Abstract
Purpose
To compare pain scores (visual analog scale) on postoperative days 1–3 and length of stay after implementing enhanced recovery after surgery (ERAS) in elderly patients undergoing multi-segments lumbar fusion surgery.
Methods
We performed a retrospective analysis of prospectively collected data, patients older than 75 years were enrolled in the study. We selected two periods, before (Pre-ERAS, n =54 patients) and after (ERAS, n =46 patients) implementation of ERAS. Data were collected on patient demographics, operative and perioperative details, 30-day readmission. The primary outcome was the length of stay (LOS), and the secondary outcomes were postoperative mean pain scores on postoperative days (POD) 1–3 and 30-day readmission rates.
Results
A total of 100 patients (46 in ERAS and 54 in pre-ERAS) were enrolled in this study. There were no significant differences in age, sex, body mass index (BMI), smoking and comorbidities between the groups. However, there was a significant difference in pain on postoperative day (POD) 1 (5.31 ± 1.98 vs 4.37 ± 0.85, p = 0.002), while there was no difference in postoperative complications. The mean LOS was significantly shorter in the ERAS than in the pre-ERAS group, it reduced from 12.29 ± 3.93 to 9.45 ± 2.72 days (p < 0.001).
Conclusion
To our knowledge, this is the first ERAS protocol used in patients (older than 75 years) undergoing polysegmental lumbar fusion surgery. Pain scores on POD 1 and LOS were significantly lower without increased adverse events after implementation of ERAS. This finding suggests that elderly people (>75 years of age) undergoing polysegmental lumbar fusion surgery could also benefit from ERAS.
Keywords: enhanced recovery after surgery, elderly, multi-segment, lumbar fusion surgery
Introduction
High living standards and life spans are associated with the increased prevalence of degenerative lumbar diseases.1 Several patients with degenerative lumbar disorders, who have failed conservative treatment consider surgical treatment in to improve quality of life. Lumbar fusion surgery is the primary treatment approach, and polysegemental lumbar fusion surgery is often associated with intense stress responses and longer functional recovery.2 These facts prevent older adults from living independently, participating in, and contributing to society, and causing declines in quality of life and increased use of healthcare resources because of poor health.3 Additionally, since age is related to more co-morbidities and muscle weakness,4 older patients are more susceptible to slow recovery and more complications after surgery.5,6
Henrik Kehlet7 introduced enhanced recovery after surgery in 1997; since then, ERAS has been implemented extensively in surgery. ERAS consists of optimizing preoperative, intraoperative, and postoperative protocols.8 The primary purpose of ERAS is to facilitate postoperative recovery and reduce postoperative complications. Compared with traditional perioperative care, ERAS significantly reduces stress responses caused by surgery, length of hospital stay, and expenses during hospitalization without increasing postoperative complications.9,10 The satisfactory outcomes after lumbar fusion surgery were observed in many studies of ERAS programs.9,11 Given these promising results, application ERAS following polysegement fusion surgery might well yield improved outcomes.
Spine surgery is often associated with more severe postoperative pain and longer functional recovery,12 and unacceptable pain may be the primary cause for prolonged inpatient admission.13 Although many literatures have reported the advantages of implementing ERAS in patients undergoing lumbar fusion,9,12,14–18 a paucity of studies compared pain scores on postoperative days 1–3 in elderly polysegement fusion surgery after the implementation of ERAS.19,20 We hypothesized that pain scores on postoperative days 1–3 and length of stay (LOS) would be significantly lower after implementing ERAS protocol.
Materials and Methods
Study Design
This is a retrospective analysis of prospectively accumulated data from the Department of Orthopedics, Xuanwu Hospital Capital Medical University. We included medical records from patients who underwent fusion surgery. In an effort to improve our perioperative care, ERAS was considered in January 2018 and implemented in January 2019.16 To increase the reliability of the data, we selected two periods, pre-ERAS (from January to December, 2017, n = 54) and ERAS (from January to December, 2020, n = 46) and the ERAS group was treated with standard ERAS items while the pre-ERAS group was performed with no unified guidelines. We enrolled patients over 75 years of age, diagnosed with lumbar disc herniation or lumbar spinal stenosis and underwent polysegement fusion surgery, defined as more than two fusion levels. Exclusion criteria were as follows: 1) loss of follow up; 2) history of previous lumbar spine surgery; and 3) emergency operations. This study was approved by the institutional review board following the declaration of Helsinki principles in Xuanwu Hospital Capital Medical University (No. 2018008). As a retrospective study and the data analysis were performed anonymously, this study was exempt from informed consent from patients.
