Abstract
It has been reported that subinhibitory concentrations (sub-MICs) of some fluoroquinolones are still capable of affecting the topological characteristics of DNA (inhibition DNA-gyrase) and that this leads to a reduction in some of the factors responsible for bacterial virulence (by means of the disruption of protein synthesis and alterations in phenotype expression), even though the microorganisms themselves are not killed. The present study investigated the ability of sub-MICs of rufloxacin, an orally absorbed monofluorinated quinolone with a long half-life (28 to 30 h), to interfere with the bacterial virulence parameters of adhesiveness, hemagglutination, hydrophobicity, motility, and filamentation, as well as their interactions with host neutrophilic defenses such as phagocytosis, killing, and oxidative bursts. It was observed that Escherichia coli adhesiveness was significantly reduced at rufloxacin concentrations of 1/32 MIC, hemagglutination and hydrophobicity were significantly reduced at concentrations of, respectively, 1/4 MIC and 1/8 MIC, and motility was significantly reduced at concentrations of 1/16 MIC; filamentation was still present at concentrations of 1/4 MIC. Phagocytosis was not affected, but killing significantly increased from 1/2 MIC to 1/8 MIC; oxidative bursts measured by means of chemiluminescence were not affected. The fact that sub-MICs are still effective in interfering with the parameters of bacterial virulence is useful information that needs to be correlated with pharmacokinetic data in order to extend our knowledge of the most effective concentrations that can be used to optimize treatment schedules, for example, single administrations, particularly in noncomplicated lower urinary tract infections.
Bacterial virulence reflects the ability of infecting microorganisms to produce pathological effects in an invaded host. The extent and severity of these effects depend on a number of bacterial cell functions, such as their invasive and adhesive capacities, fimbriation, their outermost surface characteristics and motility, their interaction with host defenses (phagocytosis and killing), the role of metabolism and the release of exocellular and endocellular products, and rates of replication.
In cases of bacterial infection antibiotic therapy is usually adopted, on the assumption that the drug concentrations at the site of infection reach the minimum bactericidal concentration, thus eliminating the virulence of the microorganisms by killing them. In contrast, although the microorganisms do not necessarily die, MICs can inhibit the growth of bacteria and, in most cases, significantly reduce their virulence (49).
With intermittent antibiotic administration, commonly used to treat bacterial infections, pharmacokinetic curves show concentrations that fluctuate on the basis of the dosing schedule. At the site of infection, these concentrations may exceed the in vitro MIC for the invading microorganism for a certain period of time, subsequently decrease more or less rapidly to values corresponding to the MIC, and finally drop to subinhibitory concentrations (sub-MICs), generally between doses. This is particularly true in the case of tissue infections, because tissue antibiotic concentrations are frequently lower than those in the blood.
A growing body of evidence (9, 13, 35, 39, 42, 50, 51) strongly suggests that antibiotic concentrations of less than the conventionally determined MICs may still be effective in reducing bacterial virulence by interfering with bacterial cell functions. These findings have generated interest in the effects of exposing bacteria to low concentrations of antibiotics (13, 34), so that pharmacodynamic and pharmacokinetic data can be correlated and therapy can thus be optimized. Rufloxacin is an orally absorbed monofluorinated quinolone with a long half-life (28 to 30 h), consistently high bactericidal concentrations at the site of infection (especially in urine), and good penetration into infected tissues (53, 54). Although some scattered information exists in the literature (5, 7, 17), the effects of sub-MICs of rufloxacin have not yet been fully investigated. The aim of the present study was therefore to investigate the activity of such concentrations on various bacterial cell functions in order to evaluate their ability to reduce bacterial virulence.
MATERIALS AND METHODS
Adhesion assay.
Two Escherichia coli strains from clinical isolates (urinary infections) and lyophilized E. coli ATCC 25922 (D.I.D., Milan, Italy) were used. The MICs were determined in Mueller-Hinton broth by using the tube macrodilution method (38). Each tube contained twofold dilutions of the antibiotic and a final bacterial inoculum of 106 CFU/ml. The tubes were incubated for 18 h at 37°C. The MIC was defined as the lowest concentration of antibiotic that prevented turbidity in the test tube after incubation.
For the adhesion assay, cell suspensions of each organism were prepared from overnight cultures (18 h) in tryptic soy broth (Sigma) at 37°C under static conditions. The organisms were harvested, washed three times in phosphate-buffered saline (PBS), and adjusted to 3 × 108 organisms/ml as determined by direct microscopic counts (interference contrast microscopy) in a Petroff-Hausser bacterial counting chamber.
Human periurethral epithelial cells were collected from the sediment of fresh urine (50 ml) of apparently healthy females and resuspended in 20 ml of PBS. The suspensions obtained from three to five subjects were pooled. The epithelial cells were then passed through a needle (0.3 mm diameter) to disrupt cell aggregates and washed three to four times to free them from debris and nonadherent bacteria by using low-speed differential centrifugation (240 × g for 10 min at 21°C).
PBS was added to the washed epithelial cell suspensions to give 105 cells/ml as determined by means of a direct microscopic count (interference contrast microscopy) in a Bürker chamber. The ability of the bacteria to adhere to epithelial cells was investigated by mixing together 1:1 volumes of standardized suspensions of bacteria (3 × 108/ml) and epithelial cells (3 × 105/ml) in polystyrene tubes. The tubes were rotated end over end at 10 rpm for 60 min at 37°C. The epithelial cells were separated from the nonadherent bacteria by means of differential centrifugation at 100 × g for 5 min. The final epithelial cell pellet was resuspended in a small quantity of PBS, placed on a round microscope coverslip, and dried.
The coverslip with the cells was fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.1) for 60 min at 4°C. After several dehydrations in alcohol, the coverslips underwent critical-point drying, were coated with 200-Å gold, and counted by using a scanning electron microscope (SEM). The adhesiveness of bacterial to epithelial cells was determined by counting the number of epithelial cells with ≥40 adhering bacteria per 100 cells (47). Each test was performed twice. Control epithelial cell suspensions were always included to provide data on the number of bacteria that were already attached (natural acquisition) when the cells were collected.
