Abstract
Objective:
To evaluate the safety and potential efficacy of autologous muscle derived cells (AMDC) for the treatment of swallowing impairment following treatment for oropharynx cancer.
Study Design:
Prospective, phase I, open label, clinical trial
Methodology:
Oropharynx cancer survivors disease free ≥ 2 years post chemoradiation were recruited. All patients had swallowing impairment but were not feeding tube dependent ((Functional Oral Intake Scale (FOIS) ≥ 5)). Muscle tissue (50–250mg) was harvested from the vastus lateralis and 150×106 AMDCs were prepared (Cook MyoSite Inc., Pittsburgh, PA). The cells were injected into four sites throughout the intrinsic tongue musculature. Participants were followed for 24 months. The primary outcome measure was safety. Secondary endpoints included objective measures on swallowing fluoroscopy, oral and pharyngeal pressure, and changes in patient-reported outcomes.
Results:
Ten individuals were enrolled. 100% (10/10) were male. The mean age of the cohort was 65(±8.87) years. No serious adverse event occurred. Mean tongue pressure increased significantly from 26.3(± 11.1) to 31.8(± 9.5) kPa (p = 0.017). The mean penetration aspiration scale (PAS) did not significantly change from 5.6(± 2.1) to 6.8(± 1.8) and the mean FOIS did not significantly change from 5.4(± 0.5) to 4.6 (± 0.7). The incidence of pneumonia was 30% (3/10) and only 10% (1/10) experienced a deterioration in swallowing function throughout 2 years of follow-up. The mean EAT-10 did not significantly change from 24.1 (± 5.57) to 21.3 (± 6.3) (p = 0.12).
Conclusion:
Results of this Phase I clinical trial demonstrate that injection of 150×106 autologous muscle derived cells into the tongue is safe and may improve tongue strength which is durable at 2 years. A blinded placebo-controlled trial is warranted.
Keywords: oropharynx cancer, Muscle Derived Cells, swallowing impairment
INTRODUCTION
Swallowing impairments following treatment for head and neck cancer are common.1,2 Consequences include reduction of quality of life, malnutrition, dehydration, depression, social isolation, aspiration pneumonia, empyema, and death. Swallowing impairments may be a consequence of tumor resection or be secondary to the late effects of radiation-induced toxicity. Radiation toxicity can cause fibrosis and weakness of the oral, laryngeal, and pharyngeal musculature as well as profound sensory neuropathy3–5. These impairments usually persist and often progress over time. Treatment options for these intractable swallowing deficits are inadequate and demonstrate limited efficacy6.
Autologous muscle-derived cell therapy (AMDC) may present an innovative therapeutic option for patients with swallowing impairment following treatment for oropharynx cancer. AMDC therapy involves the isolation and expansion of progenitor cells from skeletal muscle biopsies and their injection into the target muscle to induce fusion of the cells with existing myofibers and formation of new fibers to improve muscular function. Muscle-derived progenitor cells have recently shown benefit in treatment of stress urinary incontinence7,8, fecal incontinence9 and myocardial infarction10. In animal studies, muscle derived cells have successfully integrated within tissue to improve tongue strength and function 11–14. The purpose of this phase I open label clinical trial was to evaluate the safety and potential efficacy of AMDC for the treatment of swallowing impairment that develops following treatment for oropharynx cancer.
METHODOLOGY
The study was approved by the FDA (IND #16685) and the UC Davis Institutional Review Board (protocol #801019). Ten individuals who had undergone surgery and/or chemo- and or radiotherapy as primary treatment of oropharyngeal squamous cell cancer who presented with symptoms and findings of moderate swallowing impairment (Functional Oral Intake Scale (FOIS) score ≥ 3) were enrolled in the study. Patients qualified for the investigation only if they had completed cancer treatment at least 24 months prior to enrollment and were currently free of disease ( Refer to Table I for a complete list of inclusion and exclusion criteria).
