted of a screening of titles and abstracts to determine their eligibility and relevance to the topic: case reports, oral presentations, conferences, abstracts without a corresponding full text, reviews, meta-analyses, letters, editorials, books, non-English articles, reports describing study protocols, animal-model studies, and unrelated articles were excluded. After excluding 3469 papers, 109 articles were selected as being eligible for second-phase analysis.
The second step consisted of an analysis of the full text of articles meeting the specific inclusion criteria. Studies not including MWA as an exclusive treatment, with a sample of fewer than 10 patients, using MWA as a palliative treatment, not reporting the follow-up, combining MWA and other treatments on the same subjects, unrelated subjects, and those studies in which the patients who were affected by CRLM and/or treated with MWA could not be correctly isolated from those with other liver metastases and/or treated with other procedures (i.e., RFA) were excluded. Finally, 12 papers were included in the pooled analysis (Figure 1).
3.2. Study Characteristics
The general characteristics of these twelve papers are shown in Table 2, Table 3, Table 4 and Table 5 [20,29,30,31,32,33,34,35,36,37,38,39]. Eight studies (66.7%) were retrospective [29,30,32,34,36,37,38,39], and a total of 395 patients with CRLM were treated with MWA, 257 of whom were male (65%). The mean patients’ age was 58.9 ± 9 years. The mean lesion diameter was 17.6 ± 7.8 mm, of which 390 lesions (91.6%) were smaller than 30 mm. Percutaneous MWA treatment was performed in 108 (32.3%) of the patients, while 287 (85.9%) underwent a surgical procedure. Of the cases of surgical MWA, 34.7% were performed via the open approach and 47.5% were performed using a laparoscopic approach. Overall, 47.2% of patients presented synchronous CRLM. Regarding oncologic treatment, 70.5% and 90.4% of patients received neoadjuvant and adjuvant CHT, respectively. The type of MWA device, the MWA needle, the number of ablations per lesion, ablation time, and energy employed were reported on almost all papers; notably, there is a heterogeneity of protocols that appears to be specific for each Center (Table 4). The complications rate was 26.3%, as specified in Table 4. Serious complications (CDs ≥ 3) were reported in 8.4% of patients. The mean length of hospital stay was 5.43 days. Global survival analysis was reported in Table 5. The mean follow-up was 20.5 months (±9.6). The pooled data analysis on the oncological outcomes of MWA shows a global recurrence-free (RF) rate at 3 months, 6 months, and 1, 3, and 5 years of 95.5%, 89.5%, 65.1%, 44.6%, and 34.3%, respectively, resulting in a global RF of 37.1%. In total, the rates of patients free from local recurrence (FFLR) at 3, 6, and 12 months were 96.3%, 89.6%, and 83.7%, respectively. The overall recurrence rate was 41.36%. Intrahepatic progression during the follow-up appeared in 35.5% of patients, while 32.9% showed extra-hepatic progression. Global overall survival rates (OS) at 3 months, 6 months, and 1, 3, and 5 years were 99.3%, 97.3%, 86.7%, 59.6%, and 44.8%, respectively.
Table 2.
General characteristics of the included studies. The figure in curly brackets indicate the 95% confidence intervals.
| Study ID |
Year | Country | Study Type |
Rand. | Study Design |
Period | N-O QAS |
Sample Size, n | CRLM Patients, n |
MWA-Treated Patients, n | Ablations, n | Age, Mean ° (SD °) | Sex M/F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McEachron | 2021 | USA | Retrospective | No | Cohort | 2009–2018 | Fair | 36 | 36 | 36 | 40 ** | 52 (±12.75) |
21/15 |
| Rhaiem | 2020 | France | Prospective | No | Cohort | November 2017– December 2018 |
Poor | 19 | 19 | 19 | 23 | 67 (±10.5) |
8/11 |
| Takahashi | 2018 | USA | Retrospective | No | Case–Control | 2014–2018 | Good | 105 | 105 | 51 | 121 | NA | 33/18 |
| Shady | 2017 | USA | Retrospective | No | Case–Control | November 2019– April 2015 |
Good | 110 | 110 | 48 | 72 | NA | 35/13 |
| Yang | 2017 | China | Retrospective | No | Case–Control | January 2010– January 2016 |
Fair | 179 | 179 | 71 | 121 | 51 (±5.33) |
49/22 |
| Song | 2016 | China | Retrospective | No | Case–Control | January 2012– January 2014 |
Good | 62 | 62 | 28 | NA | NA | 15/13 |
| Eng | 2015 | USA | Retrospective | No | Cohort | January 2009– April 2013 |
Poor | 33 | 33 | 33 | 49 | 61 (±11.75) |
24/9 |
| Engstrand | 2014 | Sweden | Retrospective | No | Case–Control | October 2009– September 2012 |
Fair | 81 | 81 | 20 | 7 * (4–22 *) |
63.5 (±9.5) |
9/11 |
| Ierardi | 2013 | Italy | Prospective | No | Case–Control | May 2008– September 2011 |
Poor | 25 | 17 | 17 | 23 | 65.75 (±7.75) |
13/4 |
| Stattner | 2013 | UK | Retrospective | No | Cohort | May 2005– December 2012 |
Good | 43 | 43 | 43 | 95 | 64.5 (±11) |
32/11 |
| Shibata | 2000 | Japan | Prospective | Yes | Case–Control | December 1990– August 1887 |
Good | 30 | 30 | 14 | 58 | 61.5 (±11.26) |
8/6 |
| Seki | 1999 | Japan | Prospective | No | Cohort | January 1994– May 1997 |
Poor | 15 | 15 | 15 | 32 | 66 (±5.76) |
10/5 |
| Total | - | - | - | - | - | - | 741 | 730 | 395 | - | 58.92 (±9.19) |
257/138 |
Abbreviations—CRLM: colorectal liver metastases; MWA: microwave ablation; N-O QAS: Newcastle–Ottawa Quality Assessment Scale; Rand: randomization; SD: standard deviation; NA: missing data. *: median and range; **: intended not as MWA passages but as surgical procedures (re-treatment of 2 patients and 3 treatments on one patient); °: calculated using the Hozo and Wan method.
Table 3.
Lesion characteristics. The figures in curly brackets indicate 95% confidence intervals.
| Study ID |
MWA-Treated Patients/ Lesions, n |
Dimensions, Mean (SD), mm |
Lesions < 30 mm, n (%) |
Percutaneous/ Surgical Treatment, pt n (%) |
Surgical Open/Lap, pt n (%) |
Single/Multiple Lesions, pt n (%) |
Synch, pt n (%)/ Meta, pt n (%) |
Neoadj, n (%)/ Adj, n (%) Treatment |
|---|---|---|---|---|---|---|---|---|
| McEachron 2021 |
36/135 | 19 ° (±19.5 °) |
132 (97.8%) |
0/ 36 (100%) |
13 * (32.5% *)/ 27 * (67.5% *) |
NA/NA | 13 (36.1%)/NA | 36 (100%)/NA |
| Rhaiem 2020 |
19/23 | 15.25 ° (±2.75°) |
23 (100%) |
0/ 19 (100%) |
15 (78.9%)/ 4 (21.1%) |
15 (78.9%)/ 4 (21.1%) |
8 (42.1%)/ 11 (57.9%) |
17 (89%)/ 19 (100%) |
| Takahashi 2018 |
51/121 | 21.25 ° (±2.83 °) |
121 (100%) |
0/ 51 (100%) |
0/ 51 (100%) |
0/ 51 (100%) |
NA/NA | 28 (54.9%)/NA |
| Shady 2017 |
48/60 | 17 ° (±7.5 °) |
56 (93.3%) |
48 (100%)/ 0 |
0/0 | 40 (83.3%)/ 8 (17.7%) |
NA/NA | NA/NA |
| Yang 2017 |
71/121 | 3 ° (±0.67°) |
NA | 0/ 71 (100%) |
0/ 71 (100%) |
33 (46.5%)/ 38 (53.5%) |
NA/NA | NA/NA |
| Song 2016 |
28/NA | NA | NA | 28 (100%)/ 0 |
0/0 | 18 (64.3%)/ 10 (35.7%) |
10 (35.7%)/ 18 (64.3%) |
NA/NA |
| Eng 2015 |
33/49 | NA | 42 (85.7%) |
0/ 33 (100%) |
NA/NA | NA/NA | NA/NA | 32 (96.9%)/ 30 (90.9%) |
| Engstrand 2014 |
20/NA | NA | NA | 0/ 20 (100%) |
20 (100%)/ 0 |
0/ 20 (100%) |
18 (90%)/ 2 (10%) |
9 (45%)/ 13 (65%) |
| Ierardi 2013 |
17/23 | 34.52 ° (±13.25 °) |
1 (4.8%) |
17 (100%)/ 0 |
0/0 | 14 (82.4%)/ 3 (17.6%) |
NA/NA | NA/17 (100%) |
| Stattner 2013 |
43/95 | 20.25 ° (±5.83 °) |
NA | 0/ 43 (100%) |
43 (100%)/ 0 |
NA/NA | 27 (62.8%)/ 16 (37.2%) |
31 (72.1%)/NA |
| Shibata 2000 |
14/58 | 27 ° (±11 °) |
NA | 0/ 14 (100%) |
14 (100%)/ 0 |
0/ 14 (100%) |
NA/NA | NA/NA |
| Seki 1999 |
15/15 | 21.4 ° (±3.73 °) |
15 (100%) |
15 (100%)/ 0 |
0/0 | 15 (100%)/ 0 |
0/ 15 (100%) |
0/ 15 (100%) |
| Total | 395/700 | 17.62 ° (±7.87 °) |
390 (91.6%) {88.5–93.8%} |
108 (32.3%)/ 287 (85.9%) {27.5–37.5%}/ {81.8–89.2%} |
92 (34.7%)/ 126 (47.5%) {29.2–40.6%}/ {41.6–53.5%} |
135 (47.7%)/ 148 (52.3%) {41.9–53.5%}/ {46.5–58.1%} |
76 (47.2%)/ 62 (49.6%) {39.7–54.9%}/ {40.9–58.3%} |
153 (70.5%)/ 94 (90.4%) {64.1–76.2%}/ {83.2–94.7%} |
Abbreviations—Lap: laparoscopic; SD: standard deviation; Synch: synchronous; Meta: metachronous; Neoadj: neoadjuvant; Adj: adjuvant; pt: calculated on the number of patients; mm: millimeters; NA: missing data. *: calculated on the number of surgical treatments (data excluded from further analysis); °: calculated using the Hozo method.
