Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused global destruction since its emergence in late 2019. Over the past two years, the virus has continuously evolved in human host, leading to emergence of variants with changed viral transmission, disease severity, and evasion of immunity. Although vaccines have been developed for the coronavirus disease 2019 (COVID-19) at an unprecedently pace, the variants have constantly posed threats to the effectiveness of the approved vaccines. In this short communication, we review the key variants and discuss their implications in viral replication, transmission, and immune evasion.
Keywords: SARS-CoV-2 variants, spike mutations, neutralization, vaccine, COVID-19
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the coronavirus disease 2019 (COVID-19), has caused the global pandemic since its emergence in Wuhan, China in late 2019 [1]. As of January 5, 2022, it has caused more than 296 million infections, leading to over 5.4 million fatalities [2] . As a positive, single-strand RNA virus whose polymerase lacks proof-reading capability, the SARS-CoV-2 mutated frequently during viral replication, generating numerous viral variants, which can alter the viral infectivity, transmission, and disease severity [3]. Since spike glycoprotein on SARS-CoV-2 is responsible for binding to cellular receptor angiotensin converting enzyme 2 (ACE2), the original spike sequence of Wuhan isolate was used for vaccine development. Mutations in the spike gene have continuously emerged in different variants over time. This perspective communication (i) summarizes the key variants and their spike mutations and (ii) discusses their implications in viral replication, transmission, and immune evasion.
Dominant SARS-CoV-2 variants
Among all the SARS-CoV-2 strains, some of the notable variants obtained fitness advantages and outcompeted other variants during the viral evolution. The first dominant spike D614G substitution increased the viral replication in human upper respiratory tract and replaced almost all the primordial SARS-CoV-2 strains from June 2020 onwards [4]. After that, Alpha variant (B.1.1.7 lineage) emerged in the United Kingdom and quickly spread to many parts of the world, due to its increased binding affinity for human ACE2 receptor [5-7]. Next, Delta variant (B.1.617.2 lineage) emerged in India in October 2020, spread rapidly, and displaced the Alpha variants worldwide [8-10]. Recently, a heavily mutated Omicron variant, first detected in South Africa in November 2021, has explosively spread to many countries, leading to global Omicron surges [11-13]. Based on the previous studies on known mutations and clinical observations, the Omicron transmits much faster than the Delta variant, with a case doubling time as short as 1.5 to 3 days. In addition, the Omicron can escape many monoclonal antibodies and erode the vaccine-elicited neutralization [14-16]. Furthermore, infections with previous non-Omicron variants did not seem to elicit robust neutralization against the Omicron [17]. However, booster vaccination (e.g., the third dose of Pfizer/BionTach or Moderna vaccine) does elicit good neutralization against the Omicron [18], supporting a booster vaccination strategy. The durability of the protective immunity of booster vaccination remains to be determined against the Omicron. The high transmissibility and immune evasion have enabled Omicron to become a new dominant SARS-CoV-2 variant, reaching over 1 million cases in a single day in the United States [Fig 1].
Figure 1. The frequencies of the D614G, Alpha, Beta, Gamma, Delta and Omicron lineages over time in all genomic SARS-CoV-2 sequences.
The blue bars represent the total numbers of SARS-CoV-2 genomes. The colored lines indicate the percentage of different lineages in total SATS-CoV-2 genome. All the data sourced from GISAID database from Jan, 2020 to Dec, 2021.
