Abstract
Importance:
Dermatofibrosarcoma protuberans (DFSP) has the potential for local destruction, recurrence, and also carries a low risk of metastasis. Complete surgical resection with negative margins is considered the gold standard for treatment; however, there are cases that are unresectable due to tumor extension/size, or due to risk of cosmetic and/or functional impairment. Imatinib has been used for locally advanced or metastatic DFSP.
Objective:
The objective of this systematic review was to evaluate the usefulness of imatinib for DFSP.
Evidence review:
We conducted a systematic review on PubMed and Embase databases for articles published from September 2002 through October 2017, using the key words: “dermatofibrosarcoma” or “dermatofibrosarcoma protuberans” AND “therapy” AND “imatinib”. References within retrieved articles were also reviewed to identify additional studies. Studies on adults with histologically proven DFSP treated with imatinib as monotherapy or as an adjuvant or neoadjuvant therapy to surgery were included. Extracted data was analyzed using descriptive statistics. PRISMA guidelines were followed.
Findings:
Nine studies met inclusion criteria; 152 patients were included. The calculated mean age was 49.3 years (range 20 – 73 years). Calculated mean tumor diameter was 9.9 cm (range 1.2 – 49 cm). The COL1A1-PDGFβ translocation was present in 90.9% of patients. Complete response was seen in 5.2% of patients (8/152), partial response in 55.2% (84/152), stable disease in 27.6% (42/152), and progression in 9.2% (14/152). Four patients (4/152; 2.6%) were excluded from the analysis. There were no differences in response rate using 400 mg or 800 mg per day (p=1.0). Adverse events were present in at least 73.5% of cases; severe adverse events were present in 16.6% of cases.
Conclusions and relevance:
Imatinib is a useful directed therapy in patients with DFSP that are not surgical candidates due to disease extension or significant cosmetic or functional impairment. There seems to be no difference between 400 or 800 mg daily dose.
Keywords: dermatofibrosarcoma protuberans, sarcoma, treatment, imatinib, metastasis, advanced, dermatofibroma
Introduction:
Dermatofibrosarcoma protuberans (DFSP) is an uncommon soft tissue tumor, representing approximately 0.1% of all malignancies.1 It is the most common dermal sarcoma and has an initial indolent growth pattern.2 However, DFSP has the potential for local destruction and is associated with a 5-year 25% risk of recurrence.3 It also carries a low 2 – 5% risk of metastasis,4,5 with a 10-year disease-specific survival of 99.1%.6 Occasionally, fibrosarcomatous changes are seen, which are associated with a higher rate of local recurrence, distant metastasis and worse survival.2,3,7–9 It mainly affects middle-aged adults (30 – 50 years),3,10 and is slightly more common in females (1.14 times) and blacks (almost 2 times than whites).1,6 The most frequent location is the trunk (42 – 49%), followed by the extremities (37%), and the head and neck (13 – 16%).1,8
Complete surgical resection with negative margins is considered the gold standard for treatment of DFSP, as inadequate initial resection may result in local recurrence or disease progression.3,10 Resection may be achieved by either wide local excision (2 – 3 cm margins) or Mohs micrographic surgery (MMS).11,12 Despite surgery being the cornerstone of treatment, there are cases which are deemed unresectable due to tumor extension/size, or risk of cosmetic or functional impairment, such as tumors located on the head and neck, genitalia, or hands and feet. Similarly, some patients may be inoperable due to medical comorbidities.
Genetically, DFSP is characterized by the presence of a translocation involving distinct regions of chromosome 17 and 22 in more than 90% of cases, most commonly as a supernumerary ring chromosome.13 This t(17:22) leads to a fusion in the collagen type I alpha (COL1A1) and platelet-derived growth factor subunit β (PDGFβ) genes and results in the expression of a COL1A1-PDGFβ fusion protein.13,14 PDGFβ is a potent mitogen for mesenchymal cells with autocrine and paracrine activation of the PDGF receptor (PDFGR) on tumor cells; PDGFβ activates the Ras MAPK and PI3K-AKT-mTOR pathway leading to uncontrolled cell growth.5,13,14 The identification of the aberrant activation of the PDGF pathway led to the hypothesis that inhibition of PDGFR may have clinical efficacy in the treatment of DFSP. In 2002, the first case report showing the efficacy of a PDGFR inhibitor imatinib mesylate, a small-molecule tyrosine kinase inhibitor, in a 25-year-old patient with metastasic DFSP of the lower limb was published. The patient was severely impaired but able to walk 2 weeks after initiating treatment; surgery was performed one month later.15 Based on this initial efficacy, numerous case-series subsequently evaluated the utility of imatinib, in the treatment of DFSP.15–17 Imatinib was FDA approved for DFSP in 2006 and after the phase II study published in 2010,18 the National Comprehensive Cancer Network (NCCN) incorporated imatinib into its treatment algorithm. The NCCN guidelines recommend imatinib for metastasis/recurrences when ‘disease is unresectable, or unacceptable functional or cosmetic outcomes with resection are predicted’.19 The European consensus-based interdisciplinary guidelines also recommend imatinib for inoperable primary tumors, inoperable locally recurrent disease, and metastasic DFSP.20
Despite both guidelines’ recommendation, limited and variable data exists regarding the response rate, ideal starting dose and treatment schema of imatinib for the treatment of DFSP. The objective of this systematic review is to evaluate the usefulness of imatinib for DFSP.
Methods:
The results of this systematic review were obtained according to the guidelines for reporting systematic reviews as published in the PRISMA Statement (available in www.prisma-statement.org).
