Aminoglycosides have long been one of the commonest causes of drug-induced nephrotoxicity (137). Although a clear recognition of the patient- and treatment-related risk factors (91), combined with the once-a-day schedule and effective monitoring procedures (98), have definitely improved the situation over what prevailed in the early 1980s (115), we are still short of having brought the safety of aminoglycosides to that of the main other wide-spectrum antibiotics. Chemical research aimed at obtaining intrinsically less toxic compounds has met with only modest success, and few of the other approaches proposed to reduce the toxicities of the available agents have reached practical clinical applications. Yet, because aminoglycosides are very effective antibiotics well suited to the treatment of severe infections (35), it seems important to maintain and even develop efforts to improve their therapeutic indices. The present minireview tries to present in a prospective way the status of both the basic and the clinical research on aminoglycoside nephrotoxicity in order to clarify the main issues and to pinpoint strategies that may eventually lead to their safer use. Ototoxicity, which is the second main adverse effect of aminoglycosides and which, in contrast to nephrotoxicity, is irreversible, will not be considered here since it has already been reviewed in this journal (52) and elsewhere (10). A companion minireview (83) examines and discusses the recent research dealing with the activities of aminoglycosides and bacterial resistance.
GENERAL FEATURES OF AMINOGLYCOSIDE NEPHROTOXICITY
Nephrotoxicity induced by aminoglycosides manifests clinically as nonoliguric renal failure, with a slow rise in serum creatinine and a hypoosmolar urinary output developing after several days of treatment. Aminoglycosides are nephrotoxic because a small but sizable proportion of the administered dose (≈5%) is retained in the epithelial cells lining the S1 and S2 segments of the proximal tubules (135) after glomerular filtration (30). Aminoglycosides accumulated by these cells are mainly localized with endosomal and lysosomal vacuoles (108, 112) but are also localized with the Golgi complex (108). They elicit an array of morphological and functional alterations of increasing severity, which are presented in a summary fashion in Table 1, with their ultrastructural appearance schematically depicted in Fig. 1. We have distinguished between low and high dose effects since there is probably more than a quantitative difference between the changes seen under these two conditions (130).
TABLE 1.
Main alterations elicited by aminoglycosides in kidney cortex
| 1. At low dosesa |
| 1.1. Early alterations |
| - Accumulation of phospholipids in lysosomes and enlargement of these organelles (71, 138)b |
| - Inhibition of the activities of lysosomal phospholipases and sphingomyelinase (75) |
| - Decreased reabsorption and/or intracellular lysosomal sequestration and digestion of filtrated, low-molecular-weight proteins (e.g., lysozyme, alpha-2-macroglobulin, beta-2-microglobulinc (see references 35 and 130 for reviews) |
| - Shedding of brush-border enzymes (e.g., alanylaminopeptidase) and release of lysosomal enzymes (e.g., N-acetyl-beta-glucosaminidase)c (see reference 130 for a review) |
| 1.2. Later alterations |
| 1.2.1. Degenerative alterations |
| - Coarse granulation of epithelial cellsd (see reference 130 for a review) |
| - Focal necroses,e apoptoses (77) |
| - Increased phospholipid excretion and cast in urinef (see reference 130 for a review) |
| - Proteinuria, hypoosmotic polyuria, in humans only (see reference 35 for a review) |
| - Decreased glomerular filtration and increase in blood urea nitrogen and creatinine, without immediate signs of glomerular damage, in humans only (see reference 130 for a review) |
| 1.2.2. Regenerative lesions |
| - Tubular cell proliferation and dedifferentiation (127) |
| - Tubular dilatation (see reference 130 for a review) |
| - Interstitial proliferation (fibroblastic cells) and focal infiltration by inflammatory cells (see reference 130 for a review) |
| 2. At high doses |
| 2.1. Brush-border and apical membranes |
| - Wasting of K+, Mg2+, and Ca2+ (35, 116) |
| - Decreased reabsorption of water, HCO3−, glucose (64) |
| - Decreased of Na+ and Pi cotransport and Na+ and H+ exchange (79) |
| - Inhibition of phosphatidylinositol phospholipase C (see reference 131 for a review) |
| - Decrease of carrier-mediated dipeptide transport (113) |
| 2.2. Basolateral membrane |
| - Impairment of organic acid and bases transport (see reference 131 for a review) |
| - Inhibition of Na+/K+ ATPase (34, 131) |
| - Reduction of the electrogenic Na+ transport (126) |
| 2.3. Mitochondria |
| - Impairment of respiration and cation transport; swelling (see reference 35 for a review) |
| - Impairment of the activities of key mitochondrial enzymes in gluconeogenesis, ammoniogenesis, and tricarboxylic acid oxidation pathways (107; see reference 131 for a review) |
| 2.4. Protein synthesis and related phenomena |
| - Inhibition of protein synthesis (see reference 35 for a review) and dilatation of endoplasmic reticulum |
| - Suppression of gene expression for the Na+ and Ca2+ exchanger, Na+-dependent d-glucose transporter, and alpha, subunit of Na+/K+ ATPase (23, 47) |
| - Expression and move of heat shock proteins from nucleus to lysosomes (69) |
Low doses refer to either clinical dosages for humans or of dosages less than 20 mg/kg per day for animals.
References are given as a source for illustration of the typical effect that is described or refer to review papers; see text for more comprehensive citations.
These alterations have often been used for the early detection of aminoglycoside insult; however, their measurement is of limited practical value in diseased patients.
These cells show markedly enlarged lysosomes, with a decreased buoyant density and prominent myeloid bodies.
Electron microscopy shows widespread alteration of the cell ultrastructure and subcellular organelles, including mitochondria, endoplasmic reticulum, and nuclei.
Myeloid bodies are abundant in lumen and urine.
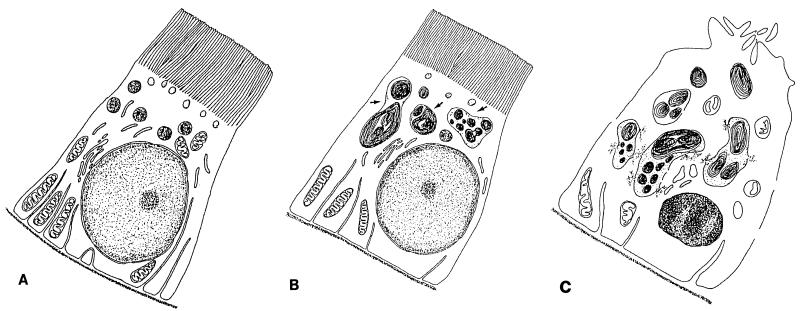
FIG. 1.
Ultrastructural alterations induced in proximal tubular cells during aminoglycoside treatment. (A) Control. Changes detected early on and at low doses (B) consist mainly of the enlargement of lysosomes, which most likely occurs by fusion of preexisting structures and which is caused by the progressive deposition of polar lipids which adopt a concentric lamellar disposition (myelin-like structures, most commonly referred to as myeloid bodies); the other subcellular structures are usually well preserved. Later changes or changes observed with high doses (C) include the apparent rupture of lysosomes (with the release of myeloid bodies in the cytosol), extensive mitochondrial swelling and damage, dilatation of the endoplasmic reticulum cisternae, shedding of the apical brush-border villi, pericellular membrane discontinuities, and the occurrence of apoptotic nuclei. These alterations do not necessarily coexist in all cells. The figure is adapted from reference 76 and is based on the typical descriptions given in references 38, 40, 71, 76, 77, 127, and 138.
EFFECTS OF CLINICAL DOSES IN ANIMALS AND HUMANS
After only a few days of administration of clinical doses to humans or of low multiples of the human therapeutic dose to animals (typically 10 to 20 mg/kg of body weight for a laboratory rat), aminoglycosides induce conspicuous and characteristic changes in lysosomes of proximal tubular cells consistent with the accumulation of polar lipids (myeloid bodies) (10, 22, 71, 138). These changes are preceded and accompanied by signs of tubular dysfunctions or alterations (release of brush-border and lysosomal enzymes; decreased reabsorption of filtered proteins; wasting of K+, Mg2+, Ca2+, and glucose; phospholipiduria; and cast excretion [for a review, see reference 35]). In humans, the occurrence of these signs may be followed by the development of overt renal failure characterized mainly by a nonoliguric and even often polyuric hypoosmotic fall in creatinine clearance (35). Progression to oliguric or anuric renal failure is infrequent, and recovery upon drug discontinuation is most often observed. Occasionally, a Fanconi’s syndrome (18) or a Bartter’s-like syndrome (74) has been observed. A correlation between the development of these clinical signs and the severity or rate of progression of the subclinical alterations remains difficult to establish mainly because of large interpatient variations. Consequently, the usefulness of monitoring the subclinical changes to detect individuals at risk has remained questionable. In animals, tubular alterations have clearly been associated with the development of focal necroses and apoptoses in the tubular epithelium, together with an extensive tubular and peritubular cell proliferation (77, 127), without an apparent change in kidney function.
EFFECTS OF HIGH DOSE IN ANIMALS
High doses (40 mg/kg or more for gentamicin) are necessary in animals to rapidly induce extended cortical necrosis and overt renal dysfunction (71, 95). At this stage, a large number of structural, metabolic, and functional alterations are observed in tubular cells (Table 1), and several of these alterations have been claimed to be responsible for cell death or dysfunction. Many of the changes observed at the level of the apical membrane (25, 45, 46, 113, 116) could, however, be merely mediated by a direct effect of the drug on this structure during its initial stages of uptake in proximal tubular cells. Conversely, other effects, such as inhibition of protein synthesis and modulation of gene expression, mitochondrial alterations, or inhibition of enzymes located on the cytosolic side of the pericellular membrane, must involve uptake and intracellular distribution of the drug to the corresponding targets.