Table 4.
The MWA apparatuses that were employed, with the ablation techniques and complications registered. The figures in curly brackets indicate 95% confidence intervals.
| Study ID |
Type of MWA Device | Type of MWA Needle |
Ablation(s) Per Lesion |
Average Ablation Time, min |
Average Energy | Operation Time, min |
Complica-tions n (%) | Complications Type, pt n |
Clavien–Dindo ≥ 3, n (%) |
Length of Stay, Days (Range) |
|---|---|---|---|---|---|---|---|---|---|---|
| McEachron 2021 |
NeuWaveTM Microwave Ablation System, Ethicon, Madison, WI, USA | Certus 140, 2.45 GHz ablation system, Certus PR XT (20 cm), or LK Max XT 25 cm probes (single, double or three probes: lesion cutoff 1.5–2.5 cm) | Single | NA | NA | NA | 8 (22.2%) |
Post-operative pain (3), tumor lysis syndrome (1) | 1 (2.8%) |
2.5 (0–28) |
| Rhaiem 2020 |
EmPrintTM Ablation System, Medtronic, Dublin, Ireland |
2.45 GHz, 14 G probes with ThermosphereTM Technology |
Single and multiple | 5 | 75 W § | NA | 6 (31.6%) |
Evisceration (1), biliary fistula (1), peritonitis due to anastomotic leakage (1), heparin-induced thrombocytopenia (1), surgical site infection (1), MHV thrombosis (1) |
0 | NA |
| Takahashi 2018 |
EmPrintTM Ablation System, Covidien, Boulder, CO, USA | 2.45 GHz, 14 Gauge antenna |
NA | 2.5–15 | 100 W | 154 (±3 *) | 7(13.7%) | NA | NA | (1–4) |
| Shady 2017 |
NeuWaveTM Microwave Ablation System; HS AMICATM; Microsulis (Angiodynamics, New York, NY, USA); and EmPrintTM Ablation System | NA | NA | NA | NA | NA | 19 (39.6%) |
PNX (11), hepatic artery- portal venous fistula (1), bowel perforation (1), bilomas (2), left portal vein thrombosis (1), sub-scapular hematoma (1), subcutaneous emphysema (1), pleural effusion (1) |
6 (12.5%) |
NA |
| Yang 2017 |
NA | NA | Single | 70 (total) | NA | NA | 9 (12.7%) |
Perihepatic fluid collection (3), ascites (3), UTI (2), pleural effusion (1) |
0 | 7 (5–19) |
| Song 2016 |
KY-2000 Microwave Ablation System (Kangyou Medical, Nanjing, Jiangsu, China) |
2450 MHz antennae of three types (0.5, 0.7 and 1.1 cm tips Ø) |
NA | NA | NA | NA | 3 (10.7%) |
Pain (3) | 2 (7.1%) |
5.9 (±0.9 *) |
| Eng 2015 |
ValleyLabTM Microwave Ablation Generator System, Covidien, Boulder, CO, USA |
NA | NA | NA | NA | NA | 18 (54.5%) |
Intra-abdominal abscess drained radiologically (4), respiratory distress (3), biliary fistula (1) |
8 (24.2%) |
6 (1–32) |
| Engstrand 2014 |
Acculis® Microwave Tissue Ablation System, Angiodynamics, Latham, NY, USA |
NA | NA | NA | NA | 235 (112–475) |
12 (60%) |
Multiple liver abscesses drained percutaneously (1), pleural effusion drained percutaneously (1), severe respiratory distress (3) |
5 (25%) |
10 (2–24) |
| Ierardi 2013 |
EvidentTM Microwave Ablation System, Covidien, USA | Straight 14.5-gauge antennas (12–17 cm of length), saline-perfused coaxial cable. Lesions of 3–4 cm Ø were treated with two antennas. Lesions of >4 cm Ø were treated with three antennas |
Single | 10 | 45 W | NA | 4 (23.5%) |
Abscess drained (1), pain (3), ascites (1) |
1 (5.9%) |
NA |
| Stattner 2013 |
Acculis® Microwave Tissue Ablation System (Microsulis Medical Ltd., Dublin, UK) | 2.65 GHz, shaft-cooled Accu2i pMTA antenna | NA | 1.5 | 100 W | NA | 15 (34.9%) |
Re-intervention (3), death (1) |
4 (9.3%) |
7 (4–80) |
| Shibata 2000 |
Microwave tissue coagulator HSD-20M (Azwell, Osaka, Japan) | For coagulation of superficial tumors: 2-cm-long electrode (0.7 mm Ø; TM-20; Azwell). For deep tumors: 20-cm-long electrode (1.6 mm Ø; TMD- 16CBL; Azwell) | NA | 2–20 | 60– 100 W |
180 (±20 *) | 2 (14.3%) |
Hepatic abscess (1), bile duct fistula (1) | 2 (14.3%) |
NA |
| Seki 1999 |
Microtaze OT-110 M, Nippon Shoji, Azwell Inc., Osaka, Japan |
MW electrode of 2.0 mm Ø, 25 cm length (MD-20 CDL- 10/25) | Multiple | 1 | 80 W | NA | 1 (6.7%) |
Pleural effusion (1) | 0 | 4 |
| Total | - | - | - | - | - | 184.16 ° (±26.45°) |
104 (26.3%) {22.2–30.9%} |
- | 29 (8.4%) {5.9– 11.8%} |
5.43 (NA) |
Abbreviations—MW: microwave; PNX: pneumothorax; UTI: urinary tract infection; NA: missing data. Ø: diameter; *: mean and standard deviation; §: an additional treatment could be performed at 45 W for 3 min for nodules between 1.5 and 2 cm or 100 W for 1–3 min for larger nodules. °: means and standard deviations, calculated using the Hozo method on the complete available data (conversions for each study are not shown).
Table 5.
