Abstract
Simple Summary
Whether it is necessary to evaluate the radiation exposure of cardiac substructures when making radiotherapy plans is one of the current research hotspots. In this cohort study of 355 patients with esophageal cancer, the radiation dose to key coronary substructures such as the left anterior descending artery V30Gy and mean left main coronary artery was closely associated with major coronary events and overall patient survival, and showed better predictive value than the mean heart dose or heart V30Gy recommended by current guidelines. Our findings suggest that, in addition to the whole heart, key coronary substructures should be contoured as organs at risk during radiotherapy plan optimization.
Abstract
Background: There is a paucity of data regarding the association between radiation exposure of heart substructures and the incidence of major coronary events (MCEs) in patients with esophageal cancer (ESOC) undergoing chemoradiation therapy. We studied radiation dosimetric determinants of MCE risk and measured their impact on patient prognosis using a cohort of ESOC patients treated at a single institution. Methods: Between March 2005 and October 2015, 355 ESOC patients treated with concurrent chemoradiotherapy were identified from a prospectively maintained and institutional-regulatory-board-approved clinical database. Dose-distribution parameters of the whole heart, the atria, the ventricles, the left main coronary artery, and three main coronary arteries were extracted for analysis. Results: Within a median follow-up time of 67 months, 14 patients experienced MCEs at a median of 16 months. The incidence of MCEs was significantly associated with the left anterior descending coronary artery (LAD) receiving ≥30 Gy (V30Gy) (p = 0.048). Patients receiving LAD V30Gy ≥ 10% of volume experienced a higher incidence of MCEs versus the LAD V30Gy < 10% group (p = 0.044). The relative rate of death increased with the left main coronary artery (LMA) mean dose (Gy) (p = 0.002). Furthermore, a mutual promotion effect of hyperlipidemia and RT on MCEs was observed. Conclusion: Radiation dose to coronary substructures is associated with MCEs and overall survival in patients with ESOC. In this study, the doses to these substructures appeared to be better predictors of toxicity outcomes than mean heart dose (MHD) or whole-heart V30Gy. These findings have implications for reducing coronary events through radiation therapy planning.
Keywords: esophageal cancer, radiotherapy, heart substructure, major coronary events, survival
1. Introduction
Radiation-induced heart disease following thoracic radiotherapy (RT) has long been reported in long-term survivors of breast cancer (BC) [1,2,3,4] and Hodgkin’s lymphoma (HL) [5,6,7]. Recently, it was found to be relatively common, with early onset, in patients with non-small cell lung cancer (NSCLC) [8,9,10] and esophageal cancer (ESOC), despite the high competing risk of death [11]. Radiation can induce a variety of pathological changes, including endothelial dysfunction, inflammation, thrombosis, and cardiac fibrosis, resulting in a variety of cardiotoxicities [12]. Coronary events are one of the important causes of cardiac mortality and morbidity among patients after RT [4,9,10,11,13].
Typically, the heart as a whole is considered as an organ at risk during thoracic RT, and the mean heart dose (MHD) has generally been used to assess the risk of coronary events in previous studies [4,14]. However, radiobiological responses in various heart substructures may be heterogeneous, and patients may have different cardiotoxicities depending on the radiation dose delivered to each individual cardiac substructure. In a group of patients with HL, Hahn et al. [15] found that the model engaging coronary artery variables was superior to the whole-heart model when analyzing ischemic cardiac events. Since the cardiac radiation exposure in ESOC patients is generally much higher than that in HL, an improved understanding of the dose–cardiotoxicity relationship while taking cardiac substructure volume exposure into consideration is particularly necessary to guide RT delivery.
Currently, the data are limited in terms of the association between radiation dose to heart substructures and major coronary events (MCEs). Our study aims to provide a detailed analysis within a modern cohort of ESOC patients treated with concurrent chemoradiotherapy at conventional radiation doses.
2. Materials and Methods
This cohort study comprised 355 patients with biopsy-confirmed esophageal adenocarcinoma or squamous cell carcinoma (SCC) that was treated with RT in prospective, single-institution biomarker or therapeutic trials in which detailed dosimetric data were maintained. Patients in this prospectively maintained database between March 2005 and October 2015 were analyzed (Supplementary Figure S1). All patients in our study underwent esophagogastroduodenoscopy (EGD) with endoscopic ultrasound, computed tomography (CT) of the chest and upper abdomen with contrast, brain imaging (CT or magnetic resonance imaging), and/or positron emission tomography (PET)/CT scans for staging, and were restaged according to the seventh edition of the American Joint Committee on Cancer TNM classification system [16]. Patients with distant metastatic disease, prior or concomitant malignancy, Eastern Cooperative Oncology Group performance status scale (ECOG) scores above 2, or those with incomplete clinical records were excluded. This study was approved by the Institutional Review Board of MD Anderson Cancer Center (protocol code: RCR02-542; date of initial approval: 13 September 2002; updated: 26 March 2021) and the Institutional Review Board waived the requirement for informed consent.
