Abstract
Weanling outbred rats were infected with Cryptococcus neoformans by direct percranial puncture and inoculation into the cranium. A lethal infection ensued. Treatment with LY295337, a depsipeptide with antifungal activity, was effective in prolonging survival and reducing fungal counts in brain tissue. Weanling rats are an acceptable model for the study of central nervous system infection with C. neoformans.
The primary model for studies of cryptococcosis has been the laboratory mouse. Mice are susceptible to infection and can be studied in large enough numbers to allow comparison of a variety of treatment regimens (1–4, 11). Athymic and SCID mice allow studies of infections of mice broadly deficient in T-lymphocyte responses (6). Also, inbred knockout mice lacking a variety of cytokines have been developed, thus permitting evaluations of disease in animals with highly focused deficiencies of individual components of the immune response. Rabbit and guinea pig models have also been used, though much less extensively (9, 10, 13). The adult rat has been utilized for studies of pulmonary infection with C. neoformans (5). Of the above-mentioned models, only the mouse has been used for percranial injection of cryptococci. The guinea pig and rabbit have also been models for injection of C. neoformans directly into the cerebrospinal fluid. These last models produce the most severe disease at the site where it occurs clinically. However, rabbits are naturally resistant to C. neoformans and must be steroid treated to maintain the infection. Rabbits are also more costly and require more space to maintain than mice and are therefore more difficult to study in large enough numbers to permit statistical comparisons. Intracerebral infection of mice is useful for a variety of studies of pharmacological agents for the treatment of cryptococcal meningitis. However, mice clear some drugs so rapidly that they are an unsuitable animal model. For example, voriconazole has a terminal half-life of 1 h in mice, 2 h in rats, and 6 h in humans (10a). Fluconazole has a terminal half-life, after an oral dose, of 5.1 h in mice and 4.0 h in rats (7). We report here the use of weanling rats as an alternative model for direct intracranial infection.
For the present studies we utilized C. neoformans 89-127, a clinical isolate obtained from the Fungus Testing Laboratory of the University of Texas Health Science Center, San Antonio. In vitro testing of LY295337 was performed according to the National Committee for Clinical Laboratory Standards method, with MICs (determined by the macrotube method) read at 24 and 72 h (8). The organisms were cultured on Sabouraud agar for 3 days at 35°C, scraped off the agar, washed three times, suspended in isotonic saline, counted in a hemacytometer, and brought to the desired concentration in isotonic saline. Male Sprague-Dawley weanling rats weighing approximately 30 g were anesthetized briefly with Metafane and infected by direct percranial puncture in the midline. The inoculum of approximately 600 cells in a volume of 0.06 ml was injected with a 27-gauge needle fastened to a tuberculin syringe with a cuff to prevent penetration of more than 1 mm. The rats were allowed to recover. Two days following infection the rats were allocated to survival studies (nine per group) or studies of tissue burden (seven per group). LY295337 was dissolved in sesame oil and administered orally once daily at 100 or 200 mg/kg of body weight in a volume of 0.2 ml. Amphotericin B was prepared in 5% glucose and administered intraperitoneally at 6 mg/kg daily. Fluconazole was dissolved in 0.3% Noble agar and administered once daily at 30 mg/kg. For the survival study, rats were treated from day 2 through day 10 and then observed through day 30 for survival. For the tissue burden study, rats were sacrificed on day 12, 2 days after the completion of therapy. Their brains were removed by an aseptic technique and homogenized in 5 ml of isotonic saline. Serial 10-fold colony count dilutions were then performed.
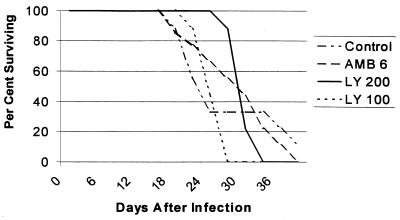
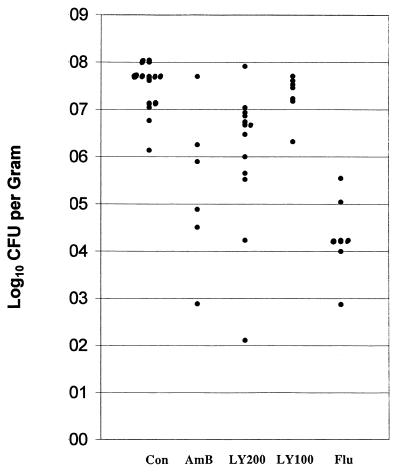
For the study of survival, the Wilcoxon and log rank tests were used, with a P value of <0.05 considered significant. As shown in Fig. 1, LY295337 at 200 mg/kg significantly prolonged survival over controls. Two studies of tissue burden, with similar infecting inocula, were combined, and the nonparametric Dunnett’s one-tailed t test was used for comparisons. For comparisons the error rate (α) was adjusted so that the overall error of the study would be ≤0.05. As shown in Fig. 2, LY295337 at 200 mg/kg, amphotericin B, and fluconazole all significantly reduced brain tissue fungal counts.
FIG. 1.
Survival after intracranial inoculation of weanling rats with 666 CFU of C. neoformans. Mice were treated orally once daily (LY295337, 100 [LY 100] or 200 [LY 200] mg/kg/day) or intraperitoneally once daily (amphotericin B, 6 mg/kg/day [AMB 6]). LY 200 was superior to the control (P = 0.03) and LY 100 (P = 0.001). LY 100 was not superior to the control (P = 0.3). AMB 6 was not superior to the control (P = 0.15, Wilcoxon test).