Survival analysis. Figures in curly brackets indicate the 95% confidence intervals.
| Study ID |
Follow-Up Mean° (SD°), Months |
3 Months RF |
6 Months RF |
1 Year RF |
3 Years RF |
5 Years RF |
Total RF |
3 Months FFLR |
6 Months FFLR |
1 Year FFLR |
|---|---|---|---|---|---|---|---|---|---|---|
| McEachron 2021 |
NA | 100% | 100% | 100% | 54% | 54% | 50% | NA | NA | NA |
| Rhaiem 2020 |
11.75 (±2.25) |
90.3% | 61.9% | 47.6% | NA | NA | NA | 94.7% | 84.2% | 78.9% |
| Takahashi 2018 |
17 (±2.25) |
97.7% | 95.8% | 91.5% | NA | NA | NA | 97.7% | 95.8% | 91.5% |
| Shady 2017 |
NA | 97% | 85.8% | 79% | NA | NA | 62% | 97% | 85.8% | 79% |
| Yang 2017 |
NA | 100% | 94.3% | 80.3% | 56.2% | 39% | 39% | NA | NA | NA |
| Song 2016 |
NA | 100% | 100% | 96.7% | 71.4% | 39.3% | 10% | NA | NA | NA |
| Eng 2015 |
17.46 (±11.51) |
96.9% | 87.9% | 66.7% | 19.3% | NA | 19.3% | NA | NA | NA |
| Engstrand 2014 |
28.25 (±11.25) |
NA | NA | NA | NA | NA | 25% | NA | NA | NA |
| Ierardi 2013 |
15.77 (±8.25) |
88.2% | 82.3% | 70.6% | NA | NA | 64.7% | 88.2% | 88.2% | 76.5% |
| Stattner 2013 |
29.25 (±20.25) |
85.7% | 85.7% | 31% | 22% | 6% | 27.9% | NA | NA | NA |
| Shibata 2000 |
NA | 100% | NA | 70% | NA | NA | 11.3% | NA | NA | NA |
| Seki 1999 |
21 (±8.09) |
73.4% | 66.7% | 60% | NA | NA | NA | NA | NA | NA |
| Total | 20.57 ° (±9.57°) |
95.5% {92.9– 97.2%} |
89.5% {85.9– 92.2%} |
65.1% {60.1– 69.7%} |
44.6% {38– 51.3%} |
34.3% {27.7– 41.5%} |
37.1% {31.9– 42.6%} |
96.3% {91.6– 98.4%} |
89.6% {83.3– 93.7%} |
83.7% {76.6– 88.9%} |
|
Study
ID |
Hepatic
Progression , pt n (%) |
Extra-Hepatic Progression,
pt n (%) |
Overall
Recurrence, pt n (%) |
3
Months OS |
6
Months OS |
1
Year OS |
3
Years OS |
5
Years OS |
||
| McEachron 2021 |
18 (50%) |
1 (2.8%) |
18 (50%) |
100% | 100% | 100% | 75% | 63% | ||
| Rhaiem 2020 |
7 (36.8%) |
NA | 7 (36.8%) |
100% | 100% | 100% | NA | NA | ||
| Takahashi 2018 |
NA | NA | 11 (21.6%) |
NA | NA | NA | NA | NA | ||
| Shady 2017 |
NA | NA | 23 (47.9%) |
NA | NA | NA | NA | NA | ||
| Yang 2017 |
NA | NA | 43 (60.6%) |
100% | 94.5% | 80.2% | 72% | 58% | ||
| Song 2016 |
NA | NA | NA | 100% | 100% | 89.1% | 71.4% | 53.6% | ||
| Eng 2015 |
10 (30.3%) |
12 (36.4%) |
13 (39.4%) |
93.9% | 90.9% | 81.8% | 45.7% | NA | ||
| Engstrand 2014 |
17 (85%) |
11 (55%) |
15 (75%) |
100% | 100% | 90% | 41.5% | NA | ||
| Ierardi 2013 |
4 (23.5%) |
2 (11.8%) |
6 (35.3%) |
100% | 100% | NA | NA | NA | ||
| Stattner 2013 |
5 (11.6%) |
22 (51.2%) |
4 (9.3%) |
100% | 100% | 82% | 40% | 12% | ||
| Shibata 2000 |
NA | NA | NA | 100% | 95% | 71% | 57% | 14% | ||
| Seki 1999 |
4 (26.6%) |
6 (40%) |
6 (40%) |
100% | 100% | 100% | NA | NA | ||
| Total | 65 (35.5%) {28.9–42.7%} |
54 (32.9%) {26.2–40.4%} |
146 (41.36%) {36.3–46.6%} |
99.3% {97.6– 99.8%} |
97.3% {94.8– 98.6%} |
86.7% {82.2– 90.2%} |
59.6% {53.3– 65.5%} |
44.8% {37.9– 51.8%} |
||
Abbreviations—RF: recurrence-free; FFLR: free from local recurrence; SD: standard deviation; NA: missing data. °: means and standard deviations calculated using the Hozo method on complete available data. OS: overall survival; DFS: disease-free survival; pt.: patients; NA: missing data.
3.3. Subgroup Analysis
To better understand the outcomes of MWA-treated patients, we sorted them into sub-categories and analyzed the outcomes of these subgroups.
CRLM with a Diameter of ≤30 mm
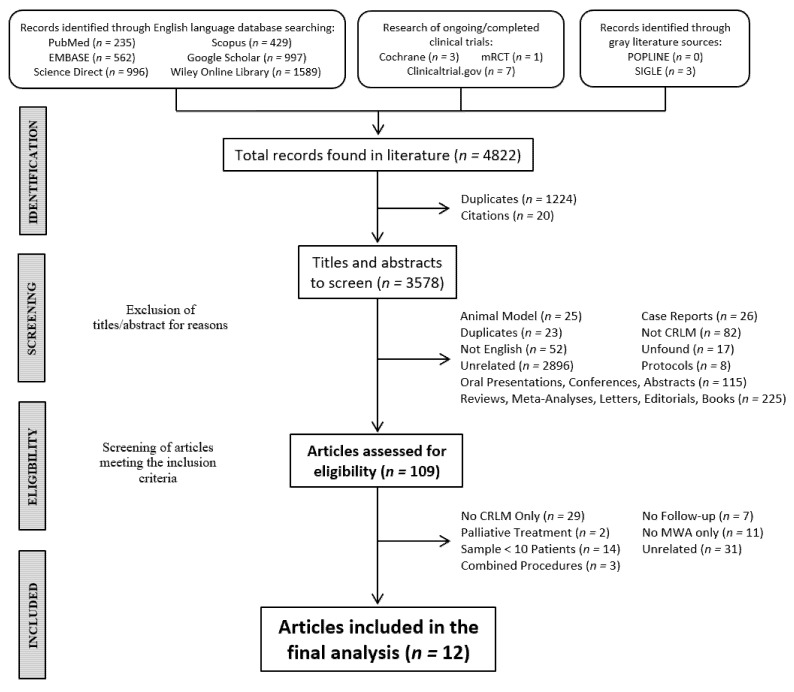
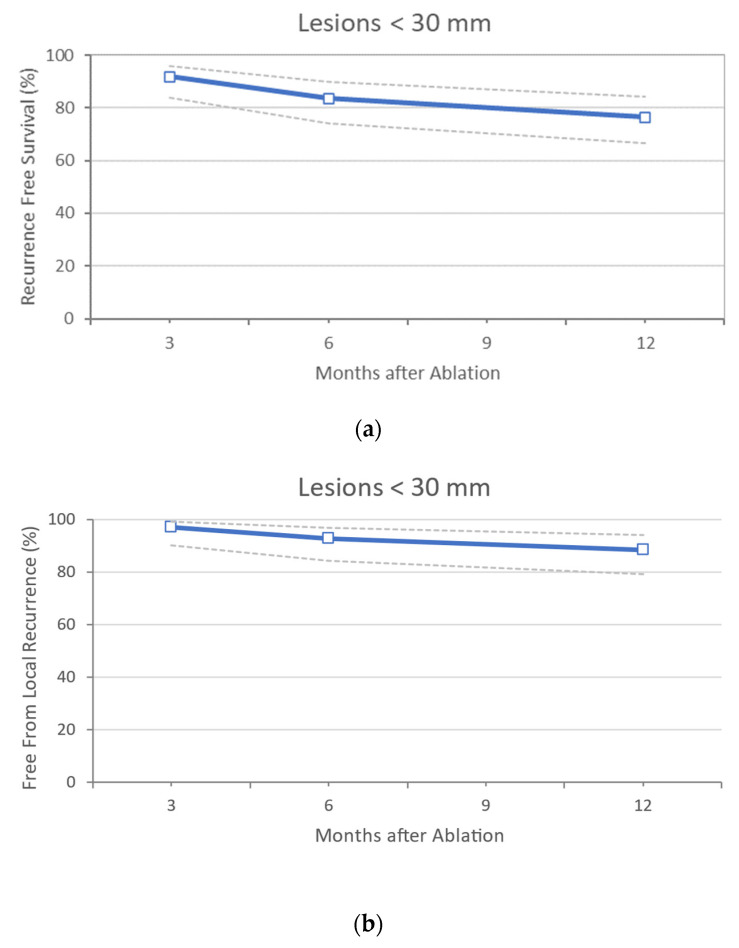
Only 3 studies report an acceptable follow-up in this cohort subcategory [20,33,38] (Table 6 and Table 7). Eighty-five patients with 159 lesions of ≤30 mm were described, with a mean reported diameter of 20.39 mm (±2.9 mm). Two series performed surgical MWA [20,38] (82.4% of the total). Of these, 64.7% benefited from a laparoscopic approach. Multifocality (i.e., presence of more than one lesion) was present in 64.7% of patients, and most CRLM were metachronous (76.5%). All patients received neoadjuvant and adjuvant treatment. In this group of analyses, 16.5% of patients experienced complications, but none of these patients had CDs ≥ 3. The RF and FFLR at 3, 6, and 12 months were 91.8%, 83.5%, 76.5%, and 97.1%, 92.9%, and 88.6%, respectively, with hepatic progression of 32.4%. The overall recurrence was 28.2% (Figure 2a,b). The OS at 3, 6, and 12 months was 100%.