All patients were treated with concurrent chemoradiotherapy strategies, either as a pre-operative treatment or as a curative treatment. About one-third of patients (35.8%) received induction chemotherapy as part of a clinical trial or due to high-risk disease burden. Chemotherapy regimens typically consisted of fluoropyrimidine and platinum- or taxane-based compounds. Ivor Lewis esophagectomy was the most commonly employed surgical approach (79%). Radiotherapy was delivered with intensity-modulated radiation therapy (IMRT) or proton beam therapy (PBT), and the standard radiation dose was 50.4 Gy (relative biological effectiveness, RBE) in 28 fractions. When the patients were treated in free-breathing mode, four-dimensional computed tomography (CT) simulation was used to track tumor motion throughout the respiratory cycle. All normal structures were contoured on time-averaged CT scans. The IMRT plans were generated by the Pinnacle treatment planning system (version 9.0, Philips, Andover, MA); the PBT plans were generated by the Eclipse planning system (Varian Medical Systems, Liverpool, NY), 92% of which were completed using passive scattering proton therapy.
The whole heart, the atria, the ventricles, the left main (LMA), and the three main coronary arteries, namely the left anterior descending artery (LAD), the left circumflex artery (LCX), and the right coronary artery (RCA) were included in our analysis. An in-house multi-atlas contouring service (MACS) software program, the auto-contouring accuracy of which was previously verified [17], was used to automatically delineate the cardiac structures on the CT images for treatment planning. The accuracy and consistency of the heart substructures were reviewed for each patient by an experienced radiation oncologist, and necessary modifications were made according to the detailed guideline published by Feng et al. [18]. Dose-volume histograms of the heart were obtained from the delivered RT plan. Based on RTOG 0617 [19], we extracted the mean dose, volume receiving ≥ 5 Gy (V5Gy), volume receiving ≥ 30 Gy (V30Gy), and volume receiving ≥ 50 Gy (V50Gy) for the whole heart and for each cardiac substructure of interest for dosimetric analyses. Planning target volume (PTV) was a geometrical concept used for treatment planning, and defined as enlarged clinical target volume (CTV), which was created from the gross tumor volume (GTV) through volume expansion using individual margins.
The primary endpoint of this study was the occurrence of MCEs after RT [4], which was defined as a diagnosis of myocardial infarction (International Classification of Diseases, 10th Revision, codes 121 to 124), coronary revascularization, or death resulting from ischemic heart disease (codes 120 to 125). These events were verified by physicians who did not know the radiation dose distribution of the whole heart and its substructures and were independently reviewed by cardiologists based on the available source documentation. The time-to-failure endpoint was calculated as the duration from the RT start date to the first occurrence of MCE. Patients without MCEs were right-censored at the last point of contact. The secondary endpoint was overall survival (OS), which was defined as the time from diagnosis to death.
Descriptive statistics were used to characterize the baseline clinic-pathological characteristics of the patients. Overall survival was estimated using the Kaplan–Meier method with Greenwood’s formula for interval estimation. Cox proportional hazards regression was used to test for associations between patient clinicopathological characteristics and the study’s endpoints (MCE and OS). The competing risk regression method of Fine and Gray [20] was used to adjust the cumulative incidence of MCE for the competing risk of non-cardiac death. Statistical significance was conferred with a p-value < 0.05. Multiple regression analyses included factors identified with a p-value < 0.1 from univariate analysis. Maximally selected rank statistics further explored the utility of applying thresholds to variables identified as statistically significant in regression analysis to classify patients into low-risk versus high-risk groups. Thresholds were selected for individual variables to maximize the log-rank statistic. All analyses were conducted by R (version 3.6.2) and SPSS (version 25.0.0).
3. Results
3.1. Patient Characteristics and MCEs
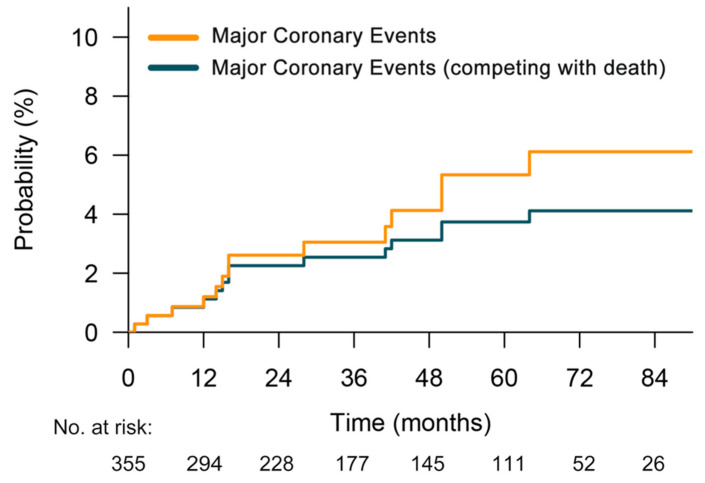
In total, 355 ESOC patients fulfilling the predefined criteria were included, with a median age of 62 years (interquartile range, 54 to 68 years). Among them, 89.6% were male and 88.2% were white. Baseline characteristics are summarized in Table 1. Within a median follow-up time of 67 months, 14 patients experienced MCEs at a median of 16 months (interquartile range, 12 to 42), which included myocardial infarction (n = 8), coronary artery bypass graft (n = 4), and atherosclerosis requiring coronary stent placement (n = 2). Details about these patients are provided in Supplementary Table S1. After accounting for non-cardiac death as a competing risk, the cumulative incidence of MCEs is shown in Figure 1. Additionally, detailed radiation dose distributions for the cardiac substructures are shown in Supplementary Figure S2.
