Abstract
The aroma of Keemun black tea (KBT) changes during storage. We investigated key aroma volatiles of fresh KBT (FKBT) and KBT stored for 1 year. Through gas chromatography–olfactometry–mass spectrometry/aroma extract dilution analysis (GC-O-MS/AEDA), 27 aroma volatiles with a flavor dilution (FD) value ≥16 were quantitated. In odor activity value (OAV) analysis, the two samples had nearly the same key aroma volatiles; (Z)-methyl epijasmonate was the exception. Dimethyl sulfide, 3-methylbutanal, 2-methylpropanal, and linalool had especially high OAVs. Except for β-damascenone, volatiles with OAVs > 1 had higher concentrations in FKBT, which revealed that most key aroma compounds were lost during storage. Sweet, malty, floral, and green/grassy aromas corresponded directly to certain compounds. Lastly, the addition test indicated that the addition of several key aroma volatiles decreasing during storage could enhance the freshness of KBT aroma, which may be a potential to control the aroma style of KBT or other teas in industry.
Keywords: Keemun black tea, GC-O-MS, storage, aroma volatile
1. Introduction
Keemun black tea (KBT) is a famous black tea produced in Keemun and surrounding regions in China. Its characteristic, complex aroma—known as the Keemun aroma—distinguishes it from other black teas. According to our evaluations, fresh KBTs (FKBT) have a strong sweet aroma with floral notes. After several months of storage at room temperature, a woody aroma forms and the tea smells notably different from fresh KBTs.
Storage has considerable effects on tea aromas. The aromas of most green teas transform negatively during room temperature storage. The freshness of the aromas fades easily and a stale aroma forms after long storage and strongly affects the green tea quality [1]. For an oolong tea such as tieguanyin, several months of room temperature storage can weaken its characteristic lavender aroma but strengthen its grassy aroma. This alteration negatively affects the tea’s quality but the use of vacuum packaging can slow the change. However, for white teas, an herbal aroma forms during storage that affects the tea positively and storage can strengthen the characteristic stale aroma of dark teas. Throughout the long history of tea drinking, both the negative and positive effects of storage on tea aromas have been understood. To date, most researchers have focused on the changes of white or dark tea volatiles during storage [2,3,4]. As far as we know, investigations about the effect of storage on black tea volatiles or odor-active compounds are blank.
In earlier research, we investigated aromatic and volatile changes during 1–20 years of storage and identified volatiles linked with storage duration [5]. However, we do not yet know which volatiles contribute to the Keemun aroma or how the key aroma compounds change during storage. Therefore, we selected FKBT and KBT stored for 1 year (1Y-KBT) with a similar matrix. Using gas chromatography–olfactometry–mass spectrometry/aroma extract dilution analysis (GC-O-MS/AEDA), standard addition methods, and sensory experiments, we attempted to identify and compare the key aroma compounds in these two teas that contribute to their main aroma nuances to comprehend aroma transformation during storage.
2. Material and Methods
2.1. Samples and Chemicals
FKBT and 1Y-KBT (produced in 2020 and 2019, respectively) were acquired from Anhui Guorun Tea Industrial Co., Ltd. (Chizhou City, Anhui, China). The 1Y-KBT sample was stored in a cabin that could keep a relatively stable storage environment. After their acquisition, the samples were stored in a clean, odorless environment at −20 °C. The distilled water used in the experiments was purchased from Watsons Water Company, Inc. (Guangzhou, China). Chromatographically pure methyl tert-butyl ether (MTBE) and pentane were purchased from Aladdin (Shanghai, China) and redistilled before use; 2-octanol and ethyl decanoate (99.5% and 99% pure) were also obtained from Aladdin. n-Alkanes (C5–C40; Sigma-Aldrich, St. Louis, MO, USA) were employed to assist in identifying volatiles by enabling the calculation of retention indices. A solid-phase extraction (SPE) cartridge (LC-Si, 500 mg/6 mL) and a solid-phase microextraction (SPME) needle (50/30 μm DVB/Carboxen/PDMS) were purchased from Supelco (St. Louis, MO, USA). All reference aroma compounds were chromatographically pure and commercially obtained, and the actual purities were confirmed through GC-MS.
2.2. Isolation of Tea Volatiles
For the isolation of volatiles, 6 g of each sample was infused in 300 mL of distilled water for 5 min, filtered, and cooled in ice water for 5 min. The infusions were extracted three times with 60 mL of 4:1 pentane:MTBE. This SPE procedure was conducted as previously described [6]. For the SPME procedure, 10 mL of each tea infusion was sealed in a 20 mL vial, equilibrated, and extracted with the SPME needle at 30 °C for 30 min.
2.3. Fractionation of Acidic and Neutral-Basic Tea Volatiles
The fractionation of acidic and neutral-basic tea volatiles followed a procedure described in a previous paper [7]. KBT volatiles were isolated and extracted as described in the previous section. Acidic volatiles were isolated by shaking the organic extract with 50 mL of aqueous sodium carbonate solution (0.5 mol/L) three times. A total of 150 mL of aqueous solution was washed with MTBE (35 mL) and acidified to pH 2 by using hydrochloric acid (32%), and the acidic volatiles were re-extracted three times with 50 mL of 4:1 pentane: MTBE. The organic phases were dried over anhydrous sodium sulfate and non-volatiles were removed using SPE as described in 2.2, which were then concentrated to 200 μL by a gentle nitrogen stream (acidic fraction). After being washed by aqueous sodium carbonate solution, the remaining organic phase contained only neutral-basic tea volatiles and the next steps were identical to the previous steps (neutral-basic fraction).
