Abstract
Two new genes (mexXY) similar to mexAB, mexCD, and mexEF and mediating multidrug resistance were cloned from the chromosome of Pseudomonas aeruginosa. Elevated ethidium extrusion was observed with Escherichia coli cells harboring the plasmid carrying mexXY. This MexXY system confers higher resistance to fluoroquinolones than the MexAB and MexCD systems, and E. coli TolC or P. aeruginosa OprM is necessary for the function of the MexXY system.
Pseudomonas aeruginosa shows significant degrees of intrinsic resistance to a wide variety of antimicrobial agents, including most β-lactams, fluoroquinolones, tetracycline, chloramphenicol, and erythromycin. This is a major problem in hospitals because P. aeruginosa is an important opportunistic pathogen and a leading cause of hospital-acquired infections. Three RND (resistance nodulation cell division) family drug efflux systems are known to exist in P. aeruginosa: MexAB-OprM (18), MexCD-OprJ (16), and MexEF-OprN (6). Recent studies have made it clear that these Mex systems, especially MexAB-OprM in wild-type cells, are mainly responsible for the intrinsic resistance of this organism to many antimicrobial agents (8, 9). Similar results demonstrating an involvement of the RND family multidrug efflux system AcrAB in mediating intrinsic resistance to many antimicrobial agents in Escherichia coli have been reported (11). Thus, it seems that RND family multidrug efflux systems are common in gram-negative bacteria and are responsible for intrinsic resistance to many antimicrobial agents. Here we report on a new multidrug efflux system, MexXY, of P. aeruginosa.
Cloning of multidrug resistance genes.
P. aeruginosa PAO1 was used as a source of chromosomal DNA. E. coli KAM3 {ΔacrAB supE hsdD5 thi (Δlac-proAB)/F′ [traD36 proAB+ lacIq lac ΔZM15]}, a derivative of TG1, was used as a host for gene cloning (13, 19). E. coli KAM3 lacks acrAB genes and is therefore hypersensitive to many antimicrobial agents (14). Chromosomal DNA was prepared from cells of P. aeruginosa (1). The DNA was partially digested with Sau3A1. The DNA fragments ranging between 4 and 10 kbp were ligated into pBR322, which had been digested with BamHI. Competent cells of E. coli KAM3 were transformed with the ligated hybrid plasmids (5) and spread onto agar plates containing L medium (7) and 1.5% agar plus one of the following antimicrobial agents: 12 μg of ethidium bromide per ml, 10 μg of erythromycin per ml, or 1 μg of chloramphenicol per ml. The plates were incubated at 37°C for 1 day. The clones formed were picked. Plasmid DNA was prepared from each of the transformants by using a miniprep kit (Qiagen Inc.) as suggested by the manufacturer. Competent cells of E. coli KAM3 were retransformed and spread on the same plates again. The plates were incubated at 37°C for 1 day. Many colonies appeared on the plates. Plasmid DNA from each of the retransformants was prepared. The ethidium bromide plates gave us the largest number of candidates (43 candidates), followed by the erythromycin plates (8 candidates), and the chloramphenicol plates (3 candidates). We prepared and digested plasmids from all of these transformants. Three patterns were seen, one of which was similar to the restriction pattern of the mexAB region and a second one that was similar to that of the mexCD region. We confirmed that the former type was mexAB and the latter type was mexCD by partial sequencing. However, the third plasmid seemed to contain a novel drug resistance gene(s). We designated the new genes mexXY as described below. The new genes were identified from all three kinds of selection plates.
We measured the MICs of many antimicrobial agents with KAM3 cells harboring plasmids carrying each type of mex gene. Cells of KAM3/pTEM4 (carrying the mexXY genes) showed resistance to acriflavine, ethidium bromide, erythromycin, and fluoroquinolones (Table 1) and some degree of resistance to tetracycline, chloramphenicol, and kanamycin (data not shown). Cells of KAM3/pTUM3 (carrying the mexAB oprM genes [17]) showed lower resistance to most of the above-mentioned antimicrobial agents than did KAM3/pTEM4 cells (data not shown). Cells of KAM3/pTEM31 (carrying the mexCD oprJ genes [16]) showed higher resistance to acriflavine and ethidium bromide than KAM3/pTEM4 cells did but lower resistance to fluoroquinolones (data not shown). Thus, this indicates that the mexXY genes are multidrug resistance genes. One of the characteristics of the MexXY system is that this system conferred higher resistance to fluoroquinolones than other Mex systems did.
TABLE 1.