ERAS Intervention
Implemented in January 2019, our ERAS program covers preoperative, intraoperative and postoperative management and most of our ERAS elements encompassed ERAS society recommendations for lumbar fusion surgery.21
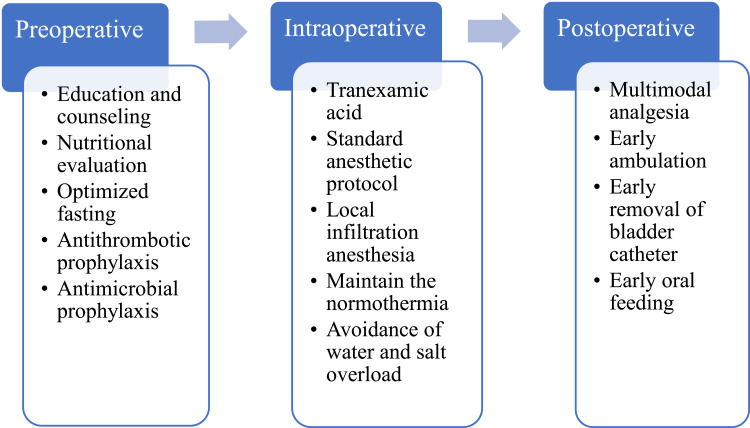
Preoperative management includes five aspects: preoperative education, nutritional counseling, optimized preoperative fasting, antithrombotic prophylaxis, and antimicrobial prophylaxis. After admission to the ward, there are trained nurse informed the patients about the multi-mode perioperative program and recommended postoperative follow-up dates. Then, Some laboratory tests were performed to determine nutritional status, and protein powder was administered if severe hypoproteinemia was present. After literature review and intense discussion, clear fluids including carbohydrate drinks were permitted up to 2 hours before surgery. Active/passive limb movements and antithrombotic stockings were used to prevent thrombosis, and routine antibiotic prophylaxis was implemented within 1 hour of the incision.
Intraoperative, tranexamic acid was used routinely to control bleeding, besides, and a combination of standard anesthetic protocol and local infiltration anesthesia was used as our anesthetic modality. In order to maintain normothermia, heated infusion fluids (37°C) is essential. Meanwhile, avoidance of water and salt overload was necessary to promote early recovery of gastrointestinal function.
Postoperative management consists of four aspects: postoperative multimodal analgesia, early ambulation, early removal of bladder catheter and early oral feeding. A multimodal analgesia pump was used routinely on postoperative days 1–2 and was usually removed on POD 3 if at an acceptable pain level. Similarly, if patients did not report nausea or vomiting, early oral feeding was encouraged in our department to promote early recovery of gastrointestinal function. In terms of early ambulation, we highly stress the importance of early mobilization, patients should be continuously encouraged to walk and avoid prolonged periods in bed and our early mobilization protocol involves sitting by the bed and walking with or without assistance.22 To prevent the chance of urinary tract infections, early removal of bladder catheter is advocated. What’s more, early removal of the bladder catheter can potentially avoid or minimize adverse events and facilitate ambulation. The simplified ERAS pathway is shown in Figure 1.
Figure 1.
The simplified ERAS pathway in our center.
Data Collection
All information relating to the patient was obtained from electronic medical records. Patient characteristics included age, gender, body mass index (BMI), smoking status, comorbidities, American Society of Anesthesiologists (ASA) classification, preoperative Oswestry Disability Index (ODI), visual analogue scale (VAS) of back, VAS of leg, 30-day readmission rates, surgical level, surgical time and estimated blood loss. Pain scores (VAS) on postoperative days (POD) 1–3 were recorded by trained nurses and pain scores were collected when pain peaked, LOS was defined as POD 1 to discharge. Complications included surgical site infection (SSI), cerebrospinal fluid leakage (CFL), urinary tract infection (UTI), deep venous thrombosis (DVT), and cerebrovascular accident (CA).