Table 6.
Sub-analysis on studies comprising lesions of <30 mm only: general data. Figures in curly brackets indicate 95% confidence intervals.
| Study ID |
Patients/ Lesions Treated, n |
Dimensi-ons, Mean (SD), mm | Percutaneous/ Surgical Treatment, pt n (%) |
Surgical Open/Lap, pt n (%) |
Single/ Multiple Lesions, pt n (%) |
Synch, pt n (%)/Meta, pt n (%) | Neoadj, n (%) ±/ Adj, n (%) Treatment |
Complica-tions n (% pt.) |
CDs ≥3, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Rhaiem 2020 |
19/23 | 15.25 ° (±2.75 °) |
0±/ 19 (100%) |
15 (78.9%) ±/ 4 (21.1%) |
15 (78.9%) ±/ 4 (21.1%) |
8 (42.1%) ±/ 11 (57.9%) |
17 (89%) ±/ 19 (100%) |
6 (38%) |
0 |
| Takahashi 2018 |
51/121 | 21.25 ° (±2.83 °) |
0±/ 51 (100%) |
0±/ 51 (100%) |
0±/ 51 (100%) |
NA/NA | 28 (54.9%)/NA | 7 (13.7%) |
NA |
| Seki 1999 |
15/15 | 21.4 (±3.73 °) |
15 (100%) ±/ 0 |
0/0 | 15 (100%) ±/ 0 |
0±/ 15 (100%) |
0±/ 15 (100%) |
1 (6.7%) |
0 |
| Total | 85/159 | 20.39° (±2.90°) |
15 (17.6%)/ 70 (82.4%) {11–27.1%} ±/ {72.3–89%} |
15 (17.7%)/ 55 (64.7%) {11–27.1%} ±/ {54.1–74%} |
30 (35.3%)/ 55 (64.7%) {26.9–45.9%} ±/ {54.1–74%} |
8 (23.5%)/ 26 (76.5%) {12.4–40%} ±/ {60–87.6%} |
45 (52.9%)/ 34 (100%) {42.4–63.2%} ±/ {89.9–100%} |
14 (16.5%) {10.1–25.8%} |
0 (0%) {0–10.2%} |
Abbreviations—SD: standard deviation; Lap: laparoscopic; Synch: synchronous; Meta: metachronous; Neoadj: neoadjuvant; Adj: adjuvant; CDs: Clavien–Dindo score; pt: calculated on the number of patients; mm: millimeters; NA: missing data. °: calculated using the Hozo method.
Table 7.
Sub-analysis on studies comprising lesions of <30 mm only: oncological data. Figures in curly brackets indicate 95% confidence intervals.
| Study ID |
3 Months RF |
6 Months RF |
1 Year RF |
3 Months FFLR |
6 Months FFLR |
1 Year FFLR |
Hepatic Progression, pt n (%) |
Overall Recurrence, pt n (%) |
3 Months OS |
6 Months OS |
1 Year OS |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Rhaiem 2020 |
90.3% | 61.9% | 47.6% | 94.7% | 84.2% | 78.9% | 7 (36.84%) |
7 (36.8%) |
100% | 100% | 100% |
|
Takahashi 2018 |
97.7% | 95.8% | 91.5% | 97.7% | 95.8% | 91.5% | NA | 11 (21.6%) |
NA | NA | NA |
|
Seki 1999 |
73.4% | 66.7% | 60% | NA | NA | NA | 4 (26.6%) |
6 (40%) |
100% | 100% | 100% |
| Total |
91.8% {83.9– 95.9%} |
83.5% {74.2– 89.9%} |
76.5% {66.4– 84.2%} |
97.1% {90.2– 99.2%} |
92.9% {84.3– 96.9%} |
88.6% {79– 94.1%} |
11 (32.4%) {19.1–49.1%} |
24 (28.2%) {19.8–38.6%} |
100% {89.9– 100%} |
100% {89.9– 100%} |
100% {89.9– 100%} |
Abbreviations—RF: recurrence-free; FFLR: free from local recurrence; OS: overall survival; pt: calculated on the number of patients; NA: missing data.
Figure 2.
(a) Sub-analysis on studies comprising lesions <30 mm only: Recurrence Free Survival data. In dotted lines are indicated the 95% confidence intervals, (b) Sub-analysis on studies comprising lesions <30 mm only: Free from Local Recurrence outcome. In dotted lines are indicated the 95% confidence intervals.
Surgical vs. Percutaneous MWA
Eight studies reported data concerning MWA conducted through a surgical approach [20,29,30,32,35,37,38,39] (Table 8 and Table 9). In this group of analyses, 602 lesions were treated in 287 patients, and the mean diameter of the lesions was 15.51 mm (±7.16 mm). The vast majority of surgically treated CRLM (96.9%) were smaller than 30 mm, and 57.8% of patients received a laparoscopic therapeutic approach. Most surgically treated MWA patients (72.6%) had more than one lesion, while 55.9% had synchronous CRLM. Complications after surgery were reported in 26.8% of patients, of which 8.5% were severe (CDs ≥ 3). The RF rates at 3 months, 6 months, and 1, 3, and 5 years were 96.3%, 90.9%, 72.7%, 40.4%, and 33.3% (3- and 5-years’ RF were analyzed in 4 and 3 studies), respectively. The FFLR at 3, 6, and 12 months were 97.1%, 92.9, and 88.6%, respectively (FFLR was analyzed in 2 studies only). Overall, 40.7% of patients experienced progression, and 37.6% had hepatic progression. The OS rates at 3 months, 6 months, and 1, 3, and 5 years were 99.2%, 96.6%, 85.6%, 58.1%, and 43.3%, respectively.
Table 8.
Sub-analysis on studies comprising a surgical vs. a radiological approach. Figures in curly brackets indicate 95% confidence intervals.
| Study ID |
Patients/ Lesions Treated, n |
Dimensions, Mean (SD), mm |
Lesions <30 mm, n (%) |
Surgical Open/Lap, pt n (%) |
Single/Multiple Lesions, pt n (%) |
Synch, pt n (%)/Meta, pt n (%) |
Complications n (%) | CDs ≥3, n (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Surgical Approach |
McEachron 2021 |
36/135 | 19 ° (±19.5 °) |
132 (97.78%) |
13* (32.5% *)/ 27* (67.5% *) |
NA | 13 (36.11%)/NA | 8 (22.2%) |
1 (2.8%) |
| Rhaiem 2020 |
19/23 | 15.25 ° (±2.75 °) |
23 (100%) |
15 (78.95%)/ 4 (21.05%) |
15 (78.95%)/ 4 (21.05%) |
8 (42,1%)/ 11 (57.89%) |
6 (38%) |
0 | |
| Takahashi 2018 |
51/121 | 21.25 ° (±2.83 °) |
121 (100%) |
0/ 51 (100%) |
0/ 51 (100%) |
NA/NA | 7 (13.7%) |
NA | |
|
Yang 2017 |
71/121 | 3 ° (±0.67°) |
NA | 0/ 71 (100%) |
33 (46.48%)/ 38 (53.52%) |
NA/NA | 9 (12.7%) |
0 | |
|
Eng 2015 |
33/49 | NA | 42 (85.71%) |
NA | NA | NA/NA | 18 (54.5%) |
8 (24.2%) |
|
|
Engstrand 2014 |
20/NA | NA | NA | 20 (100%)/ 0 |
0/ 20 (100%) |
18 (90%)/ 2 (10%) |
12 (60%) |
5 (25%) |
|
| Stattner 2013 |
43/95 | 20.25 ° (±5.83°) |
NA | 43 (100%)/ 0 |
NA | 27 (62.8%)/ 16 (37.2%) |
15 | 4 | |
| Shibata 2000 |
14/58 | 27 (±11) |
NA | 14 (100%)/ 0 |
0/ 14 (100%) |
NA/NA | 2 | 2 | |
| Total | 287/602 |
15.51 ° (±7.16°) |
318 (96.9%) {94.5–98.3%} |
92 (42.2%)/ 126 (57.8%) {35.8–48.8%}/ {51.2–64.2%} |
48 (27.4%)/ 127 (72.6%) {21.4–34.5%}/ {65.5–78.6%} |
66 (55.9%)/ 29 (35.4%) {46.9–64.7%}/ {25.9–46.2%} |
77 (26.8%) {22–32.2%} |
20 (8.5%) {5.6–12.7%} |
|
| Radiological Approach | Shady 2017 |
48/60 | 17 ° (±7.5 °) |
56 (93.33%) |
− |
40 (83.3%)/ 8 (17.67%) |
NA/NA | 19 | 6 (12%) |
| Song 2016 |
28/NA | NA | NA | − |
18 (64.3%)/ 10 (35.7%) |
10 (35.7%)/ 18 (64.3%) |
3 (10.7%) |
2 | |
| Ierardi 2013 |
17/23 | 34.52 (±13.25 °) |
1 (4.8%) |
− |
14 (82.4%)/ 3 (17.6%) |
NA/NA | 4 (23.5%) |
1 (5.9%) |
|
| Seki 1999 |
15/15 | 21.4 (±3.73 °) |
15 (100%) |
− |
15 (100%)/ 0 |
0/ 15 (100%) |
1 (6.7%) |
0 | |
| Total | 108/98 |
21.78 ° (±8.27°) |
72 (73.5%) {63.9–81.2%} |
− |
87 (80.6%)/ 21 (19.4%) {72.1–86.9%}/ {13.1–27.9%} |
10 (23.3%)/ 33 (76.8%) {13.2–37.7%}/ {62.3–86.9%} |
27 (25%) {17.8–33.9%} |
9 (8.3%) {4.5–15.1%} |
Abbreviations—SD: standard deviation; Lap: laparoscopic; Synch: synchronous; Meta: metachronous; CDs: Clavien-Dindo score; pt: calculated on the number of patients; mm: millimeters; NA: missing data. *: calculated on the number of surgical treatments (data excluded from further analysis); °: calculated using the Hozo method.