Eligibility criteria:
We included all studies published to date of adults (≥18 years old) with histologically proven DFSP treated with imatinib as monotherapy or as an adjuvant or neoadjuvant therapy to surgery, irrespective of location. Articles were eligible if written in English or Spanish. Outcomes of the studies were evaluated as complete response, partial response, no response, or progression. Most studies used the Response Evaluation Criteria In Solid Tumors (RECIST) 1.0 or 1.1 to evaluate response rate. We excluded literature reviews, single case-reports, pediatric cases, conference abstracts, animal studies, and studies lacking full-text.
Information sources and search:
We searched PubMed and Embase databases for articles published from September 2002 through October 2017, using the key words: “dermatofibrosarcoma” or “dermatofibrosarcoma protuberans” AND “therapy” AND “imatinib”. References within retrieved articles were also reviewed to identify additional studies.
Study selection:
Two reviewers (C.N-D. and S.M.) independently screened all relevant titles and abstracts for eligibility. If necessary, full-text articles were screened for eligibility. Differences in judgment were resolved with a third reviewer (K.N.) until consensus was reached (Figure 1).
Figure 1:
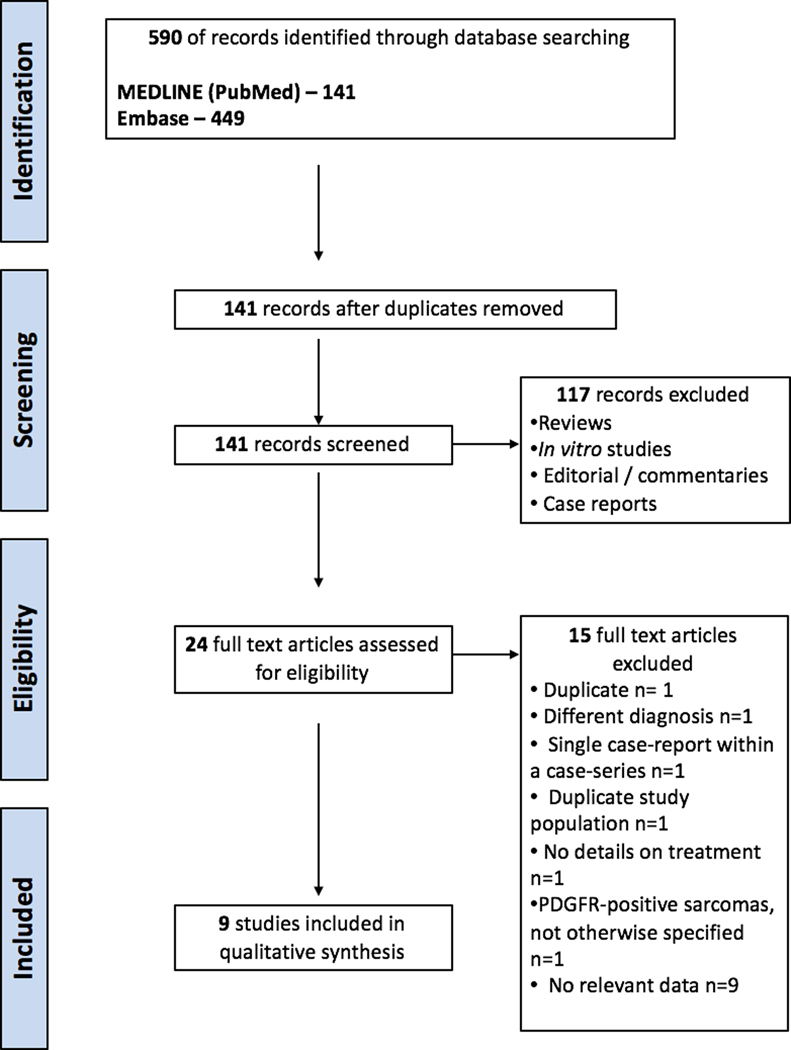
PRISMA flow diagram.
Data collection process:
Two reviewers (C.N-D. and S.M.) extracted data from the included studies independently. Disagreements were resolved by consensus; if no agreement could be reach, a third author (K.N.) was consulted.
Data extraction:
The following information was extracted from each study, when available: age, gender, location, tumor diameter, COL1A1-PDFGB translocation status, imatinib dose and schedule, treatment duration, response rate, length of followup and prognosis. Ethnicity was not reported in the studies. Most studies used the RECIST 1.0 or 1.1 to evaluate response rate. In all studies, response rate was defined by the imatinib response prior to surgery (if performed). For statistical analysis, we combined “complete or partial response rate” and “non-response or progression” as dichotomous variables in order to compare two different doses (400 mg vs 800 mg). To avoid confusion with intermediate doses, studies that used 600 mg were excluded from this subanalysis. Surgical outcome and follow-up time were extracted if available.
Adverse events reporting was less rigorous in most studies. Some used the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. For our systematic review purpose, we grouped grade 3–5 and reports stating ‘severe’ into ‘severe adverse events’ when available.
Risks of bias in individual studies:
Two reviewers (C.N-D. and S.M.) independently assessed the risk of bias with the Cochrane tool.21 Due to high-risk of publication bias, single case-reports were excluded from the analysis and only case-series were analyzed, despite also being associated to a moderate-to-high risk of bias.
Summary measures:
Data extracted was analyzed using descriptive statistics. Data is reported as medians with ranges. Response rates are reported as proportions. For summary measures and meta-analytics, reported medians with ranges were transformed to means and standard deviation with the formula published by Hozo et al.22 Data was analyzed using SPSS 23.0 (SPSS Inc., Armonk, NY, USA). For categorical variables, chi-square test was performed. A two-sided p<0.05 was considered statistically significant.