Statistical Analysis
All statistical analyses were performed using the SPSS software version 25.0 (SPSS, Inc., Armonk, NY, USA). Continuous variables were expressed as mean value ± standard deviation (mean ± SD) when the normal distribution was met, if not, median with interquartile range (IQR) was used. Continuous variables were analyzed using Student’s test. Statistical analysis for categorical variables were performed using the Chi-square test or Fisher exact test. Multivariable linear regression was performed to identify factors associated with prolonged LOS. P values < 0.05 were considered statistically significant.
Results
Baseline Characteristics
We analyzed 100 patients (46 in the ERAS group and 54 in the pre-ERAS group). The mean age was 79.06 ± 3.31 years in ERAS and 79.18 ± 2.67 years in pre-ERAS, respectively and there were no significant differences in age, smoking status and BMI between two groups. Patients in both groups underwent polysegmental fusion surgery by the same experienced team. Patients over 75 years were more vulnerable to comorbidities such as hypertension, diabetes and heart diseases. There were no significant differences in hypertension (n = 36, 78.2% in ERAS vs n = 37, 68.5% in pre-ERAS, p = 0.27), diabetes (n = 18, 39.1% in ERAS vs n = 21, 38.8% in pre-ERAS, p = 0.98) and heart diseases (n = 10, 21.7% in ERAS vs n = 12, 22.2% in pre-ERAS, p = 0.95). There were no significant differences in operative time, pre-VAS (back), pre-VAS (leg) and pre-ODI between ERAS and pre-ERAS. Although, intraoperative blood loss was lower in ERAS (581.48 ± 245.39 vs 651.08 ± 331.07mL, p=0.23), there was no statistical difference in two groups. The relevant details of baseline characteristics are shown in Table 1.
Table 1.
Patient Demographics
| Variable | ERAS | Pre-ERAS | p |
|---|---|---|---|
| Sample size | 46 | 54 | |
| Age (year) | 79.06±3.31 | 79.18±2.67 | 0.84 |
| Male/female | 16/30 | 15/39 | 0.45 |
| BMI (kg/m2) | 26.63±2.94 | 25.95±1.97 | 0.18 |
| Smoker | 4/42 | 4/50 | 0.81 |
| Comorbidities | |||
| Hypertension | 36 | 37 | 0.27 |
| Heart diseases | 10 | 12 | 0.95 |
| Diabetes | 18 | 21 | 0.98 |
| Osteoporosis | 5 | 9 | 0.41 |
| Gastrointestinal | 2 | 5 | 0.55 |
| Depression | 2 | 1 | 0.88 |
| Pre-ODI | 52.62±8.40 | 52.79±7.60 | 0.94 |
| Pre-VAS (back) | 5.26±1.18 | 5.70±1.04 | 0.14 |
| Pre-VAS (leg) | 4.83±1.55 | 5.35±1.06 | 0.14 |
| ASA | |||
| I | 2 | 3 | |
| II | 26 | 28 | |
| III | 18 | 23 | |
| No. of levels fusion | |||
| 3 | 26 | 28 | |
| 4 | 12 | 16 | |
| 5 | 8 | 10 | |
| Operative time (min) | 293.04±57.75 | 292.31±102.48 | 0.96 |
| Intraoperative blood loss (mL) | 581.48±245.39 | 651.08±331.07 | 0.23 |
Compliance with ERAS Items
Our ERAS protocol mainly consists of 14 items and the overall compliance of ERAS protocol was 90.2% (Table 2). The compliance with ERAS items were extremely high in the preoperative and intraoperative groups, however, when placing the older patients in a proactive position in the management, compliance with ERAS protocol was lower. The items with lower compliance were early ambulation (45.7%) and early removal of bladder catheter (50%), long-term chronic pain and reduced bladder function in the elderly may be the primary causes.
Table 2.