For the inhibition test, the bacteria were grown in the presence of subinhibitory concentrations of rufloxacin (37°C for 18 h), from 1/2 to 1/128 MIC (1/2, 1/4, 1/8, 1/16, 1/32, 1/64, and 1/128), or with the same amount of medium without any antibiotic (control). The bacteria were harvested by centrifugation at 470 × g and resuspended in PBS at a final concentration of 3 × 108 bacteria/ml. They were challenged with epithelial cells as in the adherence assay, and the samples were prepared for SEM analyses.
Hemagglutination assay.
Anticoagulated guinea pig and human group A erythrocytes were collected 1:1 in Alsever solution (2.05% glucose, 0.8% sodium citrate, 0.42% NaCl, 0.055% citric acid [pH 6.1]), washed three times in PBS, and finally suspended in saline; the washed erythrocytes were then stored at 4°C and used within 5 days.
The hemagglutination tests were performed by using a slightly modified version of the procedure of Evans et al. (22). Twenty microliters of a 5% (vol/vol) suspension of erythrocytes in saline and 20 μl of the bacterial suspension (109 cells/ml) were pipetted onto microscopy slides. The slides were gently rotated at room temperature for 5 min and read after 15 min.
To test for mannose sensitivity, 20 μl of 1% mannose solution was added to a duplicate slide containing undiluted bacteria. The hemagglutination test was applied to samples of each bacterial strain grown with the different sub-MICs of rufloxacin. The results were recorded as grade 3 when hemagglutination occurred in a very short period of time and was complete (coarse clumping); a reduction in clumping that gave only different degrees of fine granularity was recorded as 2, 1, or negative (22).
Hydrophobicity assay.
The hydrophobicity of the bacterial cell surfaces was measured by using the salt aggregation test (SAT) (29). Briefly, a bacterial suspension of 25 μl (5 × 109 bacterial cells/ml in 0.002 M sodium phosphate buffer [pH 6.8]) was mixed with an equal volume of ammonium sulfate [(NH4)2 SO4] ranging from 3.2 to 0.2 M (pH 6.8). The bacteria-salt solution was gently rocked for 2 min at 20°C, and aggregation was visually read against a black background.
A reaction causing optimal aggregation was regarded as positive (i.e., when most of the bacteria aggregated to give a clear solution and white aggregates with a diameter of approximately 1/10 mm). A reaction with no aggregates or only a few aggregates, i.e., not modifying the overall view of the sample, was regarded as negative. All the readings were compared with the reaction at the highest salt molarity (positive control).
Bacterial suspensions mixed with 0.0002 M sodium phosphate (pH 6.8) without the addition of salt were used as a negative control. The SAT value represented the lowest concentration of ammonium sulfate at which aggregation was observed. The test was repeated for each strain and for each sub-MIC of rufloxacin.
Motility.
The bacteria were grown overnight in tryptic soy broth (Sigma) at 37°C, and a 5-μl aliquot of the cell suspension (3 × 108 cells) was placed on the agar surface of the semisolid swarming medium (agar motility) (1% tryptone, 0.5% NaCl, 0.25% agar dissolved in distilled water [pH 7.1]) (9).
After inoculation, the assay plates (petri dishes [diameter, 9 cm], containing 10 ml of medium) were placed in a water-saturated Plexiglas incubator at 37°C. The plates were illuminated obliquely and viewed against a dark background, and the diameter of the swarming zones was measured with a ruler at regular time intervals.
Assays were performed on each strain grown overnight with the different sub-MICs of rufloxacin and inoculated onto plates with the swarming medium containing the same sub-MICs. The control plates contained no antibiotic.
Filamentation.
Changes in the morphology of bacilli do not occur at the same time or in the same ratios for all the organisms in a given population of bacteria exposed to a given concentration of an antibiotic (33), so in order to verify the exact kinetics at the time of morphological change, a 700-μl aliquot was withdrawn from the cultures at 0.5, 1, 2, 4, and 8 h and processed for SEM observation. This procedure (8) was adopted after control samples showed that the 700-μl extraction did not interfere with growth, so it was possible to compare the evolution of the changes in the same culture.
For each determination, the 700-μl aliquot was added to 2 ml of broth and centrifuged at 450 × g; the final pellet was resuspended in 100 μl of PBS (0.02 M phosphate and 0.15 M NaCl [pH 7.3]), placed on a round microscope coverslip, and dried. The coverslip was then fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.1) for 60 min at 4°C. After dehydration in graded alcohol, the coverslip underwent critical-point drying, was coated with 200-Å gold and observed in a SEM.
The microscopic fields to be counted, bacterial size, and the morphology recorded were selected by means of random scanning.
The morphological characteristics before and after incubation with the different sub-MICs of rufloxacin were classified and quantitated, with the observer unaware of the concentration of antibiotic or the duration of incubation. The lengths of the filaments and their proportions in the total number of microorganisms per 100 randomly observed bacteria were recorded; a minimum of eight different fields were examined for each sub-MIC and time. The normal length of E. coli can vary from 1.2 to 2.3 μm; during the mid-division cycle it is about 5 μm.
Organisms of up to 15 μm were classified as short filaments, and those longer than 15 μm were classified as long filaments (33).
Phagocytosis and killing. (i) Collection of human PMNs.
Peripheral venous blood was drawn from healthy adult donors into heparinized (5 U/ml) syringes. The blood (5 ml) was stratified on 3 ml of Polymorphoprep (Nycomed Pharma), and the polymorphonuclear leukocytes (PMNs) were separated by density gradient centrifugation.
When necessary, any residual erythrocytes in the granulocyte preparation were lysed with a 0.15-mol/liter of NH4Cl solution (pH 7.4). The PMNs were collected and washed in glutamine-containing RPMI 1640 (Sigma) after being passed through a 150-μm-internal-diameter needle in order to disrupt cell aggregates. They were then tested for viability by trypan blue exclusion. The final cell suspension was adjusted to the cell numbers needed for each test by counting in a Bürker chamber (interference contrast microscopy).
(ii) Phagocytosis and bacterial killing.
The phagocytic and bacterial killing capacities of the PMNs were determined by using a fluorochrome assay (acridine orange stain), which distinguishes viable and dead microorganisms intracellularly. The procedure of Bellinati-Pires et al. (4) was adopted with slight modifications. Equal volumes of PMNs (2 × 106 cell/ml) and preopsonized bacteria (2 × 107 bacteria/ml) were mixed in tubes at a ratio of 1:10 (PMN/bacteria) in a final volume of 0.5 ml. The tubes were incubated at 37°C and rotated end over end (6 rpm) for 30 min.