2.4. Gas Chromatography–Olfactometry–Mass Spectrometry
Aliquots of samples (2 μL) or SPME needles were injected manually in splitless mode. The effluent of the capillary column was split evenly (1:1) between a sniffing port (200 °C) and an MS detector (MSD). The GC-MS system employed was the Agilent 7890B-5975B (Agilent, Santa Clara, CA, USA) equipped with the Agilent DB-5MS and DB-WAX (30 m × 0.25 mm × 0.25 μm) capillary-type chromatographic columns. The GC-O-MS experiments were conducted primarily using the DB-5MS column and the DB-WAX column was used to assist in identification. The GC settings were as below. For the DB-5MS column, the oven temperature was programmed to increase from 50 °C (5 min hold) to 230 °C (2 min hold) at a rate of 5 °C/min, then to 280 °C (1 min hold) at 15 °C/min, and 50 °C (5 min hold) to 230 °C (30 min hold) at a rate of 5 °C/min for the DB-WAX column. Mass spectrometer conditions were as follows: ionization mode, electron impact (EI); ionization energy, 70 eV; ion source temperature, 230 °C; quadrupole temperature, 150 °C; and quadrupole mass spectrometer scan range, 30–350 atomic mass units (amu).
2.5. Aroma Extract Dilution Analysis
Tea volatile concentrates (acidic and neutral-basic fractions) were diluted stepwise with 1:1 (by volume) pentane:MTBE, injected, and analyzed using GC-O-MS as described above. Three trained panelists from our department participated. The flavor dilution (FD) factor of each aroma volatile was defined as the number of the greatest dilution at which its smell could be perceived.
2.6. Identification of Aroma Volatiles
The identification of aroma volatiles proceeded as described in our previous paper about KBT. Sensory descriptions of aroma volatiles were also used to assist in identification. In addition, reference chemicals were used to help identify or eliminate severely coeluted compounds.
2.7. Descriptive Sensory Analysis of KBT
Descriptive sensory analysis (DSA) was conducted using the tea infusions obtained through the procedure described in Section 2.2. To represent KBT aroma and to train the panelists, the following five aroma nuances and their corresponding chemicals were used: malty (3-methylbutanal), sweet (β-damascenone and coumarin), flowery (linalool), green/grassy (hexanal), and woody (4-oxoisophorone). Eight tea evaluators in our laboratory scored the tea aromas from 0 to 5 (with 0.5 gradation). The results are presented as averages.
2.8. Quantitation of Odor-Active Compounds through Standard Addition
Approximately 1 mg/mL stock solutions of odor-active compounds and internal standards (2-octanol and ethyl decanoate) for standard addition were prepared in absolute ethanol and stored at 4 °C. The compounds and internal standard were added to the tea infusions and stirred at 300 r/min for 10 min to equilibrate. The compounds were extracted through SE-SPE or desorbed through SPME according to their volatility. Data were collected using GC-MS in the selected-ion monitoring (SIM) or extracted-ion chromatograph mode (EIC). The qualifier and quantifier ions of the compounds are listed in Table 1. Quantitation was accomplished by building standard curves of the amount of each compound added versus the ratios of the peak areas of the quantifier ions and internal standard. Five points, including a blank, were used to build the standard curves.
Table 1.
Qualifier and quantifier ions of key aroma volatiles.
| Name | CAS | Quantifier Ion | Qualifier Ions |
|---|---|---|---|
| Dimethyl sulfide | 75-18-3 | 62 | 62; 47; 61 |
| 2-Methyl-propanal | 78-84-2 | 72 | 43; 41; 72 |
| 3-Methyl-Butanal | 590-86-3 | 44 | 44; 41; 43 |
| 2-Methyl-Butanal | 96-17-3 | 57 | 57; 58; 41 |
| Dimethyl disulfide | 624-92-0 | 94 | 94; 79; 45 |
| Hexanal | 66-25-1 | 56 | 56; 44; 41 |
| Methional | 3268-49-3 | 48 | 48; 104; 47 |
| Dimethyl trisulfide | 3658-80-8 | 126 | 126; 79; 45 |
| Benzeneacetaldehyde | 122-78-1 | 91 | 91; 120; 92 |
| Linalool | 78-70-6 | 71 | 71; 93; 55 |
| Phenylethyl alcohol | 60-12-8 | 91 | 91; 92; 122 |
| (E,Z)-2,6-Nonadienal | 557-48-2 | 69 | 41; 70; 69 |
| (E)-2-Nonenal | 18829-56-6 | 70 | 70; 43; 55 |
| Benzoic acid | 65-85-0 | 105 | 105; 122; 77 |
| (Z)-Linalool oxide (pyranoid) | 14009-71-3 | 68 | 68; 94; 59 |
| Neral | 106-26-3 | 69 | 69; 41; 39 |
| Geraniol | 106-24-1 | 69 | 69; 41; 68 |
| δ-Octalactone | 698-76-0 | 99 | 99; 71; 42 |
| Eugenol | 97-53-0 | 164 | 164; 103; 149 |
| β-Damascenone | 23726-93-4 | 69 | 69; 121; 190 |
| Vanillin | 121-33-5 | 151 | 151; 152; 81 |
| Coumarin | 91-64-5 | 146 | 146; 118; 90 |
| δ-Decalactone | 705-86-2 | 70 | 99; 71; 70 |
| Dihydroactinidiolide | 15356-74-8 | 111 | 111; 109; 43 |
| Raspberry ketone | 5471-51-2 | 107 | 107; 43; 164 |
| (Z)-Methyl epijasmonate | 39924-52-2 | 224 | 83; 151; 224 |
| Herniarin | 531-59-9 | 176 | 176; 148; 133 |
2.9. Calculation of Odor Activity Values
The odor activity values (OAVs) of the odor-active compounds were calculated by the ratio of concentrations and the thresholds in water of these compounds.
2.10. Addition Test
Three glasses of fresh tea infusions were prepared as described above and one of them was added accurate amounts of odor-active compounds to reach same concentrations in the FKBT infusion, expect for β-damascenone. The infusions were incubated at 50 °C for 10 min and presented in a random order to 10–13 assessors who orthonasally smelled them. The assessors were also asked to describe the sensory differences.