Notable mutations in the spike protein
The spike (S) glycoprotein of coronaviruses is the major surface protein presented on the viral envelope in trimer [19]. The mature S protein cleaved by furin and transmembrane serine protease 2 (TMPRSS2) mediates the virus receptor ACE2 binding, viral entry, and immune escape. Thus, the spike protein is considered as the key determinant of viral infectivity and transmissibility [20]. The D614G substitution is the first dominant mutation occurred in the S protein. A higher quantity of D614G virus was detected in the respiratory tracts in both patients and animal models, indicating the higher infectivity and increased transmissibility [21,22]. Another mutation N501Y, located at the receptor binding domain (RBD) in the S protein, was demonstrated as the most important amino acid replacement in the Alpha variant. The N501Y substitution dramatically increased the binding affinity to the human ACE2 receptor, which led to the rapid infection in the upper respiratory tract and improved the viral transmission as well [7,23]. The delta variant has a spike mutation P681R, which is located at a furin cleavage site in the S protein. The results on the human primary airway epithelial (HAE) culture and animal model demonstrate that the P681R substation enhanced the viral replication and infection, possibility through improving the furin cleavage of the full-length S protein to S1 and S2 subunits [10,24]. Many other spike amino acid changes, including L18F, 69-70 deletion, W152C, K417N/K417T, L452R, S477G/S477R, E484K/E484Q/E484P, H655Y, and N679K, were also reported to lower vaccinated serum neutralization, reduce monoclonal antibody inhibition, and/or improve viral infection and transmission [Table 1]. The new Omicron variant has more than 30 mutations in the S protein, and several of them were previously found in other variants of concern, including alpha, beta, gamma, and delta [39]. Some of these mutations, including 69-70 deletion, K417N, E484K, N501Y, N655Y, and P681H, were well studied and known to enhance infectivity, transmissibility, and immune escape, leading to high concern of severe pandemic of the ongoing Omicron surges.
Table 1.
Notable mutations that benefit the infectivity, transmissibility and immune escape of SARS-CoV-2.
| Mutations | Locations | Representative Lineages | Functions | References |
|---|---|---|---|---|
| S13I | N-terminal domain | B.1.427 | Immune escape | [25] |
| L18F | N-terminal domain | Beta, Gamma | Immune escape | [26] |
| del69-70 | N-terminal domain | Alpha, Omicron* | Immune escape/Increase transmissibility | [27] |
| W152C | N-terminal domain | B.1.427 | Immune escape | [25] |
| K417N/T | Receptor binding domain | Beta, Gamma, Omicron | Attenuate biding affinity to ACE2/Immune escape | [28] |
| N439K | Receptor binding domain | AV.1 | Increase binding affinity to ACE2/immune escape | [29] |
| N440K | Receptor binding domain | Omicron | Increase infectivity | [30] |
| L452R | Receptor binding domain | Delta | Immune escape | [31-33] |
| Y453F | Receptor binding domain | Delta | Immune escape | [34] |
| S477G/N/R | Receptor binding domain | Omicron | Immune escape | [35] |
| E484K/Q/P | Receptor binding domain | Beta, Gamma, Delta, Eta, Lota, Kappa, Mu, Omicron | Immune escape | [31,32] |
| S494P | Receptor binding domain | Alpha | immune escape | [36,37] |
| N501Y | Receptor binding domain | Alpha, Beta, Gamma, Mu, Omicron | Increase infection and transmissibility | [7,28] |
| D614G | S1 domain-C terminal | All lineages | Increase infectivity | [22] |
| H655Y | Close to S1/S2 cleavage site | Gamma, Omicron | Immune escape | [38] |
| P681H/R | Close to S1/S2 cleavage site | Alpha, Kappa, Mu, Omicron | Increase cleavage efficiency | [10] |
Omicron is underlined to indicate multiple mutations and deletions from other variants.