Table 1:
Subject Exclusion Criteria for study enrollment
| History-based Criteria |
Status-based Criteria |
|---|---|
| Simultaneously participating in another investigational drug or device study | Evidence or known high risk of recurrent or persistent cancer |
| Previously treated with an investigational device, drug, or procedure for dysphagia within 6 months prior to signing consent | Tests positive for Hepatitis B, Hepatitis C, HIV and/or Syphilis. |
| Previous treatment with a cell therapy for dysphagia. | Cannot, or is not willing to, maintain the current treatment regimen for existing conservative therapy |
| Symptoms of aspiration pneumonia prior to enrollment | Requires prophylactic antibiotics for chronic infections, or has required 2 or more courses of antibiotics for infections in the 2 months prior to signing consent |
| Dysphagia of neurogenic etiology or uncorrected congenital abnormality leading to dysphagia | Any condition, including current infection, which could lead to significant postoperative complications. |
| Neuromuscular disorder that could lead to dysphagia | Not available for, or willing to comply, with the baseline and follow-up evaluations as required by the clinical investigative plan |
| Moderate or severe fibrosis of the tongue | Pregnant, lactating, or plans to become pregnant during the course of the study. |
| Morbidly obese (BMI ≥ 35) | |
| Uncontrolled diabetes | |
| Compromised immune system due to disease state, chronic corticosteroid use, or other immunosuppressive therapy | |
| Medical condition or disorder that may limit life expectancy or that may cause deviations from the clinical investigative plan | |
| History of bleeding diathesis or uncorrectable coagulopathy | |
| Known allergy or hypersensitivity to bovine proteins or allergens, gentamicin sulfate, or ampicillin that medically warrants exclusion | |
| Any non-skin cancer that has necessitated treatment within the past 24 months. |
Participants received repeat assessment of all outcome measurements at baseline and at 3, 6, 12 and 24 months following cell therapy treatment.
Outcome Measures of Safety
The primary safety outcome measure of the study was defined as the incidence of study product-related serious adverse events (SAEs). Secondary safety outcome measures included the incidence of product-related, biopsy procedure-related, injection site-related, and injection procedure-related adverse events.
Outcome Measures of Efficacy
The primary efficacy measure was anterior tongue pressure on the Iowa Oral Performance Instrument (IOPI, Woodinville, WA). Secondary outcome measures of product efficacy included the penetration aspiration scale (PAS), pharyngeal constriction ratio (PCR), pharyngo-esophageal segment (PES) opening in the lateral fluoroscopic view (PES-L), pharyngeal transit time, and pharyngeal peak pressure on manometry. Additional secondary patient reported outcome measures included the EAT-10, SF-12, and the VHI-10 (see Table 2 for a list of all outcome measures).
Table 2.
Description of study outcome measures
| Measure | Description |
|---|---|
| Pharyngeal constriction ratio (PCR) | Ratio of the pharyngeal area measured in the lateral view at the point of maximum pharyngeal constriction to the area measured with a 1ml bolus held in the oral cavity, measured from videofluoroscopic swallow study (VFSS) |
| PES-L | Maximum opening of the pharyngoesophageal segment (PES) in the lateral view measured from VFSS. |
| Hypopharyngeal transit time | Total time of bolus passage through the hypopharynx as measured from VFSS. |
| Penetration Aspiration Scale (PAS) | A validated 8-point scale characterizing the severity of penetration or aspiration depth and response to airway invasion during VFSS |
| Anterior tongue pressure | Maximal pressure (kPa) produced by the tongue as measured by the Iowa Oral Performance Instrument (IOPI) |
| Pharyngeal Peak Pressure | Maximal pressure (mmHg) within the pharyngeal area (measured from the manometric pressure topography plot. |
| SF-12 | A validated self-administered 12-item multipurpose short form survey assessing global health-related quality of life |
| EAT-10 | A validated self-administered survey instrument to quantify dysphagia symptoms severity |
| Vocal Handicap Index (VHI) | A validated self-administered instrument quantifying symptoms of vocal dysfunction |
Videofluoroscopic Swallow Studies (VFSS)
All subjects underwent the same standardized VFSS protocol established by our center15. Participants were initially positioned in the lateral view and administered 1, 3, and then 20ml of liquid barium (60% weight/volume) in a syringe or medicine cup. This was followed by 3ml of barium paste and then a 60ml of liquid barium which the patient consumed using a straw. Then, the patient was positioned in the anterior-posterior view and given 3 and 20ml liquid barium, followed by a 13mm barium tablet. All VFSS were recorded at 30 frames per second. In addition to the Penetration-aspiration Scale (PAS)16, the following parameters were measured from the VFSS using Swallowtail software (Belldev Medical, Arlington Heights, IL): hypopharyngeal transit time, measurement of maximum pharyngo-esophageal segment (PES) opening in the lateral fluoroscopic view (PES-L), and the pharyngeal constriction ratio (PCR), a validated surrogate measure of pharyngeal strength15. All parameters were assessed during the swallow of the 20ml bolus.