Compliance to ERAS Items in Our Center
| Variable | n (%) |
|---|---|
| Preoperative | |
| Education | 46(100%) |
| Nutritional counselling | 46(100%) |
| Optimized preoperative fasting | 42(91.3%) |
| Antithrombotic prophylaxis | 46(100%) |
| Antimicrobial prophylaxis | 46(100%) |
| Intraoperative | |
| Tranexamic acid | 46(100%) |
| Standard anesthetic protocol | 46(100%) |
| Maintenance of normothermia | 46(100%) |
| Avoidance of salt and water overload | 46(100%) |
| Local infiltration analgesia | 46(100%) |
| Postoperative | |
| Early ambulation | 21(45.7%) |
| Early removal of bladder catheter | 23(50%) |
| Early oral feeding | 35(76.1%) |
| Postoperative multimodal analgesia | 46(100%) |
| Overall compliance | 90.2% |
Outcome Assessment
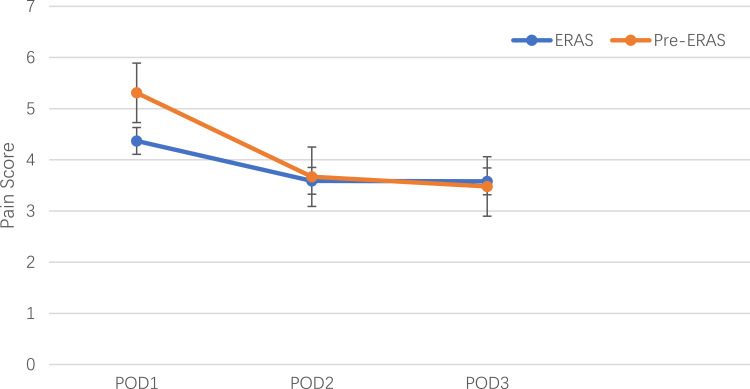
The mean pain scores on postoperative days 1–3 are shown in Figure 2. The pain scores were significantly lower in the ERAS group on POD 1 (5.31 ± 1.98 vs 4.37 ± 0.85, p = 0.002), while there were no significant differences on POD 2 (3.67 ± 0.84 vs 3.59 ± 1.05, p = 0.67) and POD 3 (3.48 ± 2.23 vs 3.58 ± 1.83, p = 0.91). The mean LOS was significantly shorter in the ERAS group than in the pre-ERAS group, and the mean LOS was declined from 12.29 ± 3.93 days to 9.45 ± 2.72 days (p < 0.001). The detailed outcomes of both groups were presented in Table 3. Complications included surgical site infection, spinal fluid leakage, urinary tract infection, deep vein thrombosis/thrombophlebitis, cerebrovascular accident and 30-day readmission, there were no significant differences between two groups. Multivariable linear regression showed that operative time (p < 0.001) and implementation of ERAS (p<0.001) were correlated with LOS, while age (p = 0.594), female sex (p = 0.298), BMI (p = 0.575), smoking (p = 0.128), comorbidities (p = 0.258) and blood loss (p = 0.098) were not related to LOS (Table 4).
Figure 2.
Pain scores on POD1–3. The pain scores were measured with VAS measuring scale. The orange and blue colors represent the Pre-ERAS and ERAS groups, respectively. Standard error was used to describe the characteristic of two groups and significance established at p < 0.05.
Abbreviation: POD, postoperative day.
Table 3.
Postoperative Outcomes Between Two Groups
| Outcome | ERAS | Pre-ERAS | p |
|---|---|---|---|
| Complications (n) | |||
| SSI | 2 | 4 | 0.52 |
| CFL | 2 | 1 | 0.47 |
| UTI | 0 | 2 | 0.15 |
| DVT | 0 | 1 | 0.35 |
| CA | 1 | 1 | 0.91 |
| LOS, mean (SD) | 9.45±2.72 | 12.29±3.93 | <0.001 |
| 30-day readmission (n) | 3 | 4 | 0.86 |
Abbreviations: SSI, surgical site infection; CFL, cerebrospinal fluid leakage; UTI, urinary tract infection; DVT, deep venous thrombosis; CA, cerebrovascular accident.
Table 4.