3. Results and Discussion
3.1. DSA Results for FKBT and 1Y-KBT
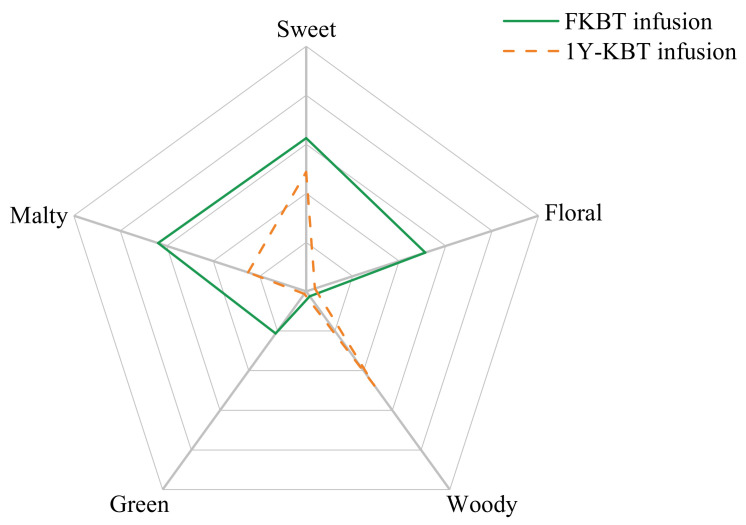
FKBT and 1Y-KBT with similar matrices were chosen for this study. As Figure 1 indicates, the aroma of the two teas greatly differed. The FKBT was characterized by a highly sweet and floral aroma, and the 1Y-KBT was characterized by a sweet and woody aroma. In addition, the FKBT had stronger malty and green/grassy scents that made it smell fresher. The aromas of the two samples reflected the effect of storage on the transformation of the Keemun aroma; these samples can be used for later experiments.
Figure 1.
Descriptive sensory analysis results of FKBT and 1Y-KBT.
3.2. Aroma Volatiles in FKBT and 1Y-KBT
According to our previous experiments, KBT has a complex volatile profile, with a high concentration of acidic volatiles. To avoid the coelution of volatiles, the acidic and neutral-basic tea volatiles were separated. First, GC-O-MS/AEDA was conducted on samples extracted through SE-SPE; all odor-active compounds with an FD value ≥16 are shown in Table 2A. For compounds that remained coeluted, reference chemicals and aroma descriptions were used to assist with identification, for example, in the confirmation of methional and elimination of dihydroactinidiolide.
Table 2.
(A) SPE-GC-O-MS/AEDA results of FKBT and 1Y-KBT. (B) SPME-GC-O-MS results of FKBT and 1Y-KBT.
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Name a | CAS | RI | Description | Fraction b | FD c | |||
| DB-5MS | DB-WAX | FKBT | 1Y-KBT | |||||
| Hexanal | 66-25-1 | 800 | 1076 | Green | NBF, AF | 16 | 8 | |
| Methional | 3268-49-3 | 902 | 1459 | Sweet | NBF | 16 | 2 | |
| Benzeneacetaldehyde | 122-78-1 | 1042 | 1649 | Floral | NBF | 64 | 64 | |
| Linalool | 78-70-6 | 1100 | 1551 | Floral | NBF | 128 | 128 | |
| Phenylethyl alcohol | 60-12-8 | 1110 | 1918 | Floral | NBF, AF | 256 | 256 | |
| (E, Z)-2,6-Nonadienal | 557-48-2 | 1152 | 1588 | Cucumber | NBF | 16 | 16 | |
| (E)-2-Nonenal | 18829-56-6 | 1157 | 1165 | Leather | NBF, AF | 32 | 32 | |
| Benzoic acid | 65-85-0 | 1165 | 2443 | Leather | NBF, AF | 8 | 32 | |
| (Z)-Linalool oxide (pyranoid) | 14009-71-3 | 1174 | 1766 | Herbal | NBF | 8 | 32 | |
| Benzeneacetic acid | 103-82-2 | 1244 | 2570 | Floral | AF | 8 | 32 | |
| Neral | 106-26-3 | 1237 | 1684 | Cucumber | NBF | 64 | 64 | |
| Geraniol | 106-24-1 | 1249 | 1851 | Floral | NBF | 512 | 128 | |
| δ-Octalactone | 698-76-0 | 1278 | 1973 | Sweet | NBF | 16 | 8 | |
| Eugenol | 97-53-0 | 1349 | 2177 | Dried orange peel | NBF | 64 | 256 | |
| β-Damascenone | 23726-93-4 | 1379 | 1378 | Sweet | NBF, AF | 512 | 1024 | |
| Vanillin | 121-33-5 | 1393 | 2578 | Sweet | AF | 128 | 64 | |
| Coumarin | 91-64-5 | 1435 | 2467 | Sweet | NBF, AF | 256 | 256 | |
| δ-Decalactone | 705-86-2 | 1491 | 2234 | Coconut | NBF | 64 | 64 | |
| Raspberry ketone | 5471-51-2 | 1548 | 3003 | Sweet | AF | 256 | 256 | |
| (Z)-Methyl epijasmonate | 39924-52-2 | 1672 | 2354 | Floral | NBF | 64 | 32 | |
| Herniarin | 531-59-9 | 1724 | 2948 | Sweet | NBF, AF | 128 | 128 | |
| (B) | ||||||||
| Name a | CAS | RI (DB-5) | Description | FKBT d | 1Y-KBT | |||
| Dimethyl sulfide | 75-18-3 | 518 | Cooked corn | √ | × | |||
| 2-Methyl-propanal | 78-84-2 | 551 | Malty | √ | √ | |||
| 3-Methyl-butanal | 590-86-3 | 647 | Malty | √ | √ | |||
| 2-Methyl-butanal | 96-17-3 | 657 | Malty | √ | √ | |||
| Dimethyl trisulfide | 3658-80-8 | 965 | Stinking | √ | √ | |||
a Aroma volatiles were identified by mass spectrometry, RI index, and GC-O data. For low abundance or co-elution compounds, reference chemicals were used. Compounds were ordered with their elution order in DB-5 column. b NBF, neutral and basic fraction; AF, acid fraction. c Aroma volatiles with FD ≥ 16 in at least one sample were presented. FKBT, fresh keemun black tea; 1Y-KBT, 1-year keemun black tea. d ‘√’ indicates compounds detected as aroma volatiles; ‘×’, contrarily.