ORIGIN OF TOXICITY
A major difficulty and a point of many controversies has been and still is ascertainment of which changes, among the numerous ones described above, are truly responsible for toxicity. It is partly the lack of unambiguous knowledge in this area which has prevented the launching of large-scale programs aimed at designing or screening new aminoglycosides on a rational basis after the trial-and-error approaches followed during the period from 1970 to 1980 had proven to be poorly successful (97).
TUBULAR NECROSIS
Histopathological studies strongly support the concept that tubular necrosis (and related phenomena) is the primary cause of functional toxicity. Frustratingly enough, however, the mechanism of this necrosis remains unsettled and cannot be unambiguously traced to a single, well-determined cause. It is, moreover, perfectly possible that no single change or alteration is important per se but that tubular cells eventually die because of the simultaneous occurrence of multiple changes (56). Three plausible lines of hypotheses have, however, been presented.
The first hypothesis assumes that aminoglycosides exert their toxicity in direct relation to their local concentration. This would therefore designate lysosomes as a key site and lysosomal alterations as a main cause of toxicity, since this is where the bulk of the tissue-bound drug is primarily stored. So far, however, the molecular and cytological links between the lysosomal alterations and cell necrosis have not been uncovered. A second hypothesis is that aminoglycosides become toxic once they are released from lysosomes. This release would take place when, and probably because, a critical threshold in lysosomal alterations and/or drug accumulation has been reached. Almost all the alterations listed in Table 1 are consistent with this hypothesis. If triggered abruptly, the release of large quantities of aminoglycosides from lysosomes could indeed cause the simultaneous development of a number of otherwise unrelated metabolic changes, many of which are capable of causing cell death. The question, then, is to distinguish between real toxic events from trivial or secondary effects, as well as from artifacts. A typical example is the inhibition of mitochondrial respiration and Ca2+ transport or lipid peroxidation, both of which were claimed to be causes of irreversible cell damage but which detailed studies eventually showed occur after cell death (31, 140). Aminoglycosides released from lysosomes could, however, act indirectly as nephrotoxins. In this connection, gentamicin was shown to chelate mitochondrial iron, forming a very oxidant Fe(II)-gentamicin complex capable of causing hair cell death (100). Finally, a third hypothesis is that the drug stored in lysosomes is intrinsically nontoxic but that, in parallel to endocytic uptake, a small amount of aminoglycoside reaches a critical, nonlysosomal target and causes toxicity (by this hypothesis, lysosomal storage could even protect the cell by retaining or diverting aminoglycosides from reaching these more crucial targets). Apical and basolateral membranes appear to be the best candidates because they are readily accessible in intact cells (from the extracellular fluids) and because changes at their levels may result in many potentially lethal effects. For instance, the changes in renal brush-border Na+ and Pi cotransport and Na+ and H+ exchange have been ascribed to an increase in membrane fluidity caused by a direct effect of gentamicin (79). Along the same lines, gentamicin was shown to cause the simultaneous inhibition of very different membrane protein species including Na+/K+ ATPase and a release of lactate dehydrogenase, resulting in an apparently multifactorial cell death process. Yet, many “membrane effects” actually require more than a simple contact of the drug with the outer part of the pericellular membrane (inhibition of Na+/K+ ATPase, for instance, occurs only if the aminoglycoside has access to the cytoplasm [34]). This clearly raises the question of a primary access of the drug to intracellular, nonlysosomal sites. Cell fractionation techniques applied to the kidney cortexes of rats treated with low doses detect aminoglycosides only in endocytic vacuoles and lysosomes (40). Yet, an autoradiographic study has suggested an early, transient occurrence of gentamicin in the cytosol of proximal tubular cells (139). In contrast, cell culture studies have always found the drug to be vacuolarly distributed, with a main localization in lysosomes, even though a recent study by confocal microscopy has shown that some of these vacuoles belong to the Golgi complex (108). The link between nonlysosomal localizations of aminoglycosides and the onset of early toxicity therefore remains an area for more investigations.
RENAL FAILURE
While the determinants of cell damage still remain undefined, more knowledge concerning the mechanisms causing the impairment of the renal function is available. Activation of the renin-angiotensin system and the ensuing local vasoconstriction appear to be primarily responsible for the decrease in glomerular filtration (45). This explains very well the aggravating effect of nonsteroidal anti-inflammatory drugs on aminoglycoside nephrotoxicity, since these drugs inhibit the production of the vasodilatatory prostaglandin PGE2 (4). An increase in proximal intratubular free-flow pressure of single nephrons, most likely related to necrotic obstruction, has also been observed (5), suggesting that the decline of glomerular filtration has a multifactorial origin and involves a combination of tubular and nontubular mechanisms. The hypoosmotic polyuria, characteristic of the aminoglycoside toxicity, has been shown to result from the decreased fluid reabsorption by proximal tubules, secondary to an impaired solute reabsorption (64, 105), evidenced by the ion-wasting phenomena described above.
EARLY COMPENSATION, REPAIR OF KIDNEY TISSUE AND POSTTOXIC ADAPTATION
The kidney has a large capacity to compensate for tubular insults so that an ongoing cell death process may long remain undetected by functional explorations. The demonstration that tubular cells undergo a marked proliferative response, even after low-dose treatments with aminoglycosides, has shed a new light on the significance of the so-called nontoxic alterations seen under these conditions (127). It may be speculated that one of the reasons why patients appear to be more sensitive to gentamicin than healthy animals (or young human volunteers) is their decreased ability to effectively regenerate and to sufficiently compensate for the spotty necrotic insults (131). The importance of regeneration for protection against renal dysfunction is clearly demonstrated by the fact that laboratory rats survive the repeated administration of relatively high daily doses of aminoglycosides (typically, 40 mg for gentamicin per kg per day for at least 42 days). After a first episode of acute tubular necrosis that occurs within 8 to 10 days and that is associated with a marked azotemia, renal function returns almost to normal, as if the kidney had become refractive (27, 28). This stage is actually related to the simultaneous occurrence of necrosis and regeneration of tubules in an asynchronous fashion (49). Regenerating cells are also less differentiated (127) and apparently less susceptible to aminoglycosides (accumulation of gentamicin is actually reduced in the cortex of animals treated for long periods of time by a mechanism that involves not a decrease in its binding but probably altered intracellular trafficking [119]).
DETERMINANTS OF THE AMINOGLYCOSIDE EARLY INSULT TO PROXIMAL TUBULES
While the molecular mechanisms of toxicity themselves are still unclear, it remains that the drugs must somehow physically interact with one or several cellular constituents to initiate the cascade of events leading to toxicity. Approaches aimed at reducing aminoglycoside toxicity should therefore preferably be targeted at preventing or modulating these early interactions. To us, two phenomena appear to be essential in this context, namely, the uptake of aminoglycosides into proximal tubular cells and their interactions with phospholipids in cells (130).
UPTAKE OF AMINOGLYCOSIDES IN TUBULAR CELLS
Aminoglycosides bind to the brush-border membrane in their cationic form (19). The initial points of attachment are probably the acidic phospholipids (and mainly phosphatidylserine, an abundant acidic phospholipid of brush borders), since modulation of the membrane content in these phospholipids results in commensurate changes in uptake (90). Quickly thereafter, aminoglycosides are transferred to the transmembrane protein megalin, with which they become internalized in endosomes (89). Megalin binds to many polybasic drugs and peptides and is expressed in the renal tubule epithelium and a few other specialized epithelial cell types including retinal and inner ear epithelia (143). It is probably responsible for the selective uptake and toxicity of aminoglycosides by these cells (109, 121) (the toxicity of intravitreal gentamicin toward retinal pigmented epithelium is a little-known but well-established fact [15]). N-Acetylneuraminic acid, the cortical content of which is increased in the kidneys during gentamicin treatment (60), could also be involved. Perhaps the most interesting aspect of this uptake process, from a toxicological point of view, is its saturable character at concentrations which are clinically relevant (apparent Km in rats, ≈15 μg/ml [39]).
INTERACTION OF AMINOGLYCOSIDES WITH PHOSPHOLIPIDS
Once transferred from endosomes to lysosomes through the physiological process of endosome-lysosome fusion, aminoglycosides will be exposed to a fairly acidic pH (≈5), at which they will be fully protonated and therefore expected to bind tightly to negatively charged structures. Among those, cellular membranes, which autophagy and heterophagy bring continuously to lysosomes, are probably a main target since they contain an average of 5 to 20% acidic phospholipids. In vitro studies show that aminoglycosides bind tightly to acidic phospholipids, primarily by electrostatic forces (20), and cause a marked decrease in the mobility of the phosphate heads in membrane bilayers (86). As shown in Fig. 2, gentamicin bound to phosphatidylinositol lies close to the interface, being inserted in the monolayer at the level of the phospho group and extending toward the hydrophobic phase up to the level of the ester linkage of the fatty acids (101, 133). The binding of aminoglycosides to lipid bilayers causes their aggregation (134) as well as the inhibition of the activities of phospholipases (48, 75). The latter is due to the neutralization of the surface negative charge which these enzymes require to fully express their activity (84, 96), as well as, perhaps, to the lesser accessibility of the substrate to the enzyme catalytic site (17). Enzyme inhibition and membrane aggregation most likely account for the conspicuous accumulation of myeloid bodies observed in lysosomes in vivo (myeloid bodies isolated from the renal cortex essentially contain phospholipids and proteins [the latter probably entrapped in the bilayers] but little cholesterol [3]). The main critical drug-related parameters involved in phospholipase inhibition appear to be (i) the energy of interaction between the drug and the surrounding negatively charged phospholipids, (ii) the drug orientation at the lipid-water interface, and (iii) the drug accessibility to the aqueous phase (85, 87). Although neither the phospholipid accumulation nor the inhibition of phospholipase activity by itself explains cell death, the extent of phospholipidosis induced by most aminoglycosides correlates rather nicely with their nephrotoxic potential (131). Moreover, as we shall see later, impairment of the binding of aminoglycosides to phospholipids or displacement of them from phospholipid layers protects against the development of both phospholipidosis and renal toxicity.