High Resolution Manometry
A 4.2mm outer diameter solid‐state manometric assembly with 36 circumferential sensors spaced at 10mm intervals was utilized for all trials (ManoScan, Given Imaging, Atlanta, GA). Before recording, the transducers were calibrated at 0 and 300 mmHg using externally applied pressure. The data acquisition frequency was 50 Hz for each sensor. The catheter was lubricated with 2% viscous lidocaine and topical nasal decongestant and anesthetic spray (phenylephrine HCl 1% with lidocaine HCl 4%) was administered. The manometry catheter was placed through the patient’s more patent nasal cavity. The catheter’s position was determined and verified manometrically. Following a brief period to allow patient acclimation, 30 seconds of baseline UES pressure was recorded at rest. The manometric evaluation consisted of 12 5ml saline bolus swallows. The first 4 swallows were conducted with the patient in a supine position and the catheter was placed so that it captured pressures spanning the esophagus. Following these 4 swallows, the patient was positioned upright, the catheter was adjusted to capture pressure changes in the pharynx, and an additional 8 swallows were recorded. Manometric data was analyzed using ManoviewTM ESO 3.0 (Given Imaging, Atlanta, GA). Pharyngeal pressure measurements were taken from the 8 swallows conducted in the upright position.
Muscle Biopsy and AMDC Preparation
The needle biopsy procedures were performed in an outpatient setting. An LOGIQ E ultrasound with linear transducer (General Electric, Boston MA ) was used to gauge the depth of the muscle and guide a 8G Spirotome™ Soft-Tissue Biopsy Needle Set (Bioncise, NV ) between the fascial layers. Local anesthesia (5–10 mL of 1% Lidocaine) was injected into the skin and subdermal superficial muscle fascia at the penetration area in the lateral thigh. A skin incision was made at the site using a scalpel, followed by the insertion of the Spirotome™. Approximately 50–250mg of muscle tissue was harvested from the vastus lateralis, placed in a hypothermic medium and sent to Cook MyoSite, Inc (Pittsburgh, PA) for subsequent culture and expansion. The isolation and cell culture processes produced the cryopreserved autologous muscle derived cells (AMDC) investigational product which was enriched in myogenic cell content and followed current good manufacturing practices to a final dose of 150 × 106 ± 20% cells. AMDC was characterized for cellular myogenic purity, myogenic differentiation capacity, viability, sterility, mycoplasma, and endotoxin prior to product release for temperature-controlled shipping and injection.
AMDC Injection Procedure
The AMDC investigational product was thawed and diluted in 2ml of physiological saline. Bilateral lingual nerve blocks were performed. A 23G spinal needle (BD, Franklin Lakes, NJ) was used to distribute the 4mL of AMDC solution within the intrinsic muscles of the tongue at four different locations. A flexible video naso-pharyngo-laryngoscope (VNL-1190STK, Pentax Medical, Montvale, NJ) was inserted through the more patent nasal cavity after the application of 2% viscous lidocaine. Transnasal endoscopy was performed to confirm needle localization at the base of tongue.
Statistical Analysis
All data was coded and recorded into SPSS Version 26.0 for the Macintosh (IBM Inc., Armonk, NY). A one-way repeated measures multivariate analysis of variance (MANOVA) was conducted to test for differences in outcome measures at each time point (3, 6, 12 and 24 months). P-values < 0.05 were considered to be statistically significant.
RESULTS
Ten individuals were enrolled. 100% (10/10) were male. The mean age of the cohort was 65 (±8.87) years. All patients had a history of oropharyngeal squamous cell cancer. All patients had undergone radiation therapy and 7 had also been treated with chemotherapy. Four patients had undergone surgical extirpation of the primary tumor. The mean number of years since completion of oropharyngeal cancer treatment was 11.5 (±7.6).