Table 1.
Patients characteristics at baseline.
| Characteristic | Total | Non-MCEs | MCEs | p-Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Sex | 0.647 | ||||||
| Male | 318 | 89.6 | 306 | 89.7 | 12 | 85.7 | |
| Female | 37 | 10.4 | 35 | 10.3 | 2 | 14.3 | |
| Age | 0.780 | ||||||
| <65 | 222 | 62.5 | 214 | 62.8 | 8 | 57.1 | |
| ≥65 | 133 | 37.5 | 127 | 37.2 | 6 | 42.9 | |
| ECOG | 0.586 | ||||||
| 0 | 148 | 41.7 | 141 | 41.3 | 7 | 50.0 | |
| 1–2 | 207 | 58.3 | 200 | 58.7 | 7 | 50.0 | |
| History of Smoking | 0.564 | ||||||
| Yes | 236 | 66.5 | 228 | 66.9 | 8 | 57.1 | |
| No | 119 | 33.5 | 113 | 33.1 | 6 | 42.9 | |
| BMI, kg/m2 | 0.587 | ||||||
| <30 | 206 | 58.0 | 199 | 58.4 | 7 | 50.0 | |
| ≥30 | 149 | 42.0 | 142 | 41.6 | 7 | 50.0 | |
| History of CAD | 0.708 | ||||||
| Yes | 57 | 16.1 | 54 | 15.8 | 3 | 21.4 | |
| No | 298 | 83.9 | 287 | 84.2 | 11 | 78.6 | |
| History of Hyperlipidemia | 0.005 | ||||||
| Yes | 200 | 56.3 | 187 | 54.8 | 13 | 92.9 | |
| No | 155 | 43.7 | 154 | 45.2 | 1 | 7.1 | |
| History of Hypertension | 0.090 | ||||||
| Yes | 218 | 61.4 | 206 | 60.4 | 12 | 85.7 | |
| No | 137 | 38.6 | 135 | 39.6 | 2 | 14.3 | |
| History of Diabetes | 0.775 | ||||||
| Yes | 89 | 25.1 | 86 | 25.2 | 3 | 21.4 | |
| No | 266 | 74.9 | 255 | 74.8 | 11 | 78.6 | |
| Tumor Location | 0.180 | ||||||
| Upper/Middle | 38 | 10.7 | 35 | 10.3 | 3 | 21.4 | |
| Distal/GEJ | 317 | 89.3 | 306 | 89.7 | 11 | 78.6 | |
| Pathology | 0.065 | ||||||
| Adenocarcinoma | 314 | 88.5 | 304 | 89.1 | 10 | 71.4 | |
| SCC | 41 | 11.5 | 37 | 10.9 | 4 | 28.6 | |
| Clinical T Stage (7th) | 0.377 | ||||||
| T1–2 | 37 | 10.4 | 37 | 10.9 | 0 | 0.0 | |
| T3–4 | 318 | 89.6 | 304 | 89.1 | 14 | 100.0 | |
| Clinical N Stage (7th) | 0.773 | ||||||
| N0 | 113 | 31.8 | 108 | 31.7 | 5 | 35.7 | |
| N+ | 242 | 68.2 | 233 | 68.3 | 9 | 64.3 | |
| Clinical Stage (7th) | 1.000 | ||||||
| Stage I/II | 122 | 34.4 | 117 | 34.3 | 5 | 35.7 | |
| Stage III | 233 | 65.6 | 224 | 65.7 | 9 | 64.3 | |
| Induction Chemotherapy | 0.592 | ||||||
| Yes | 127 | 35.8 | 123 | 36.1 | 4 | 28.6 | |
| No | 228 | 64.2 | 218 | 63.9 | 10 | 71.4 | |
| Surgery | 0.578 | ||||||
| Yes | 215 | 60.6 | 208 | 61.0 | 7 | 50.0 | |
| No | 140 | 39.4 | 133 | 39.0 | 7 | 50.0 | |
| Radiotherapy Modality | 0.397 | ||||||
| IMRT | 234 | 65.9 | 223 | 65.4 | 11 | 78.6 | |
| PBT | 121 | 34.1 | 118 | 34.6 | 3 | 21.4 | |
| PTV | 1.000 | ||||||
| <600 cc | 173 | 48.7 | 166 | 48.7 | 7 | 50.0 | |
| ≥600 cc | 182 | 51.3 | 175 | 51.3 | 7 | 50.0 | |
| Platin-based Chemotherapy | 0.581 | ||||||
| Yes | 144 | 59.4 | 137 | 40.2 | 7 | 50.0 | |
| No | 211 | 40.6 | 204 | 59.8 | 7 | 50.0 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; CAD, coronary artery disease; GEJ, gastroesophageal junction; SCC, squamous cell carcinoma; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy; PTV, Planning Target Volume.
