Abstract
DNA methyltransferase 3b (Dnmt3b) has been suggested to play a role in the host immune response during bacterial infection. Neutrophils and other myeloid cells are crucial for lung defense against Pseudomonas (P.) aeruginosa infection. This study aimed to investigate the role of Dnmt3b in neutrophils and myeloid cells during acute pneumonia caused by P. aeruginosa. Neutrophil-specific (Dnmt3bfl/flMrp8Cre) or myeloid cell-specific (Dnmt3bfl/flLysMCre) Dnmt3b-deficient mice and littermate control mice were infected with P. aeruginosa PAK via the airways. Bacteria burdens, neutrophil recruitment, and activation (CD11b expression, myeloperoxidase, and elastase levels), interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF) were measured in bronchoalveolar lavage fluid (BALF) at 6 and 24 h after infection. Our data showed that the bacterial loads and neutrophil recruitment and activation did not differ in BALF obtained from neutrophil-specific Dnmt3b-deficient and control mice, whilst BALF IL-6 and TNF levels were lower in the former group at 24 but not at 6 h after infection. None of the host response parameters measured differed between myeloid cell-specific Dnmt3b-deficient and control mice. In conclusion, dnmt3b deficiency in neutrophils or myeloid cells does not affect acute immune responses in the airways during Pseudomonas pneumonia.
Keywords: Dnmt3b, neutrophils, P. aeruginosa, pneumonia
1. Introduction
Accumulating evidence indicates that DNA methylation is involved in the regulation of host defense in bacterial infection [1]. Neonatal and adult patients with bacterial sepsis, as compared with control subjects, showed altered DNA methylation levels in blood leukocytes in genomic regions involved in immune responses [2,3]. Furthermore, the sepsis-associated aberrant DNA methylome is frequently related to dysregulated inflammatory responses and organ dysfunction [2,4].
DNA methylation is regulated by DNA methyltransferases (Dnmt’s), of which Dnmt3a and Dnmt3b mediate de novo methylation. Dnmt3b plays a major role in establishing DNA methylation patterns and, as a consequence, in gene expression [1]. Mutations in the human DNMT3B gene cause the immunodeficiency-centromeric instability-facial anomalies (ICF) syndrome [5], characterized by altered epigenetic modifications and expression of genes involved in the development and immune function [6]. In experimental sepsis in mice, inhibition of DNA methylation by 5-aza-2′-deoxycytidine, which targets Dnmt3a and Dnmt3b [7], improved survival by impeding NF-κB pathway activation [2]. Additional investigations have pointed to the role of Dnmt3b in the host response during infection. Patients with ICF syndrome normally present recurrent [8], and oftentimes severe, infections [9]. Exome sequencing showed an association of variants of DNMT3B with community-acquired P. aeruginosa infection in children [10]. We previously reported on the role of Dnmt3b in respiratory epithelial cells in Pseudomonas pneumonia, showing that targeted deletion of Dnmt3b in the bronchial epithelium-enhanced host antibacterial defense in the airways by promoting CXCL1 production and subsequent recruitment of neutrophils [11]. The role of Dntm3b partially depended on the presence of flagellin [11], an important virulence factor and Toll-like receptor 5 ligand expressed by Pseudomonas [11,12]. Epigenetic regulation of macrophage polarization by Dnmt3b has also been documented [13]. Together, these data led us to hypothesize that Dnmt3b in myeloid cells is involved in the host immune response during Pseudomonas pneumonia. To test this hypothesis, we generated neutrophil-specific or myeloid cell-specific Dnmt3b-deficient mice and infected these and littermate control mice with viable P. aeruginosa via the airways.
2. Materials and Methods
2.1. Animals
Homozygous Dnmt3bfl/fl mice [14] were crossed with Mrp8Cre mice (021614; Jackson Laboratory) [15] or LysMCre mice [16] (Jackson Laboratory, Bar Harbor, ME) to generate neutrophil cell-specific Dnmt3b-deficient (Dnmt3bfl/flMrp8Cre) mice or myeloid cell-specific Dnmt3b-deficient (Dnmt3bfl/fl LysMCre) mice, respectively. Their corresponding Dnmt3bfl/fl Cre-negative littermates (Dnmt3bfl/fl mice) were used as controls in all experiments. All genetically modified mice were backcrossed at least eight times to a C57Bl/6 background, and age and sex matched when used in experiments. Mice were used at 8–12 weeks of age. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Amsterdam.