Results:
After excluding duplicates, the search yielded 141 articles that were manually reviewed, and their references were also screened. Nine studies met the inclusion criteria and were included in the analysis (Figure 1).9,18,23–29
Demographics:
Most studies specified ‘locally unresectable’ or ‘locally advanced’ or ‘metastatic’ DFSP as their inclusion criteria; however, inclusion criteria were heterogeneous and not specified in most studies. Exclusion criteria were established only in 3 studies; in 2 studies the absence of the COL1A1-PDGFβ translocation was an exclusion criterion and the absence of metastasis was indicated in the other. The total number of patients included in the systematic review was 152; age and gender information were available for 136 patients. The calculated mean age for all the studies at diagnosis was 49.3 years (range 20 – 73 years), and the male-to-female ratio was 0.91. Tumor diameter was available for 67 patients; calculated mean tumor diameter was 9.9 cm (range 1.2 – 49 cm) and 46% (60/130) were primary tumors. For individual details see Table 1.
Table 1:
Systematic review of DFSP treated with imatinib: patient demographics and tumor characteristics.
| Author | Year | N | Median age (y, range) | Gender (male / female) | Median tumor diameter (cm, range) | Location | Primary or recurrent | Histologic subtype | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|
| Ugurel et al | 2014 | 14* | 51.3 (27 – 72) | 3 / 11 | 4.3 (1.7 – 18.3) | Trunk, extremities | 11/14 primary | 2/14 FS | Locally advanced DFSP but surgically manageable |
Metastasis |
| Wang et al | 2014 | 22 | 43 (21 – 69) | 9 / 13 | N/A | Trunk, extremities | 14/22 primary | 5/22 FS | Locally unresectable (10/22) and metastatic (12/22) |
N/A |
| Rutkowski et al | 2017 | 31 | 56 (20 – 71) | 14 / 17 | N/A | Trunk, head, extremities | 7/31 primary | 16/31 FS | Locally unresectable (16/31) and metastatic (15/31) |
N/A |
| Stacchiotti et al | 2015 | 10 | 53 (49 – 73) | 7 / 3 | N/A | Systemic metastasis | 0/10 primary | 10/10 FS | Metastatic DFSP | Absence of metastasis |
| Kerob et al | 2010 | 25 | 42.4 (35.6 – 62.1) | 13 / 12 | 4.5 (2.4 – 16) | Trunk, extremities, head and neck | 20/25 primary | All classic DFSP |
Primary or recurrent DFSP ≥ 2 cm | N/A |
| Heinrich et al | 2008 | 12 | N/A | N/A | N/A | N/A | N/A | N/A | Life-threatening malignancy refractory to standard therapy |
N/A |
| McArthur et al | 2005 | 10 | 47 (23 – 68) | 5 / 5 | N/A | Trunk, extremities, head and neck, genitalia | N/A | 2/10 FS | Locally advanced (8/10) or metastasic disease (2/10) |
N/A |
| Han et al | 2009 | 4 | N/A | N/A | 7.0 (2.4 – 7.3) | Trunk, extremities, head and neck | 2/4 primary | N/A | Significant risk of functional or aesthetic compromise after surgery |
N/A |
| Rutkowski et al • EORTC • SWOG |
2010 |
16 8 |
47.4 (23.8 – 69.6) 48.6 (28.9 – 66.1) |
11 / 5 3 / 5 |
11.7 (1.2 – 49) 4.5 (1.9 – 27.9) |
Trunk, extremities, head and neck |
4/16 primary 3/8 primary |
7/16 FS 2/8 FS |
Locally advanced/ metastasic not amenable to surgery or radiotherapy with curative intent (EORTC) or R0 resection not feasible with acceptable cosmetic or functional result | Absence of COL1A1 translocation |
| Systematic review | 152 | 49.3 (20 – 73) | 65/71 | 9.9 (1.2 – 49) | 61/130 primary | 44/152 FS |
N/A data not available in the study, FS: fibrosarcomatous.
Study with 16 patients but 2 were excluded for non DFSP diagnosis.
Cytogenetics:
All included studies were designed to evaluate imatinib’s role as a neoadjuvant therapy or as a systemic treatment for locally recurrent or metastasic disease. The COL1A1-PDGFβ translocation was present in 111 out of 122 patients (90.9%) analyzed; translocation status was not reported in 2 studies (26 patients). One study evaluated the translocation with immunohistochemistry; all other studies used fluorescence in situ hybridization (FISH) or reverse transcriptase polymerase chain reaction (rtPCR), and some studies used both (Table 2).
Table 2:
DFSP treated with imatinib: Cytogenetics.
| Author | Type | COL1A1-PDGFB translocation | Evaluation | |||
|---|---|---|---|---|---|---|
| Neoadjuvant * | Adjuvant ** | Monotherapy *** | ||||
| Ugurel et al | Neoadjuvant | Yes; for 12 weeks | In 3 patients imatinib continued >6 months after surgery | If surgery not feasible | 55.6% (5/11) | RT-PCR and/or FISH |
| Wang et al | Neoadjuvant | Yes | All received imatinib for 1 year | If surgery not feasible | N/A | N/A |
| Rutkowski et al | Neoadjuvant | Yes | Imatinib continued if margins positive after surgery | If surgery not feasible | 100% (31/31) | FISH |
| Stacchiotti et al | Neoadjuvant | Yes | If surgery not feasible | 100% (10/10) | FISH | |
| Kerob et al | Neoadjuvant | Yes; for 2 months. Surgical resection 1 week after imatinib withdrawal | - | - | 84% (21/25) | FISH |
| Heinrich et al | Monotherapy | - | - | If surgery not feasible | 100% (12/12) | Immunohistochemistry |
| McArthur et al | Neoadjuvant | Yes | - | If surgery not feasible; discontinued if disease progression | 90% (9/10) | FISH |
| Han et al | Neoadjuvant | Yes; until 4 weeks before surgery | - | - | N/A | N/A |
| Rutkowski et al • EORTC • SWOG |
Neoadjuvant |
Yes; for 14–16 weeks | Continue indefinitely after surgery at physician discretion | If surgery not feasible |
• 100% • 100% |
• RT-PCR and/or FISH • RT-PCR and/or FISH |
| Systematic review | Mainly Neoadjuvant | 90.9% (111/122) | ||||
N/A data not available in the study.