Figure 1.
Cumulative incidence plot of major coronary events after chemoradiation (yellow) and major coronary events adjusted for the competing risk of death (green).
3.2. Risk Factors for MCEs
On dosimetric analysis, LAD V30Gy (%) (hazard ratio (HR) = 1.025; 95%CI, 1.001–1.050; p = 0.048) was significantly associated with MCEs (Supplementary Table S2). We identified the optimal curve cutoff value of LAD V30Gy for predicting MCE at 10% (C-index, 0.57).
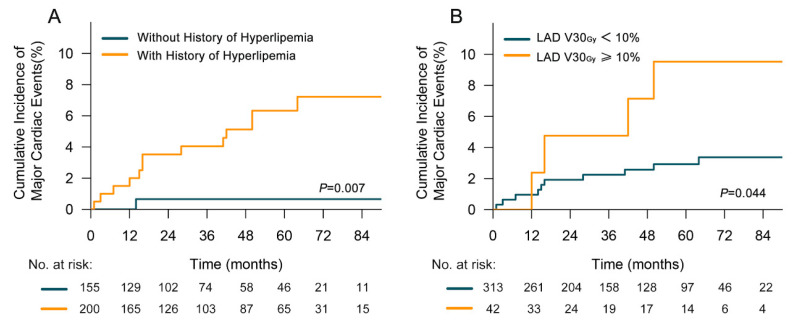
All clinicopathological factors were included in univariate analysis, and those associated with a p value < 0.1 were included in multivariate analysis. Significant correlations between MCE incidence and history of hyperlipidemia (Yes vs. No, HR = 10.522, 95%CI, 1.373–80.621; p = 0.023) as well as LAD V30Gy (≥10% vs. <10%, HR = 3.589, 95%CI, 1.124–11.462; p = 0.031) were identified (Table 2). In patients undergoing RT, a history of hyperlipidemia was associated with an increased risk of developing an MCE (2-y rates, 3.5% vs. 0.7%; 5-y rates, 6.3% vs. 0.7%; p = 0.007) (Figure 2A). Compared with patients receiving LAD V30Gy < 10%, the cumulative incidence of MCEs in patients with LAD V30Gy ≥ 10% increased significantly (2-y rates, 4.8% vs. 2.0%; 5-y rates, 9.5% vs. 2.9%; p = 0.044) (Figure 2B).
Table 2.
Univariable and multivariable analysis for time to earliest major coronary events.
| Variable | Univariable Cox Regression Analysis | Cox Multivariable Regression | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex, Male vs. Female | 0.778 | 0.174–3.476 | 0.742 | |||
| Age, ≥65 vs. <65 | 1.394 | 0.483–4.023 | 0.539 | |||
| ECOG, 1–2 vs. 0 | 0.754 | 0.264–2.151 | 0.597 | |||
| Smoking History, Yes vs. No | 0.693 | 0.240–1.997 | 0.497 | |||
| BMI, kg/m2, ≥30 vs. <30 | 1.290 | 0.452–3.681 | 0.634 | |||
| History of CAD, Yes vs. No | 1.735 | 0.483–6.232 | 0.398 | |||
| History of Hyperlipidemia, Yes vs. No | 9.748 | 1.275–74.532 | 0.028 | 10.522 | 1.373–80.621 | 0.023 |
| History of Hypertension, Yes vs. No | 4.247 | 0.950–18.991 | 0.058 | |||
| History of Diabetes, Yes vs. No | 0.853 | 0.238–3.060 | 0.808 | |||
| Tumor Location, Upper/Middle vs. Distal/GEJ | 2.767 | 0.768–9.956 | 0.119 | |||
| Pathology, SCC vs. Adenocarcinoma | 3.889 | 1.216–12.436 | 0.022 | |||
| Clinical Stage, III vs. I/II | 1.190 | 0.397–3.564 | 0.756 | |||
| Induction Chemotherapy, Yes vs. No | 0.705 | 0.221–2.247 | 0.554 | |||
| Surgery, Yes vs. No | 0.477 | 0.166–1.368 | 0.168 | |||
| Radiotherapy Technology, IMRT vs. PBT | 1.817 | 0.507–6.516 | 0.360 | |||
| PTV, ≥600 cc vs. <600 cc | 1.051 | 0.368–2.998 | 0.926 | |||
| Platin-based Chemotherapy, Yes vs. No | 1.349 | 0.473–3.849 | 0.576 | |||
| LAD, V30Gy ≥ 10% vs. <10% | 3.101 | 0.972–9.895 | 0.056 | 3.589 | 1.124–11.462 | 0.031 |
Abbreviations: CI, confidence interval; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; CAD, coronary artery disease; GEJ, gastroesophageal junction; SCC, squamous cell carcinoma; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy; PTV, Planning Target Volume; LAD, left anterior descending coronary artery.