2.2. Quantitative Reverse Transcription PCR (qRT-PCR)
Alveolar macrophages, peritoneal macrophages, and bone marrow-derived macrophages from Dnmt3bfl/fl LysMCre and Dnmt3bfl/fl were prepared as previously described [17]. Neutrophils were purified from the bone marrow of Dnmt3bfl/fl Mrp8Cre and Dnmt3bfl/fl mice using Anti-Ly-6G MicroBead Kit (Miltenyi Biotec; Bergisch Gladbach, Germany) according to manufacturer’s instructions. RNA was isolated from these cells, and the expression of Dnmt3a and Dnmt3b was measured by qRT-PCR as described before [17]. The gene-specific primers used were, 5′-CTGAGTTTGACGCTTGGCGG-3′ and 5′-AGTCCACCTCCTCACAGGGT-3′ for Dnmt3a; 5′-GTGTGGGGAAAGATCAAGGG-3′ and 5′-AACTTGCCATCACCAAACCA-3′ for Dnmt3b; 5′-AGTCAAGGGCATATCCAACA-3′ and 5′-CAGCCCCAAAATGGTTAAGGT-3′ for Hprt.
2.3. Induction of Pneumonia and Sampling of Organs
Pneumonia was induced by intranasal inoculation with 5 × 106 CFU P. aeruginosa PAK or 5 × 106 CFU flagellin-deficient PAK (PAKflic) as described [12,18]. Bronchoalveolar lavage fluid (BALF) was collected at 6 or 24 h after infection. All the procedures were performed as described [12,18].
2.4. Assays
Interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF), myeloperoxidase (MPO), and elastase were measured by enzyme-linked immunosorbent assays (ELISA; RnD Systems, Minneapolis, MN) according to manufacturer’s instructions.
2.5. Flow Cytometry
Flow cytometry was done on FACS Calibur (Becton Dickinson, Franklin Lakes, NJ, USA) after BAL cells were stained with fixable viability dye eFluor 780, rat anti mouse-CD45 PE-eFluor610 (30-F11), rat anti-mouse CD11b PE-Cy7 (clone M1/70), rat anti-mouse Siglec-F Alexa Fluor 647 (clone E50-2440), rat anti-mouse Ly-6C Alexa Fluor 700 (clone AL-21) (all from BD Biosciences), and rat anti-mouse Ly-6G FITC (clone 1A8; Biolegend, San Diego, CA). Neutrophils were identified as CD45+/Siglec-F-/CD11b+/Ly6C+/Ly6G+ cells. Data were analyzed using FlowJo software (Becton Dickinson) as described [19].
2.6. Statistical Analysis
Non-parametric variables were analyzed using the Mann–Whitney U test. Analysis was done using GraphPad Prism version 8 (Graphpad Software, San Diego, CA, USA). Statistical significance is shown as * p < 0.05.