Neoadjuvant: All studies (except Heinrich et al.) were designed as neoadjuvant trials with planned surgical resection.
Adjuvant: In most studies imatinib was also maintained after surgery for an adjuvant role.
Monotherapy: In those patient in which surgery was not feasible, imatinib was maintained as monotherapy.
No trial was designed as adjuvant in high-risk patients or in patients with close or positive margins.
Treatment dose, response rate, and duration:
All studies but one (Heinrich et al.26 study) were designed as neoadjuvant trials and imatinib was started with the intention to make tumor amenable for definitive (R0) excision. Some trials allowed for adjuvant therapy after resection and others maintained imatinib as monotherapy (Table 2). Imatinib response rates were defined pre-surgery in all studies.
The dose used in these studies ranged from 400 mg per day (2 studies) to 800 mg per day (4 studies); 2 studies used 600 mg per day. One study18 had 2 arms at different study locations, one with 400 mg and the other with 800 mg. No differences were found between these doses in that study (although no statistical tests were performed). Overall, complete response was seen in 5.2% of patients (8/152), partial response in 55.2% (84/152), stable disease in 27.6% (42/152), and progression in 9.2% (11/152) (Table 3). A complete or partial response was observed in 92/152 (60.5%) of patients and any clinical benefit in 134/152 (88.1%) of patients. We excluded 4 patients (2.6%) from the analysis due to unknown response/not evaluable. We compared studies using 400 mg and those using 800 mg and found a similar complete or partial response rate when compared to non-response or progression as dichotomous variables (67.5% [27/40 patients] for 400 mg vs 67.1% [49/73 patients] for 800 mg complete or partial response; p=1.0). Treatment duration was not clearly established; some studies used the drug for a variable period of time after surgery while others did not withdraw the drug until the time of study publication. Calculated mean treatment duration was 12 months (0.1 – 43 months).
Table 3:
DFSP treated with imatinib: response rate, adverse events and follow-up.
| Author | Dose | CR | PR | SD¶ | Progression¶ | Duration | Median treatment duration (m, range) | Median follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| Ugurel et al | 600 mg/d | 0 | 50% (7/14) | 35.7% (5/14) | 14.2% (2/14) | ≥ 6 weeks | 3.1 months (1.5 – 16.7) | 76.7 months (46.7 – 97.3+) |
| Wang et al | 400 mg/d, increased to 800 mg/d if progression | 0 | 68.1% (15/22) | 27.2% (6/22) | 4% (1/22) | N/A | 15 months (1 – 43) | 36 months (6 – 81) |
| Rutkowski et al | 800 mg/d | 0 | 68% (21/31) | 19% (6/31) | 13% (4/31) | N/A | N/A | 63.5 months |
| Stacchiotti et al | 400 mg/d, increased to 800 mg/d if progression | 0 | 80% (8/10) | 10% (1/10) | 10% (1/10) | N/A | N/A | N/A |
| Kerob et al | 600 mg/d | 0 | 36% (9/25) | 64% (16/25)†† | 0 | Up to 8 weeks | N/A | N/A |
| Heinrich et al* | 800 mg/d | 33% (4/12) | 50% (6/12) | 0 | 8.3% (1/12) | N/A | N/A | N/A |
| McArthur et al | 800 mg/d | 40% (4/10) | 50% (5/10) | 0 | 10% (1/10) | 4.6 months (0.6 – 22.9) | 4.7 months (0.69 – 22.9) | 10.6 months (1 – 27.7) |
| Han et al | 800 mg/d | 0 | 100% (4/4) | 0 | 0 | Until tumor size stabilized and continued 4 weeks after surgery | 3 months (3 – 7) | 18 – 48 months |
| Rutkowski et al - EORTC** - SWOG** |
• 800 mg/d • 400 mg/d, (increased to 800 mg if no response) |
• 0 • 0 |
• 31.3% (5/16) • 50% (4/8) |
• 37.5% (6/16) • 25% (2/8) |
• 18.7% (3/16) • 12.5% (1/8) |
• 14 weeks • 48 weeks |
• 9.2 months (0.1 – 40) • 10 months (3.6 – 11.3) |
• 31.2 months (26.4 – 35.7) • 31.2 months (26.4 – 35.7) |
| Systematic review † | 5.2% (8/152) | 55.2% (84/152) | 27.6% (42/152) | 9.2% (14/152) | Calculated mean: 12 months (0.1–43) | 39.8 months (1 – 97.3+)*** |
Some articles stated “no response” instead of “stable disease” or “progression”; in those cases we further included them on stable disease or progression group accordingly.
One patient had an unknown response
Two patients were not evaluable in the EORTC group and 1 in the SWOG group.
The different response rates sum does not add to 100% due to exclusion of 4 patients in different studies (see above).
The authors referred as “Non-respondents” (<30% decrease in size), thus we interpreted as stable disease.
Average mean was calculated from medians.
N/A data not available in the study, CR: complete response, PR: partial response, SD: stable disease.