Table 9.
Sub-analysis on studies comprising a surgical vs. a radiological approach, concerning oncological outcomes. Figures in curly brackets indicate 95% confidence intervals.
|
Study ID |
3 Months RF |
6 Months RF |
1 Year RF |
3 Years RF |
5 Years RF |
3 Months FFLR |
6 Months FFLR |
1 year FFLR |
Hepatic Progression pt n (%) |
Overall Recurrence, pt n (%) |
3 Months OS |
6 Months OS |
1 Year OS |
3 Years OS |
5 Years OS |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgical Approach |
McEachron 2021 |
100% | 100% | 100% | 54% | 54% | NA | NA | NA | 18 (50%) |
18 (50%) |
100% | 100% | 100% | 75% | 63% |
| Rhaiem 2020 |
90.3% | 61.9% | 47.6% | NA | NA | 94.7% | 84.2% | 78.9% | 7 (36.8%) |
7 (36.8%) |
100% | 100% | 100% | NA | NA | |
| Takahashi 2018 |
97.7% | 95.8% | 91.5% | NA | NA | 97.7% | 95.8% | 91.5% | NA | 11 (21.6%) |
NA | NA | NA | NA | NA | |
|
Yang 2017 |
100% | 94.3% | 80.3% | 56.2% | 39% | NA | NA | NA | NA | 43 (60.6%) |
100% | 94.5% | 80.2% | 72% | 58% | |
|
Eng 2015 |
96.9% | 87.9% | 66.7% | 19.3% | NA | NA | NA | NA | 10 (30.3%) |
13 (39.4%) |
93.9% | 90.9% | 81.8% | 45.7% | NA | |
|
Engstrand 2014 |
NA | NA | NA | NA | NA | NA | NA | NA | 17 (85%) |
15 (75%) |
100% | 100% | 90% | 41.5% | NA | |
| Stattner 2013 |
85.7% | 85.7% | 31% | 22% | 6% | NA | NA | NA | 5 (11.63%) |
4 (9.3%) |
100% | 100% | 82% | 40% | 12% | |
| Shibata 2000 |
100% | NA | 70% | NA | NA | NA | NA | NA | NA | NA | 100% | 95% | 71% | 57% | 14% | |
| Total | 96.3% {93.2– 97.9%} |
90.9% {86.7– 93.9%} |
72.7% {67– 77.7%} |
40.4% {33.6– 47.7%} |
33.3% {26.3– 41.2%} |
97.1% {90.2– 99.2%} |
92.9% {84.3– 96.9%} |
88.6% {79– 94.1%} |
57 (37.6%) {30.4–45.7%} |
111 (40.7%) {35–46.6%} |
99.2% {96.9– 99.7%} |
96.6% {93.5– 98.3%} |
85.6% {80.54– 89.50%} |
58.1% {51.1– 64.4%} |
43.3% {35.9– 50.9%} |
|
|
Radiological
Approach |
Shady 2017 |
97% | 85.8% | 79% | NA | NA | 97% | 85.8% | 79% | NA | 23 (47.9%) |
NA | NA | NA | NA | NA |
| Song 2016 |
100% | 100% | 96.7% | 71.4% | 39.3% | NA | NA | NA | NA | NA | 100% | 100% | 89.1% | 71.4% | 53.6% | |
|
Ierardi 2013 |
88.2% | 82.4% | 70.6% | NA | NA | 88.2% | 88.2% | 76.5% | 4 (23.5%) |
6 (35.3%) |
100% | 100% | NA | NA | NA | |
| Seki 1999 |
73.4% | 66.7% | 60% | NA | NA | NA | NA | NA | 4 (26.6%) |
6 (40%) |
100% | 100% | 100% | NA | NA | |
| Total | 93.5% {87.2– 96.8%} |
86.1% {78.3– 91.4%} |
79.6% {71.1– 86.2%} |
71.4% {52.9– 84.8%} |
39.3% {23.6– 57.6%} |
95.4% {87.3– 98.4%} |
86.2% {75.7– 92.5%} |
(78.5%) {67–86.7%} |
8 (25%) {13.3–42.1%} |
35 (43.8%) {33.4–54.7%} |
100% {93.9– 100%} |
100% {93.9– 100%} |
93% {81.4– 97.6%} |
71.4% {52.9– 84.8%} |
53.6% {35.8– 70.5%} |
Abbreviations—RF: recurrence-free; FFLR: free from local recurrence; OS: overall survival; pt: calculated on the number of patients; NA: missing data.
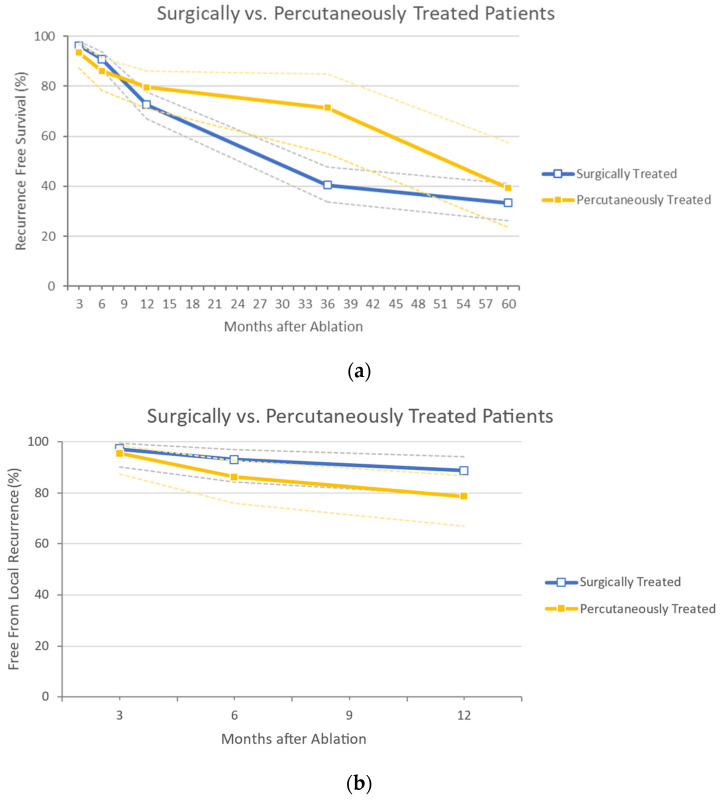
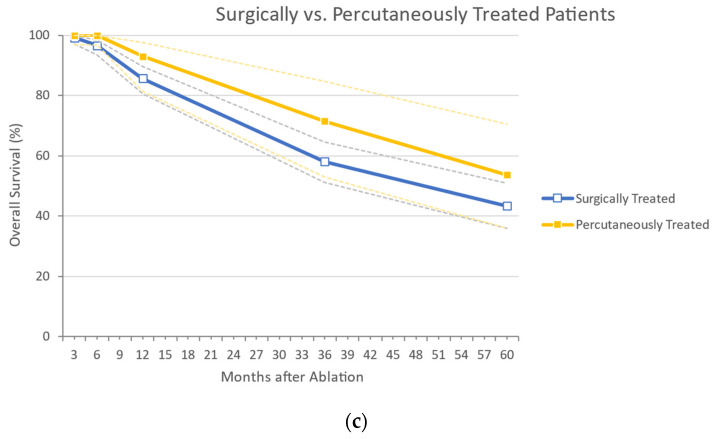
Only 4 studies report data concerning percutaneous MWA [31,33,34,36]. Overall, 98 lesions were treated in 108 patients (taking into account the missing data) [36]. The mean lesion diameter was 21.78 mm (±8.27 mm). Of all lesions, 73.5% were smaller than 30 mm. The majority of radiologically treated patients (80.6%) had a single lesion and had metachronous CRLM (76.8%). Percutaneous procedures encountered complications in 25.0% of patients, of which 8.5% were serious (CDs ≥ 3). The RF rates at 3 months, 6 months, and 1, 3, and 5 years were 93.5%, 86.1%, 79.6%, 71.4%, and 39.3%, respectively. The FFLR at 3, 6, and 12 months were 95.4%, 86.2%, and 78.5%, respectively. Overall, a recurrence was reported in 43.8% of patients, with a hepatic progression in 25.0%. The OS rates at 3 months, 6 months and 1, 3, and 5 years were 100%, 100%, 93.0%, 71.4%, and 53.6%, respectively. A graphic comparison between the two groups concerning RF, FFLR, and OS is depicted in Figure 3a–c.