No serious adverse event occurred as a result of study procedures or the AMDC investigational product. The biopsy procedure was associated with hematoma at the biopsy site in one patient and temporary thigh pain in another. The tongue injection procedure was associated with transient mild tongue swelling in three patients, temporary tongue pain in two patients and transient tongue numbness in one patient. All adverse events resolved with conservative management. Mean tongue pressure increased from 26.3 (± 11.1) kPa at baseline to 31.8 (± 9.5) kPa at 12 months post injection (p=0.017) (Table 3). Increased tongue pressures were observed in 90% (9/10) of patients (Figure 1). No significant difference was observed between baseline and 12 months post-treatment in EAT-10 scores [24.1 (± 5.6) versus 21.3 (± 6.3), respectively;] or FOIS scores [5.4 (± 0.5) versus 4.6 (± 0.7), respectively; or any of the other secondary outcome measures (Table 3). One patient experienced a deterioration in swallowing function with a change of FOIS score from 6 at baseline to 2 at 24 months. Data for 24 months was available for only 8/10 patients. In patients with available 24 months data, no significant differences were observed between the 12 and the 24-month data.
Table 3.
Statistical summary of outcome measures
| EAT-10 | VHIa | IOPIb | SF-12 | PPPc | PTTd | PES-Le | PCRf | PASg | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | 24.1 (±5.6) |
22.9 (±6.1) |
26.3 (±11.1) |
53.2 (±7.0) |
185.1 (±63) |
1.54 (±0.6) |
0.74 (±0.2) |
0.46 (±0.3) |
5.6 (±2.1) |
| 6 months post-treatment | 21.6 (±7.5) |
21.1 (±5.6) |
30.7 (±9.4) |
52.9 (±7.4) |
214.5 (±57.6) |
1.41 (±0.5) |
0.67 (±0.2) |
0.45 (±0.2) |
6.5 (±1.8) |
| 12 months post-treatment | 21.3 (±6.3) |
25.2 (±7.6) |
31.8 (±9.5) |
51.9± (7.8) |
207.7 (±83.7) |
1.41 (±0.6) |
0.84 (±0.2) |
0.45 (±0.2) |
6.8 (±1.8) |
Values are mean ±SD.
Vocal Handicap Index
Iowa Oral Performance Instrument (kPa)
Pharyngeal peak pressure (mmHg)
Pharyngeal transit time (sec)
Pharyngoesophageal segment in the lateral view (cm)
Pharyngeal constriction ratio
Penetration aspiration scale
Figure 1.
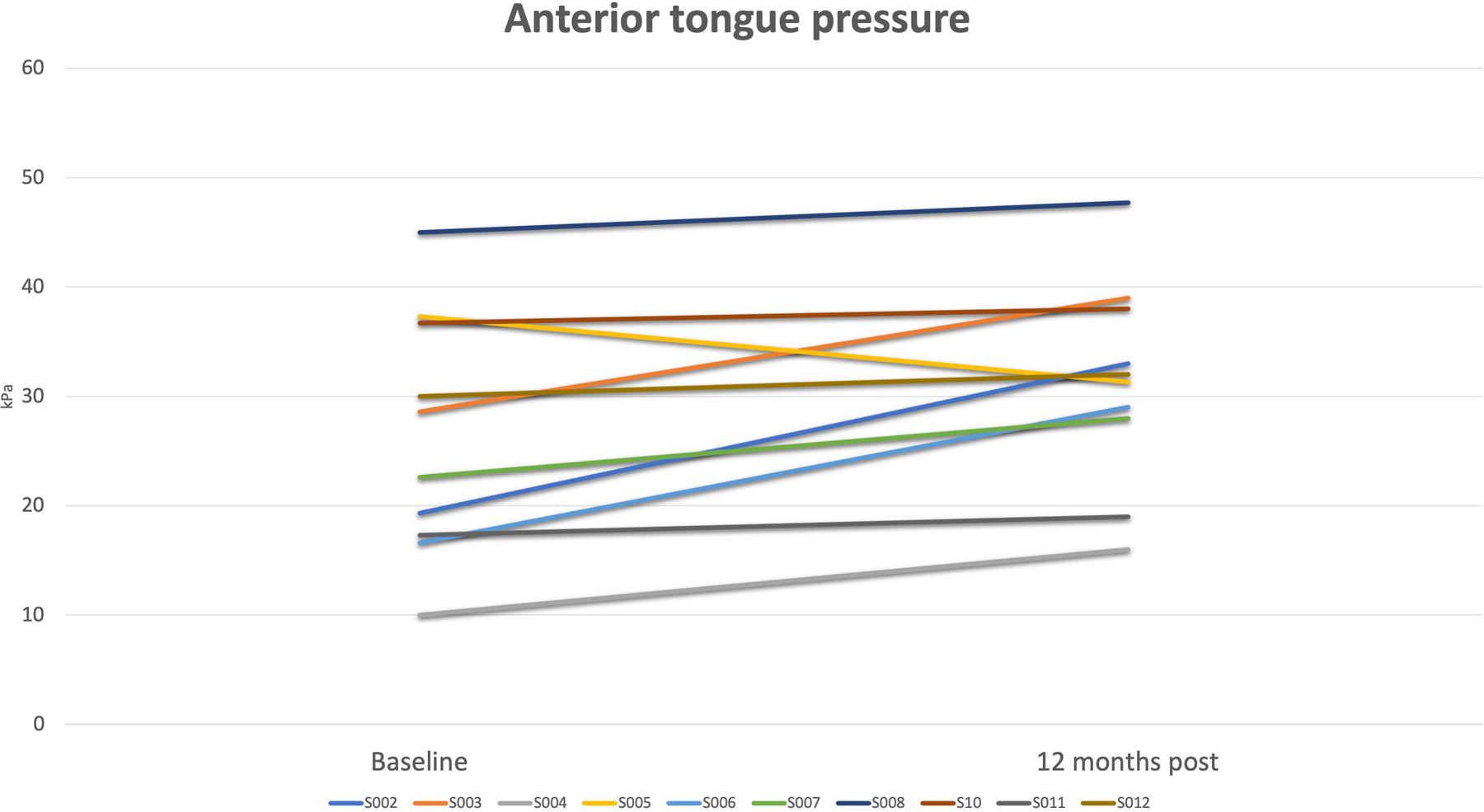
Change in anterior tongue pressures measured by IOPI in kilopascal (kPa) from baseline to 12 months post-injection
The incidence of pneumonia was 30% (3/10). Aspiration pneumonia was suspected in one (10%) of these 3 cases. In another patient, the pneumonia developed as a complication of a viral illness and the cause was unknown for the third pneumonia occurrence.
DISCUSSION