Phagocytosis was stopped by placing each tube in an ice bath and adding 0.5 ml of ice-cold medium to the bacteria-PMN suspension. Noningested bacteria were removed by means of differential centrifugation (100 × g for 7 min) and two washes. The pellet was stained with 200 μl of 14.4-mg/liter acridine orange (pH 7.2) (Sigma) in medium for 1 min. Immediately after staining, 1 ml of ice-cold Hanks’ balanced salt solution (HBSS) was added to the PMN suspension, which was then centrifuged at 160 × g for 7 min at 4°C. The cells were washed twice with ice-cold HBSS and kept in an ice bath until microscopic examination.
Acridine orange makes bacteria fluorescent, so phagocytized bacteria can be easily counted; killed bacteria are also clearly visible because they become yellow-red under UV light, whereas living bacteria are green. After staining with acridine orange to avoid overestimating phagocytosis on the basis of the E. coli CFU attached to the surface of the PMNs but not yet internalized, the techniques of Hed (27) and Goldner et al. (24) were used to quench the extracellular membrane-adherent microorganism fluorescence by crystal violet (500 μg/ml for 20 min). Since crystal violet does not penetrate PMNs, it does not alter the fluorescence of ingested microorganisms. Just before the observation of each cell sample, a drop of the cell suspension was wet mounted on a microscope slide and sealed with nail varnish. It was then immediately examined under oil immersion with a UV epifluorescence microscope (Leitz) equipped with an excitation filter at 450 to 490 nm, a beam split mirror at 510 nm, and a cut-off filter at 520 nm.
A short time interval lasting no longer than 10 min was established for each slide reading, and if this was not sufficient, another slide was prepared from the ice-cold suspension. A total of 100 PMNs was observed for each slide. The number of cells phagocytizing at least three bacteria/100 PMNs gave the percent of phagocytosis, whereas the average number of bacteria in each phagocytizing cell gave the phagocytic index. The percentage of killed bacteria was obtained by using the following formula: number of dead bacteria/number of dead + live bacteria × 100. The killing index was the average number of dead bacteria per PMN. The same procedure was followed to determine the effect of the exposure of PMNs to different sub-MICs of rufloxacin.
Measurement of oxidative burst response by chemiluminescence.
Luminol-amplified chemiluminescence (LACL) was investigated by using a slightly modified version of the procedure of Robinson et al. (43) for pathogenic organisms. In brief, 0.1 ml of PMN suspension (106 cells/ml) and 0.30 ml of HBSS with Ca2+ and Mg2+ plus 0.05 ml 10−4 M luminol (diluted from a first stock solution in dimethyl sulfoxide; Sigma) were put into a 3-ml flat-bottomed polystyrene vial. The vial was placed in a lightproof chamber of the Luminometer 1250 (Bio Orbit), and the carousel was rotated to bring the sample in line with the photomultiplier tube in order to record background activity. A suspension of preopsonized killed Candida albicans cells (2 × 107 cells/ml) in a final volume of 0.05 ml was added as a stimulus for oxidative bursts, and the resulting light output in millivolts was continuously recorded on a chart recorder and, simultaneously, by means of a digital printout set for 1- or 10-s recording integrals.
All the constituents of the mixture were kept at 37°C during the reaction by means of thermostatically controlled water passing through a polished hollow metal sample holder. No mixing took place during the recordings. The gain control was set to give a reading of 10 mV for a built-in standard. A background subtraction control zeroed the instrument prior to the addition of the opsonized cells. The patterns of LACL responses were determined by calculating the initial slopes, peaks (in millivolts), times to peak, slopes of declining response, and areas under the curves. Because the peaks correlate well with the other parameters, a first analysis is presented simply in terms of peak counts. However, since oxidant radical production has its own time course, the simple peak (which freezes measuring at a single time) does not completely characterize the phenomenon over time, so the data are also expressed as a curve. The analysis was completed by investigating the effects of sub-MICs of rufloxacin on PMN oxidative bursts.
Data analysis.
Each test was performed six to nine times for each strain and for each sub-MIC and control. Values are expressed as the means of all data ± standard errors of the means. The statistical significance of the differences was calculated by using the t test and, when necessary, analysis of variance between treatments, followed by multiple pair comparisons according to Dunnett when the differences were statistically significant. Differences were considered statistically significant when the test yielded a value of ≤0.05.
RESULTS
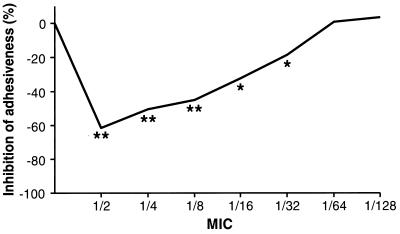
During the control test performed before antibiotic challenge, all of the bacterial strains adhered well to human epithelial cells, with a certain degree of variability in the number of bacteria per cell. The mean of the rufloxacin MICs was 1 μg/ml, and after incubation with sub-MICs, bacterial adhesiveness decreased. The data are summarized in Table 1. To normalize the susceptibilities of the different strains, the data were also expressed as percentages of inhibition versus control, as shown in Fig. 1. As expected, peak inhibition was observed at 1/2 MIC, but inhibition was statistically significant at a MIC as low as 1/32.
TABLE 1.
Effects of various subinhibitory concentrations of rufloxacin on E. coli adhesiveness to human epithelial cells
| E. coli | Control | No. of cells bearing ≥40 bacteria/100 cells at indicated subinhibitory concn
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | 1/64 | 1/128 | ||
| ATCC 25922 | 38.4 | 12.1 | 16.2 | 19.5 | 23.3 | 27.9 | 36.0 | 36.2 |
| Clinical isolate | 41.2 | 20.0 | 23.7 | 25.1 | 30.0 | 34.0 | 39.1 | 44.5 |
| Clinical isolate | 29.4 | 10.2 | 14.1 | 15.0 | 21.8 | 26.8 | 35.0 | 32.3 |
| Mean ± SD | 36.3 ± 6.2 | 14.1b ± 5.2 | 18.0b ± 5.0 | 20.1b ± 4.6 | 25.0a ± 4.3 | 29.5a ± 3.8 | 36.7 ± 2.1 | 37.6 ± 6.2 |
P ≤ 0.05.
