Abstract
Purpose
To explore the clinical characteristics, diagnosis, and treatment of severe Chlamydia psittaci pneumonia complicated by rhabdomyolysis and to improve the success rate of treatment.
Patients and Methods
The clinical characteristics, diagnosis, treatment, and outcomes of four patients with severe C. psittaci pneumonia complicated by rhabdomyolysis diagnosed by metagenomic next-generation sequencing (mNGS) in our hospital were analyzed retrospectively.
Results
All four patients were male, aged 46–64 years, and all had a history of bird contact. All patients had fever, fatigue, tea-colored urine, myalgia, and two patients were unable to walk. C. psittaci DNA was found by mNGS of the bronchoalveolar lavage fluid of all four patients. Their creatine kinase was >1000 U/L, and myoglobin, C-reactive protein, procalcitonin, and brain natriuretic peptide were significantly increased. The McMahon score of three patients was >6 points, of whom one patient suffered from acute kidney injury; he was treated with continuous renal replacement therapy and eventually died. After diagnosis, three patients were treated with doxycycline and quinolones and were discharged after recovery.
Conclusion
Psittacosis complicated by rhabdomyolysis is characterized by fever, fatigue, myalgia, and tea-colored urine, with significant increases in creatine kinase and myoglobin. The McMahon score should be applied early to assess the risk of acute kidney injury, and renal replacement therapy and renal protection therapy should be initiated in the early stage. Among severely ill patients, early use of empirical antibiotics, including quinolones, may improve the prognosis.
Keywords: Chlamydia psittaci, severe pneumonia, rhabdomyolysis, metagenomic next-generation sequencing
Introduction
Chlamydia psittaci is a specific intracellular gram-negative bacterium that mainly infects birds. The genotypes A and E can infect humans,1 with the lungs being the most common site of infection, and can lead to severe pneumonia and even death in some cases.2 Bacterial atypical pneumonia may occasionally induce rhabdomyolysis and secondary kidney damage. Causative bacteria include Francisella tularensis, Coxiella burnetii, Chlamydia pneumoniae and C. psittaci, but case reports are rare.3 Rhabdomyolysis refers to a group of clinical syndromes, such as destruction and necrosis of muscle tissue, destruction of the integrity of muscle cell membrane, damage of tissues and organs, and release of a large amount of myoglobin, creatine kinase (CK), and toxic substances into the blood, caused by various reasons.4 Only four cases of C. psittaci pneumonia complicated by rhabdomyolysis have been reported globally to date,5–7 and the data are either out of date or incomplete. C. psittaci pneumonia complicated by rhabdomyolysis is associated with multiple organ dysfunction, and has a poor prognosis, so early clinical intervention is important. In this study, we retrospectively analyzed the clinical data of four cases of severe C. psittaci pneumonia complicated by rhabdomyolysis diagnosed by metagenomic next-generation sequencing (mNGS) and summarized their clinical features to help clinicians to make an early diagnosis and to initiate empirical antibiotic treatment early in order to improve the success rate of treatment.
Metagenomic Next-Generation Sequencing
All four cases were diagnosed by metagenomic next-generation sequencing (mNGS). Bronchoalveolar lavage fluid (BALF) samples were collected by following the standards of aseptic processing procedures. Using previously described methods, mNGS analyses were completed by the BGI Genomics Institute (Shenzhen, China).8 Briefly, BALF samples (3–5 mL) were collected following standard procedures. In the laboratory, the samples were agitated at 4000 rpm for 10 min. The DNA from 0.3 mL of each sample was extracted using a TIANamp Micro DNA Kit (DP316, Tiangen Biotech, Beijing, China), following the manufacturer’s instructions. DNA libraries were constructed using DNA fragmentation, end-repair, adapter ligation, and PCR amplification. Low-quality and short (length <35 bp) reads were removed to generate high-quality sequencing data, and computational subtraction of human host sequences mapped to the human reference genome (hg19) were performed using the Burrows-Wheeler alignment. The remaining data were classified by removing low-complexity reads, and the sequences were aligned to microbial genome databases for bacteria, fungi, viruses, and parasites downloaded from the US National Center for Biotechnology Information website (ftp://ftp.ncbi.nlm.nih.gov/genomes).