Figure 2.
Cumulative incidence of major coronary events (with non-cardiac death as a competing risk) (A) for patients with or without a history of hyperlipidemia (B) for patients delivered to left anterior descending coronary artery (LAD) V30Gy < 10% and ≥10%.
3.3. Overall Survival
The median OS was 48 months for the entire group. As indicated by dosimetric analysis, the relative rate of death significantly increased with the mean LMA dose (HR = 1.014; 95%CI, 1.005–1.023; p = 0.002) and exhibited better predictive efficacy versus heart V30Gy and MHD (Supplementary Table S3). The optimal cutoff value of the mean LMA dose was determined to be 20 Gy (C-index, 0.56).
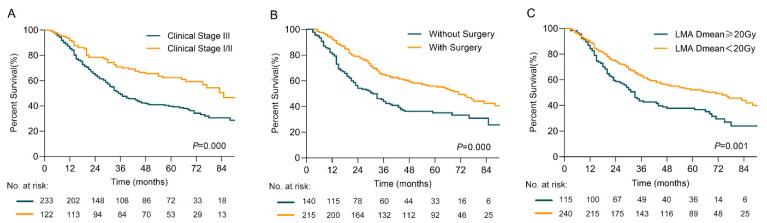
Clinical stage (III vs. I/II; HR = 1.850; 95%CI, 1.332–2.571; p < 0.001), surgery (Yes vs. No; HR = 0.542; 95%CI, 0.407–0.723; p < 0.001), and mean LMA dose (≥20 Gy vs. <20 Gy; HR = 1.488; 95%CI, 1.108–1.998; p = 0.008) were identified as independent prognostic factors for OS (Table 3). Patients with an early clinical stage (I/II vs. III; 2-y rates, 78.5% vs.64.3%; 5-y rates, 62.4% vs. 39.7%; p < 0.001), surgical treatment (Yes vs. No; 2-y rates, 79.1% vs. 54.2%; 5-y rates, 55.9% vs. 35.1%; p < 0.001), and a mean LMA dose < 20 Gy (mean LMA dose < 20 Gy vs. ≥20 Gy; 2-y rates, 74.3% vs. 58.6%; 5-y rates, 52.3% vs. 37.8%; p = 0.001) demonstrated prolonged OS (Figure 3A–C).
Table 3.
Univariable and multivariable analysis for overall survival.
| Variable | Univariable Cox Regression Analysis | Cox Multivariable Regression | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex, Male vs. Female | 1.584 | 0.935–2.683 | 0.087 | |||
| Age, ≥65 vs. <65 | 1.061 | 0.793–1.420 | 0.688 | |||
| ECOG, 1–2 vs. 0 | 0.980 | 0.739–1.299 | 0.887 | |||
| Smoking History, Yes vs. No | 1.168 | 0.864–1.578 | 0.314 | |||
| BMI, kg/m2, ≥30 vs. <30 | 0..925 | 0.697–1.229 | 0.593 | |||
| History of CAD, Yes vs. No | 1.585 | 1.115–2.252 | 0.010 | |||
| History of Hyperlipidemia, Yes vs. No | 0.872 | 0.659–1.155 | 0.340 | |||
| History of Hypertension, Yes vs. No | 1.364 | 1.015–1.832 | 0.039 | |||
| History of Diabetes, Yes vs. No | 1.296 | 0.950–1.766 | 0.102 | |||
| Tumor Location, Upper/Middle vs. Distal/GEJ | 1.220 | 0.783–1.902 | 0.379 | |||
| Pathology, SCC vs. Adenocarcinoma | 1.364 | 0.896–2.076 | 0.148 | |||
| Clinical Stage, III vs. I/II | 1.863 | 1.353–2.564 | 0.000 | 1.850 | 1.332–2.571 | <0.001 |
| Induction Chemotherapy, Yes vs. No | 0.879 | 0.655–1.180 | 0.390 | |||
| Surgery, Yes vs. No | 0.557 | 0.420–0.738 | 0.000 | 0.542 | 0.407–0.723 | <0.001 |
| Radiotherapy Technology, IMRT vs. PBT | 1.047 | 0.776–1.411 | 0.764 | |||
| PTV, ≥600 cc vs. <600 cc | 1.319 | 0.995–1.748 | 0.054 | |||
| Platin-based Chemotherapy, Yes vs. No | 0.955 | 0.719–1.269 | 0.751 | |||
| Mean LMA Dose, ≥20 Gy vs. <20 Gy | 1.594 | 1.196 | 0.001 | 1.488 | 1.108–1.998 | 0.008 |
Abbreviations: CI, confidence interval; HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; CAD, coronary artery disease; GEJ, gastroesophageal junction; SCC, squamous cell carcinoma; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy; PTV, Planning Target Volume; LMA, left main coronary artery.
Figure 3.
Overall survival rates (A) for patients with clinical stage I/II or III (B) for patients with or without surgery (C) for patients with 20 Gy mean left main coronary artery (LMA) dose cutoff.