Surgery after imatinib:
For those with available data, 70 patients out of 116 patients (60.3%) underwent surgery after imatinib. In the most recent study with the largest follow-up time (63.5 months), 13 patients (47%) underwent surgery with >1 cm wide margins (after a median time of 4 months of neoadjuvant imatinib) and 9/13 (69%) patients were disease-free on follow-up after discontinuation of imatinib.9 In a previous study, there was only 1 relapse when surgery was performed (in a total of 13 patients; mostly with 0.5 – 2 cm margins) after a median treatment duration of 3.1 months on imatinib, with a mean follow-up of >76.7 months. In the same study, one patient was on 16.7 months of imatinib with complete response; no definitive surgery was done due to patient refusal.25 In another study, 4/10 unresectable patients were able to receive complete surgical resection after imatinib; the drug was maintained for 1 year after surgery, with no signs of recurrence after imatinib withdrawal.27 On the other hand, another study showed that all DFSP patients relapsed after surgery and imatinib withdrawal; the study included only metastatic cases, however.28 Although it was not assessed in all studies, DFSP with fibrosarcomatous transformation had a similar initial response to imatinib but may have a shorter sustained response (Table 4).9
Table 4:
DFSP treated with imatinib: treatment outcomes.
| Author | Surgery after imatinib | Disease free | Surgical margins | Any adverse event | Severe adverse events | Death by DFSP | Type of study |
|---|---|---|---|---|---|---|---|
| Ugurel et al | 13/14¶ | 2 deaths, one because of distant metastasis and one by other reason. | 0.5 - >2.0 cm | N/A | 25% (4/16); 2 were withdrawn because of AE | 1/14 patients | Prospective, single arm |
| Wang et al | 4/22, all received imatinib for 1 year after surgery, currently free of recurrence | Median PFS 19 months (7 – 51). 3-year OS 17/22 (77.3%) |
N/A | 54.5% (12/22) | 13.6% (3/22)** | 5/22 patients | Retrospective, single arm |
| Rutkowski et al | 13/31 (after median 4 months neoadjuvant), negative margins in 8/13 | 5-year PFS 58%† 5-year OS 64% 9/13 disease free after surgery and imatinib discontinuation |
>1 cm | >77% (24/31) | 19% (6/31); no drug withdrawals reported | 9/31 patients | Retrospective, single arm |
| Stacchiotti et al | 5/10, imatinib withdrawn, all relapsed | PFS 11 months (range 2 – 25 m) | N/A | N/A | 30% (3/10), 1 was withdrawn because of AE | N/A | Retrospective, single arm |
| Kerob et al | 25/25, 1 week after imatinib withdrawal | N/A | N/A | 88% (22/25) | 12% (3/25)** | N/A | Prospective, single arm |
| Heinrich et al | N/A | TTP 23.9 months (7.7 - present) | N/A | N/A | N/A | N/A | Prospective, single arm |
| McArthur et al | 6/10, all disease free after 1 year | N/A | N/A | N/A | N/A | 1/10 patients | Prospective, single arm |
| Han et al | 4/4, all disease free | N/A | Mohs | 100% (4/4) | 0/4 | 0/4 | Retrospective, single arm |
| Rutkowski et al • EORTC • SWOG |
• N/A* • N/A* |
• TTP 20.4 months (7.7 to present) • TTP 20.4 months (7.7 to present) |
N/A N/A |
• >68.8% (11/16) • >62% (5/8) |
• 6.25%** (1/16) • 0/8 |
• 5/16 patients • 0/8 |
Prospective, single arm |
| Systematic review | 60% (70/116) | N/A | 0.5 – >2.0 cm | >73.5% (78/106) | 16.6% (20/120) | 21/105 |
5-year PFS rate was 93% for classic DFSP and 33% for fibrosarcomatous DFSP.
One patient refuse to undergo surgery and was treated for 16.7 months until complete response
Data on surgery is not available but it was allowed after 14–16 weeks of treatment if feasible (see table 2 for details)
Definitive drug-withdrawal.
N/A data not available in the study. PFS: progression free survival, OS: overall survival, TTP: time to progression, AE: adverse effects.
Adverse events:
Occurrence of any adverse event was present in at least 73.5% of cases (78/106). Severe adverse events (grade 3 to 4, defined as those that may potentially cause severe damage or death and/or lead to the suspension of the drug) were present in 16.6% of cases (20/120). Of these 20 severe adverse events, 10 out of 20 (50%) led to the discontinuation of the drug. The others were manageable either with dose reduction or other specific measures (Table 4). In one study, 35% of patients initially treated with 800 mg/d were reduced to 400 – 600 mg/d due to adverse events.9
Prognosis:
Regarding mortality, in this selected group of patients with locally advanced or metastatic DFSP treated with imatinib, 20% died of the disease (21/105) (Table 4).
Risk of bias of individual studies:
Due to the methodology of the studies included (case-series), there is a moderate-to-high risk of bias. No quantitative measurements are possible.
Discussion:
In this systematic review of DFSP treated with imatinib, we found complete/partial response rates of 60.5%, a stable disease rate of 28.2%, and a progression rate of only 7.9%. In other words, 85 – 90% of patients with advanced DFSP demonstrate some degree of clinical benefit with imatinib; in addition, few patients show progression of disease on imatinib (9.2%), at least in the follow-up period reported in most studies. Herein we corroborated what was hypothesized by Rutkowski et al. in their preliminary series, that there does not seem to be a difference between starting treatment with 400 mg/d or 800 mg/d in terms of efficacy.18 However, 400 mg/d may be favored as a starting dose due to its lower incidence of adverse effects. The European consensus-based interdisciplinary guideline on DFSP also recommends starting with lower doses (400 – 600 mg/d) as they are better tolerated.20 However, if no response occurs at 400 mg/d, increasing the dose to 600 – 800 mg/d may be considered on a case-by-case discussion. Despite these promising findings, we highlight the low rate of complete responses (5.9%) found in this group of locally advanced or metastasic DFSP patients; this is relevant given the fact that this neoplasm affects mainly young adults with a long life expectancy.3 Risk of progression has been reported to be as high as 45.8%, even after an initial favorable response.18 We believe that imatinib can achieve its role with curative intent only when given as a neoadjuvant therapy and subsequent complete removal with negative margins is accomplished.