P ≤ 0.01.
FIG. 1.
Percent inhibition of E. coli adherence to human epithelial cells after incubation with different sub-MICs of rufloxacin.
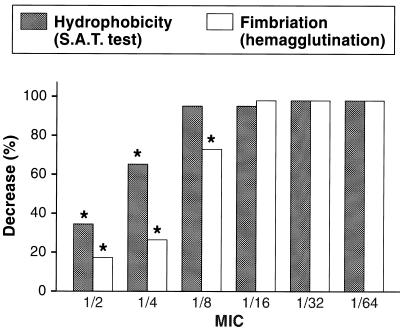
The inhibition of hemagglutination and hydrophobicity were significant as low as 1/4 and 1/8 MIC, respectively (Fig. 2). The different radii of the swarming zone read at different times are a measure of the motility of E. coli strains. Incubation with 1/2 and 1/4 MICs completely inhibited bacterial motility, and this inhibition was significant as low as 1/16 MIC (Table 2).
FIG. 2.
Effects of various rufloxacin sub-MICs of E. coli hemagglutination and hydrophobicity (S.A.T., salt aggregation test).
TABLE 2.
Mean values of swarming zone diameter (mm) at different hours for E. coli incubated with different sub-MICs of rufloxacin
| Time (h) | Control | Mean ± SD of swarming zone diameter (mm) for indicated subinhibitory concn
|
||||
|---|---|---|---|---|---|---|
| 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | ||
| 0 | 6.08 ± 0.87 | 6.00 ± 0.93 | 6.13 ± 0.83 | 6.13 ± 1.03 | 6.13 ± 1.00 | 6.13 ± 1.00 |
| 1 | 6.71 ± 1.01 | 6.25 ± 0.84 | 6.42 ± 0.70 | 6.75 ± 1.06 | 6.71 ± 0.81 | 6.83 ± 1.07 |
| 2 | 17.48 ± 3.42 | 6.75b ± 0.66 | 11.25b ± 2.86 | 14.75b ± 2.90 | 16.17a ± 3.30 | 17.00 ± 2.84 |
| 3 | 32.58 ± 8.25 | 6.88b ± 0.74 | 13.25b ± 2.42 | 20.17b ± 4.11 | 29.33b ± 7.84 | 30.92 ± 6.19 |
| 4 | 55.67 ± 13.20 | 6.88b ± 0.74 | 14.50b ± 2.54 | 29.08b ± 9.92 | 49.08b ± 13.40 | 51.75 ± 11.22 |
| 5 | 76.58 ± 9.93 | 6.96b ± 0.81 | 16.33b ± 4.50 | 37.75b ± 15.09 | 68.42b ± 16.23 | 73.50 ± 12.11 |
| 6 | 84.17 ± 2.12 | 7.17b ± 0.96 | 17.25b ± 7.55 | 46.00b ± 18.48 | 78.83a ± 8.81 | 81.83a ± 4.47 |
P ≤ 0.05.
P ≤ 0.01.
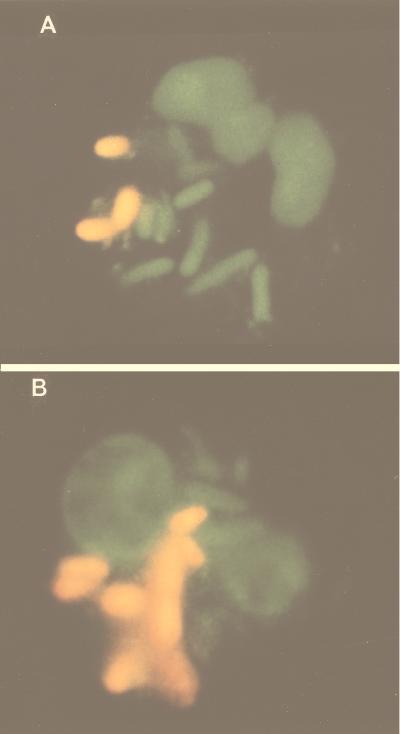
The morphological changes induced by the sub-MICs of rufloxacin consisted of filamentation. The exposure of E. coli to 1/2 MIC and, to a lesser extent, even to 1/4 MIC was capable of inducing filamentation within 2 to 4 h (Table 3). The above parameters relate to the bacteria themselves, but there are also host-bacteria interaction parameters, such as phagocytosis and killing. These were investigated by using fluorescence microscopy and acridine orange fluorochrome. Figure 3A and B show neutrophils with phagocytized E. coli. The green bacteria are still alive, whereas those that are orange-red have been killed. Rufloxacin sub-MICs did not change the percentage of phagocytosis or the phagocytic index but significantly increased the killing of bacteria as low as 1/8 MIC (Table 4).
TABLE 3.
Ratios of normal-length bacteria, short filaments, long filaments, and ghosts for every 100 bacteria at different times and concentrations
| Time (h) and cell typea | Control | Subinhibitory conc
|
||||
|---|---|---|---|---|---|---|
| 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | ||
| 1 | ||||||
| N | 95 | 93 | 94 | 98 | 95 | 94 |
| S | 5 | 7 | 6 | 2 | 5 | 6 |
| L | 0 | 0 | 0 | 0 | 0 | 0 |
| G | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | ||||||
| N | 92 | 54 | 67 | 91 | 91 | 89 |
| S | 6 | 20 | 14 | 4 | 6 | 8 |
| L | 2 | 21 | 17 | 5 | 3 | 3 |
| G | 0 | 5 | 2 | 0 | 0 | 0 |
| 4 | ||||||
| N | 91 | 51 | 61 | 93 | 92 | 88 |
| S | 7 | 16 | 13 | 3 | 4 | 8 |
| L | 2 | 23 | 20 | 2 | 4 | 2 |
| G | 0 | 10 | 6 | 2 | 0 | 2 |
| 8 | ||||||
| N | 88 | 61 | 73 | 89 | 89 | 87 |
| S | 7 | 12 | 10 | 6 | 7 | 6 |
| L | 3 | 21 | 13 | 5 | 3 | 4 |
| G | 2 | 6 | 4 | 0 | 1 | 3 |
N, normal-length bacteria; S, short filaments; L, long filaments; G, ghosts (cells flattened and disrupted due to the loss of cytoplasm).
FIG. 3.