Despite differences in FD, all of the investigated odor-active compounds were present in both samples. Compatible with our understanding of the Keemun aroma and with the DSA results, the most frequent aroma descriptor obtained from GC-O was ‘sweet’. The various sweet aroma components included sulfur compounds, lactones, aldehydes, and ketones. Among them, lactones were the most abundant. In studies on odor-active compounds in black tea [8,9,10,11,12,13,14,15], raspberry ketone and herniarin have been reported as the primary sweet compounds; β-damascenone and vanillin, also common sweet compounds in black tea, were detected in this experiment. However, furanone, another common sweet component, was not detected in the current GC-O-MS experiments or in our previous study of KBT.
Compounds with a floral aroma were the second most abundant, including alcohols, aldehydes, acids, and esters. The third-most common compounds were all aldehydes with a cucumber-like aroma; among them, (E,Z)-2,6-nonadienal has frequently been described as smelling cucumber-like in GC-O studies. Unexpectedly, another component with a cucumber-like aroma, neral, was discovered but it has not been reported. The other compounds were described as green, leather, herbal, or orange-peel-like; except for benzoic acid, all such others have been reported as odor-active compounds in black tea as cited above.
Although a woody aroma was characteristic of KBT after storage, no odor-active compounds were described as woody. We previously analyzed aroma volatiles in KBT stored for 3 and 15 years through GC-O-MS and similarly discovered no woody aroma volatiles (data not shown). After 1Y-KBT concentrate was dripped onto a smelling strip, a woody aroma was smelled; therefore, the extraction method and solvent used may be appropriate. In our previous study of the key volatiles related to the storage of KBT, we observed that the concentrations of some volatiles formed through the degradation of lipids and carotenoids increased during storage. We therefore inferred woody aroma volatiles to be among them; however, in GC-O-MS, most of these volatiles did not contribute to the KBT aroma.
Highly volatile compounds cannot be analyzed through methods based on solvent extraction because of the loss during the concentration and cover by the solvent peak. Therefore, a static headspace technique or SPME is generally used to trap the highly volatile compounds and supply GC-O data. In the present study, SPME was employed. Due to the matrix differences between the samples and differences among the volatiles in their degree of absorption, AEDA was not applied in the SPME-GC-O-MS and we recorded only whether aroma volatiles were perceived. Differences in the highly volatile compounds between the two samples were quantitated. As Table 2B indicates, four highly volatile compounds existed in both the FKBT and 1Y-KBT: 2-methylpropanal, 3-methylbutanal, 2-methylbutanal, and dimethyl trisulfide. Dimethyl sulfide was present only in FKBT. In addition, dimethyl disulfide was a common odor-active sulfur compound [16,17,18] and dihydroactinidiolide exhibited a linear increase during storage [5]; thus, these two volatiles were also quantitated.
3.3. Quantitative Differences between FKBT and 1Y-KBT
The standard addition method could be used for the accurate quantitation of volatile compounds, which could avoid the matrix effect and mimic losses during extraction and concentration [19]. The SIM mode of GC-MS improved the accuracy of quantification. The concentrations of the aroma volatiles with an FD value ≥16 in both KBT infusions are presented in Table 3A. The regression correlation coefficients (R2) of compounds quantified through standard addition ranged from 0.9888 to 0.9999 (benzeneacetic acid was semi-quantified using an internal standard because of the lack of a reference chemical), which was acceptable for precise quantification. Table 3B contains the OAVs of these compounds. Compared with FD, the quantitative analysis more directly indicated the differences between FKBT and 1Y-KBT. The concentrations of aroma volatiles changed dramatically after 1 year of storage; the ratios of the compound concentrations in FKBT to those in 1Y-KBT ranged from 0.3 to 10.9. After OAV calculation, FKBT and 1Y-KBT had 15 and 14 compounds, respectively, with an OAV > 1; all of the compounds, apart from (Z)-methyl epijasmonate were shared between them. Furthermore, all the aroma volatiles with an OAV > 1, apart from β-damascenone, had a higher OAV in FKBT than in 1Y-KBT.
Table 3.
(A) Concentrations of key aroma volatiles in FKBT and 1Y-KBT infusions. (B) OAVs of key aroma volatiles in FKBT and 1Y-KBT infusions.