The existence of skeletal muscle stem/progenitor cells (aka ‘satellite cells’) beneath the basal lamina of normal myofibers has been well established as the resident source of homeostasis, growth and muscle repair17. With the potential for self-renewal, durable proliferation, and differentiation, transplantation of these cells and their myoblast progeny has been investigated for therapeutic purposes to augment weakened muscle tissue and improve function7–10,18.
Several studies have reported the formation of new muscle fibers following the injection of muscle-derived progenitor cells in a rodent hemiglossectomy model11–13. Kim et al. (2003) injected a hydrogel-myoblast composite in a rat hemiglossectomy model and observed significant increase in tongue weight, evidence of neovascularization and possible neurotization12. Their results were supported by a study conducted by Bunaprasert et al. (2003), who reported that rats receiving myoblast transplantation, whether via gel suspension or in the form of undifferentiated or differentiated constructs, showed superior quality of the muscular regenerate compared to controls. 11 Similarly, Luxameechanporn et al. (2006) described that the introduction of myoblast/collagen constructs resulted in regeneration of muscle and less scarring in a rat tongue reconstruction model19. Kuhn et al. (2017) later demonstrated survival of human muscle-derived cells (MDC) injected into the tongue of immune deficient mice post-hemiglossectomy13. Animals injected with MDCs weighed significantly more than control animals 12 weeks after treatment, suggesting improved alimentation. Plowman et al. (2014) examined the effects of autologous muscle-derived stem cells in a denervated ovine tongue model14. Sternocleidomastoid muscle biopsies were acquired from the sheep to create the autologous muscle cell cultures, which were injected into the tongue. Results suggested that the muscle cells survive for at least a 2-month period, and their fusion with partially denervated myofibers enlarged the myofibers. Additionally, a 27% increase in maximal tongue contractile force and a 54% increase in maximum base of tongue pressure was observed in one animal. The only previous application of myoblast transplantation for swallowing impairment in humans was conducted by Périé et al. (2014) in patients with oculopharyngeal muscular dystrophy (OPMD). Autologous myoblasts were in injected into the pharyngeal constrictor muscles at the time of cricopharyngeus muscle myotomy. No adverse events occurred during the 2 year follow up period and no functional degradation of the swallow was observed in 10/12 patients20.