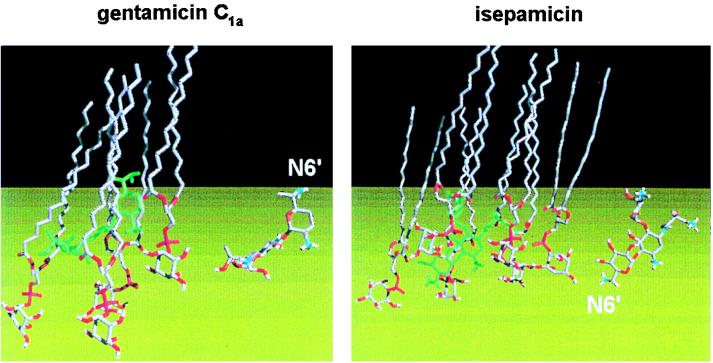
FIG. 2.
Skeleton views of the mode of assembly of gentamicin C1a and isepamicin (in green) with phosphatidylinositol (hydrocarbon is in grey and oxygen and phosphorus atoms are in red to clearly indicate the polar domain). The two isolated drug molecules, with the same orientation, are shown on the right for ease of identification of their various parts (carbons are in grey, oxygens are in red, and nitrogens are in blue; see Fig. 1 of the companion minireview [83] for the chemical structures of isepamicin and gentamicin C1a). The molecular modeling approach suggests that the orientations and the positions of the two drugs inserted in the phosphatidylinositol monolayer are entirely different. First, the N-6′ amino groups are oriented in opposite directions (toward the lipophilic phase in the case of gentamicin and toward the water phase for isepamicin. Second, gentamicin lies above the plane of the inositol moieties and far away from the water phase (bottom), whereas isepamicin is readily accessible and is probably therefore more easily displaceable. Similar differences have been noted between kanamycin A and amikacin (133). Since both isepamicin and amikacin are characterized by a side chain at position N-1, these differences have been ascribed to the presence of this side chain (note that the orientation and position of gentamicin C1a are very akin to those of gentamicin B, the parent, unsubstituted compound of isepamicin, and kanamycins). Such changes in position and orientation are thought to explain the lower inhibitory potential of amikacin and isepamicin toward the activities of phospholipases (for discussions, see references 16, 110, and 131). As outlined in this minireview and elsewhere (130, 133), inhibition of phospholipases is probably an important, early event in aminoglycoside nephrotoxicity. The figure was adapted from reference 133, with permission.
REDUCING OR PROTECTING AGAINST AMINOGLYCOSIDE NEPHROTOXICITY
The goal of reducing or protecting against aminoglycoside nephrotoxicity has attracted much effort and attention over the last decade. Based on the considerations discussed so far, these efforts can be subdivided into several types of approaches, as illustrated in Table 2.
TABLE 2.
Main approaches toward reduction of aminoglycoside nephrotoxicitya
| Mechanism | Compound |
|---|---|
| I. Decrease or prevention of drug accumulation by kidneys | |
| Intracellular complexation of aminoglycosides | |
| Polyanionic compounds | Dextran sulfate (59) |
| Inositol hexasulfate (67) | |
| Acidic drugs | Piperacillin (44) |
| Latamoxef-moxalactam (68) | |
| Fosfomycin (33, 54) | |
| Pyridoxal-5′-phosphate (114) | |
| Competition with or decrease in aminoglycoside binding to brush border membrane | |
| Raising the urine pH | Bicarbonate (19, 29) |
| Competitors | Ca2+ (diet supplementation [51] or vitamin D-induced hypercalcemia [21]) |
| Lysine (81) | |
| Aminoglycosides (as their own competitors) (39) | |
| Increase in exocytosis | Fleroxacin (9) |
| II. Prevention or decrease of lysosomal phospholipase inhibition | |
| Derivatives with lesser intrinsic bindingb | |
| N substitution | Amikacin (75), isepamicin (133), arbekacin,c 1-N- and 6′-N-peptidic and aminoacid derivative of kanamycin A and netilmicin (72) |
| Other substitution | 6"-substituted kanamycin B (88) |
| Fluorinated derivativesc | 5, 3"′ or 3′ fluoro derivatives of tobramycin, dibekacin, arbekacin, or kanamycinc |
| Disaccharidic aminoglycosides | Astromicin (fortimicin) (73) |
| Dactimicin (2-N"-formidoyl-astromicin) (53, 73) | |
| Coadministration of agent preventing intralysosomal phospholipidosis | |
| Intralysosomal sequestration of aminoglycosides | Polyaspartic acid (55, 62) |
| Increase of membrane negative charge | Daptomycin (41) |
| Other | Torbafylline (32) |
| III. Protection against necrosis and other gross cellular alterations | |
| Antioxidants | Deferroxamine (11) |
| Methimazole (24) | |
| Sairei-to (94) | |
| Vitamin E + selenium, vitamin C (1, 57) | |
| Lower copper feeding (58) | |
| Antioxidant and multifactorial factors | Lipoic acid (107) |
| IV. Protection against vascular and glomerular effects | |
| Suppression of renin-angiotensin activation | Deoxycortisone and saline drinking (45) |
| Protection against Ca2+ influx | Ca2+ channel blockers (80) |
| Undefined mechanism | Platelet activation antagonists (104) |
| V. Increase in kidney regeneration capabilities | |
| Unspecific mitogenic effect | Ulinastatin (92) |
| Growth factors | Fibroblast growth factor 2 (78) |
| Heparin-binding epidermal growth factor (106) |
References refer to publications dealing with the proposed mechanism; see text for further details on the extent and characterization of the protection.
See reference 83 for structures.
Mechanism is assumed on the basis of the substitution made (see reference 83 for a discussion and references to original papers), but it has not actually examined.
Decreasing or preventing aminoglycoside accumulation by kidneys.
Decreasing or preventing aminoglycoside accumulation by the kidneys would represent one of the most simple and radical approaches to reduce aminoglycoside nephrotoxicity, since it should lead to success whatever the targets of aminoglycosides are in the kidney. Aminoglycoside accumulation could be reduced either by impairing their uptake or by enhancing their release. Reduction of uptake has been obtained by two strategies. The first one is aimed at complexing the aminoglycosides extracellularly, and the second one is aimed at competing with or decreasing drug binding to the brush-border membrane. Table 2 illustrates the compounds used in this context. Unfortunately, these approaches could not be translated into clinical applications because of a lack of efficacy and/or because of intrinsic toxicity (21, 81). Yet, the strategy based on competition for binding eventually led to the recognition that aminoglycosides could be their own competitors. Early studies with animals (103) indeed revealed that administration of the daily dose of gentamicin as a single dose (thus creating one high daily peak level) was considerably less toxic than administration of the same daily dose divided into three doses per day or by continuous infusion (38). An explanation for this unexpected behavior came from the finding that aminoglycoside uptake by kidney tubular cells is saturable (39), so that much of the drug that passes in the lumen will not be reabsorbed if the drug is too concentrated. Because saturation was shown to occur at a clinically meaningful range of concentrations, this observation triggered a large number of studies comparing the toxicities of various drug administration schedules. Almost at the same time, animal and clinical studies demonstrated that a high, transient peak level in serum was also a critical determinant of aminoglycoside efficacy (see reference 35 for a review). The once-daily dosing mode of administration of aminoglycosides was therefore clinically tested in the late 1980s in a limited series of clinical trials that were at first cautious (118, 123, 132), but thereafter, it was tested with almost all indications for aminoglycosides (see reference 35 for a review and references 2, 6, 7, 13, and 43 for meta-analyses plus the large number of references cited therein). The once-daily regimen is now widely accepted (36), and it is in the official package insert recommendations for netilmicin and amikacin in several countries in Europe and elsewhere, even though discordant voices are still heard (12). Beyond its potential impact on the toxicity and activity of aminoglycosides, the once-daily dosing regimen also offers interesting pharmacoeconomic and practical advantages (93) and makes drug monitoring easier (98). A further development could be the implementation of once-daily administration at a specific hour of the day since there might be important circadian variations in the glomerular filtration rate and, hence, the availability of the aminoglycoside to the kidney (a recent clinical study has indicated that the administration of gentamicin during the midnight to 7 a.m. period was probably more likely to cause toxicity than administration during other periods of the day [8, 99]).
Preventing or decreasing the lysosomal phospholipidosis induced by the cell-associated aminoglycosides.
A reduction in lysosomal phospholipidosis could be achieved either by use of an aminoglycoside modified to bind less tightly to phospholipids at an acidic pH (Fig. 3) or by the administration of an agent that would prevent the binding of the antibiotic to phospholipids. Both strategies have been followed.
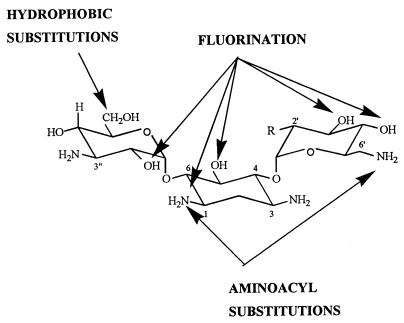
FIG. 3.
Structural modifications made in kanamycin A (R = OH) or kanamycin B (R = NH2) (these aminoglycosides are the most frequently used for chemical modifications) to obtain a modulation of the potential of the drug to cause lysosomal phospholipidosis in comparison with the structure of the corresponding parent compound. The figure is from references 65, 72, 88, 111, 122, 128, and 129.
(i) Aminoglycoside modifications.