| (A) | |||||
|---|---|---|---|---|---|
| Name | CAS | Concentration (μg/L) | Ratios (FKBT:1Y-KBT) | ||
| FKBT | 1Y-KBT | ||||
| Dimethyl sulfide | 75-18-3 | 379 | 37 | 10.4 | |
| 2-Methyl-propanal | 78-84-2 | 584 | 145 | 4.0 | |
| 3-Methyl-Butanal | 590-86-3 | 715 | 65 | 10.9 | |
| 2-Methyl-Butanal | 96-17-3 | 824 | 108 | 7.6 | |
| Dimethyl disulfide | 624-92-0 | 0.87 | 0.11 | 7.9 | |
| Hexanal | 66-25-1 | 135 | 32 | 4.2 | |
| Methional | 3268-49-3 | 4.2 | 1.0 | 4.1 | |
| Dimethyl trisulfide | 3658-80-8 | 0.14 | 0.06 | 2.4 | |
| Benzeneacetaldehyde | 122-78-1 | 511 | 445 | 1.1 | |
| Linalool | 78-70-6 | 149 | 59 | 2.5 | |
| Phenylethyl alcohol | 60-12-8 | 416 | 519 | 0.8 | |
| (E,Z)-2,6-nonadienal | 557-48-2 | 1.5 | 0.8 | 1.9 | |
| (E)-2-Nonenal | 18829-56-6 | 0.70 | 0.37 | 1.9 | |
| Benzoic acid | 65-85-0 | 9.9 | 31 | 0.3 | |
| (Z)-Linalool oxide (pyranoid) | 14009-71-3 | 620 | 344 | 1.8 | |
| Benzeneacetic acid | 103-82-2 | 4.8 | 21 | 0.2 | |
| Neral | 106-26-3 | 4.2 | 2.2 | 1.9 | |
| Geraniol | 106-24-1 | 311 | 138 | 2.2 | |
| δ-Octalactone | 698-76-0 | 13 | 13 | 0.9 | |
| Eugenol | 97-53-0 | 0.52 | 0.73 | 0.7 | |
| β-Damascenone | 23726-93-4 | 0.25 | 0.43 | 0.6 | |
| Vanillin | 121-33-5 | 10 | 12 | 0.9 | |
| Coumarin | 91-64-5 | 28 | 17 | 1.7 | |
| δ-Decalactone | 705-86-2 | 4.5 | 2.2 | 2.0 | |
| Dihydroactinidiolide | 15356-74-8 | 23 | 40 | 0.6 | |
| Raspberry ketone | 5471-51-2 | 0.29 | 0.90 | 0.3 | |
| (Z)-Methyl epijasmonate | 39924-52-2 | 2.8 | 0.56 | 5.1 | |
| Herniarin | 531-59-9 | 19 | 21 | 0.9 | |
| (B) | |||||
| Name | CAS | OAV | |||
| FKBT | 1Y-KBT | ||||
| Dimethyl sulfide | 75-18-3 | 1264 | 122 | ||
| 3-Methyl-Butanal | 590-86-3 | 595 | 54 | ||
| 2-Methyl-propanal | 78-84-2 | 307 | 76 | ||
| Linalool | 78-70-6 | 257 | 102 | ||
| 2-Methyl-Butanal | 96-17-3 | 187 | 25 | ||
| Geraniol | 106-24-1 | 97 | 43 | ||
| Benzeneacetaldehyde | 122-78-1 | 81 | 71 | ||
| β-Damascenone | 23726-93-4 | 61 | 108 | ||
| (E,Z)-2,6-Nonadienal | 557-48-2 | 49 | 26 | ||
| Dimethyl trisulfide | 3658-80-8 | 14 | 6 | ||
| Hexanal | 66-25-1 | 14 | 3 | ||
| Methional | 3268-49-3 | 10 | 2 | ||
| Coumarin | 91-64-5 | 3 | 2 | ||
| (E)-2-Nonenal | 18829-56-6 | 2 | 1 | ||
| (Z)-Methyl epijasmonate | 39924-52-2 | 1 | <1 | ||
| Dimethyl disulfide | 624-92-0 | <1 | <0.1 | ||
| Phenylethyl alcohol | 60-12-8 | <1 | <1 | ||
| Vanillin | 121-33-5 | <1 | <1 | ||
| Eugenol | 97-53-0 | <1 | <1 | ||
| δ-Decalactone | 705-86-2 | <1 | <0.1 | ||
| δ-Octalactone | 698-76-0 | <1 | <1 | ||
| (Z)-Linalool oxide (pyranoid) | 14009-71-3 | <1 | <0.1 | ||
| Benzoic acid | 65-85-0 | <1 | <1 | ||
| Dihydroactinidiolide | 15356-74-8 | <0.1 | <0.1 | ||
| Neral | 106-26-3 | <0.1 | <0.1 | ||
| Raspberry ketone | 5471-51-2 | <0.1 | <0.1 | ||
| Herniarin | 531-59-9 | <0.1 | <0.1 | ||
| Benzeneacetic acid | 103-82-2 | <0.1 | <0.1 | ||
Dimethyl sulfide, 2-methylpropanal, 3-methylbutanal, and 2-methylbutanal, which were detected through SPME-GC-O-MS, were measured at >300 μg/L in the FKBT infusion much higher than in the 1Y-KBT infusion. The ratios of the concentrations of these four volatiles in FKBT to those in 1Y-KBT were between 4.0 and 10.9. After OAV calibration, the OAVs of all four of these volatiles exceeded 100 in the FKBT infusion. In particular, dimethyl sulfide had an OAV > 1000. Although the concentrations of these volatiles were much lower in the 1Y-KBT infusion, they contributed greatly to the aroma. These four compounds are commonly detected in various teas and products that undergo the Maillard reaction and have clear metabolic pathways [20]. Some evidence indicates that these compounds exhibit an increasing trend with infusion time [8,16].
Except for dimethyl sulfide, the concentrations of the sulfide compounds (dimethyl disulfide, methional, and dimethyl trisulfide) were also higher in the FKBT infusion than in the 1Y-KBT infusion; the ratios were 7.9, 4.1, and 2.4, respectively. In an earlier analysis of KBT after 1 to 20 years of storage, dimethyl disulfide was detected in only 1Y-KBT and neither methional nor dimethyl trisulfide were detected in any stored samples. Methional is a Strecker aldehyde of methionine and both dimethyl disulfide and dimethyl trisulfide are further oxidation products of methional [20]. Methional’s aroma has been described as cooked potato in several reports [21,22] but we linked it with the characteristic sweet aroma of high-grade FKBT in our GC-O-MS experiment. The concentration of methional in the FKBT infusion was 4.2 μg/L and those of both methyl disulfide and dimethyl trisulfide were <1.0 μg/L. The OAVs of methional and dimethyl trisulfide were >1 in both the FKBT and 1Y-KBT infusions, indicating that these compounds contributed to the aromas.