3. Results
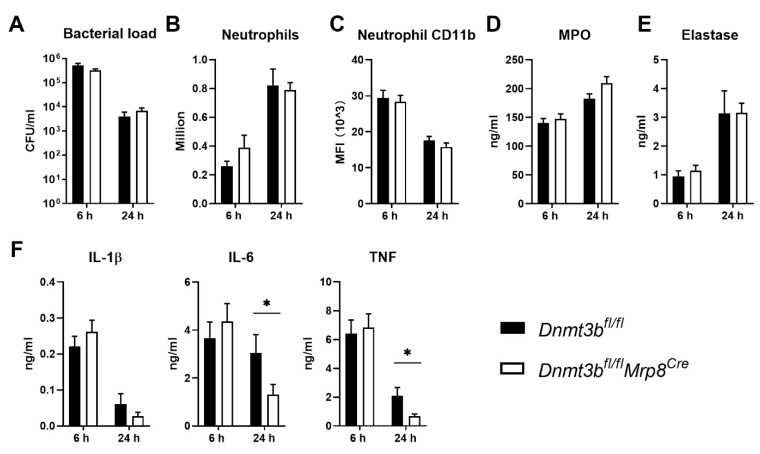
Dnmt3b is a de novo methyltransferase that has been widely studied as a regulator of DNA methylation [20]. More recent studies implicated Dnmt3b in the regulation of the host immune response during bacterial infection in general and P. aeruginosa infection in particular [2,10,11]. Neutrophils are the first cells recruited upon infection of the airways by P. aeruginosa, where they play a crucial role in host defense against this bacterium [21,22]. To determine the role of Dnmt3b in neutrophils during Pseudomonas pneumonia, we crossed Mrp8Cre mice, which specifically targets floxed gene segments in neutrophils [23] with Dnmt3bfl/fl mice to generate neutrophil-specific Dnmt3b knockout (Dnmt3bfl/flMrp8Cre) and littermate control (Dnmt3bfl/fl) mice, and infected these with PAK via the airways. Dnmt3b but not Dnmt3a mRNA level in neutrophils of naïve Dnmt3bfl/flMrp8Cre mice was significantly lower than that of littermate control mice (Figure S1), confirming the neutrophil-specific knockout of Dnmt3b in Dnmt3bfl/flMrp8Cre mice. Bacterial loads and neutrophil numbers in BALF were similar in both mouse strains at 6 and 24 h after infection (Figure 1A,B). Likewise, neutrophil activation, determined by quantification of cell surface CD11b expression and by measuring the neutrophil degranulation products MPO and elastase in BALF was comparable in Dnmt3bfl/flMrp8Cre and control mice at both time points after Pseudomonas infection (Figure 1C–E). BALF levels of the proinflammatory cytokines IL-1β, IL-6, and TNF were similar in both mouse strains at 6 h after infection. At 24 h, cytokine concentrations had decreased in both groups but to a greater extent in Dnmt3bfl/flMrp8Cre mice than in control mice (significantly so for IL-6 and TNF; Figure 1F). Taken together, these data indicate that neutrophil Dnmt3b does not affect key immune responses or antibacterial defense during P. aeruginosa pneumonia.
Figure 1.
Neutrophil-specific Dnmt3b deficiency does not affect the acute host response during Pseudomonas aeruginosa pneumonia. Neutrophil-specific Dnmt3b-deficient mice (Dnmt3bfl/flMrp8Cre) and littermate control (Dnmt3bfl/fl) mice were infected with viable PAK (5 × 106 CFU) via the airways and euthanized 6 or 24 h after infection. (A) Bacterial counts (colony forming units, CFU), (B) neutrophil numbers, (C) neutrophil CD11b expression, (D) myeloperoxidase (MPO), (E) elastase, (F) IL-β, IL-6, TNF levels, all in bronchoalveolar lavage fluid (BALF). Data are shown as bar graphs with mean + SEM, n = 8. * p < 0.05.
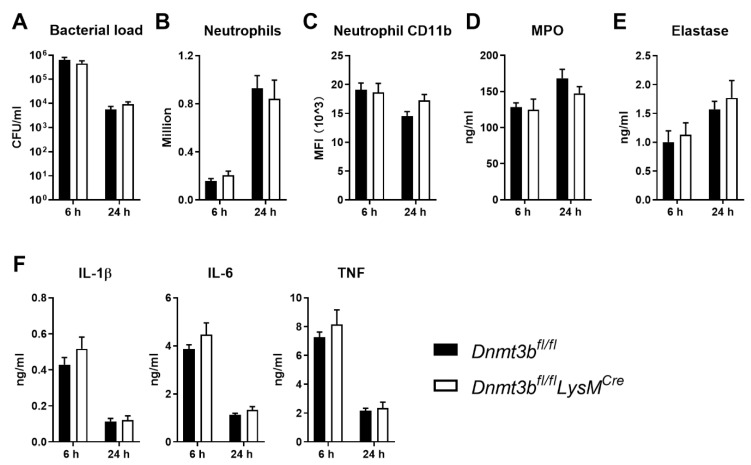
Considering that Dnmt3b affects macrophages polarization [13], we next induced Pseudomonas pneumonia in Dnmt3bfl/fl LysMCre mice (in which Dnmt3b is predominately deleted in macrophages as confirmed at mRNA levels (Figure S2)), and partially in neutrophils and monocytes [23]) and control mice. Both at 6 and 24 h after infection, bacterial burdens, neutrophil recruitment, neutrophil activation (CD11b expression, MPO and elastase production in the BALF), and pro-inflammatory cytokines (IL-1β, IL-6 and TNF) levels were similar in both mouse strains (Figure 2A–F). Flagellin is a crucial virulence factor expressed by P. aeruginosa that potentially activates the respiratory epithelium [12]. We speculated that a possible role of myeloid Dntm3b could be obscured by a dominant effect of flagellin on epithelial cells during infection of the airways with P. aeruginosa. To test this possibility, we infected Dnmt3bfl/fl LysMCre and control mice with flagellin-deficient PAK (PAKflic). Six hours after infection with PAKflic bacterial burdens, neutrophil recruitment, and activation, and cytokine concentrations in BALF were similar in both mouse strains (Figure S3). Collectively, these data suggest that Dnmt3b in myeloid cells does not play a significant role in the host response during pneumonia caused by acute P. aeruginosa infection.