As explained in the companion minireview (83), a series of 1-N-substituted derivatives of gentamicins and kanamycins were synthesized in the late 1970s to obtain molecules resistant to the bacterial enzymes that inactivate the parent compounds. Retrospectively, it was found that all derivatives in which the N-1 atom has been made nonionizable (i.e., by substitution of the amino function with an acyl side chain such as in amikacin) show reduced levels of binding to acidic phospholipids together with a lesser inhibitory potency toward lysosomal phospholipases (16). Among them, amikacin, isepamicin, and arbekacin have been successfully developed and have been proved to cause less intense renal changes in animals as well as in humans when they are tested under strictly comparable conditions with other currently used aminoglycosides (14, 22, 70, 75, 82). For amikacin and isepamicin, this effect has been ascribed to a lesser degree of interaction of the amino functions of these drugs with the phospho group of negatively charged phospholipid (17, 110) and to changes in the orientation of the drug bound to the lipid layer (133) (see the illustration of isepamicin in Fig. 2; these models, however, have recently been challenged [17]). Efforts have therefore been made accordingly to rationally design new aminoglycosides with similar and, it is hoped, even more favorable properties. This led to the synthesis of derivatives of kanamycin B substituted at position C-6" with halogen atoms, a pseudo-halogen group (azido), or increasingly bulkier alkyl chains via an intermediate N, S, or O atom, yielding the corresponding 6"-amino, -amido, -thioalkyl, or -alkoxy derivatives (88), as well as to derivatives of netilmicin and kanamycin A substituted at positions N-1 and N-6′ with amino acids (72), but with only modest success. In a similar context, the disaccharidic aminoglycosides astromicin (fortimicin A) and dactimicin (2-N"-formimidoyl-astromicin) have been shown to bind less tightly to phospholipid bilayers and to be weaker inhibitors of lysosomal phospholipases (73). Their lesser nephrotoxicities observed in animals could, however, mainly be due to their lower levels of accumulation in the renal cortex (53).
Of greater interest are probably the derivatives of tobramycin, dibekacin, arbekacin, or kanamycin with a fluorine atom at position 5, 3′, or 3"′ (65, 111, 122, 128, 129). These were originally made to confer resistance to aminoglycoside-inactivating enzymes, but for these derivatives chemical and biophysical considerations suggest a reduced level of binding to phospholipids because of a decreased basicity of the vicinal amino group through an inductive effect. These compounds showed increased 50% lethal doses, but experimental data on their binding to phospholipids are not available.
(ii) Prevention of aminoglycoside binding to phospholipids.
Polyaspartic acid has emerged as a very successful protectant against aminoglycoside-induced nephrotoxicity from the screening of various polymers that are likely to impair the binding of aminoglycosides to kidney membrane vesicles (142). In experimental studies with animals, the coadministration of polyaspartic acid with gentamicin or amikacin was shown to protect against the development of phospholipidosis and phospholipiduria (61), as well against all early and late signs of aminoglycoside nephrotoxicity (26, 37). Actually, both polyaspartic acid and the aminoglycoside reach the lysosomes by endocytosis (55) and form ion-pair complexes within these organelles due to the acidic pH prevailing therein. In vitro studies demonstrated that polyaspartic acid prevents aminoglycoside binding to negatively charged phospholipids bilayers and thereby makes the drug unable to inhibit the activities of lysosomal phospholipases (62). Further studies showed that polyaspartic acid also protects against gentamicin-induced alterations of phospholipid metabolism in cultured cells (102) and of electrophysiological alterations in cultured human proximal tubular cells (125). Polyaspartic acid also prevents impairment by gentamicin of homotypic fusion of renal cell endosomes (42) and blocks the process of aminoglycoside-induced aggregation of negatively charged liposomes (134), all events which had been directly related to the binding of gentamicin to phospholipids. In vivo studies have now defined the limits and the duration of the protection afforded by polyaspartic acid (120). Moreover, pharmacokinetic evaluations have shown that polyaspartic acid increases the penetration of gentamicin in the so-called deep peripheral compartment (which most likely represents the intracellular drug-polyaspartic acid complex [141] and which suggests that the antibiotic is stored in a nontoxic form). A protective effect of polyaspartic acid against ototoxicity has also been demonstrated (50). A movement toward large-scale toxicological studies and clinical applications of polyaspartic acid therefore appears to be warranted but is still hindered by the lack of a clear definition of the precise type of polymer which needs to be used. It must indeed, at the same time, be filtratable through the glomerulus, bind effectively to gentamicin (66), remain sufficiently stable in the kidney to afford significant protection (61), and not causing renal toxicity per se, as was shown for polymers that are too stable (63).
Daptomycin (LY 146032), which contains three Asp residues, also colocalizes in the lysosomes of the renal cortex with gentamicin and protects against lysosomal alterations in vivo (124). In vitro, it increases the negative charge density of membranes, while at the same time affecting the lipid packing (41), two effects which counteract those of gentamicin and facilitate the access of the catalytic site of the phospholipases to their lipidic substrate. Torbafylline (HWA-448), an analog of the vasculoactive agent pentoxifylline, also protects against gentamicin-induced phospholipidosis, but its mode of action is unknown (32).
Other means of protection.
Among the other main approaches used so far to reduce or to protect against aminoglycoside nephrotoxicity (Table 2), the most consistent effects have been observed with the use of antioxidants and especially deferroxamine. On the basis of the finding that gentamicin forms complexes with mitochondrial Fe2+ to catalyze the formation of free oxygen radicals (see above), iron chelators were tested and were proven to be effective in the prevention of aminoglycoside-induced ototoxicity (109, 117). Extension of this finding to nephrotoxicity appears to be possible (136), but biophysical and biochemical considerations (100) suggest that the protective effect of deferroxamine may be critically dependent on the dosage of gentamicin. Other compounds were also used on account of their antioxidant effects (Table 2), but the mechanisms have not always been unambiguously established. Means of protection based on a correction of the functional abnormalities or on an increase in cell regeneration capabilities have also been attempted, but no clinical application has so far been made.
CONCLUSIONS
The study of aminoglycoside nephrotoxicity has clearly identified several critical mechanisms, the knowledge of which may allow for the safer use of these drugs. Among the various approaches applicable to the presently available aminoglycosides, only once-a-day dosing has already been brought successfully to the clinic. Other protective approaches such as the coadministration of polyaspartic acid or deferroxamine deserve preclinical and clinical development, and many more could certainly be explored. Our progress in molecular modeling and an improved knowledge of the meaningful differences in structure-activity and structure-toxicity relationships for aminoglycosides (see the companion minireview [83]) could also bring us, before too long new, intrinsically less toxic aminoglycosides.
ACKNOWLEDGMENTS
M.-P.M.-L. is Chercheur Qualifié of the Belgian Fonds National de la Recherche Scientifique. Support was received from the Belgian Fonds de la Recherche Scientifique Médicale (grants 3.4589.96, 3.4516.94, and 9.4514.92), the Fonds National de la Recherche Scientifique (grant 9.4546.94), the Actions de Recherches Concertées 94/99-172 of the Direction Générale de la Recherche Scientifique-Communauté Française de Belgique of Belgium, and the French nonprofit organization (Association-loi 1901) Vaincre les Maladies Lysosomales.
We thank R. Brasseur (Centre de Biophysique Moléculaire Numérique, Faculté des Sciences Agronomiques de Gembloux, Gembloux, Belgium) for performing computer-aided conformational analysis of aminoglycoside-phosphatidylinositol interactions.