Building on the therapeutic potential demonstrated in these previous studies, this current study aimed to examine the safety and potential efficacy of AMDC treatment in improving tongue function for humans with swallowing impairment following treatment for oropharyngeal cancer. Treatment for oropharyngeal cancer frequently has a negative impact on posttreatment swallowing function, characterized by oral transit difficulties, weak pharyngeal constriction, reduced PES opening, tracheal aspiration and pharyngeal residue. For some patients, these impairments are chronic and are associated with poor quality of life, recurrent episodes of aspiration pneumonia and mortality6,21–23.
The results of this phase I study suggest that lingual cell therapy with autologous muscle derived progenitor cells is safe with no significant study or product related adverse events. In regard to potential AMDC efficacy, the majority (90%) of patients in this study demonstrated an increase in tongue pressure. At the onset of the study, all participants demonstrated below average tongue pressures, compared to the average tongue pressures in healthy adults of equivalent age ((26.3 (±11.1) kPa versus 49.5 (±11.2) kPa))24. By six months post-treatment, the study cohort demonstrated a significant improvement in tongue strength, which was maintained at the 24-month follow-up period. This persistent improvement in oral tongue function may be secondary to the formation of new myofibers or the repair of existing fibrotic myofibers and suggests that AMDC may be beneficial in improving skeletal muscle function in the tongue after treatment of oropharynx cancer. It is plausible that a larger dose of AMDC may result in a greater impact on tongue strength. The dose of 150 × 106 AMDC was chosen as the initial dose in this phase 1 trial because it has demonstrated safety in clinical human studies for other medical applications 8,25. This investigation was the initial phase of a dose escalation study. Phase 2 is currently underway and involves an increased dose of 300 × 106 AMDC.
No significant difference was observed in measures of swallow function or patient reported outcomes for swallowing, voice and QOL. Only one patient experienced a deterioration in swallow function during the 2-year study period. Given the progressive nature of the late effects of radiation toxicity on the oropharyngeal swallow, the lack of deterioration of swallow function observed throughout the two-year period in most participants may signify treatment benefit.
The main limitations of this study are the small sample size and open label design which preclude a definitive assessment of treatment safety and efficacy in larger and more diverse population. This study was also underpowered to identify a treatment effect for all of the primary efficacy endpoints. Nonetheless, the data from this phase I trial suggest that lingual cell therapy with autologous muscle derived progenitor cells is safe and may be efficacious in the treatment of late radiation toxicity to the tongue. This study supports the need for a double-blind, placebo-controlled, adequately powered, randomized clinical trial.
Conclusions
Results of this phase I clinical trial demonstrate that injection of 150×106 autologous muscle derived cells into the tongue is safe and may improve tongue strength in oropharynx cancer survivors. A double-blind, placebo-controlled randomized clinical trial is warranted.
Funding:
Authors are supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award KL2 TR001859. Further funding was provided by T32 Cardio NIH T32-HL086350, and Denny & Jeanene Dickenson Fellowship. The clinical trial was supported by an Investigator Initiated research grant from Cook MyoSite Incorporated (Pittsburgh, PA).
Footnotes
This work will be presented as an oral presentation at the Virtual Combined Otolaryngology Spring Meetings (COSM) on April 8th 2021.
Disclosure: Emanuele Canestrari and Ron J Jankowski are employees of Cook MyoSite, Inc.