Figure 2.
Myeloid cell Dnmt3b deficiency does not affect the acute host response during Pseudomonas aeruginosa pneumonia. Myeloid cell-specific Dnmt3b-deficient mice (Dnmt3bfl/flLysMCre) and littermate control (Dnmt3bfl/fl) mice were infected with viable PAK (5 × 106 CFU) via the airways and euthanized 6 or 24 h after infection. (A) Bacterial counts (colony forming units, CFU), (B) neutrophil numbers, (C) neutrophil CD11b expression, (D) myeloperoxidase (MPO), (E) elastase, (F) IL-β, IL-6 and TNF levels, all in bronchoalveolar lavage fluid (BALF). Data are shown as bar graphs with mean + SEM, n = 8. Differences between groups were not significant.
4. Discussion
P. aeruginosa is one of the most frequent causative pathogens in nosocomial pneumonia [24]. Both neutrophils and macrophages play vital roles in host defense against pulmonary P. aeruginosa infection [24]. Whilst genetic studies in humans have implicated Dnmt3b in host defense to P. aeruginosa infection [8,9,10], the data presented here strongly argue against the role of myeloid Dnmt3b in Pseudomonas pneumonia. Previously, it was demonstrated that knock-down of Dnmt3b in macrophages resulted in decreased expression and secretion of pro-inflammatory cytokines in response to in vitro stimulation with lipopolysaccharide [13], a major component of the outer membrane of gram-negative bacteria like P. aeruginosa. Opposite to these results, we show here that the absence of Dnmt3b in macrophages does not impact on cytokine production during Pseudomonas infection in vivo. Interestingly, we have recently shown that Dnmt3b in bronchial epithelial cells impairs the clearance of P. aeruginosa from the airways by a mechanism that involves inhibition of mucosal responses to bacterial flagellin by an effect on epithelial cell DNA methylation [11]. Further research into the cell-specific role of Dnmt3b in host defense to infection is, therefore, warranted.
In conclusion, the results of the present study indicate that Dnmt3b in myeloid cells is dispensable for host defense against pulmonary P. aeruginosa infection and does not regulate the acute immune response in the airways during Pseudomonas-induced pneumonia. These findings suggest that Dnmt3b functions in a cell-specific way during the host response in pneumonia caused by P. aeruginosa.
Acknowledgments
We thank Marieke S. ten Brink for helping with the animal experiments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells11050787/s1, Figure S1: Dnmt3b and Dnmt3a mRNA levels in neutrophils of Dnmt3bfl/flMrp8Cre mice and their littermate control mice (Dnmt3bfl/fl); Figure S2: tDnmt3b (A) and Dnmt3a mRNA levels (B) in alveolar macrophages (AMs), peritoneal macrophages (PM) and bone marrow-derived macrophages (BMDMs) of Dnmt3bfl/flLysMCre mice and their littermate control mice (Dnmt3bfl/fl); Figure S3: Myeloid cell Dnmt3b deficiency does not affect the host response during acute pneumonia caused by flagellin deficient Pseudomonas aeruginosa.