Neutralization of the SARS-CoV-2 variants and vaccine strategy
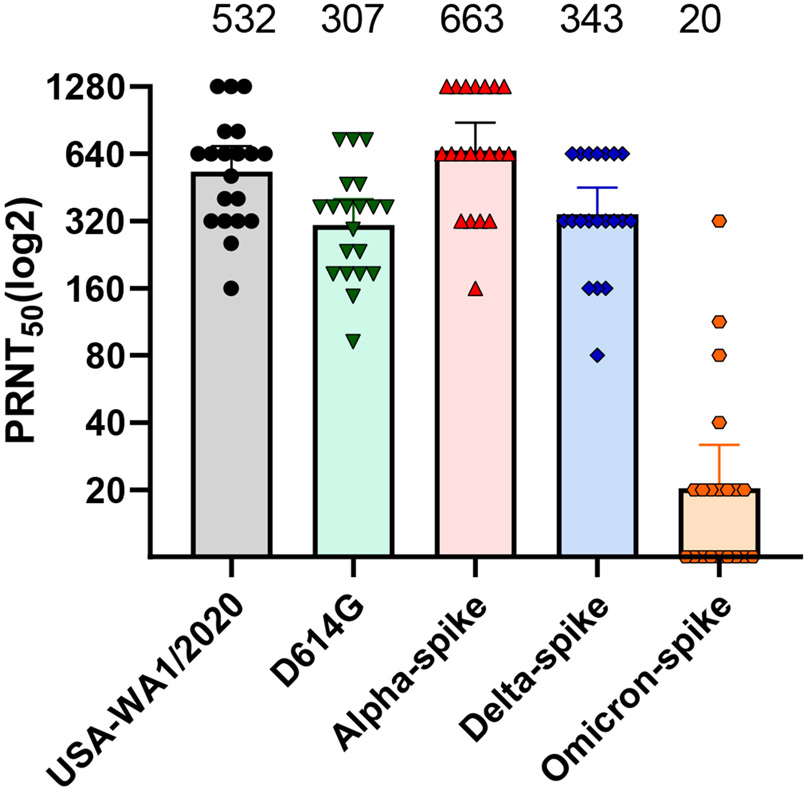
Several COVID-19 vaccines have been approved and immunized globally to develop the herd immunity against COVID-19 [40]. The Pfizer/BioNtech BNT162b2 nucleoside-modified mRNA vaccine is one of the most common ones and widely introduced in the North America and Europe [41]. Due to the high mutation frequency of SARS-CoV-2 S protein, some of the newly emerged variants decreased their susceptibilities to neutralization by antibodies generated by vaccination or natural infection. A number of approaches have been used to measure the neutralization sensitivity of different variants to human sera, including pseudotype virus expressing SARS-CoV-2 spike protein, clinical viral isolate, and chimeric SARS-CoV-2 bearing the spike protein from different variants [13,14,42]. Among these approaches, the chimeric SARS-CoV-2 bearing variant spike has two major advantages. First, this approach does not need to wait for the isolation of clinical virus strains; as soon as the spike sequence becomes available, the variant spike sequence can be synthesized and engineered into the original SARS-CoV-2 backbone [43]. Second, the chimeric virus represents an authentic SARS-CoV-2, which contrasts with pseudovirus approach. The neutralization titer (PRNT50) against different recombinant viruses can be easily tested and compared among all the variants accurately. The Alpha variants had higher infection and transmission efficiency, but the neutralization titers were roughly equivalent to the WT strain [42]. The D614G and representative Delta variants exhibited modestly reduced neutralization titers in comparison with the WT virus [44,45]. Unfortunately, two doses of Pfizer vaccine are not sufficient to induce robust antibody neutralization against Omicron variant [46]. The above studies were all performed using the same set of human sera taken at 2 or 4 weeks after the two doses of Pfizer vaccine [Fig 2]. Many other studies also demonstrate the neutralization titers against Omicron of infected and vaccinated persons were significantly reduced when compared with the WT virus [17,18]. However, as mentioned above, the third dose of Pfizer vaccine increases the magnitude and breadth of neutralization, leading to robust neutralization against the Omicron variant [18,46,47]. So far, the neutralization level remained robust up to 4 months after the third dose of Pfizer vaccine; however, the durability of neutralization beyond 4 months post dose 3 remains to be determined [46]. These results support a two-pronged vaccine strategy against Omicron and other newly emerged variants: (i) Booster vaccination using the currently approved safe vaccines; (ii) modify the vaccine spike sequence to match the Omicron and new variants. The mRNA vaccine technology allows a rapid modification of spike sequence. Real world vaccine effectiveness, together with laboratory studies, are needed to guide the implementation of this two-pronged vaccine strategy.
Figure 2. Serum neutralization titers of different lineages of SARS-CoV-2.
The D614G mutation or spike genes from different lineages of SARS-CoV-2 were engineered into USA-WA1/2020 backbone. The 50% plaque reduction neutralization testing (PRNT50) with the use of 20 samples obtained from 15 trial participants after the administration of the second dose of the BNT162b2 vaccine. Each data point represents the geometric mean PRNT50 obtained with a serum sample against the indicated virus. The heights of bars and the numbers over the bars indicate geometric mean titers. The error bars indicate 95% confidence intervals.