REFERENCES
- 1.Ademuyiwa O, Ngaha E O, Ubah F O. Vitamin E and selenium in gentamicin nephrotoxicity. Hum Exp Toxicol. 1990;9:281–288. doi: 10.1177/096032719000900504. [DOI] [PubMed] [Google Scholar]
- 2.Ali M Z, Goetz M B. A meta-analysis of the relative efficacy and toxicity of single daily dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:796–809. doi: 10.1093/clinids/24.5.796. [DOI] [PubMed] [Google Scholar]
- 3.Appelkvist E L, Soderstrom M, Nassberger L, Damberg C, Dallner G, DePierre J W. Characterization of the lipid and protein contents of myelin bodies isolated from the renal cortex of gentamicin-treated rats. Biochem Biophys Res Commun. 1991;181:894–901. doi: 10.1016/0006-291x(91)91275-h. [DOI] [PubMed] [Google Scholar]
- 4.Assael B M, Chiabrando C, Gagliardi L, Noseda A, Bamonte F, Salmona M. Prostaglandins and aminoglycoside nephrotoxicity. Toxicol Appl Pharmacol. 1985;78:386–394. doi: 10.1016/0041-008x(85)90244-3. [DOI] [PubMed] [Google Scholar]
- 5.Aynedjian H S, Nguyen D, Lee H Y, Sablay L B, Bank N. Effects of dietary electrolyte supplementation on gentamicin nephrotoxicity. Am J Med Sci. 1988;295:444–452. doi: 10.1097/00000441-198805000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Bailey T C, Little J R, Littenberg B, Reichley R M, Dunagan W C. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:786–795. doi: 10.1093/clinids/24.5.786. [DOI] [PubMed] [Google Scholar]
- 7.Barza M, Ioannidis J P, Cappelleri J C, Lau J. Single or multiple daily doses of aminoglycosides: a meta-analysis. Br Med J. 1996;312:338–345. doi: 10.1136/bmj.312.7027.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauchamp D, Guimont C, Grenier L, LeBrun M, Tardif D, Gourde P, Bergeron M G, Thilbault L, Labrecque G. Time-restricted feeding schedules modify temporal variation of gentamicin experimental nephrotoxicity. Antimicrob Agents Chemother. 1997;41:1468–1474. doi: 10.1128/aac.41.7.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauchamp D, Laurent G, Grenier L, Gourde P, Zanen J, Heuson-Stiennon J A, Bergeron M G. Attenuation of gentamicin induced nephrotoxicity in rats by fleroxacin. Antimicrob Agents Chemother. 1997;41:1237–1245. doi: 10.1128/aac.41.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Begg E J, Barclay M L. Aminoglycosides—50 years on. Br J Clin Pharmacol. 1995;39:597–603. [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Ismail T H, Ali B H, Bashir A A. Influence of iron, deferroxamine and ascorbic acid on gentamicin-induced nephrotoxicity in rats. Gen Pharmacol. 1994;25:1249–1252. doi: 10.1016/0306-3623(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 12.Bertino J S, Rotschafer J C. Editorial response: single daily dosing of aminoglycosides. A concept whose time has not yet come. Clin Infect Dis. 1997;24:820–823. doi: 10.1093/clinids/24.5.820. [DOI] [PubMed] [Google Scholar]
- 13.Blaser J, Konig C. Once-daily dosing of aminoglycosides. Eur J Clin Microbiol Infect Dis. 1995;14:1029–1038. doi: 10.1007/BF01590935. [DOI] [PubMed] [Google Scholar]
- 14.Blum D. An overview of the safety of isepamicin in adults. J Chemother. 1995;7(Suppl. 2):87–93. [PubMed] [Google Scholar]
- 15.Brown G C, Eagle R C, Shakin E P, Gruber M, Arbizio V V. Retinal toxicity of intravitreal gentamicin. Arch Ophthalmol. 1990;108:1740–1744. doi: 10.1001/archopht.1990.01070140094037. [DOI] [PubMed] [Google Scholar]
- 16.Carlier M B, Laurent G, Claes P J, Vanderhaeghe H J, Tulkens P M. Inhibition of lysosomal phospholipases by aminoglycoside antibiotics: in vitro comparative studies. Antimicrob Agents Chemother. 1983;23:440–449. doi: 10.1128/aac.23.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrier D, Chartrand N, Matar W. Comparison of the effects of amikacin and kanamycins A and B on dimyristoylphosphatidylglycerol bilayers. Biochem Pharmacol. 1997;53:401–408. doi: 10.1016/s0006-2952(96)00765-4. [DOI] [PubMed] [Google Scholar]
- 18.Casteels-Van Daele M, Corbeel L, Van de Casseye W, Standaert L. Gentamicin-induced Fanconi syndrome. J Pediatr. 1980;97:507–508. doi: 10.1016/s0022-3476(80)80230-7. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 19.Chiu P J S, Miller G H, Long J F, Waitz J A. Renal uptake and nephrotoxicity of gentamicin during urinary alkalinization in rats. Clin Exp Pharmacol Physiol. 1979;6:317–326. doi: 10.1111/j.1440-1681.1979.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 20.Chung L, Kaloyanides G, McDaniel R, McLaughin A, McLaughin S. Interaction of gentamicin and spermine with bilayer membranes containing negatively-charged phospholipids. Biochemistry. 1985;24:442–452. doi: 10.1021/bi00323a030. [DOI] [PubMed] [Google Scholar]
- 21.Cohen R, Johnson K, Humes H D. Potentiation of aminoglycoside nephrotoxicity by vitamin-D-induced hypercalcemia. Miner Electrolyte Metab. 1988;14:121–128. [PubMed] [Google Scholar]
- 22.De Broe M E, Paulus G J, Verpooten G A, Roels F, Buyssens N, Wedeen R, Tulkens P M. Early effects of gentamicin, tobramycin and amikacin on the human kidney. Kidney Int. 1984;25:643–652. doi: 10.1038/ki.1984.69. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez J H, Hale C C, Qulali M. Studies of renal injury. I. Gentamicin toxicity and expression of basolateral transporters. Am J Physiol. 1996;270:F245–F253. doi: 10.1152/ajprenal.1996.270.2.F245. [DOI] [PubMed] [Google Scholar]
- 24.Elfarra A A, Duescher R J, Sausen P J, O’Hara T M, Cooley A J. Methimazole protection of rats against gentamicin-induced nephrotoxicity. Can J Physiol Pharmacol. 1994;72:1238–1244. doi: 10.1139/y94-176. [DOI] [PubMed] [Google Scholar]
- 25.Elliott W C, Patchin D S. Aminoglycoside-mediated calciuresis. J Pharmacol Exp Ther. 1992;262:151–156. [PubMed] [Google Scholar]
- 26.Elliott W C, Patchin D S. Effects and interactions of gentamicin, polyaspartic acid and diuretics on urine calcium concentration. J Pharmacol Exp Ther. 1995;273:280–284. [PubMed] [Google Scholar]
- 27.Elliott W C, Houghton D C, Gilbert D N, Baines-Hunter J, Bennett W M. Gentamicin nephrotoxicity. I. Degree and permanence of acquired insensitivity. J Lab Clin Med. 1982;100:501–512. [PubMed] [Google Scholar]
- 28.Elliott W C, Houghton D C, Gilbert D N, Baines-Hunter J, Bennett W M. Gentamicin nephrotoxicity. II. Definition of conditions necessary to induce acquired insensitivity. J Lab Clin Med. 1982;100:513–525. [PubMed] [Google Scholar]
- 29.Elliott W C, Parker R A, Houghton D C, Gilbert D N, Porter G A, DeFehr J, Bennett W M. Effect of sodium bicarbonate and ammonium chloride ingestion in experimental gentamicin nephrotoxicity in rats. Res Commun Chem Pathol Pharmacol. 1980;28:483–495. [PubMed] [Google Scholar]
- 30.Fabre J, Rudhardt M, Blanchard P, Regamey C, Chauvin P. Persistence of sisomicin and gentamicin in renal cortex and medulla compared with other organs and serum of rats. Kidney Int. 1976;10:444–449. doi: 10.1038/ki.1976.131. [DOI] [PubMed] [Google Scholar]
- 31.Fauconneau B, Tallineau C, Huguet F, Piriou A. Gentamicin-induced kidney damage and lipid peroxidation in rats. Toxicol Lett. 1995;76:127–134. doi: 10.1016/0378-4274(94)03205-l. [DOI] [PubMed] [Google Scholar]
- 32.Ford D M, Thieme R E, Lamp C A, Covington S J, Molitoris B A. HWA-448 reduces gentamicin toxicity in LLC-PK1 cells. J Pharmacol Exp Ther. 1995;274:29–33. [PubMed] [Google Scholar]
- 33.Fujita K, Fujita H M, Aso Y. Protective effect of fosfomycin against renal accumulation of aminoglycoside antibiotics. Jpn J Antibiot. 1983;36:3392–3394. [PubMed] [Google Scholar]
- 34.Fukuda Y, Malmborg A S, Aperia A. Gentamicin inhibition of Na+, K+-ATPase in rat kidney cells. Acta Physiol Scand. 1991;141:27–34. doi: 10.1111/j.1748-1716.1991.tb09040.x. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert D N. Aminoglycosides. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 279–306. [Google Scholar]
- 36.Gilbert D N. Editorial response: meta-analyses are no longer required for determining the efficacy of single daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:816–819. doi: 10.1093/clinids/24.5.816. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert D N, Woods C A, Kohlhepp S J, Kohnen P W, Houghton D C, Finkbeiner H C, Bennett W M. Polyaspartic acid prevents experimental aminoglycoside nephrotoxicity. J Infect Dis. 1989;159:945–953. doi: 10.1093/infdis/159.5.945. [DOI] [PubMed] [Google Scholar]
- 38.Giuliano R A, Paulus G J, Verpooten G A, Pattijn V M, Pollet D E, Nouwen E J, De Broe M E. Recovery of cortical phospholipidosis and necrosis after acute gentamicin loading in rats. Kidney Int. 1984;26:838–847. doi: 10.1038/ki.1984.226. [DOI] [PubMed] [Google Scholar]
- 39.Giuliano R A, Verpooten G A, Verbist L, Wedeen R, De Broe M E. In vivo uptake kinetics of aminoglycosides in the kidney cortex of rats. J Pharmacol Exp Ther. 1986;236:470–475. [PubMed] [Google Scholar]