Microphotography (epifluorescence) of PMN phagocytosis and killing. (A) Unexposed E. coli. (B) E. coli after exposure to 1/2 MIC rufloxacin (living bacteria are green; dead bacteria are orange-red).
TABLE 4.
Effects on the phagocytosis and intracellular killing of E. coli exposed to rufloxacin sub-MICs
| Exposure | Phagocytosis
|
Killing
|
||
|---|---|---|---|---|
| % | Index | % | Index | |
| Control | 82.67 ± 4.51 | 6.27 ± 1.43 | 37.09 ± 3.76 | 2.33 ± 0.57 |
| Rufloxacin MIC | ||||
| 1/2 | 82.20 ± 7.74 | 5.46 ± 2.55 | 52.56b ± 12.15 | 2.92b ± 1.12 |
| 1/4 | 83.07 ± 2.81 | 6.34 ± 1.96 | 46.24b ± 6.00 | 2.83a ± 0.80 |
| 1/8 | 83.73 ± 3.20 | 6.26 ± 1.48 | 42.41b ± 5.95 | 2.56 ± 0.80 |
| 1/16 | 82.70 ± 4.27 | 6.31 ± 1.40 | 38.17 ± 4.90 | 2.41 ± 0.57 |
| 1/32 | 80.23 ± 3.32 | 6.47 ± 1.93 | 36.15 ± 3.80 | 2.32 ± 0.66 |
P ≤ 0.05.
P ≤ 0.01.
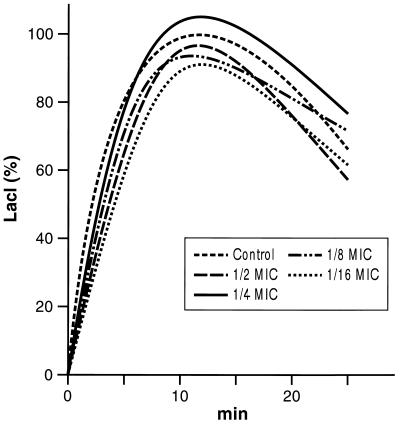
Possible interference with oxidative bursts was investigated by exposing the neutrophils to different sub-MICs of rufloxacin. The findings reported in Table 5 and Fig. 4 show that the rufloxacin sub-MICs did not interfere with this important feature.
TABLE 5.
Means ± standard deviations of LACL (millivolts) of PMNs (peaks) before and after incubation with different sub-MICs of rufloxacin
| Control | Subinhibitory concn
|
||||
|---|---|---|---|---|---|
| 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | |
| 21.34 ± 7.05 | 19.07 ± 5.10 | 22.50 ± 4.80 | 21.09 ± 5.60 | 20.85 ± 4.80 | 22.10 ± 5.78 |
FIG. 4.
LACL curves of PMNs before and after incubation with different sub-MICs of rufloxacin (curves are shown only down to 1/16 MIC to avoid a crowded figure).
DISCUSSION
Bacterial virulence is a result of the specific properties of both bacterial and host cells and their reciprocal interactions. Adhesion is considered to be the first step in the sequence of events leading to colonization and subsequent infection (1, 3). The ability to adhere is an important determinant of virulence (21), and the exposure of bacteria to sub-MICs of antibiotics generally weakens this ability (14, 30, 45, 47). This effect has been observed in the case of both quinolones and fluoroquinolones; sub-MICs of oxolinic acid (26), ciprofloxacin (52), pefloxacin (18), enoxacin (6, 49), rufloxacin (7), and lomefloxacin (6) lead to a significant decrease in the adhesion of E. coli.
Our rufloxacin findings are similar to those found with other quinolones, but they add further information about the extent of the effect. We observed significantly reduced adhesion at concentrations ranging from 1/2 to 1/32 MIC (corresponding to 0.03 μg/ml), whereas the majority of previous studies considered only 1/2 or 1/4 MIC.
Sub-MICs of antibiotics can exert their antiadhesive effects in different ways. They may inhibit the synthesis or expression of adhesins on the bacterial cell surface, lead to the formation of functionally aberrant adhesins, cause the release of adhesins from the surface of bacterial cells, or modify bacterial shape in a such way as to interfere with the ability of the microorganisms to approach receptors on animal cell surfaces (31, 32).
It has been reported that the fluoroquinolones pefloxacin and enoxacin inhibit adhesion on the basis of the first mechanism of adhesion inhibition (10, 18), so it is likely that rufloxacin also reduces the number of adhesins and thus the possibility of bacterial anchorage (or junction). This is also partially correlated with hemagglutination (an indirect measure of fimbriation) and hydrophobicity.
At 1/2 MIC (and to a lesser extent also at 1/4 MIC), rufloxacin is capable of inducing morphological changes in E. coli, such as different levels of filamentation. Maximum filamentation (≅40%) was achieved after 2 h of incubation of bacteria with the 1/2 MIC of rufloxacin (7). Quinolone-induced filamentation has also been observed with the use of pefloxacin (18), enoxacin (25, 49), lomefloxacin (49), ciprofloxacin (16, 25, 52), oxolinic acid (2, 26), and nalidixic acid (26).
The mechanism of filamentation differs from the well-known penicillin-binding protein inhibition induced by β-lactam antibiotics, probably because initial DNA-gyrase inhibition followed by alterations in DNA topology and synthesis (12, 20, 48, 49) provide the signal necessary for the induction of an SOS response pathway that inhibits cell division (28, 41, 46). In addition to filamentation, the SOS response can involve vacuolation and the leakage of intracellular material (28), which is probably a secondary mechanism of death (19).
The fact that rufloxacin sub-MICs affect some important functions of E. coli is also confirmed by the significant reduction in swarming observed after treatment with 1/2 to 1/16 MIC. Together with adhesiveness, this function is correlated with the pathogenicity of bacteria, because the inhibition of motility also reduces the possibility of the formation of new colonies and the spread of infection from the first point of contact.
It has been shown that the sub-MICs of various antimicrobial agents induce morphological and biochemical changes in bacteria, thus making them more susceptible to phagocytosis and killing (36). There are some data in the literature concerning the effect of quinolone sub-MICs on neutrophil phagocytosis and the killing of gram-positive bacteria (23, 40), but only a few investigations have explored their effects on E. coli. Pre-exposure of E. coli-encapsulated strains to 1/2 MICs of fleroxacin and ciprofloxacin has been found to enhance phagocytosis (6), and sub-MICs of ciprofloxacin can increase the killing of E. coli by neutrophils (37). As for rufloxacin, none of the three strains investigated showed any modification in phagocytosis, but killing was significantly increased in two strains for which the MIC was as low as 1/8 and in one strain for which the MIC was as low as 1/4.