It has been already two years since the pandemic of COVID-19, different SARS-CoV-2 variants have taken turns to dominate the pandemic transmission and surges. The current Omicron surge may not be the last one. Although some of these variants obtained the ability to escape the immune protection from vaccination and/or natural infection [43-45,48], There are compelling evidence demonstrating that vaccination minimizes the risk of severe disease, including lower rates of hospitalization and deaths [49,50]. Thus, mass immunization and more booster shots with highly effective and safe vaccines, together with masking and social distance, will continue to be the most effective strategy to end the COVID-19 pandemic in the future.
Acknowledgments
We thank the colleagues at University of Texas Medical Branch (UTMB) for helpful discussions. P.-Y.S. was supported by NIH grants HHSN272201600013C, AI134907, AI145617, and UL1TR001439, and awards from the Sealy & Smith Foundation, the Kleberg Foundation, the John S. Dunn Foundation, the Amon G. Carter Foundation, the Gilson Longenbaugh Foundation, and the Summerfield Robert Foundation.
Footnotes
Competing interests
P.-Y.S. have filed a patent on the reverse genetic system of SARS-CoV-2. The Shi laboratory has received funding support in sponsored research agreements from Pfizer, Gilead, Novartis, GSK, Merck, IGM Biosciences, and Atea Pharmaceuticals. P.Y.S. is a member of the Pfizer COVID Antiviral Medical Board, a member of the Scientific Advisory Board of AbImmune, and a founder of FlaviTech.
Reference
- 1.WHO Coronavirus (COVID-19) Dashboard. [https://covid19.who.int] Accessed on 4 January 2022.
- 2.Home—Johns Hopkins Coronavirus Resource Center. [https://coronavirus.jhu.edu/] Accessed on 4 January 2022.
- 3.Astrezeneca. The Natural Evolution of SARS-CoV-2: How Science Responds to These Challenges. [https://www.astrazeneca.com/what-science-can-do/topics/disease-understanding/the-natural-evolution-of-sars-cov-2.html] Accessed on 4 January 2022.
- 4.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182(4): 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung K, Shum MH, Leung GM, Lam TT, Wu JT. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill 2021, 26(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claro IM, da Silva Sales FC, Ramundo MS, Candido DS, Silva CAM, de Jesus JG, et al. Local Transmission of SARS-CoV-2 Lineage B.1.1.7, Brazil, December 2020. Emerg Infect Dis 2021, 27(3): 970–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Liu J, Plante KS, Plante JA, Xie X, Zhang X, et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan SR, Spratt AN, Cohen AR, Naqvi SH, Chand HS, Quinn TP, et al. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J Autoimmun 2021, 124: 102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira I, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599(7883): 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Liu J, Johnson BA, Xia H, Ku Z, Schindewolf C, et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. bioRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021, 398(10317): 2126–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena SK, Kumar S, Ansari S, Paweska JT, Maurya VK, Tripathi AK, et al. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) Variant of Concern and its global perspective. J Med Virol 2021. [DOI] [PubMed] [Google Scholar]
- 13.Sheward DJ, Kim C, Ehling RA, Pankow A, Dopico XC, Martin D, et al. Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron). bioRxiv 2021. [Google Scholar]
- 14.Cele S, Jackson L, Khan K, Khoury D, Moyo-Gwete T, Tegally H, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv 2021 [Google Scholar]
- 15.Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and monoclonal antibodies. medRxiv 2021. [Google Scholar]
- 16.Cameroni E, Saliba C, Bowen JE, Rosen LE, Culap K, Pinto D, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. bioRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou J, Xia H, Xie X, Kurhade C, Machado RRG, Weaver SC, et al. Neutralization against Omicron SARS-CoV-2 from previous non-Omicron infection. bioRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfizer and BioNTech Provide Update on Omicron Variant. [https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant] Accessed on 4 January 2022.