- 40.Giurgea-Marion L, Toubeau G, Laurent G, Heuson-Stiennon J, Tulkens P M. Impairment of lysosome-pinocytic vesicle fusion in rat kidney proximal tubules after treatment with gentamicin at low doses. Toxicol Appl Pharmacol. 1986;86:271–285. doi: 10.1016/0041-008x(86)90058-x. [DOI] [PubMed] [Google Scholar]
- 41.Gurnani K, Khouri H, Couture M, Bergeron M G, Beauchamp D, Carrier D. Molecular basis of the inhibition of gentamicin nephrotoxicity by daptomycin: an infrared spectroscopic investigation. Biochem Biophys Acta. 1995;1237:86–94. doi: 10.1016/0005-2736(95)00082-e. [DOI] [PubMed] [Google Scholar]
- 42.Hammond T G, Majewski R R, Kaysen J H, Goda F O, Navar G L, Pontillon F, Verroust P J. Gentamicin inhibits rat renal cortical homotypic endosomal fusion: role of megalin. Am J Physiol. 1997;272:F117–F123. doi: 10.1152/ajprenal.1997.272.1.F117. [DOI] [PubMed] [Google Scholar]
- 43.Hatala R, Dinh T T, Cook D J. Single daily dosing of aminoglycosides in immunocompromised adults: a systematic review. Clin Infect Dis. 1997;24:810–815. doi: 10.1093/clinids/24.5.810. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi T, Watanabe Y, Kumano K, Kitayama R, Yasuda T, Saikawa I, Shimizu K. Protective effect of piperacillin against nephrotoxicity of cephaloridine and gentamicin in animals. Antimicrob Agents Chemother. 1988;32:912–918. doi: 10.1128/aac.32.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hishida A, Nakajima T, Yamada M, Kato A, Honda N. Roles of hemodynamic and tubular factors in gentamicin-mediated nephropathy. Renal Fail. 1994;16:109–116. doi: 10.3109/08860229409044852. [DOI] [PubMed] [Google Scholar]
- 46.Hori R, Okuda M, Ohishi Y, Yasuhara M, Takano M. Surface binding and intracellular uptake of gentamicin in the cultured kidney epithelial cell line (LLC-PK1) J Pharmacol Exp Ther. 1992;261:1200–1205. [PubMed] [Google Scholar]
- 47.Horio M, Fukuhara Y, Orita Y, Nakanishi T, Nakahama H, Moriyama T, Kamada T. Gentamicin inhibits Na+ dependent d-glucose transport in rabbit kidney brush-border membrane vesicles. Biochim Biophys Acta. 1986;858:153–160. doi: 10.1016/0005-2736(86)90301-9. [DOI] [PubMed] [Google Scholar]
- 48.Hostetler K Y, Hall L B. Aminoglycoside antibiotics inhibit lysosomal phospholipase A and C from rat liver in vitro. Biochim Biophys Acta. 1982;710:506–509. doi: 10.1016/0005-2760(82)90136-9. [DOI] [PubMed] [Google Scholar]
- 49.Houghton D C, Lee D, Gilbert D N, Bennett W M. Chronic gentamicin nephrotoxicity. Continued tubular injury with preserved glomerular filtration function. Am J Pathol. 1986;123:183–194. [PMC free article] [PubMed] [Google Scholar]
- 50.Hulka G F, Prazma J, Brownlee R E, Pulver S, Pillsbury H C. Use of poly-l-aspartic acid to inhibit aminoglycoside cochlear ototoxicity. Am J Otol. 1993;14:352–359. [PubMed] [Google Scholar]
- 51.Humes H D, Sastrasinh M, Weinberg J M. Calcium is a competitive inhibitor of gentamicin-renal membrane binding interactions and dietary calcium supplementation protects against gentamicin nephrotoxicity. J Clin Invest. 1984;73:134–147. doi: 10.1172/JCI111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutchin T, Cortopassi G. Proposed molecular and cellular mechanism for aminoglycoside ototoxicity. Antimicrob Agents Chemother. 1994;38:2517–2520. doi: 10.1128/aac.38.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inouye S, Niizato T, Shomura T, Kitasato I. Nephrotoxicity of dactimicin, a novel pseudodisaccharide aminoglycoside possessing the N-formimidoyl group, compared with that of astromicin, amikacin and other aminoglycoside antibiotics in animals. Drugs Exp Clin Res. 1989;15:189–209. [PubMed] [Google Scholar]
- 54.Inouye S, Niizato T, Takeda U, Koeda T. Protective effect of fosfomycin on the experimental nephrotoxicity induced by dibekacin. J Pharmacobio-dyn. 1982;5:659–669. doi: 10.1248/bpb1978.5.659. [DOI] [PubMed] [Google Scholar]
- 55.Kállay Z, Tulkens P M. Uptake and subcellular distribution of poly-l-aspartic acid, a protectant against aminoglycoside-induced nephrotoxicity, in rat kidney cortex. In: Bach P H, Lock E A, editors. Nephrotoxicity: extrapolation from in vitro to in vivo, and animals to man. London, United Kingdom: Plenum Press; 1989. pp. 189–192. [Google Scholar]
- 56.Kaloyanides G J. Aminoglycoside-induced functional and biochemical defects in the renal cortex. Fundam Appl Toxicol. 1984;4:930–943. doi: 10.1016/0272-0590(84)90231-8. [DOI] [PubMed] [Google Scholar]
- 57.Kavutcu M, Canbolat O, Ozturk S, Olcay E, Ulutepe S, Ekinci C, Durak I. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin-treated guinea pigs: effects of vitamins E and C. Nephron. 1996;72:269–274. doi: 10.1159/000188853. [DOI] [PubMed] [Google Scholar]
- 58.Kays S E, Crowell W A, Johnson M A. Influence of copper nutrition on gentamicin nephrotoxicity in rats. Magnesium Trace Elem. 1990;9:294–302. [PubMed] [Google Scholar]
- 59.Kikuchi S, Aramaki Y, Nonaka H, Tsuchiya S. Effects of dextran sulphate on renal dysfunctions induced by gentamicin as determined by the kidney perfusion technique in rats. J Pharm Pharmacol. 1991;43:292–293. doi: 10.1111/j.2042-7158.1991.tb06690.x. [DOI] [PubMed] [Google Scholar]
- 60.Kishore B K, Ibrahim S, Tulkens P M. Increased levels of protein- and lipid-bound sialic acids in the renal cortex of rats injected with low doses of gentamicin. Toxicol Lett. 1990;51:59–65. doi: 10.1016/0378-4274(90)90225-b. [DOI] [PubMed] [Google Scholar]
- 61.Kishore B K, Ibrahim S, Lambricht P, Laurent G, Maldague P, Tulkens P M. Comparative assessment of poly-l-aspartic and poly-l-glutamic acids against gentamicin-induced renal lysosomal phospholipidosis, phospholipiduria and cell proliferation in rats. J Pharmacol Exp Ther. 1992;262:424–432. [PubMed] [Google Scholar]
- 62.Kishore B K, Kállay Z, Lambricht P, Laurent G, Tulkens P M. Mechanism of protection afforded by polyaspartic acid against gentamicin-induced phospholipidosis. I. Polyaspartic acid binds and displaces gentamicin from negatively-charged phospholipid layers. J Pharmacol Exp Ther. 1990;255:867–874. [PubMed] [Google Scholar]
- 63.Kishore B K, Lambricht P, Laurent G, Maldague P, Wagner R, Tulkens P M. Mechanism of protection afforded by polyaspartic acid against gentamicin-induced phospholipidosis. II. Comparative in vitro and in vivo studies with poly-l-aspartic, poly-l-glutamic and poly-d-glutamic acids. J Pharmacol Exp Ther. 1990;255:875–885. [PubMed] [Google Scholar]
- 64.Klotman P E, Yarger W E. Reduction of renal blood flow and proximal bicarbonate reabsorption in rats by gentamicin. Kidney Int. 1983;24:638–643. doi: 10.1038/ki.1983.205. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi Y, Tsuchiya T, Ohgi T, Taneichi N, Koyama Y. Study on fluorination of 2,3-dideoxy-2,3-(N-tosylepimino)-alpha-d-allopyranosides, and synthesis of 3′-deoxy-3′-fluoro-kanamycin B and 3′,4′-dideoxy-3′-fluorokanamycin B. Carbohydr Res. 1992;230:89–105. doi: 10.1016/s0008-6215(00)90515-9. [DOI] [PubMed] [Google Scholar]
- 66.Kohlhepp S J, McGregor D N, Cohen S J, Kohlhepp M E, Gilbert D N. Determinants of the in vitro interaction of polyaspartic acid and aminoglycoside antibiotics. J Pharmacol Exp Ther. 1992;263:1464–1470. [PubMed] [Google Scholar]
- 67.Kojima R, Suzuki Y. Studies on the nephrotoxicity of aminoglycoside antibiotics and protection from these effects (9): protective effect of inositol hexasulfate against tobramycin-induced nephrotoxicity. Jpn J Pharmacol. 1990;53:347–358. doi: 10.1254/jjp.53.347. [DOI] [PubMed] [Google Scholar]
- 68.Kojima R, Ito M, Suzuki Y. Studies on the nephrotoxicity of aminoglycoside antibiotics and protection from these effects (6): a mechanism for the suppressive action of latamoxef on intrarenal tobramycin level. Jpn J Pharmacol. 1990;53:111–120. doi: 10.1254/jjp.53.111. [DOI] [PubMed] [Google Scholar]
- 69.Komatsuda A, Wakui H, Imai H, Miura A B, Itoh H, Tashima Y. Expression of 90-kDa heat shock protein within cellular crescents in human diseased kidneys. Nephrology. 1996;2:87–91. [Google Scholar]
- 70.Kondo S. Development of arbekacin and synthesis of new derivatives stable to enzymatic modifications by methicillin-resistant Staphyloccus aureus. Jpn J Antibiot. 1994;47:561–574. [PubMed] [Google Scholar]
- 71.Kosek J C, Mazze R I, Cousins M J. Nephrotoxicity of gentamicin. Lab Invest. 1974;30:48–57. [PubMed] [Google Scholar]
- 72.Kotretsou S, Mingeot-Leclercq M-P, Constantinou-Kokotou V, Brasseur R, Georgiadis M P, Tulkens P M. Synthesis and antimicrobial and toxicological studies of amino acid and peptide derivatives of kanamycin A and netilmicin. J Med Chem. 1995;38:4710–4719. doi: 10.1021/jm00023a011. [DOI] [PubMed] [Google Scholar]