FIG. 2.
CFU of C. neoformans per gram of brain measured at 12 days after infection and treatment days 2 through 10. Mice were treated once daily with LY295337 orally at 200 mg/kg (LY200, n = 13) or 100 mg/kg (LY100, n = 7), amphotericin B intraperitoneally at 6 mg/kg (AmB, n = 6), or fluconazole at 30 mg/kg (Flu, n = 7). All regimens but LY100 reduced fungal brain tissue counts significantly below controls (Con) (P < 0.05, Dunnett’s one-tailed t test).
In part because of its inefficacy against aspergillosis, LY295337 has not been further developed as an antifungal drug. There are no kinetic data available. Nevertheless, the present studies demonstrate that a progressive meningeal infection can be induced by direct percranial inoculation of C. neoformans. It is important to use weanling rats, because shortly after this stage the cranium becomes too thick for facile injection of organisms. This model has also shown that two commercial antifungal drugs (and the investigational drug) can be used successfully for treatment of the fungal infection. In mice fluconazole is commonly effective at doses of ≥5 mg/kg/day (14). Mice may also be successfully treated with doses of amphotericin B ranging upward from 0.38 mg/kg/day (12). Thus, the effective dose ranges of these two commercial drugs are similar in mice and rats.
Acknowledgments
We acknowledge Lilly Pharmaceuticals for their support of this study and supply of LY295337.
REFERENCES
- 1.Craven P C, Graybill J R, Jorgensen J H. Ketoconazole therapy of murine cryptococcal meningitis. Am Rev Respir Dis. 1982;125:696–700. doi: 10.1164/arrd.1982.125.6.696. [DOI] [PubMed] [Google Scholar]
- 2.Ding J C, Bauer M, Diamond D M, Leal M A E, Johnson D, Williams B K, Thomas A M, Najvar L, Graybill J R, Larsen R A. Effect of severity of meningitis on fungicidal activity of flucytosine combined with fluconazole in a murine model of cryptococcal meningitis. Antimicrob Agents Chemother. 1997;41:1589–1593. doi: 10.1128/aac.41.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez E P, Patino M M, Graybill J R, Tarbit M H. Treatment of cryptococcal meningitis in mice with fluconazole. J Antimicrob Chemother. 1986;18:261–270. doi: 10.1093/jac/18.2.261. [DOI] [PubMed] [Google Scholar]
- 4.Graybill J R, Ahrens J. R 51211 (itraconazole) therapy of murine cryptococcosis. J Med Vet Mycol. 1984;22:445–453. doi: 10.1080/00362178485380721. [DOI] [PubMed] [Google Scholar]
- 5.Graybill J R, Ahrens J, Nealon T, Paque R. Pulmonary cryptococcosis in the rat. Am Rev Respir Dis. 1983;127:636–640. doi: 10.1164/arrd.1983.127.5.636. [DOI] [PubMed] [Google Scholar]
- 6.Graybill J R, Mitchell L, Drutz D J. Host defense in cryptococcosis. Protection of nude mice by thymus transplantation. J Infect Dis. 1979;140:546–552. doi: 10.1093/infdis/140.4.546. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey M J, Jevons S, Tarbit M H. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother. 1985;28:648–653. doi: 10.1128/aac.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeasts. Proposed standard M27-P. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 9.Perfect J R, Durack D T. Treatment of experimental cryptococcal meningitis with amphotericin B, 5-fluorocytosine, and ketoconazole. J Infect Dis. 1982;146:429–435. doi: 10.1093/infdis/146.3.429. [DOI] [PubMed] [Google Scholar]
- 10.Perfect J R, Durack D T. Comparison of amphotericin B and N-d-ornithyl amphotericin B methyl ester in experimental cryptococcal meningitis and Candida albicans endocarditis with pyelonephritis. Antimicrob Agents Chemother. 1985;28:751–755. doi: 10.1128/aac.28.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Pfizer, Inc. Data on file. Groton, Conn: Pfizer, Inc.; 1997. [Google Scholar]
- 11.Restrepo B I, Ahrens J, Graybill J R. Efficacy of SCH39304 in murine cryptococcosis. Antimicrob Agents Chemother. 1989;33:1242–1246. doi: 10.1128/aac.33.8.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sneller M R, Hariri A, Sorenson W G, Larsh H W. Comparative study of trichothecin, amphotericin B, and 5-fluorocytosine against Cryptococcus neoformans in vitro and in vivo. Antimicrob Agents Chemother. 1977;12:390–394. doi: 10.1128/aac.12.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Cutsem, J., F. Van Gerven, and P. A. J. Janssen. 1987. Activity of orally, topically, and parenterally administered itraconazole in the treatment of superficial and deep mycoses: animal models. Rev. Infect. Dis. 9(Suppl. 1):s15–s32. [DOI] [PubMed]
- 14.Velez J D, Allendoerfer R, Luthger M, Rinaldi M G, Graybill J R. Correlation of in vitro azole susceptibility with in vivo response in a murine model of cryptococcal meningitis. J Infect Dis. 1993;168:508–510. doi: 10.1093/infdis/168.2.508. [DOI] [PubMed] [Google Scholar]