- 19.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181(2): 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Science Alliance 2020, 3(9): e202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, Thao TTN, Hoffmann D, Taddeo A, Ebert N, Labroussaa F, et al. SARS-CoV-2 spike D614G change enhances replication and transmission. Nature 2021, 592(7852): 122–127. [DOI] [PubMed] [Google Scholar]
- 22.Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 2021, 592(7852): 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, Zhu Y, Niu Z, Zhou L, Sun Q. SARS-CoV-2 N501Y variants of concern and their potential transmission by mouse. Cell Death & Differentiation 2021, 28(10): 2840–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito A, Irie T, Suzuki R, Maemura T, Nasser H, Uriu K, et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCallum M, Bassi J, De Marco A, Chen A, Walls AC, Di Iulio J, et al. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern. Science 2021, 373(6555): 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCallum M, De Marco A, Lempp FA, Tortorici MA, Pinto D, Walls AC, et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 2021, 184(9): 2332–2347 e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy KR, Rennick LJ, Nambulli S, Robinson-McCarthy LR, Bain WG, Haidar G, et al. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 2021, 371(6534): 1139–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramanathan M, Ferguson ID, Miao W, Khavari PA. SARS-CoV-2 B.1.1.7 and B.1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect Dis 2021, 21(8): 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson EC, Rosen LE, Shepherd JG, Spreafico R, da Silva Filipe A, Wojcechowskyj JA, et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell 2021, 184(5): 1171–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tandel D, Gupta D, Sah V, Harinivas Harshan K. N440K variant of SARS-CoV-2 has Higher Infectious Fitness. bioRxiv. [Google Scholar]
- 31.Starr TN, Greaney AJ, Dingens AS, Bloom JD. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Reports Medicine 2021, 2(4): 100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184(9): 2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Davis BD, Chen SS, Sincuir Martinez JM, Plummer JT, Vail E. Emergence of a Novel SARS-CoV-2 Variant in Southern California. JAMA 2021, 325(13): 1324–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann M, Zhang L, Kruger N, Graichen L, Kleine-Weber H, Hofmann-Winkler H, et al. SARS-CoV-2 mutations acquired in mink reduce antibody-mediated neutralization. Cell Rep 2021, 35(3): 109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591(7851): 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenig PA, Das H, Liu H, Kummerer BM, Gohr FN, Jenster LM, et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science 2021, 371(6530). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greaney AJ, Starr TN, Gilchuk P, Zost SJ, Binshtein E, Loes AN, et al. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain that Escape Antibody Recognition. Cell Host Microbe 2021, 29(1): 44–57 e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Jackson CB, Mou H, Ojha A, Rangarajan ES, Izard T, et al. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.GISAID. Tracking of variants. [https://www.gisaid.org/hcov19variants/] Accessed on 4 January 2022.
- 40.Kaur SP, Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res 2020, 288(198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020, 383(27): 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing Activity of BNT162b2-Elicited Serum. N Engl J Med 2021, 384(15): 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie X, Muruato A, Lokugamage KG, Narayanan K, Zhang X, Zou J, et al. An Infectious cDNA Clone of SARS-CoV-2. Cell Host Microbe 2020, 27(5): 841–848 e843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou J, Xie X, Fontes-Garfias CR, Swanson KA, Kanevsky I, Tompkins K, et al. The effect of SARS-CoV-2 D614G mutation on BNT162b2 vaccine-elicited neutralization. NPJ Vaccines 2021, 6(1): 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Liu Y, Xia H, Zou J, Weaver SC, Swanson KA, et al. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature 2021, 596(7871): 273–275. [DOI] [PubMed] [Google Scholar]
- 46.Xia H, Zou J, Kurhade C, Cai H, Yang Q, Cutler M, et al. Neutralization of Omicron SARS-CoV-2 by 2 or 3 doses of BNT162b2 vaccine. bioRxiv 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falsey AR, Frenck RW Jr., Walsh EE, Kitchin N, Absalon J, Gurtman A, et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N Engl J Med 2021, 385(17): 1627–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med 2021, 27(4): 620–621. [DOI] [PubMed] [Google Scholar]
- 49.Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021. MMWR Morb Mortal Wkly Rep 2021, 70(38): 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021, eabm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]