Among the six volatiles with a floral aroma, benzeneacetaldehyde was slightly more abundant in the FKBT infusion and the concentrations of linalool, geraniol, and (Z)-methyl epijasmonate were 2.5, 2.2, and 5.1 times higher in the FKBT infusion; except for that of (Z)-methyl epijasmonate, the concentrations of the compounds were all >100 μg/L. The concentration of phenylethyl alcohol was slightly lower in the FKBT infusion than in the 1Y-KBT infusion but both concentrations were >400 μg/L. These results generally support our previous conclusions that storage has a negative effect on floral compounds, especially linalool, geraniol, and (Z)-methyl epijasmonate. Linalool, geraniol, and benzeneacetaldehyde contributed greatly to the floral aroma of the FKBT infusion, especially linalool, which had an OAV of 257. Although it was considerably reduced during storage, the OAV of linalool remained at 102 in the 1Y-KBT infusion. (Z)-methyl epijasmonate, a key aroma volatile in tea, contributed only slightly to the aroma of the FKBT infusion, with an OAV of 1. The acidic aroma volatiles exhibited similar trends. The concentrations of benzeneacetic acid and benzoic acid in the 1Y-KBT infusion were 4.4 and 3.1 times higher, respectively, than in the FKBT infusion.
Hexanal, the only green/grassy aroma volatile with an FD value ≥16, is derived from lipid oxidation during tea processing [20]. The concentration of this compound in the FKBT infusion (165 μg/L) was 4.2-folds higher than in the 1Y-KBT infusion. The OAVs of hexanal in both KBT infusions were higher than 1.
The concentrations of all eight sweet aroma volatiles in both KBT infusions were <30 μg/L. Methional, coumarin, and δ-decalactone were more abundant in the FKBT infusion and the other five sweet volatiles were more abundant in the 1Y-KBT infusion. The concentrations of δ-octalactone, vanillin, and herniarin in the FKBT infusion were all 0.9 times lower than those in the 1Y-KBT infusion and the concentrations of β-damascenone and raspberry ketone were 0.6 and 0.3 times lower than those in the 1Y-KBT infusion. Among the sweet aroma volatiles, only β-damascenone, methional, and coumarin had OAVs > 1. Though low concentrations of β-damascenone were present in the KBT infusions (both <1 μg/L), its OAVs were still much higher (61 and 108 in FKBT and 1Y-KBT, respectively) than those of methional and coumarin because of its extremely low threshold.
β-Damascenone receives tremendous attention because it can easily achieve high FD in GC-O experiments and has a high OAV due to its extremely low threshold. β-damascenone exists in various teas, fruits, coffees, honeys, wines, and other foods and drinks [23]. The sensory effect of β-damascenone in wine is especially concentrated. On the basis of GC-O results and OAVs, many researchers have concluded that β-damascenone contributes greatly to wine’s aroma. However, sensory tests have indicated that the threshold of β-damascenone is easily affected by the matrix. Its thresholds in water and wine are 0.004 and 7 µg/L, respectively, but its concentration in wine is 1–1.5 µg/L; thus, β-damascenone does not affect wine’s aroma directly and may contribute only indirectly to the fruity aroma of wine [24]. Compared with studies on the sensory effect of β-damascenone in wine, those on its effect in tea aroma are few. Research studies about the β-damascenone sensory effect indicated to us that checking the actual sensory effect of aroma volatiles was necessary.
The concentrations of two cucumber-like aroma volatiles, namely (E,Z)-2,6-nonadienal and neral, in the FKBT infusion were 1.9-folds higher than those in the 1Y-KBT infusion, but still <5 μg/L. The OAVs of (E,Z)-2,6-nonadienal were 49 and 26 in the FKBT and 1Y-KBT infusions, respectively, but neral made no contribution to either KBT infusion aroma, with an OAV <0.1. In addition, (E)-2-nonenal, frequently detected in various teas through GC-O, contributed slightly to both KBT aromas, with OAVs of 2 and 1 in the FKBT and 1Y-KBT infusions, respectively. Although dihydroactinidiolide was reported to be an aroma volatile in several teas [3,25,26], we did not perceive its aroma. However, the concentration of dihydroactinidiolide in the FKBT infusion was only 60% of that in the 1Y-KBT infusion according to standard addition, supporting our previous conclusion that dihydroactinidiolide was a reliable indicator of storage duration.
3.4. Additional Test to Understand Aroma Transformation during Storage
Additional tests were designed to further investigate the transformation of aroma during KBT storage. Considering all aroma volatiles with OAVs > 1, apart from β-damascenone, were more abundant in the FKBT than in the 1Y-KBT infusion, we deduced that the loss of key aroma volatiles during storage may contribute to the transformation of KBT aroma. Therefore, to evaluate the effects of individual compounds on the aroma, aroma compounds with an OAV of >1 in the 1Y-KBT infusion were added to match the levels of those in the FKBT infusion and β-damascenone was added to 150% of the level in the 1Y-KBT infusion for triangle testing to determine significant differences.
As illustrated in Table 4, several aroma volatiles significantly inhibited the woody aroma, especially 3-methylbutanal, dimethyl sulfide, and benzeneacetaldehyde; (E,Z)-2,6-nonadienal, 3-methylbutanal, dimethyl sulfide, and (E,Z)-2,6-nonadienal also made the infusion feel fresher. In addition, the addition of benzeneacetaldehyde, linalool, and geraniol increased the strength of the floral aroma of the infusion and both β-damascenone and methional made the infusion aroma sweeter. The sensory evaluation results indicated a direct contribution of these compounds to the KBT aroma.
Table 4.