4. Discussion
To our knowledge, this is the first study to demonstrate the relationship between MCEs and the radiation exposure of the cardiac substructures in a large cohort of ESOC patients. Our previous study in ESOC, in which the heart was analyzed as an entire organ, showed the correlation between cardiac toxicity and RT. [11]. In this study, we showed that the radiation doses to coronary substructures such as the LAD and LMA are important predictors of subsequent MCEs and OS, and are superior in this prediction compared with the dose to the whole heart as the method of prediction. Our results are consistent with a recent report in a large cohort of non-small cell lung cancer (NSCLC) patients treated with chemoradiation [21]. In that study, the authors found that the LAD V15Gy ≥10% was strongly predictive of major adverse cardiac events (MACE), including coronary and heart failure events, cardiac death (adjusted HR 13.9; 95% CI, 1.23–157.21), and all-cause mortality. The dose cutoff for ESOC being higher than that for NSCLC (V30Gy for ESOC vs. V15Gy for NSCLC) may be related to the underlying comorbidities of patients with NSCLC vs. those with ESOC. These studies stress the importance of evaluating and avoiding radiation dosing to the key coronary substructures during RT planning for thoracic cancers such as ESOC and NSCLC.
At present, the NCCN Clinical Practice Guidelines in Oncology for Esophageal and Esophagogastric Junction recommended MHD and heart V30Gy to be the primary indices for the risk assessment of cardiac toxicity. However, highly inhomogeneous doses to the small volumes of the heart may result in diverse heart injuries. Nilsson et al. [22] demonstrated through coronary angiography that the location and severity of coronary artery stenoses were associated with the anticipated hotspot areas for radiation in women with breast cancer. Van et al. [14] found that the MHD-based normal tissue complication probability (NTCP) model for acute coronary events could be improved in terms of calibration and discrimination by replacing MHD with LV-V5Gy,. A recent publication from Canada evaluating ischemic-only late cardiotoxicity in a group of HL patients indicated that the best predictive model included age, LAD V5Gy, and LCX V20Gy as variables [15]. In our study, LAD V30Gy exhibited better prediction of MCEs than either MHD or heart V30Gy, and patients with LAD V30Gy ≥10% had a significantly increased risk of MCEs, which occurred notably earlier, at a median of 16 months after RT. Accordingly, we recommend that key coronary substructures such as the LAD should be contoured as organs at risk, along with the whole heart, for RT plan optimization.
The other major finding of interest in our study was that among several cardiac risk factors and pre-existing coronary disease, hyperlipidemia was the only independent predictor of MCEs. Hyperlipidemia is a known risk factor for atherosclerosis and increases the incidence of coronary events [23]. Radiation exposure tends to accelerate this process. Mancuso et al. [24] showed that both chronic low dose rate and acute exposure of the coronary arteries to irradiation accelerate atherosclerosis in apolipoprotein E-deficient (ApoE−/−) mice. In the same model of spontaneous atherogenesis, Hoving et al. [25] also observed such an expedited process in the carotid arteries. Notably, in contrast to ApoE−/− mice, none of the irradiated or control wild-type C57BL/6J mice developed atherosclerotic plaques within the 30-week follow-up period. These studies and others [26] that showed a mutual promotion effect between hyperlipidemia and RT on coronary events support the findings of our study. In addition to reducing radiation exposure to the critical coronary substructures, we suggest that patients with a history of hyperlipidemia may benefit significantly from optimized lipid management during and after RT.
Accumulating evidence shows that an excessive cardiac dose potentially contributes to cardiotoxicity, which is indicative of poor OS [9,10,11,27,28]. In the Radiation Therapy Oncology Group (RTOG) 0617 study [19], 74 Gy delivered in 2 Gy daily fractions for patients with stage III NSCLC was no better than 60 Gy, and even potentially harmful, with corresponding increases in heart V5Gy and heart V30Gy that were both significantly associated with a greater risk of death. A close relationship between radiation exposure of the heart and OS was also found in our study. We further demonstrated that the radiation dose to the key cardiac substructures, such as LMA, performed as a better predictor of OS than MHD or heart V30Gy. Sparing these key substructures, in particular when making RT plans, could potentially improve patient outcomes.
This study should be interpreted in the context of some limitations. First, we were limited by the nature of a retrospective study. Although the events were reviewed from the patients’ medical records and adjudicated by cardiologists, we may have undercounted the incidence of MCEs if some of them occurred elsewhere and were not documented in the electronic medical records at MD Anderson Cancer Center (MDACC), or if the patients did not return for follow-up at MDACC. Second, attributed to potential inter-observer variation [29] and the impact of the motion of the heart and its substructures [30], the reconstruction of small structures such as coronary arteries might be less reliable. In order to minimize such uncertainties, we used a validated in-house software program for delineation, and the delineation was checked by a single radiation oncologist with a common contouring guideline. Lastly, given the smaller number of events, further subgroup analysis was not possible. We look forward to validating our findings in larger, prospective, cooperative group clinical trials in the future.