Figure 3.
(a) Sub-analysis on studies comprising surgical vs. radiological approach, concerning Recurrence Free Survival outcomes. In dotted lines are indicated the 95% confidence intervals, (b) Sub-analysis on studies comprising surgical vs. radiological approach, concerning Free from Local Recurrence outcomes. In dotted lines are indicated the 95% confidence intervals, (c) Sub-analysis on studies comprising surgical vs. radiological approach, concerning Overall Survival outcomes. In dotted lines are indicated the 95% confidence intervals.
Surgical MWA: Open vs. Laparoscopic Approach
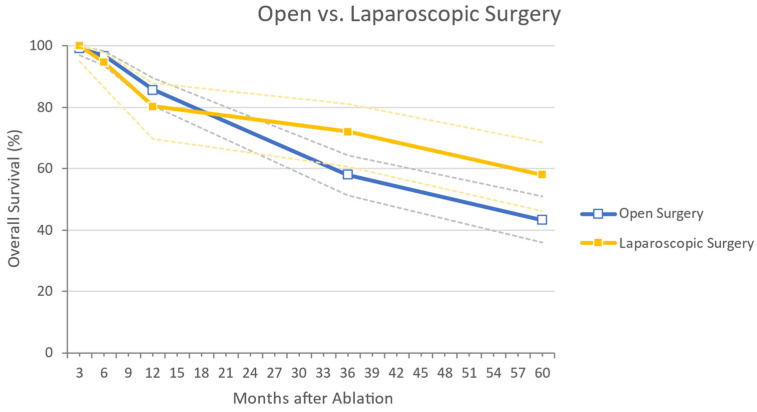
Three studies analyzed the data concerning laparotomic surgery for MWA [30,35,37] (Table 10 and Table 11). In this group, 153 lesions were treated in 77 patients. The mean lesion diameter was 22.81 mm (±7.79 mm). All patients presented with more than one lesion. Open surgery resulted in complications for 37.7% of patients, of which 14.3% were severe (CDs ≥ 3). The RF rates at 3 and 12 months were 89.5% and 40.4%, respectively. The overall recurrence was 30.2%; the OS rates at 3 months, 6 months, and 1, 3, and 5 years were 100%, 98.7%, 81.8%, 42.9%, and 12.3%, respectively.
Table 10.
Sub-analysis on studies comprising lesions treated by open vs. laparoscopic surgical approach: general data. Figures in curly brackets indicate 95% confidence intervals.
| Study ID | Patients/ Lesions Treated, n |
Dimensions, Mean (SD), mm |
Single/Multiple Lesions, pt n (%) |
Complications, n (%) |
CDs ≥ 3, n (%) | |
|---|---|---|---|---|---|---|
| Open | Engstrand 2014 |
20/NA | NA | 0/ 20 (100%) |
12 (60%) |
5 (25%) |
| Stattner 2013 |
43/95 | 20.25 ° (±5.83 °) |
NA | 15 | 4 | |
| Shibata 2000 |
14/58 | 27 (±11) |
0/ 14 (100%) |
2 | 2 | |
| Total | 77/153 | 22.81 ° (±7.79 °) |
0 (0%)/34 (100%) {0–10.2%}/{89.9–100%} |
29 (37.7%) {27.7–48.8%} |
11 (14.3%) {8.2–23.8%} |
|
| Laparoscopic | Takahashi 2018 |
51/121 | 21.25 ° (±2.83 °) |
0 51 (100%) |
7 (13.7%) |
NA |
|
Yang 2017 |
71/121 | 3 ° (±0.67 °) |
33 (46.5%)/ 38 (53.5%) |
9 (12.7%) |
0 | |
| Total | 122/242 | 10.63 ° (±1.57 °) |
33 (27.1%)/89 (73%) {19.9–35.5%}/{64.5–80%} |
16 (13.1%) {8.2–20.3%} |
- |
Figures in squared brackets indicated 95% confidence intervals. Abbreviations—SD: standard deviation; CDs: Clavien–Dindo score; pt: calculated on the number of patients; mm: millimeters; NA: missing data. °: calculated using the Hozo method using complete available data.
Table 11.
Sub-analysis on studies comprising lesions treated by an open vs. a laparoscopic surgical approach: oncological outcomes. Figures in curly brackets indicate 95% confidence intervals.
| Study ID | 3 Months RF |
6 Months RF |
1 Year RF |
Overall Recurrence, pt n (%) |
3 Months OS |
6 Months OS |
1 Year OS |
3 Years OS |
5 Years OS |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Open | Engstrand 2014 |
NA | NA | NA | 15 (75%) |
100% | 100% | 90% | 41.5% | NA |
| Stattner 2013 |
85.7% | 85.7% | 31% | 4 (9.3%) |
100% | 100% | 82% | 40% | 12% | |
| Shibata 2000 |
100% | NA | 70% | NA | 100% | 95% | 71% | 57% | 14% | |
| Total | 89.5% {78.9– 95.1%} |
- | 40.4% {28.6– 53.3%} |
19 (30.2%) {20.2–42.4%} |
100% {95.3– 100%} |
98.7% {93– 99.8%} |
81.8% {71.8– 88.9%} |
42.9% {32.4– 54%} |
12.3% {6.1– 23.3%} |
|
| Laparoscopic | Takahashi 2018 |
97.7% | 95.8% | 91.5% | 11 (21.6%) |
NA | NA | NA | NA | NA |
| Yang 2017 |
100% | 94.3% | 80.3% | 43 (60.6%) |
100% | 94.5% | 80.2% | 72% | 58% | |
| Total | 99.2% {95.5– 99.9%} |
95.1% {89.7– 97.7%} |
85.5% {77.9– 90.5%} |
54 (44.3%) {35.8–53.1%} |
- | - | - | - | - |
Abbreviations—RF: recurrence-free; OS: overall survival; pt: calculated on the number of patients; NA: missing data.
Only 2 studies reported data on laparoscopic MWA [38,39]. In this group, 242 lesions were treated in 122 patients. The mean lesion diameter was 10.63 mm (±1.57 mm). The majority (73.0%) had more than one lesion. Laparoscopically treated patients showed a complication rate of 13.1%; data concerning complications with CDs ≥ 3 were available for one study only, and no further analysis was possible. The RF rates at 3, 6, and 12 months were 99.2%, 95.1%, and 85.5%, respectively; the overall recurrence rate was 44.3%. Data concerning OS rates were available for one study only, and no further analysis was possible. A graphic comparison between two groups concerning OS is depicted in Figure 4.
Figure 4.
Sub-analysis on studies comprising lesions treated by open vs. laparoscopic surgical approach, concerning Overall Survival outcomes. In dotted lines are indicated the 95% confidence intervals.
4. Discussion
Surgical resection with a parenchymal sparing technique is the gold standard of care for CRLM [40,41]. Unfortunately, for oncological reasons and due to the patients’ condition, few patients are candidates for curative-intent surgery. In this setting, when percutaneous thermal ablation techniques have been used in non-selected patients, oncological data from thermal ablation seemed to not be optimal when compared with a curative-intent surgical approach [32]. Modern CHT and target therapies for CRLM increased not only the resection rates but also the number of patients with complex CRLM and a high risk of recurrence in patients [32,40,42]. The main challenge is to define which patients can benefit most from surgery and thermal ablation techniques without increasing the morbidity and mortality rates [43].
Tabuse first developed the surgical technique of microwave coagulation in 1979 and applied it to the transection of hepatic parenchyma by coagulating the tumor tissue in many organs [44]. In the past, RFA and MWA have increased their usefulness in the context of CRLM treatment.