Histopathology response to imatinib-treated DFSP samples most frequently shows cellular depleted areas replaced by hyalinized fibrotic stroma; early signs of response include variable inflammatory component with scattered viable tumoral cells. Tumor density is decreased, and no necrosis is evident after treatment.23,25,28,29 Pre-imatinib tumor cellular and nuclear pleomorphism, Ki67 positivity, and mitotic rate are not associated with clinical response.25 Recently, Tazzari et al. demonstrated that imatinib-treated samples of fibrosarcomatous transformed DFSP up-regulated pro-inflammatory genes, which in turn increased cytokines, IFN-γ and HLA antigens, together with dense CD4+ and CD8+ T-cell infiltrates (all negative before treatment), suggesting a dual role of imatinib inhibiting molecular pathways (PDGFR) and modulating immune response.30 PD-L1 expression was evident after imatinib treatment, opening a potential window of opportunity for PD-1/PD-1L blocking agents.30,31
As reported in most of the studies reviewed herein and as stated in the NCCN guidelines,19 we recommend cytogenetics evaluation of t(17:22) by either FISH or rt-PCR as part of the DFSP diagnosis workup. However, imatinib may still be considered in cases without the specific translocation as there have been reports of response to imatinib.25,29 More studies are needed to evaluate the utility of testing for the COL1A1-PDGFB translocation prior to imatinib initiation and its role in DFSP response to imatinib. In one study including 25 patients, 2 patients without the translocation were non-responders, however, this difference was not statistically significant (p=0.53).29 Another study including 10 patients showed no response in 1 patient with metastatic DFSP lacking the t(17:22) translocation; however, follow-up was short (32 days) due to the patient’s early death.23 Since data is limited, we do not know how patients will respond in the absence of the mutation. We previously published a case that showed good response to 400 mg/d imatinib despite yielding a completely different mutation.32
Imatinib adverse events in this systematic review were common but usually mild and tolerable. The overall adverse event rate of imatinib for DFSP treatment was more than 73.5%. Severe adverse events were present in 16.6% in this systematic review with 50% of these leading to drug withdrawal. However, reporting of adverse events was not homogeneous and few studies used standard systems such as the CTCAE v4.0.
For a more comprehensive understanding of imatinib adverse events, we can extrapolate from imatinib used in thousands of patients with other tumors such as gastrointestinal stromal tumors (GIST) and Chronic myeloid leukemia.33,34 Most adverse events occur in the first 8 weeks of treatment.34 The most common adverse events are mucocutaneous (7 – 88.9%) and include edema (11 – 86%), maculo-papular rash (40%), pruritus (7 – 26%), alopecia (7 – 15%), xeroderma (<7%), lichenoid reactions, pigmentary disorders of skin (hypopigmentation most commonly), nails (hyperpigmentation) and mucosa (hyperpigmentation, 4%), psoriasiform reactions, pityriasis rosea-like rash, acute generalized exanthematous pustulosis, Stevens-Johnson syndrome/Toxic epidermal necrolysis, neutrophilic dermatosis, and photosensitivity, among others.35,36 Other relevant systemic adverse events include fever (6 – 41%), muscle cramps (49%), abdominal pain (37%) and diarrhea (45%), nausea and vomiting (50%), fatigue (39%), headache (37%), musculoskeletal pain, anemia (4%), neutropenia (17%), hyperglycemia (10%), electrolyte disturbance (1 – 10%), thrombocytopenia (9%), and liver enzyme elevation (5%).37,38
One of the most relevant predictors of severe adverse events and severity is the imatinib dose;33,34 in a randomized trial using 400 mg/day or 400 mg twice daily in GIST patients, 7% of treatment interruptions were due to toxic effects, similar to our results (5.4%). Despite 41% having at least one grade 3–4 event, about two-thirds did not need a dose reduction.34 In summary, compared to other systemic anti-cancer therapies such as chemotherapy, imatinib is remarkably well tolerated and most side effects are manageable with supportive care and dose adjustment, enabling patients with imatinib-sensitive diseases (GIST, DFSP, chronic myeloid leukemia) to be treated for years with good outcomes.
Surgery remains the main curative treatment for DFSP. In locally advanced or metastatic cases that were initially not amenable to surgery, 60% were able to undergo surgery after imatinib treatment; of these patients, only 5 studies (32/70 [45.7%] patients who underwent surgery) reported follow-up. In 2 studies, patients who had surgery (6/10 and 4/4 patients had surgery) are all disease-free after the procedure.23,24 In the most recent study, 9 out of 13 patients who underwent surgery with >1 cm margins were disease-free at the time of resection.9 In another study, there was a single relapse (n=13) after discontinuation of imatinib (neoadjuvant, surgery, adjuvant) with a mean follow-up of >76.7 months.25
Finally, in a study of 10 unresectable locally advanced tumors (>10 cm and adjacent to vital organs or surgery causing unacceptable aesthetic results), after neoadjuvant imatinib, 4 patients were amenable to surgical resection and imatinib was maintained for 1 year after surgery with no relapses after discontinuation (median follow-up 36 months; range 6 – 81 months).27 In a case series including 22 patients, 4 previously unresectable patients underwent surgery with “complete resection” and received imatinib for a year after with no signs of recurrence or metastasis. However, follow-up is still short (median 36 months) so definitive treatment response duration is not currently evaluable.27 Future studies should assess the duration of DFSP response to imatinib and timing of discontinuation.
