To the Editor,
Patients with lymphoma with coronavirus disease 2019 (COVID-19) require special consideration because of their deficient immune status and exposure to antitumor treatment.
In November 2014, a 78-year-old male was diagnosed with mantle cell lymphoma (MCL) by palatine tonsil biopsy. His previous treatment history included R-CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, and prednisone), BORID (bortezomib, rituximab, and dexamethasone), and R-BAC (rituximab, bendamustine, and cytarabine). In September 2017, he presented with stridor due to bilateral tonsillar mass, and MCL relapse was diagnosed. Ibrutinib 560 mg/day was started and complete remission was achieved. In May 2020, he presented with diarrhea and dyspnea. His vital signs were as follows: a temperature of 38°C, a pulse of 112 beats per minute, and a respiratory rate of 22 breaths per minute. His oxygen saturation on ambient air was 91%. Real-time reverse transcriptase-polymerase chain reaction assay detected the presence of severe acute respiratory syndrome coronavirus 2 RNA in the nasopharyngeal swab. Chest computed tomography showed patchy peripheral ground-glass opacities in both lungs, findings consistent with severe COVID-19 pneumonia. Complete blood count (CBC) showed the following: hemoglobin level of 11 g/dL, total leukocyte count of 1 580 cells/mm3, neutrophil count of 800 cells/mm3, lymphocyte count of 428 cells/mm3, and platelet count of 212,000 cells/mm3. The following laboratory tests were abnormal: C-reactive protein level of 5.67 mg/dL (range: 0–0.5), ferritin level of 660 ug/L (range: 23–336), D-dimer level of 1.24 mg/L (range: 0–0.5). Hydroxychloroquine, levofloxacin, and prophylactic low-molecular-weight heparin were initiated.1 Because of grade 3 neutropenia and fever, ibrutinib was stopped. At 6 days after the discontinuation of ibrutinib, he presented with left orbital swelling and a mass lesion over the left hard palate (Figure 1.a,b). His CBC showed hematological recovery. Thus, dexamethasone 8 mg/day for 4 days and ibrutinib 560 mg/day were restarted. At 1 week later, orbital swelling and the mass lesion over the hard palate mass regressed. At 2 weeks later, symptoms associated with COVID-19 pneumonia completely regressed. Currently, under the treatment of ibrutinib, MCL is still in remission, and the patient is free of COVID-19 infection.
FIG. 1. a, b.
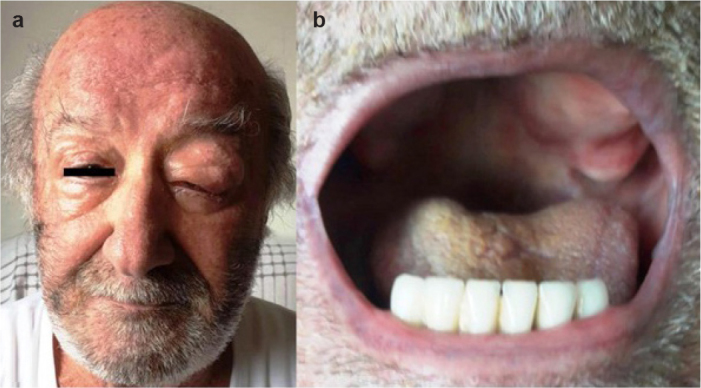
The appearance of the lesions after the interruption of ibrutinib treatment. Mass lesion over the left orbit (a) and left hard palate (b).
The interruption of ibrutinib during the course of MCL treatment has not been extensively evaluated. Studies require a continuous dosing even after remission has been achieved. Taking into consideration the potential toxicities of continuous therapy, more research is warranted to evaluate shorter durations of therapy, depth of response, and planned dose interruptions in responding patients.2 In this patient who had ongoing long-lasting remission with ibrutinib, we had to interrupt ibrutinib because of hematological toxicity at the time of diagnosis of COVID-19 pneumonia. Yet, shortly after cessation, the disease recurred with mass lesions over the orbit and hard palate. Recently, a study suggested that targeting excessive host inflammation with a Bruton kinase inhibitor is a therapeutic strategy in severe COVID-19.3 Ibrutinib is associated with an increased incidence of bleeding and proarrhythmia, which may worsen the outcome in severe COVID-19.4 Our data support that ibrutinib can be used with careful monitoring in COVID-19.
Footnotes
Patient Consent for Publication: Informed consent was obtained from the patient for publication.
Author Contributions: Concept - F.K., R.D.K.; Design - F.K., R.D.K.; Data Collection and/or Processing - İ.Y.H.; Writing - F.K., İ.Y.H., R.D.K.; Critical Review - R.D.K.
Conflict of Interest: The authors have no conflicts of interest to declare.
Funding: The authors declared that this study has received no financial support
REFERENCES
- 1.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens DM, Spurgeon SE. Ibrutinib in mantle cell lymphoma patients: glass half full? Evidence and opinion. Ther Adv Hematol. 2015;6(5):242–252. doi: 10.1177/2040620715592569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5(48):eabd0110. doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26(2):e233–e240. doi: 10.3747/co.26.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]