Level of Evidence: Level 3
REFERENCES
- 1.Rinkel RN, Verdonck-de Leeuw IM, Doornaert P, et al. Prevalence of swallowing and speech problems in daily life after chemoradiation for head and neck cancer based on cut-off scores of the patient-reported outcome measures SWAL-QOL and SHI. Eur Arch Otorhinolaryngol 2016;273(7):1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RV, Kotz T, Beitler JJ, Wadler S. Long-term swallowing problems after organ preservation therapy with concomitant radiation therapy and intravenous hydroxyurea: initial results. Arch Otolaryngol Head Neck Surg 2000;126(3):384–389. [DOI] [PubMed] [Google Scholar]
- 3.Mehdizadeh OB, Dhar SI, Evangelista L, Nativ-Zeltzer N, Bewley AF, Belafsky PC. Prevalence of profound laryngeal sensory neuropathy in head and neck cancer survivors with feeding tube-dependent oropharyngeal dysphagia. Head Neck 2020;42(5):898–904. [DOI] [PubMed] [Google Scholar]
- 4.Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA. Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia 2014;29(2):223–233. [DOI] [PubMed] [Google Scholar]
- 5.Scott SI, Kathrine Ø Madsen A, Rubek N, et al. Long-term quality of life & functional outcomes after treatment of oropharyngeal cancer. Cancer Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer 2012;118(23):5793–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barakat B, Franke K, Schakaki S, Hijazi S, Hasselhof V, Vögeli TA. Stem cell applications in regenerative medicine for stress urinary incontinence: A review of effectiveness based on clinical trials. Arab J Urol 2020;18(3):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski RJ, Tu LM, Carlson C, et al. A double-blind, randomized, placebo-controlled clinical trial evaluating the safety and efficacy of autologous muscle derived cells in female subjects with stress urinary incontinence. International Urology and Nephrology 2018;50(12):2153–2165. [DOI] [PubMed] [Google Scholar]
- 9.Frudinger A, Marksteiner R, Pfeifer J, Margreiter E, Paede J, Thurner M. Skeletal muscle-derived cell implantation for the treatment of sphincter-related faecal incontinence. Stem Cell Res Ther 2018;9(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Wang L, Zhang F, et al. Nine-year follow-up of local implantation of autologous skeletal myoblasts in a patient with coronary heart disease. Am J Case Rep 2013;14:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunaprasert T, Hadlock T, Marler J, et al. Tissue engineered muscle implantation for tongue reconstruction: a preliminary report. Laryngoscope 2003;113(10):1792–1797. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Hadlock T, Cheney M, Varvares M, Marler J. Muscle tissue engineering for partial glossectomy defects. Arch Facial Plast Surg 2003;5(5):403–407. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn MA, Black AB, Siddiqui MT, Nolta JA, Belafsky PC. Novel murine xenograft model for the evaluation of stem cell therapy for profound dysphagia. Laryngoscope 2017;127(10):E359–e363. [DOI] [PubMed] [Google Scholar]
- 14.Plowman EK, Bijangi-Vishehsaraei K, Halum S, et al. Autologous myoblasts attenuate atrophy and improve tongue force in a denervated tongue model: a pilot study. Laryngoscope 2014;124(2):E20–26. [DOI] [PubMed] [Google Scholar]
- 15.Leonard R, Rees CJ, Belafsky P, Allen J. Fluoroscopic surrogate for pharyngeal strength: the pharyngeal constriction ratio (PCR). Dysphagia 2011;26(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 1996;11(2):93–98. [DOI] [PubMed] [Google Scholar]
- 17.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 2012;139(16):2845–2856. [DOI] [PubMed] [Google Scholar]
- 18.Negroni E, Gidaro T, Bigot A, Butler-Browne GS, Mouly V, Trollet C. Invited review: Stem cells and muscle diseases: advances in cell therapy strategies. Neuropathol Appl Neurobiol 2015;41(3):270–287. [DOI] [PubMed] [Google Scholar]
- 19.Luxameechanporn T, Hadlock T, Shyu J, Cowan D, Faquin W, Varvares M. Successful myoblast transplantation in rat tongue reconstruction. Head Neck 2006;28(6):517–524. [DOI] [PubMed] [Google Scholar]
- 20.Périé S, Trollet C, Mouly V, et al. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol Ther 2014;22(1):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Høxbroe Michaelsen S, Grønhøj C, Høxbroe Michaelsen J, Friborg J, von Buchwald C. Quality of life in survivors of oropharyngeal cancer: A systematic review and meta-analysis of 1366 patients. Eur J Cancer 2017;78:91–102. [DOI] [PubMed] [Google Scholar]
- 22.Hunter KU, Lee OE, Lyden TH, et al. Aspiration pneumonia after chemo-intensity-modulated radiation therapy of oropharyngeal carcinoma and its clinical and dysphagia-related predictors. Head Neck 2014;36(1):120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suarez-Cunqueiro MM, Schramm A, Schoen R, et al. Speech and swallowing impairment after treatment for oral and oropharyngeal cancer. Arch Otolaryngol Head Neck Surg 2008;134(12):1299–1304. [DOI] [PubMed] [Google Scholar]
- 24.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci 1995;50(5):M257–262. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman MR. Contemporary application of autologous muscle-derived cells for urinary sphincter regeneration. World Journal of Urology 2020;38(9):2095–2099. [DOI] [PubMed] [Google Scholar]