This study is the first systematic review focused only on the MWA of CRLM. In this review of 12 studies, we pooled 395 patients undergoing MWA for CRLM and reported the pooled analyses of OS, RF, and FFLR at 3 months, 6 months, and 1, 3, and 5 years.
A general observation of this systemic review was the performance of surgical MWA in 62.9% of patients. This underlines the need to explore other frontiers for CRLM treatment options, other than hepatic resection [20,29,30,32,35,37,38,39]. It was also reported that the complication rate was 26.8%, of which cases only 8.5% were severe (CDs ≥ 3). These data were in concordance with the literature data concerning outcomes after liver resection for CRLM [45]. The pooled analyses of the oncological outcome of MWA showed OS survival rates at 1, 3, and 5 years of 86.7%, 59.6%, and 44.8%, respectively. This was also comparable with OS rates reported after the curative liver resection of CRLM [45]. The RF rates at 1, 3, and 5 years after MWA were 65.1%, 44.6%, and 34.3%, respectively, which were better than the previously reported data [45].
The present results, as well as the reported literature data, show a common intention to treat CRLM that are less than 3 cm (91.6% of lesions) as a general principle of the advantageous influence of factors of the MWA [46,47]. In this subset of lesions, despite the presence of only 3 studies that analyzed oncological follow-up, 16.5% of patients experienced low-impact complications (CDs ≥ 3 = 0%). These results show a better safety profile than surgical resection and/or MWA from the Sweden Nationwide Registry, reporting severe complication rates of 16.4% and 7.0%, respectively [48].
Pooled analyses for RF rates and FFLR after MWA at 3, 6, and 12 months showed 91.8%, 83.5%, and 76.5% and 97.1%, 92.9%, and 88.6%, respectively, which was encouraging. In addition, hepatic progression rates of 32.4% and overall recurrence rates of 28.2% were in concordance with the data observed after resection. Interestingly, the OS rates after MWA for CRLM (100% at 3, 6, and 12 months) were encouraging and seemed better than surgical resection [48,49].
Analyzing the subgroup of surgical vs. radiological MWA approaches, no difference concerning complications was found. However, local hepatic control was more satisfactory after following the surgical approach, compared to radiological procedures. Indeed, the global RF and OS rates did not reflect the efficacy of treatment but, above all, the biological characteristics of CRLM. These results might be explained by the possible favorable tumor location for MWA and not for surgical resections, which are likely to be more complicated to treat in the same way as for MWA. As the rate of local recurrence seems dependent upon tumor location and its vessel proximity, in order to minimize the “heat sink effect”, pedicle clamping during MWA has been used successfully [20,32]. In terms of comparing surgical MWA, only the laparoscopic approach ensured a good RF rate. We believe that these results might be due to the fact that smaller-sized CRLM were treated using the laparoscopic approach (10 mm vs. 22.8 mm in an open approach) [50,51].
Despite our efforts to create a homogeneous comparison group, we acknowledge that our systematic review suffers from several limitations. Only 4 studies were prospective; in addition, some papers reported a heterogeneous number and size of ablated lesions, showing a general lack of standardization and follow-up protocols. Therefore, these results should be interpreted with caution, considering that most of them were extracted from clinically heterogeneous studies.
Finally, we believe that MWA represents a promising technique in the treatment of CRLM. When used appropriately, especially in selected patients with tumors less than 3 cm in size, the oncological results are promising. RFA and MWA are not mutually exclusive but they are additional, with the advantage of being able to perform MWA near the large blood vessels. For the bigger biliary ducts, the use of thermal ablation remains absolutely contraindicated. This extends the use of intraoperative MWA, which can be performed safely using a laparoscopy. Resection with or without MWA can achieve encouraging oncological results, with low morbidity allowing patients to receive their systemic drugs more quickly; thus, their care is not interrupted. We are also impatient to discover the final results of the ongoing prospective randomized COLLISION trial (Colorectal liver metastases: surgery versus thermal ablation trial) [52].
5. Conclusions
Our findings indicate that MWA could be a valid tool for CRLM treatment, especially for deep lesions and those smaller than 3 cm. The surgical approach for MWA could improve local control and reduce complications.
Author Contributions
Write the work: A.M. and F.P. Analyzed the data: A.M., F.P. and R.M. Designed the work: A.M., R.R. and T.P. Collected the data: A.M., F.P., A.D., A.T., A.R.A.-S. Revised and approved the work: A.M., R.R., R.K. and T.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elferink M.A., de Jong K.P., Klaase J.M., Siemerink E.J., de Wilt J.H. Metachronous metastases from colorectal cancer: A population-based study in North-East Netherlands. Int. J. Colorectal Dis. 2015;30:205–212. doi: 10.1007/s00384-014-2085-6. [DOI] [PubMed] [Google Scholar]
- 2.Engstrand J., Nilsson H., Stromberg C., Jonas E., Freedman J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi: 10.1186/s12885-017-3925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris E.J., Forman D., Thomas J.D., Quirke P., Taylor E.F., Fairley L., Cottier B., Poston G. Surgical management and outcomes of colorectal cancer liver metastases. Br. J. Surg. 2010;97:1110–1118. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- 4.Hackl C., Neumann P., Gerken M., Loss M., Klinkhammer-Schalke M., Schlitt H.J. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg R.M. Therapy for metastatic colorectal cancer. Oncologist. 2006;11:981–987. doi: 10.1634/theoncologist.11-9-981. [DOI] [PubMed] [Google Scholar]
- 6.Gillams A., Khan Z., Osborn P., Lees W. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc. Intervent. Radiol. 2013;36:724–730. doi: 10.1007/s00270-012-0500-3. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K., Shimada H., Matsumoto C., Matsuo K., Takeda K., Nagano Y., Togo S. Impact of the degree of liver resection on survival for patients with multiple liver metastases from colorectal cancer. World J. Surg. 2008;32:2057–2069. doi: 10.1007/s00268-008-9610-0. [DOI] [PubMed] [Google Scholar]
- 8.Kingham T.P., Tanoue M., Eaton A., Rocha F.G., Do R., Allen P., De Matteo R.P., D’Angelica M., Fong Y., Jarnagin W.R. Patterns of recurrence after ablation of colorectal cancer liver metastases. Ann. Surg. Oncol. 2012;19:834–841. doi: 10.1245/s10434-011-2048-x. [DOI] [PubMed] [Google Scholar]
- 9.White R.R., Avital I., Sofocleous C.T., Brown K.T., Brody L.A., Covey A., Getrajdman G.I., Jarnagin W.R., Dematteo R.P., Fong Y. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J. Gastrointest. Surg. 2007;11:256–263. doi: 10.1007/s11605-007-0100-8. [DOI] [PubMed] [Google Scholar]
- 10.Otto G., Duber C., Hoppe-Lotichius M., Konig J., Heise M., Pitton M.B. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann. Surg. 2010;251:796–803. doi: 10.1097/SLA.0b013e3181bc9fae. [DOI] [PubMed] [Google Scholar]
- 11.Adam R., Hagopian E.J., Linhares M., Krissat J., Savier E., Azoulay D., Kunstlinger F., Castaing D., Bismuth H. A comparison of percutaneous cryosurgery and percutaneous radiofrequency for unresectable hepatic malignancies. Arch. Surg. 2002;137:1332–1339. doi: 10.1001/archsurg.137.12.1332. [DOI] [PubMed] [Google Scholar]
- 12.Gillams A.R., Lees W.R. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur. Radiol. 2009;19:1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 13.Solbiati L., Ahmed M., Cova L., Ierace T., Brioschi M., Goldberg S.N. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: Local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 14.Van Tilborg A.A., Meijerink M.R., Sietses C., Van Waesberghe J.H., Mackintosh M.O., Meijer S., Van Kuijk C., Van Den Tol P. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: A potentially curative intervention. Br. J. Radiol. 2011;84:556–565. doi: 10.1259/bjr/78268814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J., Liang P., Yu X., Liu F., Chen L., Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: Results in ex vivo and in vivo porcine livers. Eur. J. Radiol. 2011;79:124–130. doi: 10.1016/j.ejrad.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Gravante G., Ong S.L., Metcalfe M.S., Strickland A., Dennison A.R., Lloyd D.M. Hepatic microwave ablation: A review of the histological changes following thermal damage. Liver Int. 2008;28:911–921. doi: 10.1111/j.1478-3231.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 17.Wright A.S., Sampson L.A., Warner T.F., Mahvi D.M., Lee F.T., Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 18.Dupuy D.E. Microwave ablation compared with radiofrequency ablation in lung tissue-is microwave not just for popcorn anymore? Radiology. 2009;251:617–618. doi: 10.1148/radiol.2513090129. [DOI] [PubMed] [Google Scholar]