5. Conclusions
In conclusion, the radiation dose to the key coronary substructures was closely associated with MCEs and overall patient survival. Therefore, this parameter may be used as a better predictor of coronary events than MHD or heart V30Gy for ESOC patients. RT plan optimization and dedicated dose constraints for these crucial substructures of the heart may be the best option to reduce cardiac injury and prolong patient survival. Moreover, hyperlipidemia was an aggravating factor for MCEs in the presence of RT in our study, which suggests that optimization of lipid management may be particularly important during and after RT, especially in patients with thoracic cancers such as ESOC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14051304/s1, Figure S1: CONSORT diagram; Figure S2: Distribution of median dose indices (A) for heart chambers (B) for left main and three main coronary arteries; Table S1: Details of patients with major coronary events; Table S2: Dosimetric univariable and multivariable analysis for time to earliest major coronary event; Table S3: Dosimetric univariable and multivariable analysis for overall survival.
Author Contributions
Conceptualization, X.W. and S.H.L.; methodology, X.W. and S.H.L.; software, X.W.; validation, X.W., B.P.H. and S.H.L.; formal analysis, X.W.; investigation, X.W., N.L.P., J.-i.A., S.W.Y. and A.D.; resources, X.W. and S.H.L.; data curation, S.H.L.; writing—original draft preparation, X.W.; writing—review and editing, X.W., N.L.P., B.P.H., J.-i.A., K.T.N., S.W.Y., J.H., A.D. and S.H.L.; visualization, X.W.; supervision, X.W. and S.H.L.; project administration, X.W.; funding acquisition, S.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
S.H.L. is supported by NCI Cancer Center Support Grant (CCSG) CA016672, MD Anderson Multidisciplinary Research Program Grant, NIH/NCI 1P01CA261669-01 and the Cancer Prevention & Research Institute of Texas (RP200670). N.L.P. is supported by the NIH/NCI 1P01CA261669-01, the Cancer Prevention & Research Institute of Texas (RP200670), and the Sabin Family Foundation Fellowship. J.-i.A. is supported by NIH, U01AI156921. K.T.N. is a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar in Cancer Research and supported by CPRIT RR190077, NCI L30CA253796 and NCI K08CA263313. J.H. is supported by NIH/NCI: CA 233610. A.D. is supported by the Ting Tsung and Wei Fong Chao Distinguished Chair, and has received research support from the MD Anderson Multidisciplinary Research Program Grant.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of MD Anderson Cancer Center (protocol code: RCR02-542; date of initial approval: 13 September 2002; updated: 26 March 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective design of this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (S.H.L.) upon reasonable request.
Conflicts of Interest
The authors declare no potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 2.Bouillon K., Haddy N., Delaloge S., Garbay J.R., Garsi J.P., Brindel P., Mousannif A., Le M.G., Labbe M., Arriagada R., et al. Long-term cardiovascular mortality after radiotherapy for breast cancer. J. Am. Coll. Cardiol. 2011;57:445–452. doi: 10.1016/j.jacc.2010.08.638. [DOI] [PubMed] [Google Scholar]
- 3.McGale P., Darby S.C., Hall P., Adolfsson J., Bengtsson N.O., Bennet A.M., Fornander T., Gigante B., Jensen M.B., Peto R., et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden, Radiotherapy and oncology. J. Eur. Soc. Ther. Radiol. Oncol. 2011;100:167–175. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Darby S.C., Ewertz M., McGale P., Bennet A.M., Blom-Goldman U., Bronnum D., Correa C., Cutter D., Gagliardi G., Gigante B., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 5.Aleman B.M., van den Belt-Dusebout A.W., Klokman W.J., Veer M.B.V., Bartelink H., van Leeuwen F.E. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J. Clin. Oncol. 2003;21:3431–3439. doi: 10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 6.van Nimwegen F.A., Schaapveld M., Janus C.P., Krol A.D., Petersen E.J., Raemaekers J.M., Kok W.E., Aleman B.M., van Leeuwen F.E. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern. Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 7.van Nimwegen F.A., Schaapveld M., Cutter D.J., Janus C.P., Krol A.D., Hauptmann M., Kooijman K., Roesink J., van der Maazen R., Darby S.C., et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J. Clin. Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 8.Ning M.S., Tang L., Gomez D.R., Xu T., Luo Y., Huo J., Mouhayar E., Liao Z. Incidence and Predictors of Pericardial Effusion After Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017;99:70–79. doi: 10.1016/j.ijrobp.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dess R.T., Sun Y., Matuszak M.M., Sun G., Soni P.D., Bazzi L., Murthy V.L., Hearn J.W.D., Kong F.M., Kalemkerian G.P., et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2017;35:1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K., Eblan M.J., Deal A.M., Lipner M., Zagar T.M., Wang Y., Mavroidis P., Lee C.B., Jensen B.C., Rosenman J.G., et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J. Clin. Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Palaskas N.L., Yusuf S.W., Abe J.I., Lopez-Mattei J., Banchs J., Gladish G.W., Lee P., Liao Z., Deswal A., et al. Incidence and Onset of Severe Cardiac Events After Radiotherapy for Esophageal Cancer. J. Thorac. Oncol. 2020;15:1682–1690. doi: 10.1016/j.jtho.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapio S. Pathology and biology of radiation-induced cardiac disease. J. Radiat. Res. 2016;57:439–448. doi: 10.1093/jrr/rrw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darby S.C., Cutter D.J., Boerma M., Constine L.S., Fajardo L.F., Kodama K., Mabuchi K., Marks L.B., Mettler F.A., Pierce L.J., et al. Radiation-related heart disease: Current knowledge and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Bogaard V.A., Ta B.D., van der Schaaf A., Bouma A.B., Middag A.M., Bantema-Joppe E.J., van Dijk L.V., van Dijk-Peters F.B., Marteijn L.A., de Bock G.H., et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients with Breast Cancer Treated with Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J. Clin. Oncol. 2017;35:1171–1178. doi: 10.1200/JCO.2016.69.8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn E., Jiang H., Ng A., Bashir S., Ahmed S., Tsang R., Sun A., Gospodarowicz M., Hodgson D. Late Cardiac Toxicity after Mediastinal Radiation Therapy for Hodgkin Lymphoma: Contributions of Coronary Artery and Whole Heart Dose-Volume Variables to Risk Prediction. Int. J. Radiat. Oncol. Biol. Phys. 2017;98:1116–1123. doi: 10.1016/j.ijrobp.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Edge S.B., Compton C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R., Liao Z., Pan T., Milgrom S.A., Pinnix C.C., Shi A., Tang L., Yang J., Liu Y., Gomez D., et al. Cardiac atlas development and validation for automatic segmentation of cardiac substructures, Radiotherapy and oncology. J. Eur. Soc. Ther. Radiol. Oncol. 2017;122:66–71. doi: 10.1016/j.radonc.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng M., Moran J.M., Koelling T., Chughtai A., Chan J.L., Freedman L., Hayman J.A., Jagsi R., Jolly S., Larouere J., et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S., Bogart J., Hu C., Forster K., Magliocco A., et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine J.P., Gray R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 21.Atkins K.M., Chaunzwa T.L., Lamba N., Bitterman D.S., Rawal B., Bredfeldt J., Williams C.L., Kozono D.E., Baldini E.H., Nohria A., et al. Association of Left Anterior Descending Coronary Artery Radiation Dose With Major Adverse Cardiac Events and Mortality in Patients With Non-Small Cell Lung Cancer. JAMA Oncol. 2020;7:206–219. doi: 10.1001/jamaoncol.2020.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson G., Holmberg L., Garmo H., Duvernoy O., Sjogren I., Lagerqvist B., Blomqvist C. Distribution of coronary artery stenosis after radiation for breast cancer. J. Clin. Oncol. 2012;30:380–386. doi: 10.1200/JCO.2011.34.5900. [DOI] [PubMed] [Google Scholar]
- 23.Malakar A.K., Choudhury D., Halder B., Paul P., Uddin A., Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell Physiol. 2019;234:16812–16823. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso M., Pasquali E., Braga-Tanaka I., 3rd, Tanaka S., Pannicelli A., Giardullo P., Pazzaglia S., Tapio S., Atkinson M.J., Saran A. Acceleration of atherogenesis in ApoE-/- mice exposed to acute or low-dose-rate ionizing radiation. Oncotarget. 2015;6:31263–31271. doi: 10.18632/oncotarget.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoving S., Heeneman S., Gijbels M.J., Poele J.A.T., Russell N.S., Daemen M.J., Stewart F.A. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE (-/-) mice. Int. J. Radiat. Oncol. Biol. Phys. 2008;71:848–857. doi: 10.1016/j.ijrobp.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Tribble D.L., Barcellos-Hoff M.H., Chu B.M., Gong E.L. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler. Thromb. Vasc. Biol. 1999;19:1387–1392. doi: 10.1161/01.ATV.19.6.1387. [DOI] [PubMed] [Google Scholar]
- 27.Chun S.G., Hu C., Choy H., Komaki R.U., Timmerman R.D., Schild S.E., Bogart J.A., Dobelbower M.C., Bosch W., Galvin J.M., et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speirs C.K., DeWees T.A., Rehman S., Molotievschi A., Velez M.A., Mullen D., Fergus S., Trovo M., Bradley J.D., Robinson C.G. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017;12:293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzen E.L., Taylor C.W., Maraldo M., Nielsen M.H., Offersen B.V., Andersen M.R., O’Dwyer D., Larsen L., Duxbury S., Jhitta B., et al. Inter-observer variation in delineation of the heart and left anterior descending coronary artery in radiotherapy for breast cancer: A multi-centre study from Denmark and the UK, Radiotherapy and oncology. J. Eur. Soc. Ther. Radiol. Oncol. 2013;108:254–258. doi: 10.1016/j.radonc.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Tan W., Xu L., Wang X., Qiu D., Han G., Hu D. Estimation of the displacement of cardiac substructures and the motion of the coronary arteries using electrocardiographic gating. OncoTargets Ther. 2013;6:1325–1332. doi: 10.2147/OTT.S52101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (S.H.L.) upon reasonable request.