Multivariable Linear Regression for LOS
| Variable | Multivariable Linear Regression for LOS | p |
|---|---|---|
| Coefficient (95% CI) | ||
| Age | 0.052(−0.140–0.244) | 0.594 |
| Female | −0.701(−2.031–0.629) | 0.298 |
| BMI | 0.065(−0.165–0.295) | 0.575 |
| Smoker | 0.762(0.046–0.516) | 0.128 |
| Comorbidities | 0.906(−0.674–2.487) | 0.258 |
| Operative time | 0.018(0.010–0.026) | 0.000 |
| Blood loss | 0.002(0.000–0.004) | 0.098 |
| ERAS | −3.118(−4.263–1.973) | 0.000 |
Discussion
In recent years, the popularity of “healthy aging” has been associated with patients with lumbar disc herniation and lumbar spinal stenosis who failing conservative treatment and opting for surgery. As a result, the number of multi-segment lumbar fusion surgery is gradually increasing.20,23,24 However, multi-segment lumbar fusion is often associated with intense stress responses caused by surgery and delayed long-term functional recovery. Notably, there is an increased risk of postoperative complications.25 In previous studies, the age range of the included population was mostly from 65 to 75 years.12,26 With the improvement of living standards and the extension of life, there is an increasing proportion of the elderly population and more attention is needed to pay to patients older than 75 years. Therefore, we selected patients aged 75 years and older, despite the high risk of surgery and more comorbidities, results showing that patients older than 75 years could also benefit from ERAS protocol.
According to our study, we found a significant decrease in LOS without causing an increase in postoperative complications suggesting that ERAS protocol in the elderly undergoing multi-segment lumbar fusion surgery is feasible, which is consistent with other studies.27,28 There was a significant reduction in VAS on POD1 after implementation of ERAS protocol, however, there was an insignificant reduction on POD 2 and POD 3. Generally, multimodal analgesia pump was removed on POD 3, hence, the pain scores were somewhat higher, contrast to traditional perioperative care. Multi-segment lumbar fusion surgery is often related to prolonged periods in bed, which not only prolongs the LOS, but increases the risk of postoperative complications and muscle weakness. After the implementation of ERAS protocol, in addition to multimodal analgesia, the importance of early mobilization is strongly emphasized. There were literatures stressing the safety of early mobilization.12,29 Our early mobilization protocol involved a transition from sitting by the bed to walking with or without assistance after surgery, usually on the POD 2, if the pain was acceptable In this study, we found that early mobilization did not increase pain scores on POD 2 or POD 3. In the process of implementing ERAS program, patients should be placed the proactive position in their management although there may be a reduction in compliance to ERAS items. In our study, the items with lower compliance were early ambulation (45.7%) and early removal of bladder catheter on (50.0%), because of the reduction of bladder function and the longer functional recovery of spine surgery. However, according to postoperative complications, there were two patient developing UTI, manifesting the feasibility of early removal bladder catheter. Further study was implemented to identify the specific day to ambulate and remove bladder catheter in advanced age patients in our center.
The present study has some limitations. Firstly, this was a single-center study, with relatively small sample sizes and a lack of long-term follow-up data, both of which limit the generalizability of our findings. Second, we did not record the duration of symptoms, which might affect the results’ credibility. Finally, this was a retrospective cohort study, due to the characteristic of multi-segment spine surgery, the severe stress response and longer functional recovery, as well as the feature of the elderly population, patients with no more than 2 fusion levels were excluded in this study which may have introduced analytical bias. Therefore, a prospective study with large sample sizes and multicenter studies are needed to validate our findings.
Conclusions
In this retrospective cohort study, we found a significant reduction in pain scores on POD1 and LOS in older individuals who underwent polysegmental lumbar fusion surgery after implementing ERAS protocol. Older adults (>75 years) could also benefit from our ERAS protocol, which showing that our ERAS protocol was safe and effective. Further investigations with larger sample sizes are needed to validate our findings.
Acknowledgments
We thank the staff at the Department of Orthopedics, Xuanwu Hospital Capital Medical University, and all the patients who participated in the study.
Funding Statement
This work was supported by the Beijing Municipal Health Commission (Jing 2019–2).
Data Sharing Statement
The data used to support the findings of this study were included within the article.