Susceptibilities of study strains to different compounds and effect of tolC and oprM on the function of MexXY
| Compound | MIC (μg/ml) for E. coli strain tested
|
||||||
|---|---|---|---|---|---|---|---|
| KAM3 | KAM3/pTEM4 (mexXY)a | N43 | N43/pTEM4 (mexXY) | N43 tolC::Tn10 | N43 tolC::Tn10/pTEM4 (mexXY) | N43 tolC::Tn10/ pTEM4/pPMM2 (mexXY oprM) | |
| Acriflavine | 2 | 16 | 4 | 32 | 4 | 4 | 32 |
| Ethidium bromide | 1 | 32 | 4 | 64 | 4 | 2 | 32 |
| Erythromycin | 1 | 32 | 8 | 128 | 2 | 2 | 128 |
| Norfloxacin | 0.016 | 0.13 | 0.03 | 0.125 | 0.03 | 0.016 | 0.125 |
| Ofloxacin | 0.016 | 0.13 | 0.016 | 0.06 | 0.016 | 0.008 | 0.125 |
| Tetraphenyl phosphonium | NTb | NT | 16 | 512 | 16 | 16 | NT |
Genes carried on the plasmids are shown in parentheses.
NT, not tested.
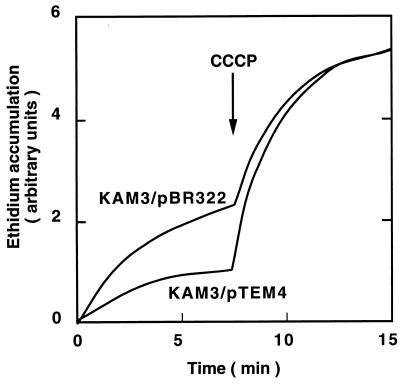
We measured ethidium efflux with KAM3/pBR322 cells and KAM3/pTEM4 cells to clarify whether the mexXY are genes for a multidrug efflux system. We observed a lower intracellular ethidium level before addition of an H+ conductor, CCCP (carbonyl cyanide m-chlorophenylhydrazone), than after its addition with KAM3/pBR322 cells (Fig. 1). This indicates that an ethidium efflux pump driven by an electrochemical potential of H+ is still present in KAM3 cells. We observed a much lower intracellular ethidium level before the addition of CCCP with KAM3/pTEM4 cells than with KAM3/pBR322 cells. The intracellular ethidium level increased after the addition of CCCP and reached the same level as that in the case of KAM3/pBR322 cells. Thus, it is clear that the mexXY genes confer to the cells H+-driven ethidium efflux ability.
FIG. 1.
Accumulation of ethidium in host cells and in transformed cells. E. coli KAM3/pBR322 and KAM3/pTEM4 cells were grown in L medium supplemented with 40 mM potassium lactate. Ethidium bromide was added to cell suspensions of KAM3/pBR322 and KAM3/pTEM4 at a final concentration of 10 μM. Accumulation of ethidium was monitored continuously by measuring the fluorescence of ethidium in cells, at the excitation and emission wavelengths of 500 and 580 nm, respectively. After 7.5 min (arrow), CCCP was added to the suspensions at a final concentration of 100 μM.
Sequences of genes and products.
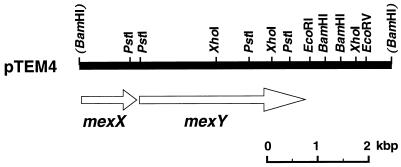
We determined the nucleotide sequence (20) of the DNA insert in pTEM4. We found two open reading frames (ORFs) oriented in the same direction preceded by Shine-Dalgarno sequences in the nucleotide sequence determined (Fig. 2). We designated the first ORF mexX and the second ORF mexY. Amino acid sequences were deduced from the mexX and mexY nucleotide sequences. The deduced MexX and MexY sequences consist of 389 and 1,046 residues, respectively. The calculated molecular masses are 41,444 and 113,116 Da, respectively. We found a promoter-like sequence in the upstream region from the mexX gene. It seems that mexX and mexY are in one operon. No other ORF was found in the region downstream (about 1 kbp in length) from the mexY gene.
FIG. 2.
Restriction map of DNA insert carried on pTEM4. The DNA region derived from the P. aeruginosa chromosomal DNA and carried on pTEM4 is shown as a horizontal solid bar. Restriction sites on pTEM4 are indicated. Locations of mexX and mexY are indicated by open arrows.
Characteristics of the primary structure.
A homology search of sequence databases (GenBank and SwissProt) revealed that MexX has 30 to 40% sequence identity and 40 to 60% similarity with MexA (17), MexC (16), MexE (6), AcrA (10), AcrE (12), and putative YhiU (15) and that MexY has 40 to 50% identity and 60 to 70% similarity with MexB (17), MexD (16), MexF (6), AcrB (10), AcrD (12), AcrF (12), and putative YhiV (15). Thus, it is clear that MexXY is a member of the RND family.
Hydropathy values were calculated along amino acid sequences of MexX and MexY by the method of Eisenberg et al. (3). Judging from the hydropathy patterns, MexX is a hydrophilic protein with one hydrophobic domain in its N-terminal region (data not shown). The N-terminal hydrophobic region seems to be a signal sequence (24). Immediately following the hydrophobic core region of the signal sequence is the sequence L-L-G-C, which is very similar to the sequence L-L-A-G-C, which has been reported to be a consensus sequence of the signal cleavage site of lipoproteins of gram-negative bacteria (14). Therefore, it is likely that the MexX is a lipoprotein anchored in the membrane by its lipid portion. It has been reported that AcrE (EnvC) is a lipoprotein of the cytoplasmic membrane of E. coli (21). On the other hand, MexY seems to be an intrinsic membrane protein with many hydrophobic domains.