- 73.Lambricht P. Ph.D thesis. Louvain, Belgium: Université Catholique de Louvain; 1992. Evaluation systématique et prospective du potentiel toxique de nouveaux aminoglycosides; p. 168. [Google Scholar]
- 74.Landau D, Kher K K. Gentamicin induced Bartter-like syndrome. Pediatr Nephrol. 1997;11:737–740. doi: 10.1007/s004670050378. [DOI] [PubMed] [Google Scholar]
- 75.Laurent G, Carlier M B, Rollman B, Van Hoof F, Tulkens P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem Pharmacol. 1982;31:3861–3870. doi: 10.1016/0006-2952(82)90303-3. [DOI] [PubMed] [Google Scholar]
- 76.Laurent G, Kishore B K, Tulkens P M. Aminoglycoside-induced phospholipidosis and nephrotoxicity. Biochem Pharmacol. 1990;40:2383–2392. doi: 10.1016/0006-2952(90)90078-y. [DOI] [PubMed] [Google Scholar]
- 77.Laurent G, Maldague P, Carlier M B, Tulkens P. Increased renal DNA synthesis in vivo after administration of low doses of gentamicin to rats. Antimicrob Agents Chemother. 1983;24:586–593. doi: 10.1128/aac.24.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonard I, Zanen J, Nonclercq D, Toubeau G, Heuson-Stiennon J A, Beckers J F, Laurent G. Modification of immunoreactive EGF and EGF receptor after acute tubular necrosis induced by tobramycin or cisplatin. Renal Fail. 1994;16:583–608. doi: 10.3109/08860229409044887. [DOI] [PubMed] [Google Scholar]
- 79.Levi M, Cronin R E. Early selective effects of gentamicin on renal brush-border membrane Na-Pi cotransport and Na-H exchange. Am J Physiol. 1990;258:F1379–F1387. doi: 10.1152/ajprenal.1990.258.5.F1379. [DOI] [PubMed] [Google Scholar]
- 80.Lortholary O, Blanchet F, Nochy D, Heudes D, Seta N, Amirault P, Carbon C. Effects of diltiazem on netilmicin-induced nephrotoxicity in rabbits. Antimicrob Agents Chemother. 1993;37:1790–1798. doi: 10.1128/aac.37.9.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malis C D, Racusen L C, Solez K, Whelton A. Nephrotoxicity of lysine and of a single dose of aminoglycoside in rats given lysine. J Lab Clin Med. 1984;103:660–676. [PubMed] [Google Scholar]
- 82.Miller G H, Chiu P J S, Waitz J A. Biological activity of Sch 21420, the 1-N-S-alphahydroxybeta aminopropionyl-derivative of gentamicin B. J Antibiot. 1978;31:688–696. doi: 10.7164/antibiotics.31.688. [DOI] [PubMed] [Google Scholar]
- 83.Mingeot-Leclercq M P, Glupczynsky Y, Tulkens P M. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother. 1999;43:727–737. doi: 10.1128/aac.43.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mingeot-Leclercq M P, Laurent G, Tulkens P M. Biochemical mechanism of aminoglycoside-induced inhibition of phosphatidylcholine hydrolysis by lysosomal phospholipases. Biochem Pharmacol. 1988;37:591–599. doi: 10.1016/0006-2952(88)90130-x. [DOI] [PubMed] [Google Scholar]
- 85.Mingeot-Leclercq M P, Piret J, Brasseur R, Tulkens P M. Effect of acidic phospholipids on the activity of lysosomal phospholipases and on their inhibition by aminoglycoside antibiotics. I. Biochemical analysis. Biochem Pharmacol. 1990;40:489–497. doi: 10.1016/0006-2952(90)90547-x. [DOI] [PubMed] [Google Scholar]
- 86.Mingeot-Leclercq M P, Schanck A, Ronveaux-Dupal M F, Deleers M, Brasseur R, Ruysschaert J M, Tulkens P M. Ultrastructural, physico-chemical and conformational study of the interactions of gentamicin and bis(beta-diethylaminoethylether)hexestrol with negatively charged phospholipid bilayers. Biochem Pharmacol. 1989;38:729–741. doi: 10.1016/0006-2952(89)90225-6. [DOI] [PubMed] [Google Scholar]
- 87.Mingeot-Leclercq M-P, Tulkens P M, Brasseur R. Accessibility of aminoglycosides, isolated and in interaction with phosphatidylinositol, to water: a conformational analysis using the concept of molecular hydrophobicity potential. Biochem Pharmacol. 1992;44:1967–1975. doi: 10.1016/0006-2952(92)90099-5. [DOI] [PubMed] [Google Scholar]
- 88.Mingeot-Leclercq M P, Van Schepdael A, Brasseur R, Busson R, Vanderhaeghe H J, Claes P J, Tulkens P M. New derivatives of kanamycin B obtained by modifications and substitutions in position 6". II. In vitro and computer-aided toxicological evaluation, with respect to interactions with phosphatidylinositol. J Med Chem. 1991;34:1476–1482. doi: 10.1021/jm00108a036. [DOI] [PubMed] [Google Scholar]
- 89.Moestrup S K, Cui S, Vorum H, Bregengard C, Bjorn S E, Norris K, Christensen E I. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest. 1995;96:1404–1413. doi: 10.1172/JCI118176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molitoris B A, Simon F R. Renal cortical brush-border and basolateral membranes: cholesterol and phospholipid composition and relative turn-over. J Membr Biol. 1985;83:207–215. doi: 10.1007/BF01868695. [DOI] [PubMed] [Google Scholar]
- 91.Moore R D, Lietman P S, Smith C R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 92.Nakakuki M, Yamasaki F, Shinkawa T, Kudo M, Watanabe M, Mizota M. Protective effect of human ulinastatin against gentamicin-induced acute renal failure in rats. Can J Physiol Pharmacol. 1996;74:104–111. [PubMed] [Google Scholar]
- 93.Nicolau D P, Freeman C D, Belliveau P P, Nightingale C H, Ross J W, Quintilliani R. Experience with once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother. 1995;39:650–655. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohno I, Shibasaki T, Nakano H, Matsuda H, Matsumoto H, Misawa T, Sakai O. Effect of Sairei-to on gentamicin nephrotoxicity in rats. Arch Toxicol. 1993;67:145–147. doi: 10.1007/BF01973686. [DOI] [PubMed] [Google Scholar]
- 95.Parker R A, Bennett W H, Porter G A. Animal models in the study of aminoglycoside nephrotoxicity. In: Whelton A, Neu H C, editors. The aminoglycosides: microbiology, clinical use and toxicology. New York, N.Y: Marcel Dekker, Inc.; 1982. pp. 235–267. [Google Scholar]
- 96.Piret J, Kishore B K, Tulkens P M. Effect of substrate organization on the activity and on the mechanism of gentamicin-induced inhibition of rat liver lysosomal phospholipase A1. Biochem Pharmacol. 1992;43:895–898. doi: 10.1016/0006-2952(92)90258-k. [DOI] [PubMed] [Google Scholar]
- 97.Price K E. Aminoglycoside research 1975–1985: prospects for development of improved agents. Antimicrob Agents Chemother. 1986;29:543–548. doi: 10.1128/aac.29.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prins J M, Weverling G J, de Blok K, van Ketel R J, Speelman P. Validation and nephrotoxicity of a simplified once-daily aminoglycoside schedule and guidelines for monitoring therapy. Antimicrob Agents Chemother. 1996;40:2494–2499. doi: 10.1128/aac.40.11.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prins J M, Weverling G J, van Ketel R J, Speelman P. Circadian variations in serum levels and the renal toxicity of aminoglycosides in patients. Clin Pharmacol Ther. 1997;62:106–111. doi: 10.1016/S0009-9236(97)90156-9. [DOI] [PubMed] [Google Scholar]
- 100.Priuska E M, Schacht J. Mechanism and prevention of aminoglycoside ototoxicity: outer hair cells as targets and tools. Ear Nose Throat J. 1997;76:164–171. [PubMed] [Google Scholar]
- 101.Ramsammy L S, Kaloyanides G J. The effect of gentamicin on the biophysical properties of phosphatidic acid liposomes is influenced by the O C⩵O group of the lipid. Biochemistry. 1988;27:8249–8254. doi: 10.1021/bi00421a039. [DOI] [PubMed] [Google Scholar]
- 102.Ramsammy L, Josepovitz C, Lane B, Kaloyanides G J. Polyaspartic acid inhibits gentamicin-induced perturbation of phospholipid metabolism. Am J Physiol. 1990;258:C1141–C1149. doi: 10.1152/ajpcell.1990.258.6.C1141. [DOI] [PubMed] [Google Scholar]
- 103.Reiner N E, Bloxham D D, Thompson W L. Nephrotoxicity of gentamicin and tobramycin given once daily or continuously in dogs. J Antimicrob Chemother. 1978;4(Suppl. A):85–101. doi: 10.1093/jac/4.suppl_a.85. [DOI] [PubMed] [Google Scholar]
- 104.Rodriguez-Barbero A, Lopez-Novoa J M, Arevalo M. Involvement of platelet-activating factor in gentamicin nephrotoxicity in rats. Exp Nephrol. 1997;5:47–54. [PubMed] [Google Scholar]
- 105.Safirstein R, Miller P, Kahn T. Cortical and papillary absorptive defects in gentamicin nephrotoxicity. Kidney Int. 1983;24:526–533. doi: 10.1038/ki.1983.189. [DOI] [PubMed] [Google Scholar]
- 106.Sakai M, Zhang M Z, Homma T, Garrick B, Abraham J A, McKanna J A, Harris R C. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. J Clin Invest. 1997;99:2128–2138. doi: 10.1172/JCI119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sandhya P, Mohandass S, Varalakshmi P. Role of dlalpha-lipoic acid in gentamicin induced nephrotoxicity. Mol Cell Biochem. 1995;145:11–17. doi: 10.1007/BF00925707. [DOI] [PubMed] [Google Scholar]
- 108.Sandoval R, Leiser J, Molitoris B A. Aminoglycoside antibiotics traffic to the Golgi complex in LLC-PK1 cells. J Am Soc Nephrol. 1998;9:167–174. doi: 10.1681/ASN.V92167. [DOI] [PubMed] [Google Scholar]