Ethics Approval and Consent to Participate
The study protocol was approved by the ethics review board of Xuanwu Hospital Capital Medical University. We have obtained written informed consent from all study participants. All of the procedures were performed in accordance with the Declaration of Helsinki and relevant policies in China.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Adogwa O, Elsamadicy A, Vuong V, et al. Geriatric comanagement reduces perioperative complications and shortens duration of hospital stay after lumbar spine surgery: a prospective single-institution experience. J Neurosurg Spine. 2017;27(6):670–675. doi: 10.3171/2017.5.SPINE17199 [DOI] [PubMed] [Google Scholar]
- 2.Dietz N, Sharma M, Adams S. Enhanced Recovery After Surgery (ERAS) for Spine Surgery: a systematic review. World Neurosurg. 2019;130:415–426. doi: 10.1016/j.wneu.2019.06.181 [DOI] [PubMed] [Google Scholar]
- 3.Bailes AH, Navlani R, Koscumb S, et al. Use of healthcare resources in patients with low back pain and comorbid depression or anxiety. Spine J. 2021;21(9):1440–1449. doi: 10.1016/j.spinee.2021.03.031 [DOI] [PubMed] [Google Scholar]
- 4.Boon K, Bislenghi G, D’Hoore A, et al. Do older patients (> 80 years) also benefit from ERAS after colorectal resection? A safety and feasibility study. Aging Clin Exp Res. 2021;33(5):1345–1352. doi: 10.1007/s40520-020-01655-4 [DOI] [PubMed] [Google Scholar]
- 5.Chan DKH, Ang JJ, Tan JKH, et al. Age is an independent risk factor for increased morbidity in elective colorectal cancer surgery despite an ERAS protocol. Langenbecks Arch Surg. 2020;405(5):673–689. doi: 10.1007/s00423-020-01930-y [DOI] [PubMed] [Google Scholar]
- 6.Li G, Patil CG, Lad SP, et al. Effects of age and comorbidities on complication rates and adverse outcomes after lumbar laminectomy in elderly patients. Spine. 2007;33(11):1250–1255. doi: 10.1097/BRS.0b013e3181714a44 [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617. doi: 10.1093/bja/78.5.606 [DOI] [PubMed] [Google Scholar]
- 8.Debono B, Sabatier P, Boniface G, et al. Implementation of enhanced recovery after surgery (ERAS) protocol for anterior cervical discectomy and fusion: a propensity score-matched analysis. Eur Spine J. 2021;30(2):560–567. doi: 10.1007/s00586-020-06445-0 [DOI] [PubMed] [Google Scholar]
- 9.Debono B, Corniola MV, Pietton R, et al. Benefits of enhanced recovery after surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurg Focus. 2019;46(4):E6. doi: 10.3171/2019.1.FOCUS18669 [DOI] [PubMed] [Google Scholar]
- 10.Staartjes VE, de Wispelaere MP, Schroder ML. Improving recovery after elective degenerative spine surgery: 5-year experience with an enhanced recovery after surgery (ERAS) protocol. Neurosurg Focus. 2019;46(4):E7. doi: 10.3171/2019.1.FOCUS18646 [DOI] [PubMed] [Google Scholar]
- 11.D’Astorg H, Fiere V, Dupasquier M, et al. Enhanced recovery after surgery (ERAS) protocol reduces LOS without additional adverse events in spine surgery. Orthop Traumatol Surg Res. 2020;106(6):1167–1173. doi: 10.1016/j.otsr.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 12.Smith J, Probst S, Calandra C, et al. Enhanced recovery after surgery (ERAS) program for lumbar spine fusion. Perioper Med. 2019;8(1):4. doi: 10.1186/s13741-019-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones DB, Abu-Nuwar MRA, Ku CM, et al. Less pain and earlier discharge after implementation of a multidisciplinary enhanced recovery after surgery (ERAS) protocol for laparoscopic sleeve gastrectomy. Surg Endosc. 2020;34(12):5574–5582. doi: 10.1007/s00464-019-07358-w [DOI] [PubMed] [Google Scholar]
- 14.Dagal A, Bellabarba C, Bransford R, et al. Enhanced perioperative care for major spine surgery. Spine. 2019;44(13):959–966. doi: 10.1097/BRS.0000000000002968 [DOI] [PubMed] [Google Scholar]