Table 6 summarizes the known data concerning the interactions of quinolone sub-MICs with different virulence parameters. This aspect of nonfluorinated quinolones has not been investigated. The ciprofloxacin, enoxacin, and pefloxacin monofluorinated quinolones have been studied in terms of some parameters, but rufloxacin has been better characterized. The only difluorinated quinolone that has been partially studied is lomefloxacin (11), and trifluorinated quinolones need to be further investigated. Comparison of the data reveals some interesting similarities in effects. Adhesiveness and fimbriation are reduced, and filamentation is increased; phagocytosis remains almost unaffected, but killing is increased; finally, neutrophil oxidative bursts do not vary.
TABLE 6.
Overall data on the activity of sub-MICs of different quinolones and fluoroquinolones on various bacterial virulence factors
| Quinolone | Effect of subinhibitory concn on indicated virulence factora
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Adhesiveness | Fimbriation | Hydrophobicity | Filamentation | Motility | Phagocytosis | Killing | LACL | |
| Non-F | ||||||||
| Nalidixic acid | ? | ? | ? | > | ? | ? | ? | ? |
| Oxolinic acid | < | ? | ? | > | ? | ? | ? | ? |
| Pipemidic acid | ? | ? | ? | ? | ? | ? | ? | ? |
| Cinoxacin | ? | ? | ? | ? | ? | ? | ? | ? |
| Rosoxacin | ? | ? | ? | ? | ? | ? | ? | ? |
| Mono-F | ||||||||
| Amifloxacin | ? | ? | ? | ? | ? | ? | ? | ? |
| Ciprofloxacin | < | ? | ? | > | ? | > | > | = |
| Enoxacin | < | < | ? | > | ? | ? | > | ? |
| Norfloxacin | ? | ? | ? | ? | ? | ? | ? | = |
| Ofloxacin | ? | ? | ? | ? | ? | ? | ? | = |
| Pefloxacin | < | < | ? | > | ? | > | ? | ? |
| Rufloxacin | < | < | < | > | < | = | > | = |
| Di-F | ||||||||
| Lomefloxacin | < | < | = | > | ? | ? | > | ? |
| Sparfloxacin | ? | ? | ? | ? | ? | ? | ? | < |
| Tri-F | ||||||||
| Fleroxacin | ? | ? | ? | > | ? | ≶ | ? | ? |
| Temafloxacin | ? | ? | ? | ? | ? | ? | ? | < |
| Tosulfoxacin | ? | ? | ? | ? | ? | ? | ? | ? |
?, data not found in literature; >, increase; <, decrease; =, no effect.
Previous studies have demonstrated that exposure to low concentrations of quinolones may reduce the ability of pathogens to cause clinical symptoms by decreasing the level of production of virulence factors (10, 15). The success of quinolone treatment in patients with sub-MIC levels in their blood (44) supports the clinical significance of our findings. These data must be interpreted in relation to pharmacokinetic behavior when the effectiveness of concentrations reaching mucosal surfaces and other tissues during therapy is considered.
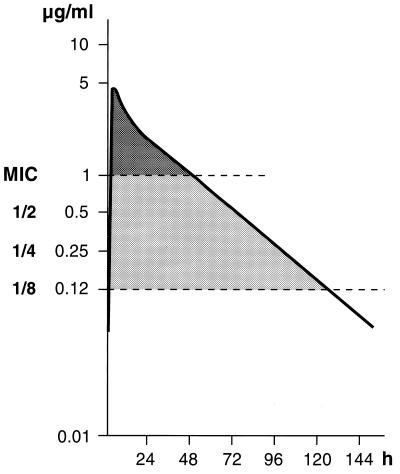
The usual therapeutic dose of rufloxacin is 400 mg, and our MIC for E. coli was 1 μg/ml. The pharmacokinetic curve of a single dose of 400 mg (53) indicates that sub-MICs occur after about 48 h (Fig. 5). Given that the average overall effects of sub-MICs of rufloxacin can still be considered significant at levels as low as 1/8 MIC (which corresponds to 0.12 μg/ml), interpolation of this value with the pharmacokinetic curve shows that the effects of sub-MICs may last as long as (approximately) 132 h after drug administration (rufloxacin has a half-life of 28 to 30 h).
FIG. 5.
Mean plasma levels after a single 400-mg oral dose of rufloxacin and interpolation with 1 and 1/8 MIC of rufloxacin.
Such information can be used to optimize treatment schedules; for example, single administrations for the treatment of noncomplicated lower urinary tract infections such as cystitis.
ACKNOWLEDGMENTS
The technical collaboration of G. Poli and T. Zuccotti is greatly appreciated. We gratefully acknowledge the helpful comments made by V. Gianelle of the Centro Microscopia Elettronica, Azienda U.S.S.L. 38, Milan, Italy. We also gratefully acknowledge Bracco, Milan, Italy, for the kind gift of rufloxacin.
This study was partially supported (60%) by a grant from MURST.