- 109.Schacht J. Aminoglycoside ototoxicity: prevention in sight? Otolaryngol Head Neck Surg. 1997;117:1–4. doi: 10.1177/019459989811800518. [DOI] [PubMed] [Google Scholar]
- 110.Schanck A, Brasseur R, Mingeot-Leclercq M P, Tulkens P M. Interactions of aminoglycosides with phosphatidylinositol: a 15N nuclear magnetic resonance study. Magn Reson Chem. 1992;30:11–15. [Google Scholar]
- 111.Shitara T, Kobayashi Y, Tsuchiya T, Umezawa S. Synthesis of 5-deoxy-5-fluoro and 5-deoxy-5,5-difluoro derivatives of kanamycin B and its analogs. Study on structure-toxicity relationships. Carbohydr Res. 1992;232:273–290. doi: 10.1016/0008-6215(92)80060-e. [DOI] [PubMed] [Google Scholar]
- 112.Silverblatt F J, Kuehn C. Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int. 1979;15:335–345. doi: 10.1038/ki.1979.45. [DOI] [PubMed] [Google Scholar]
- 113.Skopicki H A, Zikos D, Sukowski E J, Fisher K A, Peterson D R. Gentamicin inhibits carrier-mediated dipeptide transport in kidney. Am J Physiol. 1996;270:F531–F538. doi: 10.1152/ajprenal.1996.270.3.F531. [DOI] [PubMed] [Google Scholar]
- 114.Smetana S, Khalef S, Kopolovic G, Bar-Khayim Y, Birk Y, Kacew S. Effect of interaction between gentamicin and pyridoxal-5-phosphate on functional and metabolic parameters in kidneys of female Sprague-Dawley rats. Renal Fail. 1992;14:147–153. doi: 10.3109/08860229209039124. [DOI] [PubMed] [Google Scholar]
- 115.Smith C R, Lipsky J J, Laskin O L, Hellmann D B, Mellits E D, Longstreth J, Lietman P S. Double-blind comparison of the nephrotoxicity and auditory toxicity of gentamicin and tobramycin. N Engl J Med. 1980;302:1106–1109. doi: 10.1056/NEJM198005153022002. [DOI] [PubMed] [Google Scholar]
- 116.Somermeyer M G, Knauss T C, Weinberg J M, Humes H D. Characterization of Ca2+transport in rat renal brush-border membranes and its modulation by phosphatidic acid. Biochem J. 1983;214:37–46. doi: 10.1042/bj2140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song B B, Anderson D J, Schacht J. Protection from gentamicin ototoxicity by iron chelators in guinea pig in vivo. J Pharmacol Exp Ther. 1997;282:1–9. [PubMed] [Google Scholar]
- 118.Sturm A W. Netilmicin in the treatment of gram-negative bacteremia: simple daily versus multiple daily dosage. J Infect Dis. 1989;159:931–937. doi: 10.1093/infdis/159.5.931. [DOI] [PubMed] [Google Scholar]
- 119.Sundin D P, Meyer C, Dahl R, Geerdes A, Sandoval R, Molitoris B A. Cellular mechanism of aminoglycoside tolerance in long-term gentamicin treatment. Am J Physiol. 1997;41:C1309–C1318. doi: 10.1152/ajpcell.1997.272.4.C1309. [DOI] [PubMed] [Google Scholar]
- 120.Swan S K, Gilbert D N, Kohlhepp S J, Kohnen P W, Bennett W M. Pharmacologic limits of the protective effect of polyaspartic acid on experimental gentamicin nephrotoxicity. Antimicrob Agents Chemother. 1993;37:347–348. doi: 10.1128/aac.37.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tabatabay C A, Young L H, D’Amico D J, Kenyon K R. Immunocytochemical localization of gentamicin in the rabbit retina following intravitreal injection. Arch Ophthalmol. 1990;108:723–726. doi: 10.1001/archopht.1990.01070070109046. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi Y, Ueda C, Tsuchiya T, Kobayashi Y. Study on flurination-toxicity relationships. Syntheses of 1-N-[(2R,3R)- and (2R,3S)-4-amino-3-fluoro-2-hydroxybutanoyl] derivatives of kanamycins. Carbohydr Res. 1993;249:57–76. doi: 10.1016/0008-6215(93)84060-j. [DOI] [PubMed] [Google Scholar]
- 123.ter Braak E W, de Vries P J, Bouter K P, van der Vegt S G, Dorrestein G C, Nortier J W, Verbrugh H A. Once-daily dosing regimen for aminoglycoside plus beta-lactam combination therapy of serious bacterial infections: comparative trial with netilmicin plus ceftriaxone. Am J Med. 1990;89:58–65. doi: 10.1016/0002-9343(90)90099-y. [DOI] [PubMed] [Google Scholar]
- 124.Thibault N, Grenier L, Simard M, Bergeron M G, Beauchamp D. Attenuation by daptomycin of gentamicin-induced experimental nephrotoxicity. Antimicrob Agents Chemother. 1994;38:1027–1035. doi: 10.1128/aac.38.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Todd J H, Hottendorf G H. Poly-l-aspartic acid protects cultured human proximal tubule cells against aminoglycoside-induced electrophysiological alterations. Toxicol Lett. 1997;90:217–221. doi: 10.1016/s0378-4274(96)03858-1. [DOI] [PubMed] [Google Scholar]
- 126.Todd J H, Sens D A, Hazen-Martin D J, Bylander J E, Smyth B J, Sens M A. Aminoglycoside antibiotics alter the electrogenic transport properties of cultured human proximal tubule cells. Toxicol Pathol. 1992;20:608–616. doi: 10.1177/019262339202000408. [DOI] [PubMed] [Google Scholar]
- 127.Toubeau G, Laurent G, Carlier M B, Abid S, Maldague P, Heuson-Stiennon J A, Tulkens P M. Tissue repair in rat kidney cortex after short treatment with aminoglycosides at low doses: a comparative biochemical and morphometric study. Lab Invest. 1986;54:385–393. [PubMed] [Google Scholar]
- 128.Tsuchiya T, Shitara T, Umezawa S, Takeuchi T, Hamada M, Tomono N, Umemura E. Synthesis of low-toxicity, 5-deoxy-5-fluoro and 5-deoxy-5,5-difluoro derivatives of arbekacin and its analogs, and study of structure-toxicity relationships. Carbohydr Res. 1993;240:307–312. doi: 10.1016/0008-6215(93)84194-b. [DOI] [PubMed] [Google Scholar]
- 129.Tsuchiya T, Takahashi Y, Kobayashi Y, Umezawa S, Umezawa H. Synthesis of 3′-deoxy-3′-fluorokanamycins A and B active against resistant bacteria. J Antibiot (Tokyo) 1985;38:1287–1290. doi: 10.7164/antibiotics.38.1287. [DOI] [PubMed] [Google Scholar]
- 130.Tulkens P M. Experimental studies on nephrotoxicity of aminoglycosides at low doses: mechanisms and perspectives. Am J Med. 1986;80(Suppl. 6B):105–114. doi: 10.1016/0002-9343(86)90487-0. [DOI] [PubMed] [Google Scholar]
- 131.Tulkens P M. Nephrotoxicity of aminoglycosides. Toxicol Lett. 1989;46:107–123. doi: 10.1016/0378-4274(89)90121-5. [DOI] [PubMed] [Google Scholar]
- 132.Tulkens P M, Clerckx-Braun F, Donnez J, Ibrahim S, Kallay Z, Jacqmin P, Gersdorff M. Safety and efficacy of aminoglycosides once-a-day: experimental data and randomized, controlled evaluation in patients suffering from pelvic inflammatory disease. J Drug Dev. 1988;1(Suppl. 3):71–82. [Google Scholar]
- 133.Tulkens P M, Mingeot-Leclercq M P, Laurent G, Brasseur R. Conformational and biochemical analysis of the interactions phospholipids-aminoglycoside antibiotics in relation with their toxicity. In: Brasseur R, editor. Molecular description of biological membrane components by computer-aided conformational analysis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 63–93. [Google Scholar]
- 134.Van Bambeke F, Tulkens P M, Brasseur R, Mingeot-Leclercq M-P. Aminoglycoside antibiotics induce aggregation but not fusion of negatively-charged liposomes. Eur J Pharmacol. 1995;289:321–333. doi: 10.1016/0922-4106(95)90110-8. [DOI] [PubMed] [Google Scholar]
- 135.Vandewalle A, Farman N, Morin J P, Fillastre J P, Hatt P Y, Bonvalet J P. Gentamicin incorporation along the nephron: autoradiographic study on isolated tubules. Kidney Int. 1981;19:529–539. doi: 10.1038/ki.1981.50. [DOI] [PubMed] [Google Scholar]
- 136.Walker P D, Shah S V. Gentamicin enhanced production of hydrogen peroxide by renal cortical mitochondria. Am J Physiol. 1987;253:C495–C499. doi: 10.1152/ajpcell.1987.253.4.C495. [DOI] [PubMed] [Google Scholar]
- 137.Walker R J, Duggin G G. Drug nephrotoxicity. Annu Rev Pharmacol Toxicol. 1988;28:331–345. doi: 10.1146/annurev.pa.28.040188.001555. [DOI] [PubMed] [Google Scholar]
- 138.Watanabe M. Drug-induced lysosomal changes and nephrotoxicity in rats. Acta Pathol Jpn. 1978;28:867–889. doi: 10.1111/j.1440-1827.1978.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 139.Wedeen R P, Batuman V, Cheeks C, Marquet E, Sobel H. Transport of gentamicin in rat proximal tubule. Lab Invest. 1983;48:212–223. [PubMed] [Google Scholar]
- 140.Weinberg J M. The role of cell calcium overload in nephrotoxic renal tubular cell injury. Am J Kidney Dis. 1986;8:284–291. doi: 10.1016/s0272-6386(86)80099-3. [DOI] [PubMed] [Google Scholar]
- 141.Whittem T, Parton K, Turner K. Effect of polyaspartic acid on pharmacokinetics of gentamicin after single intravenous dose in the dog. Antimicrob Agents Chemother. 1996;40:1237–1241. doi: 10.1128/aac.40.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Williams P D, Hottendorf G H. Inhibition of renal membrane binding and nephrotoxicity of gentamicin by polyasparagine and polyaspartic acid in the rat. Res Commun Chem Pathol Pharmacol. 1985;47:317–320. [PubMed] [Google Scholar]
- 143.Zheng G, Bachinsky D R, Stamenkovic I, Strickland D K, Brown D, Andres G, McCluskey R T. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP) J Histochem Cytochem. 1994;42:531–542. doi: 10.1177/42.4.7510321. [DOI] [PubMed] [Google Scholar]