- 15.Echeverria-Villalobos M, Stoicea N, Todeschini AB, et al. Enhanced Recovery After Surgery (ERAS): a perspective review of postoperative pain management under ERAS pathways and its role on opioid crisis in the United States. Clin J Pain. 2020;36(3):219–226. doi: 10.1097/AJP.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Lu S, Kong C, et al. Impact of compliance with an enhanced recovery after surgery program on the outcomes among elderly patients undergoing lumbar fusion surgery. Clin Interv Aging. 2020;15:2423–2430. doi: 10.2147/CIA.S286007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angus M, Jackson K, Smurthwaite G, et al. The implementation of enhanced recovery after surgery (ERAS) in complex spinal surgery. J Spine Surg. 2019;5(1):116–123. doi: 10.21037/jss.2019.01.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rolving N, Nielsen CV, Christensen FB, et al. Does a preoperative cognitive-behavioral intervention affect disability, pain behavior, pain, and return to work the first year after lumbar spinal fusion surgery? Spine. 2015;40(9):593–600. doi: 10.1097/BRS.0000000000000843 [DOI] [PubMed] [Google Scholar]
- 19.Qu L, Liu B, Zhang H, et al. Management of postoperative pain after elective craniotomy: a prospective randomized controlled trial of a neurosurgical Enhanced Recovery after Surgery (ERAS) program. Int J Med Sci. 2020;17(11):1541–1549. doi: 10.7150/ijms.46403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brusko GD, Kolcun JPG, Heger JA, et al. Reductions in length of stay, narcotics use, and pain following implementation of an enhanced recovery after surgery program for 1- to 3-level lumbar fusion surgery. Neurosurg Focus. 2019;46(4):E4. doi: 10.3171/2019.1.FOCUS18692 [DOI] [PubMed] [Google Scholar]
- 21.Debono B, Wainwright TW, Wang MY, et al. Consensus statement for perioperative care in lumbar spinal fusion: Enhanced Recovery After Surgery (ERAS (R)) Society recommendations. Spine J. 2021;21(5):729–752. doi: 10.1016/j.spinee.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Wang Q, Kong C, et al. Enhanced recovery after surgery (ERAS) program for elderly patients with short-level lumbar fusion. J Orthop Surg Res. 2020;15(1):299. doi: 10.1186/s13018-020-01814-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalinin A, Goloborodko V, Shepelev V, et al. Accelerated recovery program for patients with polysegmental degenerative lumbar spine disease. Sovremennye tekhnologii v meditsine. 2021;13(2):74–81. doi: 10.17691/stm2021.13.2.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang S, Zhang F, Ma J, et al. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. doi: 10.1016/j.arr.2019.100978 [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Ding W, Yang D, et al. Prevalence and risk factors of deep vein thrombosis in patients undergoing lumbar interbody fusion surgery: a single-center cross-sectional study. Medicine. 2015;94(48):e2205. doi: 10.1097/MD.0000000000002205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Lu S, Kong C, et al. Comparative short-term outcomes of enhanced recovery after surgery (ERAS) program and non-ERAS traditional care in elderly patients undergoing lumbar arthrodesis: a retrospective study. BMC Musculoskelet Disord. 2021;22(1):283. doi: 10.1186/s12891-021-04166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soffin E, Vaishnav A, Wetmore D, et al. Design and implementation of an Enhanced Recovery After Surgery (ERAS) program for minimally invasive lumbar decompression spine surgery: initial experience. Spine. 2019;44(9):E561–E570. doi: 10.1097/BRS.0000000000002905 [DOI] [PubMed] [Google Scholar]
- 28.Garg B, Mehta N, Bansal T, et al. Design and implementation of an enhanced recovery after surgery protocol in elective lumbar spine fusion by posterior approach: a retrospective, comparative study. Spine. 2021;46(12):E679–E687. doi: 10.1097/BRS.0000000000003965 [DOI] [PubMed] [Google Scholar]
- 29.Rupich K, Missimer E, O’Brien D, et al. The benefits of implementing an early mobility protocol in postoperative neurosurgical spine patients. Am J Nurs. 2018;118(6):46–53. doi: 10.1097/01.NAJ.0000534851.58255.41 [DOI] [PubMed] [Google Scholar]