Addition tests’ results to explain woody aroma.
| Added Compounds a | Correct Answers/Panelists b | p Value c | Significant Level d | Difference Description |
|---|---|---|---|---|
| 3-Methyl butanal | 11/12 | 0.000 | *** | More fresh, less woody |
| Dimethyl sulfide | 10/12 | 0.001 | ** | More fresh, less woody |
| Benzeneacetaldehyde | 9/11 | 0.001 | ** | More floral, less woody |
| (E,Z)-2,6-Nonadienal | 9/11 | 0.001 | ** | More fresh, less woody |
| (E)-2-Nonenal | 7/10 | 0.020 | * | More fatty, less woody |
| Linalool | 7/11 | 0.039 | * | More floral, less woody |
| β-Damascenone (+50%) | 7/11 | 0.039 | * | Sweeter, less woody |
| Methional | 7/11 | 0.039 | * | Sweeter, less woody |
| Geraniol | 7/11 | 0.039 | * | More floral, less woody |
| 2-Methyl-propanal | 6/12 | 0.178 | - | - |
| Hexanal | 5/10 | 0.213 | - | - |
| Coumarin | 5/10 | 0.213 | - | - |
| 2-Methyl butanal | 4/10 | 0.441 | - | - |
| Dimethyl trisulfide | 4/10 | 0.441 | - | - |
a Aroma volatiles were added in 1Y-KBT infusion individually, same as the concentrations in FKBT infusion, and β-damascenone was added to150% level than original. b Number of correct answers/all addition test participants. c The statistical significance (p value) of the addition test was evaluated by binomial test tables. d ***, very highly significant (p < 0.001); **, highly significant (0.001 ≤ p ≤ 0.01); *, significant (0.01< p ≤ 0.05); and -, not significant (p > 0.05).
4. Conclusions
GC-O-MS/AEDA and quantitative experiments with FKBT and 1Y-KBT demonstrated that approximately two-thirds of the aroma volatiles were more abundant in FKBT; these included high-volatility compounds, sulfide compounds, and some floral volatiles. Approximately one-third of the aroma volatiles were more abundant in 1Y-KBT, including benzeneacetic acid, benzoic acid, and raspberry ketone. However, most compounds with an OAV > 1 were more abundant in the FKBT infusion, with the exception of β-damascenone. In addition, we understood the transformation of KBT aroma through additional testing, in which we observed that the additions of several key aroma volatiles (including 3-methylbutanal, dimethyl sulfide, benzeneacetaldehyde, (E,Z)-2,6-nonadienal, and so on) weakened the woody aroma and enhanced the freshness of the KBT aroma, which indicated that controlling the storage environment to prevent the loss of these key aroma volatiles was very important to make KBT fresh.
Acknowledgments
The authors express their appreciation to Yin Tianji for generously providing the keemun black tea samples.
Author Contributions
Conceptualization, Z.L.; formal analysis, M.T.; data curation, M.T.; writing—original draft preparation, M.T.; writing—review and editing, M.T., W.G. and W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key Research and Development Program of China (2021YFD1601103); the National Key Research and Development Program of China (2017YFD0400800); and Modern Agriculture Tea Industry System of Anhui.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dai Q., Jin H., Gao J., Ning J., Yang X., Xia T. Investigating volatile compounds’ contributions to the stale odour of green tea. Int. J. Food Sci. Technol. 2020;55:1606–1616. doi: 10.1111/ijfs.14387. [DOI] [Google Scholar]
- 2.Zhu Y., Kang S., Yan H., Lv H.-P., Zhang Y., Lin Z. Enantiomeric distributions of volatile lactones and terpenoids in white teas stored for different durations. Food Chem. 2020;320:126632. doi: 10.1016/j.foodchem.2020.126632. [DOI] [PubMed] [Google Scholar]
- 3.Xu S., Zeng X., Wu H., Shen S., Yang X., Deng W.-W., Ning J. Characterizing volatile metabolites in raw Pu’er tea stored in wet-hot or dry-cold environments by performing metabolomic analysis and using the molecular sensory science approach. Food Chem. 2021;350:129186. doi: 10.1016/j.foodchem.2021.129186. [DOI] [PubMed] [Google Scholar]
- 4.Qi D., Miao A., Cao J., Wang W., Chen W., Pang S., He X., Ma C. Study on the effects of rapid aging technology on the aroma quality of white tea using GC-MS combined with chemometrics: In comparison with natural aged and fresh white tea. Food Chem. 2018;265:189–199. doi: 10.1016/j.foodchem.2018.05.080. [DOI] [PubMed] [Google Scholar]
- 5.Meng T., Zhipeng X., Ai H., Jiayu C., Tianji Y., Zhengquan L. Effect of 1–20 years storage on volatiles and aroma of Keemun congou black tea by solvent extraction-solid phase extraction-gas chromatography-mass spectrometry. LWT. 2021;136:110278. doi: 10.1016/j.lwt.2020.110278. [DOI] [Google Scholar]
- 6.Feng Z., Li Y., Li M., Wang Y., Zhang L., Wan X., Yang X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019;285:347–354. doi: 10.1016/j.foodchem.2019.01.174. [DOI] [PubMed] [Google Scholar]
- 7.Tatsu S., Matsuo Y., Nakahara K., Hofmann T., Steinhaus M. Key Odorants in Japanese Roasted Barley Tea (Mugi-Cha)—Differences between Roasted Barley Tea Prepared from Naked Barley and Roasted Barley Tea Prepared from Hulled Barley. J. Agric. Food Chem. 2020;68:2728–2737. doi: 10.1021/acs.jafc.9b08063. [DOI] [PubMed] [Google Scholar]
- 8.Schuh C., Schieberle P. Characterization of the Key Aroma Compounds in the Beverage Prepared from Darjeeling Black Tea: Quantitative Differences between Tea Leaves and Infusion. J. Agric. Food Chem. 2006;54:916–924. doi: 10.1021/jf052495n. [DOI] [PubMed] [Google Scholar]