- 19.Simon C.J., Dupuy D.E., Mayo-Smith W.W. Microwave ablation: Principles and applications. Radiographics. 2005;25((Suppl. 1)):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 20.Rhaiem R., Kianmanesh R., Minon M., Tashkandi A., Aghaei A., Ledoux G., Hoeffel C., Bouche O., Sommacale D., Piardi T. Microwave Thermoablation of Colorectal Liver Metastases Close to Large Hepatic Vessels Under Pringle Maneuver Minimizes the “Heat Sink Effect”. World J. Surg. 2020;44:1595–1603. doi: 10.1007/s00268-020-05379-4. [DOI] [PubMed] [Google Scholar]
- 21.de Baere T., Deschamps F., Briggs P., Dromain C., Boige V., Hechelhammer L., Abdel-Rehim M., Auperin A., Goere D., Elias D. Hepatic malignancies: Percutaneous radiofrequency ablation during percutaneous portal or hepatic vein occlusion. Radiology. 2008;248:1056–1066. doi: 10.1148/radiol.2483070222. [DOI] [PubMed] [Google Scholar]
- 22.Tombesi P., Di Vece F., Sartori S. Radiofrequency, microwave, and laser ablation of liver tumors: Time to move toward a tailored ablation technique? Hepatoma Res. 2015;1:52–57. [Google Scholar]
- 23.Correa-Gallego C., Fong Y., Gonen M., D’Angelica M.I., Allen P.J., DeMatteo R.P., Jarnagin W.R., Kingham T.P. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann. Surg. Oncol. 2014;21:4278–4283. doi: 10.1245/s10434-014-3817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rethlefsen M.L., Kirtley S., Waffenschmidt S., Ayala A.P., Moher D., Page M.J., Koffel J.B. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst Rev. 2021;10:39. doi: 10.1186/s13643-020-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D., de Santibanes E., Pekolj J., Slankamenac K., Bassi C., et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 26.The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. [(accessed on 3 May 2020)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 27.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eng O.S., Tsang A.T., Moore D., Chen C., Narayanan S., Gannon C.J., August D.A., Carpizo D.R., Melstrom L.G. Outcomes of microwave ablation for colorectal cancer liver metastases: A single center experience. J. Surg. Oncol. 2015;111:410–413. doi: 10.1002/jso.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engstrand J., Nilsson H., Jansson A., Isaksson B., Freedman J., Lundell L., Jonas E. A multiple microwave ablation strategy in patients with initially unresectable colorectal cancer liver metastases—A safety and feasibility study of a new concept. Eur. J. Surg. Oncol. 2014;40:1488–1493. doi: 10.1016/j.ejso.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Ierardi A.M., Floridi C., Fontana F., Chini C., Giorlando F., Piacentino F., Brunese L., Pinotti G., Bacuzzi A., Carrafiello G. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol. Med. 2013;118:949–961. doi: 10.1007/s11547-013-0968-1. [DOI] [PubMed] [Google Scholar]
- 32.McEachron K.R., Ankeny J.S., Robbins A., Altman A.M., Marmor S., D’Souza D., Schat R., Spilseth B., Jensen E.H. Surgical microwave ablation of otherwise non-resectable colorectal cancer liver metastases: Expanding opportunities for long term survival. Surg. Oncol. 2021;36:61–64. doi: 10.1016/j.suronc.2020.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Seki T., Wakabayashi M., Nakagawa T., Imamura M., Tamai T., Nishimura A., Yamashiki N., Inoue K. Percutaneous microwave coagulation therapy for solitary metastatic liver tumors from colorectal cancer: A pilot clinical study. Am. J. Gastroenterol. 1999;94:322–327. doi: 10.1111/j.1572-0241.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- 34.Shady W., Petre E.N., Do K.G., Gonen M., Yarmohammadi H., Brown K.T., Kemeny N.E., D’Angelica M., Kingham P.T., Solomon S.B., et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 2018;29:268–275.e261. doi: 10.1016/j.jvir.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata T., Niinobu T., Ogata N., Takami M. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276–284. doi: 10.1002/1097-0142(20000715)89:2<276::AID-CNCR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Song P., Sheng L., Sun Y., An Y., Guo Y., Zhang Y. The clinical utility and outcomes of microwave ablation for colorectal cancer liver metastases. Oncotarget. 2017;8:51792–51799. doi: 10.18632/oncotarget.15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stattner S., Jones R.P., Yip V.S., Buchanan K., Poston G.J., Malik H.Z., Fenwick S.W. Microwave ablation with or without resection for colorectal liver metastases. Eur. J. Surg. Oncol. 2013;39:844–849. doi: 10.1016/j.ejso.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi H., Kahramangil B., Kose E., Berber E. A comparison of microwave thermosphere versus radiofrequency thermal ablation in the treatment of colorectal liver metastases. HPB. 2018;20:1157–1162. doi: 10.1016/j.hpb.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Yang B., Li Y. A comparative study of laparoscopic microwave ablation with laparoscopic radiofrequency ablation for colorectal liver metastasis. J. BUON. 2017;22:667–672. [PubMed] [Google Scholar]
- 40.Evrard S., Torzilli G., Caballero C., Bonhomme B. Parenchymal sparing surgery brings treatment of colorectal liver metastases into the precision medicine era. Eur. J. Cancer. 2018;104:195–200. doi: 10.1016/j.ejca.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 41.Moris D., Ronnekleiv-Kelly S., Rahnemai-Azar A.A., Felekouras E., Dillhoff M., Schmidt C., Pawlik T.M. Parenchymal-Sparing Versus Anatomic Liver Resection for Colorectal Liver Metastases: A Systematic Review. J. Gastrointest. Surg. 2017;21:1076–1085. doi: 10.1007/s11605-017-3397-y. [DOI] [PubMed] [Google Scholar]
- 42.Meijerink M.R., Puijk R.S., van Tilborg A., Henningsen K.H., Fernandez L.G., Neyt M., Heymans J., Frankema J.S., de Jong K.P., Richel D.J., et al. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc. Intervent. Radiol. 2018;41:1189–1204. doi: 10.1007/s00270-018-1959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi H., Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg. Nutr. 2020;9:49–58. doi: 10.21037/hbsn.2019.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabuse K. A new operative procedure of hepatic surgery using a microwave tissue coagulator. Nihon Geka Hokan. 1979;48:160–172. [PubMed] [Google Scholar]
- 45.Zhao Q., Cheng Z., Han Z., Liu F., Yu X., Tan X., Han B., Dou J., Yu J., Liang P. Percutaneous Microwave Ablation Versus Open Surgical Resection for Colorectal Cancer Liver Metastasis. Front. Oncol. 2021;11:638165. doi: 10.3389/fonc.2021.638165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S.K., Rhim H., Kim Y.S., Koh B.H., Cho O.K., Seo H.S., Kim Y. Radiofrequency thermal ablation of hepatic tumors: Pitfalls and challenges. Abdom Imaging. 2005;30:727–733. doi: 10.1007/s00261-005-0304-x. [DOI] [PubMed] [Google Scholar]
- 47.van Duijnhoven F.H., Jansen M.C., Junggeburt J.M., van Hillegersberg R., Rijken A.M., van Coevorden F., van der Sijp J.R., van Gulik T.M., Slooter G.D., Klaase J.M., et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann. Surg. Oncol. 2006;13:651–658. doi: 10.1245/ASO.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Tinguely P., Dal G., Bottai M., Nilsson H., Freedman J., Engstrand J. Microwave ablation versus resection for colorectal cancer liver metastases—A propensity score analysis from a population-based nationwide registry. Eur. J. Surg. Oncol. 2020;46:476–485. doi: 10.1016/j.ejso.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Di Martino M., Rompianesi G., Mora-Guzman I., Martin-Perez E., Montalti R., Troisi R.I. Systematic review and meta-analysis of local ablative therapies for resectable colorectal liver metastases. Eur. J. Surg. Oncol. 2020;46:772–781. doi: 10.1016/j.ejso.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Della Corte A., Ratti F., Monfardini L., Marra P., Gusmini S., Salvioni M., Venturini M., Cipriani F., Aldrighetti L., De Cobelli F. Comparison between percutaneous and laparoscopic microwave ablation of hepatocellular carcinoma. Int. J. Hyperthermia. 2020;37:542–548. doi: 10.1080/02656736.2020.1769869. [DOI] [PubMed] [Google Scholar]
- 51.Yun D., Kim S., Song I., Chun K. Comparative analysis of Laparoscopic versus open surgical radiofrequency ablation for malignant liver tumors. Korean J. Hepatobiliary Pancreat. Surg. 2014;18:122–128. doi: 10.14701/kjhbps.2014.18.4.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puijk R.S., Ruarus A.H., Vroomen L., van Tilborg A., Scheffer H.J., Nielsen K., de Jong M.C., de Vries J.J.J., Zonderhuis B.M., Eker H.H., et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer. 2018;18:821. doi: 10.1186/s12885-018-4716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]