Requirement for TolC or OprM.
OprM, an outer membrane protein, is necessary for the function of the MexAB system. The oprM gene is located just downstream from the mexAB genes (18). OprJ is necessary for the function of MexCD. The oprJ gene is adjacent to the mexCD genes (16). There are only 400 bp between the termination codon of mexY and a BamHI site, which is in the downstream end of the DNA region necessary for conferring drug resistance. No ORF corresponding to an outer membrane protein or to any other protein was found in this 400-bp region or in the downstream region (1 kbp in length) from mexXY. The MexXY system is functional in E. coli KAM3. This suggests that an outer membrane protein of E. coli such as TolC, which is required for the functioning of the AcrAB system (4), may be utilized by the MexXY system as the outer membrane component. We tested this possibility. E. coli N43 (F− lac ara mal xyl mtl gal rpsL acrA1) tolC::Tn10 and its parent N43 (4, 25) were used for this purpose. E. coli N43 lacks the AcrAB system like KAM3. Cells of N43 were hypersensitive to many antimicrobial agents, but, as anticipated, N43/pTEM4 became resistant to many antimicrobial agents (Table 1). Cells of N43 tolC::Tn10 were also hypersensitive to many antimicrobial agents. However, N43 tolC:: Tn10/pTEM4 cells were still hypersensitive to many antimicrobial agents (Table 1). This means that the MexXY system in N43 tolC::Tn10 was not functional whereas that in N43 was functional. Thus, we conclude that TolC is necessary for the MexXY system to function in E. coli. It has been reported that MexCD functions in E. coli cells in conjunction with TolC (22). We also tested whether the TolC could be replaced with the OprM of P. aeruginosa in E. coli. Plasmid pTEM4 carrying the mexXY genes and plasmid pPMM2 carrying the oprM gene were used for this purpose. pPMM2 (vector, pACYC184) is a derivative of pTUM3 (pBR322) and carries oprM but not mexAB. Either pTEM4 or pPMM2 alone, or both, were introduced into N43 tolC::Tn10 cells. The sensitivity to many antimicrobial agents was then tested. Introduction of both mexXY and oprM into N43 tolC::Tn10 cells resulted in an increase in the MICs of many antimicrobial agents (Table 1). Introduction of either mexXY or oprM alone, however, resulted in no increase in the MICs. Thus, it seems that OprM forms a functional multidrug efflux pump together with MexXY in E. coli cells. It has been reported that OprM and OprJ are interchangeable (23). Thus, it seems that OprJ and OprN could be alternative outer membrane proteins for the MexXY system. However, since OprJ and OprN are not expressed in wild-type P. aeruginosa (6, 16), it is very likely that OprM is the most probable candidate to form a complex with MexXY and function as a multidrug efflux pump in wild-type P. aeruginosa. Very recently it has been reported that OprM can be expressed and function in a drug efflux capacity independent of MexAB in P. aeruginosa (26). This OprM-dependent and MexAB-independent system is responsible for resistance to quinolones, erythromycin, and tetracycline (26). This substrate specificity is the same as that of the MexXY system.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been submitted to the DDBJ/EMBL/GenBank nucleotide sequence databases under accession no. AB015853.
Acknowledgments
We thank Joe A. Fralick for providing us with E. coli N43 and N43 tolC::Tn10 and Manuel F. Varela for critically reading the manuscript.
This work was supported in part by a grant from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Berns K L, Thomas C A J. Isolation of high molecular weight DNA from Haemophilus influenzae. J Mol Biol. 1965;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- 2.Bolhuis H, Molenaar D, Poelarends G, van Veen H W, Poolman B, Driessen A J M, Konings W N. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J Bacteriol. 1994;176:6957–6964. doi: 10.1128/jb.176.22.6957-6964.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenberg D, Schwarz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- 4.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 6.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 7.Lennox E S. Transduction of linked genetic characters of host by bacteriophage P1. Virology. 1955;1:190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- 8.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and resistance in Gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 13.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother. 1998;42:1778–1782. doi: 10.1128/aac.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura K, Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979;18:1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Nesha S, Yamagishi J, Li X, Nishino T. Overexpression of mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 17.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 18.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. p. A.12. [Google Scholar]
- 20.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seiffer D, Klein J R, Plapp R. EnvC, a new lipoprotein of the cytoplasmic membrane of Escherichia coli. FEMS Microbiol Lett. 1993;107:175–178. doi: 10.1111/j.1574-6968.1993.tb06026.x. [DOI] [PubMed] [Google Scholar]
- 22.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srikumar R, Li X-Z, Poole K. Inner membrane efflux components are responsible for beta-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi K, Yu F, Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 25.Young K K, Edlin G. Physical and genetical analysis of bacteriophage T4 generalized transduction. Mol Gen Genet. 1983;192:241–246. doi: 10.1007/BF00327673. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q, Li X-Z, Srikumar R, Poole K. Contribution of outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob Agents Chemother. 1998;42:1682–1688. doi: 10.1128/aac.42.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]