Limitations:
Most studies had less than 30 patients and a wide heterogeneity in study design. There were different treatment doses and schemas (even within the same study). Treatment intent was not clear in most studies (i.e. neoadjuvant, adjuvant, monotherapy). Some studies used the drug 12 weeks before surgery and completely suspended the drug after the procedure, while others used it as a neoadjuvant therapy and then continued its use as adjuvant for up to 12 months.17,27
The use of neoadjuvant drugs in solid tumors may theoretically create ‘pockets’ of non-contiguous tumor that may be falsely read as negative margins in surgical excisions. The only way of proving this principle and clinical relevance of this potential risk is with long-term follow-up. Limited follow-up time for most of the studies included (mean follow-up in this systematic review was 39.8 months [3.3 years]) precludes definitive conclusions since DFSP is a slow-growing neoplasm that may recur years later. The optimal duration of therapy remains uncertain. Clinicians and patients should consider the cost of the drug that may need to be continued for a long period of time. Also, the role of the COL1A1-PDGFB translocation and its determination by cytogenetics or FISH to select imatinib candidates and predict the clinical response of DFSP is lacking.
Finally, there was considerable risk of bias according to GRADE guidelines, as case-series have the possibility of publication bias. The current information is insufficient to perform an evidence-based recommendation on treatment duration; however, in the authors’ experience, we use the drug as monotherapy only for unresectable disease (for an undefined duration, until maximum response) or as neoadjuvant therapy until the tumor is surgically resectable, and then at least 3 months post-operatively. Role of imatinib in the setting of narrow or positive margins has not been studied.
Conclusions and future directions:
Imatinib is a useful neoadjuvant directed therapy in select patients with DFSP that are not direct candidates for surgery due to its local extension or due to possible secondary cosmetic or functional impairment. Imatinib may improve quality of life and functionality in patients with advanced disease.9 However, as the criteria for an ‘unresectable DFSP’ can vary considerably, use of imatinib therapy should be carefully guided through a multidisciplinary approach. Review of available studies suggest that starting doses of 400 or 800 mg per day are equally effective for the treatment of locally advanced or metastatic DFSP, but with fewer adverse effects at the lower dose. Prospective randomized trials are needed to define the most appropriate treatment schema for these patients.
Key points:
Question: What is the efficacy of imatinib in the treatment of metastatic or locally advanced dermatofibrosarcoma protuberans (DFSP)?
Findings: In this systematic review we found that imatinib is associated with a complete or partial response of 60.5% in advanced cases, irrespective of 400 mg or 800 mg daily dose.
Meaning: Imatinib is a safe and effective therapy for advanced DFSP with 400 mg/day starting dose.
Acknowledgments
Dr(s) Navarrete-Dechent and Nehal had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Navarrete-Dechent, and Nehal.
Acquisition, analysis, and interpretation of data: Navarrete-Dechent, Mori, Barker, Dickson, and Nehal.
Drafting of the manuscript: Navarrete-Dechent, Mori, Barker, Dickson, and Nehal.
Critical revision of the manuscript for important intellectual content: Navarrete-Dechent, Mori, Barker, Dickson, and Nehal.
Statistical analysis: Navarrete-Dechent.
Obtained funding: N/A.
Administrative, technical, or material support: Navarrete-Dechent and Nehal.
Study supervision: Nehal.
Founding source: This research is funded in part by a grant from the National Cancer Institute / National Institutes of Health (P30-CA008748) made to the Memorial Sloan Kettering Cancer Center.
Abbreviations:
- DFSP
dermatofibrosarcoma protuberans
- MMS
Mohs micrographic surgery
- FS
fibrosarcomatous variant DFSP
- COL1A1
collagen type I alpha
- PDGFβ
platelet-derived growth factor subunit β
- NCCN
National Comprehensive Cancer Network
- FISH
fluorescence in situ hybridization
- rtPCR
reverse transcriptase polymerase chain reaction
Footnotes
Consent for publication: The authors consent the publication of this submission (manuscript and figures).
Prior presentation: none.
IRB status: N/A.
Conflict of interest: The authors have not conflict of interest to declare.