Author Contributions
W.Q.: Conceptualization, Methodology, Investigation, Formal analysis, Writing—original draft, Writing—review & editing. X.B.: Conceptualization, Investigation, Writing—review & editing. C.v.V.: Supervision, Writing—review & editing. A.F.d.V.: Supervision, Writing—review & editing. B.P.S.: Formal analysis, Supervision, Writing—review & editing. T.v.d.P.: Conceptualization, Supervision, Writing—original draft, Writing—review & editing, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
Wanhai Qin was funded by the Chinese Scholarship Council (CSC #20160617115); Xanthe Brands was funded by the Netherlands Organization for Health Research and Development (ZonMW #50-53000-98-139).
Institutional Review Board Statement
All animal studies were conducted according to the guidelines of the Declaration of Helsinki, and the animal care and use protocol adhered to the Dutch Experiments on Animals Act and European Directive of 22 September 2010 (Directive 2010/63/EU), in addition to the Directive of 6 May 2009 (Directive 2009/41/EC). The animal stud-ies were reviewed and approved by the Central Authority for Scientific Procedures on Animals, and the Animal Care and Use Committee and the Animal Welfare Body of the Academic Medi-cal Center, Amsterdam; approval codes DIX17-4125-62.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed in this study are included in the manuscript.
Conflicts of Interest
The authors declare no commercial or financial conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Qin W., Scicluna B.P., van der Poll T. The Role of Host Cell DNA Methylation in the Immune Response to Bacterial Infection. Front. Immunol. 2021;12:696280. doi: 10.3389/fimmu.2021.696280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao L., Zhu T., Lang X., Jia S., Yang Y., Zhu C., Wang Y., Feng S., Wang C., Zhang P., et al. Inhibiting DNA Methylation Improves Survival in Severe Sepsis by Regulating NF-κB Pathway. Front. Immunol. 2020;11:1360. doi: 10.3389/fimmu.2020.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorente-Pozo S., Navarrete P., Garzón M.J., Lara-Cantón I., Beltrán-García J., Osca-Verdegal R., Mena-Mollá S., García-López E., Vento M., Pallardó F.V., et al. DNA Methylation Analysis to Unravel Altered Genetic Pathways Underlying Early Onset and Late Onset Neonatal Sepsis. A Pilot Study. Front. Immunol. 2021;12:622599. doi: 10.3389/fimmu.2021.622599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binnie A., Walsh C.J., Hu P., Dwivedi D.J., Fox-Robichaud A., Liaw P.C., Tsang J., Batt J., Carrasqueiro G., Gupta S., et al. Epigenetic Profiling in Severe Sepsis: A Pilot Study of DNA Methylation Profiles in Critical Illness. Crit. Care Med. 2020;48:142–150. doi: 10.1097/CCM.0000000000004097. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich M., Jackson K., Weemaes C. Immunodeficiency, centromeric region instability, facial anomalies syndrome (ICF) Orphanet J. Rare Dis. 2006;1:2. doi: 10.1186/1750-1172-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin B., Tao Q., Peng J., Soo H.M., Wu W., Ying J., Fields C.R., Delmas A.L., Liu X., Qiu J., et al. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum. Mol. Genet. 2008;17:690–709. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- 7.Oka M., Meacham A.M., Hamazaki T., Rodić N., Chang L.J., Terada N. De novo DNA methyltransferases Dnmt3a and Dnmt3b primarily mediate the cytotoxic effect of 5-aza-2’-deoxycytidine. Oncogene. 2005;24:3091–3099. doi: 10.1038/sj.onc.1208540. [DOI] [PubMed] [Google Scholar]
- 8.Sterlin D., Velasco G., Moshous D., Touzot F., Mahlaoui N., Fischer A., Suarez F., Francastel C., Picard C. Genetic, Cellular and Clinical Features of ICF Syndrome: A French National Survey. J. Clin. Immunol. 2016;36:149–159. doi: 10.1007/s10875-016-0240-2. [DOI] [PubMed] [Google Scholar]