REFERENCES
- 1.Abraham S N, Beachey E M. Host defence against adhesion of bacteria to mucosal surfaces. In: Gallin J J, Fauci A S, editors. Advances in host defence mechanism. New York, N.Y: Raven Press; 1985. pp. 68–83. [Google Scholar]
- 2.Amaral L, Schwarz U, Lorian V. Penicillin-binding proteins of filaments of Escherichia coli induced by low concentrations of nalidixic acid, oxalinic acid, novobiocin, nitrofurantoin. Drugs Exp Clin Res. 1986;12:653–656. [PubMed] [Google Scholar]
- 3.Beachey E M, Eisestein B I. Bacterial adherence in infections diseases. Kalamazoo, Mich: Upjohn Company; 1982. [Google Scholar]
- 4.Bellinati-Pires R, Melki S E, Colletto G M D D, Carneiro-Sampaio M M S. Evaluation of a fluorochrome assay for assessing the bactericidal activity of neutrophils in human phagocyte dysfunctions. J Immunol Methods. 1989;119:189–196. doi: 10.1016/0022-1759(89)90395-5. [DOI] [PubMed] [Google Scholar]
- 5.Braga P C. Effects of subinhibitory concentrations of seven macrolides and four fluoroquinolones on adhesion of Staphylococcus aureus to human mucosal cells. Chemotherapy. 1994;40:304–310. doi: 10.1159/000239211. [DOI] [PubMed] [Google Scholar]
- 6.Braga P C, Piatti G. Influence of enoxacin sub-MICs on the adherence of Staphylococcus aureus and Escherichia coli to human buccal and urinary epithelial cells. Chemotherapy. 1992;38:261–266. doi: 10.1159/000239009. [DOI] [PubMed] [Google Scholar]
- 7.Braga P C, Piatti G. Favourable effects of sub-MIC rufloxacin concentrations in decreasing the pathogen-host cell adhesion. Pharmacol Res. 1993;28:11–19. doi: 10.1006/phrs.1993.1105. [DOI] [PubMed] [Google Scholar]
- 8.Braga P C, Piatti G. Kinetics of filamentation of Escherichia coli induced by different sub-MICs of ceftibuten at different times. Chemotherapy. 1993;39:272–277. doi: 10.1159/000239136. [DOI] [PubMed] [Google Scholar]
- 9.Braga P C, Dal Sasso M, Maci S, Reggio S, Piatti G. Influence of subinhibitory concentrations of brodimoprim and trimethoprim on the adhesiveness, hydrophobicity, hemagglutination and motility of Escherichia coli. Chemotherapy. 1995;41:50–58. doi: 10.1159/000239324. [DOI] [PubMed] [Google Scholar]
- 10.Burnham J C. Mediation by enoxacin of adherence of Escherichia coli to uroepithelial cells. Rev Infect Dis. 1988;10(Suppl.):S175. [Google Scholar]
- 11.Burnham J C, Sonstein S A. Program and abstracts of the 30th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1990. Anti-virulence effects of lomefloxacin and other quinolones, abstr. 1010; p. 254. [Google Scholar]
- 12.Chapman J S, Georgopapadakou N H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988;32:438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chopra I, Linton A. The antibacterial effects of low concentrations of antibiotics. Adv Microbiol Physiol. 1986;28:212–259. doi: 10.1016/s0065-2911(08)60240-4. [DOI] [PubMed] [Google Scholar]
- 14.Costerton J W. Effects of antibiotics on adherent bacteria. In: Sabath L D, editor. Action of antibiotics in patients. Vienna, Austria: Hans Huber; 1981. pp. 160–175. [Google Scholar]
- 15.Courthright J B, Turowski D A, Sonstein S A. Alteration of bacterial DNA structure, gene expression and plasmid encoded antibiotic resistance following exposure to enoxacin. J Antimicrob Chemother. 1988;21(Suppl. B):1–18. doi: 10.1093/jac/21.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- 16.Crosby H A, Bron J F, Penn C W, Elliott T S J. Antibiotic-induced release of endotoxin from bacteria in vitro. J Med Microbiol. 1994;40:23–30. doi: 10.1099/00222615-40-1-23. [DOI] [PubMed] [Google Scholar]
- 17.Cuffini A M, Tullio V, Allocco A, Paizis G, De Leo C, Carlone N A. Effect of rufloxacin upon non-specific immune defences: in-vitro, ex-vivo and in-vivo results. J Antimicrob Chemother. 1994;34:545–553. doi: 10.1093/jac/34.4.545. [DOI] [PubMed] [Google Scholar]
- 18.Desnottes J F, Le Roy D, Diallo N. Effect of subminimal inhibitory concentrations of pefloxacin on the piliation and adherence of E. coli. Drugs Exp Clin Res. 1988;14:629–634. [PubMed] [Google Scholar]
- 19.Diver J N, Wise R. Morphological and biochemical changes in Escherichia coli after exposure to ciprofloxacin. J Antimicrob Chemother. 1986;18(Suppl. D):31–41. doi: 10.1093/jac/18.supplement_d.31. [DOI] [PubMed] [Google Scholar]
- 20.Dougherty T J, Saukkonen J J. Membrane permeability changes associated with DNA gyrase inhibitors in Escherichia coli. Antimicrob Agents Chemother. 1985;28:200–206. doi: 10.1128/aac.28.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douglas L J. Adhesion of Candida species to epithelial surfaces. Crit Rev Microbiol. 1987;15:27–43. doi: 10.3109/10408418709104446. [DOI] [PubMed] [Google Scholar]
- 22.Evans D J, Jr, Evans D G, Young L S, Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980;12:235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forsgren A, Bergkvist P J. Effect of ciprofloxacin on phagocytosis. Eur J Clin Microbiol. 1985;4:575–578. doi: 10.1007/BF02013398. [DOI] [PubMed] [Google Scholar]
- 24.Goldner M, Farkas-Himsley H, Kormendy A, Skinner M. Bacterial phagocytosis monitored by fluorescence and extracellular quenching: ingestion and intracellular killing. Lab Med. 1993;14:291–294. [Google Scholar]
- 25.Guan L, Burnham J C. Postantibiotic effect of Cl-960, enoxacin and ciprofloxacin on Escherichia coli: effect on morphology and hemolysin activity. J Antimicrob Chemother. 1992;29:529–538. doi: 10.1093/jac/29.5.529. [DOI] [PubMed] [Google Scholar]
- 26.Hammami A, Agueda S, Archambaud M. Etudes in vitro des effects de l’acide oxolinique à concentratios sub-inhibitrices sur l’activité de hemagglutinés et de l’adhesion aux cellules uro-epithéliales des Escherichia coli isolés des urines. Pathol Biol (Paris) 1987;35:545–550. [PubMed] [Google Scholar]
- 27.Hed J. The extinction of fluorescence by crystal violet and its use to differentiate between attached and ingested microorganisms in phagocytosis. FEMS Microbiol Lett. 1977;1:357–361. [Google Scholar]