References:
- 1.Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol. 2007;56(6):968–973. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101(11):2503–2508. [DOI] [PubMed] [Google Scholar]
- 3.Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88(12):2711–2720. [PubMed] [Google Scholar]
- 4.Acosta AE, Velez CS. Dermatofibrosarcoma Protuberans. Curr Treat Option On. 2017;18(9). [DOI] [PubMed] [Google Scholar]
- 5.Thway K, Noujaim J, Jones RL, Fisher C. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol. 2016;25:64–71. [DOI] [PubMed] [Google Scholar]
- 6.Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol Surg. 2016;42 Suppl 1:S24–31. [DOI] [PubMed] [Google Scholar]
- 7.Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous (“high-grade”) dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22(5):576–587. [DOI] [PubMed] [Google Scholar]
- 8.Criscito MC, Martires KJ, Stein JA. Prognostic Factors, Treatment, and Survival in Dermatofibrosarcoma Protuberans. JAMA Dermatol. 2016;152(12):1365–1371. [DOI] [PubMed] [Google Scholar]
- 9.Rutkowski P, Klimczak A, Lugowska I, et al. Long-term results of treatment of advanced dermatofibrosarcoma protuberans (DFSP) with imatinib mesylate - The impact of fibrosarcomatous transformation. Eur J Surg Oncol. 2017;43(6):1134–1141. [DOI] [PubMed] [Google Scholar]
- 10.Fields RC, Hameed M, Qin LX, et al. Dermatofibrosarcoma protuberans (DFSP): predictors of recurrence and the use of systemic therapy. Ann Surg Oncol. 2011;18(2):328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutkowski P, Debiec-Rychter M. Current treatment options for dermatofibrosarcoma protuberans. Expert Rev Anticancer Ther. 2015;15(8):901–909. [DOI] [PubMed] [Google Scholar]
- 12.Lowe GC, Onajin O, Baum CL, et al. A Comparison of Mohs Micrographic Surgery and Wide Local Excision for Treatment of Dermatofibrosarcoma Protuberans With Long-Term Follow-up: The Mayo Clinic Experience. Dermatol Surg. 2017;43(1):98–106. [DOI] [PubMed] [Google Scholar]
- 13.Sirvent N, Maire G, Pedeutour F. Genetics of dermatofibrosarcoma protuberans family of tumors: from ring chromosomes to tyrosine kinase inhibitor treatment. Genes Chromosomes Cancer. 2003;37(1):1–19. [DOI] [PubMed] [Google Scholar]
- 14.Simon MP, Pedeutour F, Sirvent N, et al. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet. 1997;15(1):95–98. [DOI] [PubMed] [Google Scholar]
- 15.Rubin BP, Schuetze SM, Eary JF, et al. Molecular targeting of platelet-derived growth factor B by imatinib mesylate in a patient with metastatic dermatofibrosarcoma protuberans. J Clin Oncol. 2002;20(17):3586–3591. [DOI] [PubMed] [Google Scholar]
- 16.Bashir S, Tariq M, Aslam HM, et al. Orbital dermatofibrosarcoma protuberans with intracranial extension preceded by recurrent leiomyoma of the orbit: a case report. J Med Case Rep. 2015;9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontecilla NM, Kittler NW, Geskin L, et al. Recurrent dermatofibrosarcoma protuberans treated with neoadjuvant imatinib mesylate followed by Mohs micrographic surgery. JAAD Case Rep. 2017;3(6):467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rutkowski P, Van Glabbeke M, Rankin CJ, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol. 2010;28(10):1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Dermatofibrosarcoma protuberans (Version 1.2018). 2017; https://www.nccn.org/professionals/physician_gls/pdf/dfsp.pdf. Accessed October 27, 2017, 2017. [DOI] [PubMed] [Google Scholar]
- 20.Saiag P, Grob JJ, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51(17):2604–2608. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen L, Paludan-Muller AS, Laursen DR, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. 2016;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23(4):866–873. [DOI] [PubMed] [Google Scholar]
- 24.Han A, Chen EH, Niedt G, Sherman W, Ratner D. Neoadjuvant imatinib therapy for dermatofibrosarcoma protuberans. Arch Dermatol. 2009;145(7):792–796. [DOI] [PubMed] [Google Scholar]
- 25.Ugurel S, Mentzel T, Utikal J, et al. Neoadjuvant imatinib in advanced primary or locally recurrent dermatofibrosarcoma protuberans: a multicenter phase II DeCOG trial with long-term follow-up. Clin Cancer Res. 2014;20(2):499–510. [DOI] [PubMed] [Google Scholar]
- 26.Heinrich MC, Joensuu H, Demetri GD, et al. Phase II, open-label study evaluating the activity of imatinib in treating life-threatening malignancies known to be associated with imatinib-sensitive tyrosine kinases. Clin Cancer Res. 2008;14(9):2717–2725. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Luo Z, Chen J, et al. Target therapy of unresectable or metastatic dermatofibrosarcoma protuberans with imatinib mesylate: an analysis on 22 Chinese patients. Medicine (Baltimore). 2015;94(17):e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stacchiotti S, Pantaleo MA, Negri T, et al. Efficacy and Biological Activity of Imatinib in Metastatic Dermatofibrosarcoma Protuberans (DFSP). Clin Cancer Res. 2016;22(4):837–846. [DOI] [PubMed] [Google Scholar]
- 29.Kerob D, Porcher R, Verola O, et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin Cancer Res. 2010;16(12):3288–3295. [DOI] [PubMed] [Google Scholar]
- 30.Tazzari M, Indio V, Vergani B, et al. Adaptive Immunity in Fibrosarcomatous Dermatofibrosarcoma Protuberans and Response to Imatinib Treatment. J Invest Dermatol. 2017;137(2):484–493. [DOI] [PubMed] [Google Scholar]
- 31.Ugurel S, Becker JC. Imatinib in Dermatofibrosarcoma: Targeted Therapy or Immunotherapy? J Invest Dermatol. 2017;137(2):277–279. [DOI] [PubMed] [Google Scholar]
- 32.Saab J, Rosenthal IM, Wang L, et al. Dermatofibrosarcoma Protuberans-Like Tumor With COL1A1 Copy Number Gain in the Absence of t(17;22). Am J Dermatopathol. 2017;39(4):304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358(9291):1421–1423. [DOI] [PubMed] [Google Scholar]
- 34.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–1134. [DOI] [PubMed] [Google Scholar]
- 35.Pretel-Irazabal M, Tuneu-Valls A, Ormaechea-Perez N. Adverse skin effects of imatinib, a tyrosine kinase inhibitor. Actas Dermosifiliogr. 2014;105(7):655–662. [DOI] [PubMed] [Google Scholar]
- 36.Ransohoff JD, Kwong BY. Cutaneous Adverse Events of Targeted Therapies for Hematolymphoid Malignancies. Clin Lymphoma Myeloma Leuk. 2017. [DOI] [PubMed] [Google Scholar]
- 37.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–1061. [DOI] [PubMed] [Google Scholar]
- 38.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]