- 9.Kang S., Yan H., Zhu Y., Liu X., Lv H.P., Zhang Y., Dai W.D., Guo L., Tan J.F., Peng Q.H., et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019;121:73–83. doi: 10.1016/j.foodres.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Z., Wang H., Niu Y., Liu Q., Zhu J., Chen H., Ma N. Characterization of aroma compositions in different Chinese congou black teas using GC-MS and GC-O combined with partial least squares regression. Flavour Fragr. J. 2017;32:265–276. doi: 10.1002/ffj.3378. [DOI] [Google Scholar]
- 11.Mao S., Lu C., Li M., Ye Y., Wei X., Tong H. Identification of key aromatic compounds in Congou black tea by partial least-square regression with variable importance of projection scores and gas chromatography-mass spectrometry/gas chromatography-olfactometry. J. Sci. Food Agric. 2018;98:5278–5286. doi: 10.1002/jsfa.9066. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Chen D., Jiang H., Sun H., Zhang C., Zhao H., Li X., Yan F., Chen C., Xu Z. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography-mass spectrometry and olfactometry and sensory analysis. Food Chem. 2019;274:130–136. doi: 10.1016/j.foodchem.2018.08.124. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Sun H., Qu D., Yan F., Jin W., Jiang H., Chen C., Zhang Y., Li C., Xu Z. Identification and characterization of key aroma compounds in Chinese high altitude and northernmost black tea (Camellia sinensis) using distillation extraction and sensory analysis methods. Flavour Fragr. J. 2020;35:666–673. doi: 10.1002/ffj.3605. [DOI] [Google Scholar]
- 14.Baba R., Nakamura M., Kumazawa K. Identification of the Potent Odorants Contributing to the Characteristic Aroma of Darjeeling Black Tea Infusion. Nippon Shokuhin Kagaku Kogaku Kaishi. 2017;64:294–301. doi: 10.3136/nskkk.64.294. [DOI] [Google Scholar]
- 15.Guth H., Grosch W. Identification of potent odourants in static headspace samples of green and black tea powders on the basis of aroma extract dilution analysis (AEDA) Flavour Fragr. J. 1993;8:173–178. doi: 10.1002/ffj.2730080402. [DOI] [Google Scholar]
- 16.Flaig M., Qi S.C., Wei G., Yang X., Schieberle P. Characterisation of the key aroma compounds in a Longjing green tea infusion (Camellia sinensis) by the sensomics approach and their quantitative changes during processing of the tea leaves. Eur. Food Res. Technol. 2020;246:2411–2425. doi: 10.1007/s00217-020-03584-y. [DOI] [Google Scholar]
- 17.Flaig M., Qi S., Wei G., Yang X., Schieberle P. Characterization of the Key Odorants in a High-Grade Chinese Green Tea Beverage (Camellia sinensis; Jingshan cha) by Means of the Sensomics Approach and Elucidation of Odorant Changes in Tea Leaves Caused by the Tea Manufacturing Process. J. Agric. Food Chem. 2020;68:5168–5179. doi: 10.1021/acs.jafc.0c01300. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J., Chen F., Wang L., Niu Y., Yu D., Shu C., Chen H., Wang H., Xiao Z. Comparison of Aroma-Active Volatiles in Oolong Tea Infusions Using GC-Olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015;63:7499–7510. doi: 10.1021/acs.jafc.5b02358. [DOI] [PubMed] [Google Scholar]
- 19.Schieberle P., Molyneux R.J. Quantitation of sensory-active and bioactive constituents of food: A Journal of Agricultural and Food Chemistry perspective. J. Agric. Food Chem. 2012;60:2404–2408. doi: 10.1021/jf2047477. [DOI] [PubMed] [Google Scholar]
- 20.Ho C.-T., Zheng X., Li S. Tea aroma formation. Food Sci. Hum. Wellness. 2015;4:9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- 21.Lan Y.-B., Xiang X.-F., Qian X., Wang J.-M., Ling M.-Q., Zhu B.-Q., Liu T., Sun L.-B., Shi Y., Reynolds A.G., et al. Characterization and differentiation of key odor-active compounds of ‘Beibinghong’ icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution. Food Chem. 2019;287:186–196. doi: 10.1016/j.foodchem.2019.02.074. [DOI] [PubMed] [Google Scholar]
- 22.Neiens S.D., Steinhaus M. Odor-Active Compounds in the Special Flavor Hops Huell Melon and Polaris. J. Agric. Food Chem. 2018;66:1452–1460. doi: 10.1021/acs.jafc.7b05859. [DOI] [PubMed] [Google Scholar]
- 23.Sefton M.A., Skouroumounis G.K., Elsey G.M., Taylor D.K. Occurrence, sensory impact, formation, and fate of damascenone in grapes, wines, and other foods and beverages. J. Agric. Food Chem. 2011;59:9717–9746. doi: 10.1021/jf201450q. [DOI] [PubMed] [Google Scholar]
- 24.Pineau B., Barbe J.-C., Van Leeuwen C., Dubourdieu D. Which Impact for β-Damascenone on Red Wines Aroma? J. Agric. Food Chem. 2007;55:4103–4108. doi: 10.1021/jf070120r. [DOI] [PubMed] [Google Scholar]
- 25.Pang X., Yu W., Cao C., Yuan X., Qiu J., Kong F., Wu J. Comparison of Potent Odorants in Raw and Ripened Pu-Erh Tea Infusions Based on Odor Activity Value Calculation and Multivariate Analysis: Understanding the Role of Pile Fermentation. J. Agric. Food Chem. 2019;67:13139–13149. doi: 10.1021/acs.jafc.9b05321. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y., Lv H.P., Shao C.Y., Kang S., Zhang Y., Guo L., Dai W.D., Tan J.F., Peng Q.H., Lin Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018;108:74–82. doi: 10.1016/j.foodres.2018.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.