- 28.Huisman O, D’Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981;290:797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- 29.Ljungh Å, Wadström T. Salt aggregation test for measuring cell surface hydrophobicity of urinary Escherichia coli. Eur J Clin Microbiol. 1982;1:388–393. doi: 10.1007/BF02019940. [DOI] [PubMed] [Google Scholar]
- 30.Lorian V. Some effects of subinhibitory concentrations of antibiotics on bacteria. Bull N Y Acad Med. 1975;51:1046–1055. [PMC free article] [PubMed] [Google Scholar]
- 31.Lorian V, Ernst J. Effects of antibiotic on bacterial structure and their pathogenecity. Pathol Biol. 1987;35:1370–1376. [PubMed] [Google Scholar]
- 32.Lorian V, Ernst J, Amaral L. The post-antibiotic effect defined by bacteria morphology. J Antimicrob Chemother. 1989;23:485–491. doi: 10.1093/jac/23.4.485. [DOI] [PubMed] [Google Scholar]
- 33.Lorian V, Tosch W, Joyce D. Weight and morphology of bacteria exposed to antibiotics. In: Adam D, Hahn H, Opferkuch W, editors. The influence of antibiotics on the host-parasite relationship II. Heidelberg, Germany: Springer-Verlag; 1985. pp. 65–72. [Google Scholar]
- 34.Löwdin G, Odenholt-Tornqvist I D, Bengtsson S, Cars O. A new method to determine postantibiotic effect and effects of subinhibitory antibiotic concentrations. Antimicrob Agents Chemother. 1993;37:2200–2205. doi: 10.1128/aac.37.10.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandell L A, Afnan M. Mechanisms of interaction among subinhibitory concentrations of antibiotics, human polymorphonuclear neutrophils, and gram-negative bacilli. Antimicrob Agents Chemother. 1991;35:1291–1297. doi: 10.1128/aac.35.7.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milatovic D. Effect of subinhibitory antibiotic concentrations on the phagocytosis of Staphylococcus aureus. Eur J Clin Microbiol. 1982;1:97–101. doi: 10.1007/BF02014199. [DOI] [PubMed] [Google Scholar]
- 37.Milatovic D. Antibiotics and phagocytosis. Eur J Clin Microbiol. 1983;2:414–425. doi: 10.1007/BF02013898. [DOI] [PubMed] [Google Scholar]
- 38.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 39.Odenholt-Tornqvist I O, Löwdin E, Cars O. Pharmacodynamic effects of subinhibitory concentrations of β-lactam antibiotics in vitro. Antimicrob Agents Chemother. 1991;35:1834–1839. doi: 10.1128/aac.35.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peman J, Canton E, Hernandez M T, Gobernado M. Intraphagocytic killing of Gram-positive bacteria by ciprofloxacin. J Antimicrob Chemother. 1994;34:965–974. doi: 10.1093/jac/34.6.965. [DOI] [PubMed] [Google Scholar]
- 41.Phillips I, Culebras E, Moreno F, Baquero F. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother. 1987;20:632–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- 42.Raponi G, Keller N, Overbeek B P, Rosenberg-Arska M, van Kessel K P M, Verhoef J. Enhanced phagocytosis of encapsulated Escherichia coli strains after exposure to sub-MICs of antibiotics is correlated to changes of the bacterial cell surface. Antimicrob Agents Chemother. 1990;34:332–336. doi: 10.1128/aac.34.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson P, Wakefield D, Breit S N, Easter J F, Penny R. Chemiluminescent response to pathogenic organisms: normal human polymorphonuclear leukocytes. Infect Immun. 1984;43:744–752. doi: 10.1128/iai.43.2.744-752.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaeffer A J, Amundsen S K, Schmidt L N. Adherence of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1979;24:753–759. doi: 10.1128/iai.24.3.753-759.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schifferli D M, Beachey E M. Bacterial adhesion: modulation by antibiotics with primary targets other than protein synthesis. Antimicrob Agents Chemother. 1988;32:1609–1613. doi: 10.1128/aac.32.11.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoemaker J M, Gayda R C, Markovitz A. Regulation of cell division in Escherichia coli: SOS induction and cellular location of the Sul A protein, a key to lon-associated filamentation and death. J Bacteriol. 1984;158:551–561. doi: 10.1128/jb.158.2.551-561.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibl A M. Effect of antibiotics on adherence of microorganisms to epithelial cell surfaces. Rev Infect Dis. 1995;7:51–56. doi: 10.1093/clinids/7.1.51. [DOI] [PubMed] [Google Scholar]
- 48.Smith J T, Lewin C S. Chemistry and mechanisms of action of the quinolone antibacterials. In: Andriole V T, editor. The quinolones. New York, N.Y: Academic Press; 1988. [Google Scholar]
- 49.Sonstein S A, Burnham J C. Effect of low concentrations of quinolone antibiotics on bacterial virulence mechanisms. Diagn Microbiol Infect Dis. 1993;16:277–289. doi: 10.1016/0732-8893(93)90078-l. [DOI] [PubMed] [Google Scholar]
- 50.Sous H, Hirsch I. Bactericidal activity of phenoxymethyl-penicillin in an in-vitro model simulating tissue kinetics. J Antimicrob Chemother. 1985;15(Suppl. A):233–239. doi: 10.1093/jac/15.suppl_a.233. [DOI] [PubMed] [Google Scholar]
- 51.Svanborg-Eden C, Sandberg T, Alestig K. Decrease in adhesion of E. coli to human urinary tract epithelial cells in vitro by subinhibitory concentrations of ampicillin. Infection. 1978;6(Suppl. 1):121–124. [Google Scholar]
- 52.Vrane S J, Zagar Z, Kurbel S. Influence of subinhibitory concentrations of ceftazidime, ciprofloxacin and azithromycin on the morphology and adherence of P-fimbriated Escherichia coli. J Chemother. 1996;8:254–260. doi: 10.1179/joc.1996.8.4.254. [DOI] [PubMed] [Google Scholar]
- 53.Wise R, Johnson J, O’Sullivan N, Andrews J M, Imbimbo B P. Pharmacokinetics and tissue penetration of rufloxacin, a long acting quinolone antimicrobial agent. J Antimicrob Chemother. 1991;28:905–909. doi: 10.1093/jac/28.6.905. [DOI] [PubMed] [Google Scholar]
- 54.Wise R, Andrews J M, Imbimbo B P, Honeybourne D. The penetration of rufloxacin into sites of potential infection in the respiratory tract. J Antimicrob Chemother. 1993;32:861–866. doi: 10.1093/jac/32.6.861. [DOI] [PubMed] [Google Scholar]