- 9.Cerbone M., Wang J., Van der Maarel S.M., D’Amico A., Agostino A., Romano A., Brunetti-Pierri N. Immunodeficiency, centromeric instability, facial anomalies (ICF) syndrome, due to ZBTB24 mutations, presenting with large cerebral cyst. Am. J. Med. Genet. A. 2012;158:2043–2046. doi: 10.1002/ajmg.a.35486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asgari S., McLaren P.J., Peake J., Wong M., Wong R., Bartha I., Francis J.R., Abarca K., Gelderman K.A., Agyeman P., et al. Exome Sequencing Reveals Primary Immunodeficiencies in Children with Community-Acquired Pseudomonas aeruginosa Sepsis. Front. Immunol. 2016;7:357. doi: 10.3389/fimmu.2016.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin W., Brands X., Van’t Veer C., de Vos A.F., Sirard J.C., JTH Roelofs J.J.T.H., Scicluna B.P., Van Der Poll T. Bronchial epithelial DNA methyltransferase 3b dampens pulmonary immune responses during Pseudomonas aeruginosa infection. PLoS Pathog. 2021;17:e1009491. doi: 10.1371/journal.ppat.1009491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayan A., Rumbo M., Carnoy C., Sirard J.C. Compartmentalized Antimicrobial Defenses in Response to Flagellin. Trends Microbiol. 2018;26:423–435. doi: 10.1016/j.tim.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Yang X., Wang X., Liu D., Yu L., Xue B., Shi H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol. Endocrinol. 2014;28:565–574. doi: 10.1210/me.2013-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodge J.E., Okano M., Dick F., Tsujimoto N., Chen T., Wang S., Ueda Y., Dyson N., Li E. Inactivation of Dnmt3b in mouse embryonic fibroblasts results in DNA hypomethylation, chromosomal instability, and spontaneous immortalization. J. Biol. Chem. 2005;280:17986–17991. doi: 10.1074/jbc.M413246200. [DOI] [PubMed] [Google Scholar]
- 15.Passegué E., Wagner E.F., Weissman I.L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119:431–443. doi: 10.1016/j.cell.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Clausen B.E., Burkhardt C., Reith W., Renkawitz R., Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 17.Qin W., Brands X., Matsumoto H., Butler J.M., van’t Veer C., de Vos A.F., Roelofs J., Scicluna B.P., van der Poll T. Role of Myeloid Tet Methylcytosine Dioxygenase 2 in Pulmonary and Peritoneal Inflammation Induced by Lipopolysaccharide and Peritonitis Induced by Escherichia coli. Cells. 2021;11:82. doi: 10.3390/cells11010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin W., Brands X., van’t Veer C., de Vos A.F., Scicluna B.P., van der Poll T. Bronchial Epithelial Tet2 Maintains Epithelial Integrity during Acute Pseudomonas aeruginosa Pneumonia. Infect. Immun. 2020;89:e00603–e00620. doi: 10.1128/IAI.00603-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Porto A.P., Liu Z., de Beer R., Florquin S., de Boer O.J., Hendriks R.W., van der Poll T., de Vos A.F. Btk inhibitor ibrutinib reduces inflammatory myeloid cell responses in the lung during murine pneumococcal pneumonia. Mol. Med. 2019;25:3. doi: 10.1186/s10020-018-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- 21.Chen S.M., Cheng D.S., Williams B.J., Sherrill T.P., Han W., Chont M., Saint-Jean L., Christman J.W., Sadikot R.T., Yull F.E., et al. The nuclear factor kappa-B pathway in airway epithelium regulates neutrophil recruitment and host defence following Pseudomonas aeruginosa infection. Clin. Exp. Immunol. 2008;153:420–428. doi: 10.1111/j.1365-2249.2008.03707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovewell R.R., Patankar Y.R., Berwin B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L591–L603. doi: 10.1152/ajplung.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abram C.L., Roberge G.L., Hu Y., Lowell C.A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods. 2014;408:89–100. doi: 10.1016/j.jim.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran C.S., Bolig T., Torabi-Parizi P. Mechanisms and Targeted Therapies for Pseudomonas aeruginosa Lung Infection. Am. J. Respir. Crit. Care Med. 2018;197:708–727. doi: 10.1164/rccm.201705-1043SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are included in the manuscript.