Case Presentations
Case 1
The patient, a 64-year-old man, had a history of diabetes. Five days before presenting to our clinic, he developed fever, fatigue, myalgia, cough, and expectoration, and became unable to walk. His highest body temperature was 39°C. Two days before presenting, he experienced nausea, vomiting, and dizziness. He visited a local Hospital. Chest computed tomography (CT) revealed consolidation in the lower lobe of the left lung. His white blood cell (WBC) count was 12.47×109/L, neutrophil percentage 82.6%, and his C-reactive protein (CRP) and procalcitonin (PCT) levels were 142.4 mg/L and 5.86 ng/mL, respectively. His clinical condition worsened, and he was transferred to our hospital after tracheal intubation. On admission his temperature was 37.8°C. Physical examination revealed wet rales in both lungs. Blood tests revealed elevated WBC count, absolute neutrophil count and neutrophil percentage; elevated blood glucose (20.5 mmol/L), glycosylated hemoglobin (HbA1c) (10.9%), CRP, PCT, CK, myoglobin, brain natriuretic peptide (BNP), creatinine, urea, fibroblast, D-dimer, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) levels; and low serum calcium, HCO3 and estimated glomerular filtration rate (eGFR)level (Table 1).;. Urinalysis revealed: proteinuria, 1+; glycosuria, 3+; hematuria, 3+; erythrocyte sediment, 67.1/μL; urinary casts, 2.3/μL; and epithelial cells, 19.8/μL. Chest CT showed lung consolidation in the left lower lobe with a small left-sided pleural effusion (Figure 1A). After admission, mechanical ventilation was initiated. The patient was treated with intravenous piperacillin/tazobactam (4.5 g 8-hourly), which was switched to intravenous meropenem (1 g 8-hourly) 2 days later. On day 2 of admission, bronchoalveolar lavage was performed by fiberoptic bronchoscopy, and BALF was sent for mNGS. On day 3, the patient developed anuria. He was diagnosed with acute kidney injury (AKI) and treated with continuous renal replacement therapy (CRRT). On day 4, the patient died of multiple organ failure. On the day of death, the mNGS identified C. psittaci (sequence number 1255). When questioned about the patient’s history of exposure to birds, family members reported that the patient had been exposed to parrots 10 days before the disease onset.
Table 1.
Clinical Characteristics and Laboratory Data of the Four Patients
| Items | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Sex, age (years) | Male, 64 | Male, 54 | Male, 46 | Male, 56 |
| History of bird contact | Parrots | Dead birds | Geese | Chickens |
| Underlying diseases | Diabetes | Coronary heart disease, hypertension | Hypertension | None |
| Symptoms | Fever, fatigue, muscle soreness, cough, expectoration, nausea, and vomiting | Fever, fatigue, muscle soreness, cough, poor consciousness | Fever, fatigue, muscle soreness, cough, expectoration, shortness of breath, diarrhea | Fever, fatigue, cough, expectoration |
| HCO3 (normal: 22–28 mmol/L) | 19.54 | 29.6 | 24.4 | 22.3 |
| WBC (normal: 4–10×109/L) | 13.31 | 3.2 | 6.17 | 5.88 |
| Absolute neutrophil count(normal:2–7×109/L) | 12.05 | 2.28 | 5.45 | 4.76 |
| Neutrophil percentage (normal: 50–70%) | 90.5 | 88.2 | 88.3 | 80.6 |
| CRP (normal: 0–8 mg/L) | 89 | 229.5 | 179.96 | 147.79 |
| PCT (normal: 0–5 ng/mL) | 4.75 | 4.26 | 1.16 | 1.15 |
| CK (normal: 26–174 U/L) | 20,252 | 7659 | 2363 | 5066 |
| CK-MB (normal:0–24U/L) | 23 | 19 | 20 | 17 |
| High-sensitivity troponin T(normal:0–14ng/L) | 13 | 11 | 12 | 11.53 |
| Myoglobin (normal: 0–110 ng/mL) | 823.13 | 613.5 | 867.5 | 534.21 |
| BNP (normal: 0–100 pg/mL) | 4246 | 1669 | 1461 | 1388 |
| AST (normal: 13–40 U/L) | 348 | 258 | 81.7 | 141 |
| ALT (normal: 5–40 U/L) | 70 | 72 | 48.4 | 141 |
| LDH (normal: 0–247 U/L) | 576 | 669 | 461 | 388 |
| Cr (normal: 59–104 µmol/L) | 178 | 257 | 111 | 137 |
| Urea (normal: 3.2–7.1 µmol/L) | 11.34 | 12.02 | 8.21 | 10.3 |
| eGFR(normal:80–120 mL/min) | 34.69 | 23.87 | 69.69 | 50.36 |
| Ca (normal: 2.25–2.75 mmol/L) | 1.87 | 1.74 | 1.68 | 1.85 |
| P (normal: 0.96–1.62 mmol/L) | 1.42 | 0.51 | 0.55 | 0.76 |
| Fibroblasts (normal: 2–4 g/L) | 12.54 | 6.65 | 4.71 | 6.58 |
| D-dimer (normal: 0–0.5 mg/L) | 2.26 | 2.13 | 3.44 | 1.3 |
| mNGS C. psittaci reads (n) | 1255 | 1178 | 1563 | 3102 |
| McMahon score | 9 | 9.5 | 5 | 8 |
Abbreviations: AST, aspartate aminotransferase; BNP, brain natriuretic peptide; CK, creatine kinase; Cr, creatinine; CRP, C-reactive protein; LDH, lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cell.
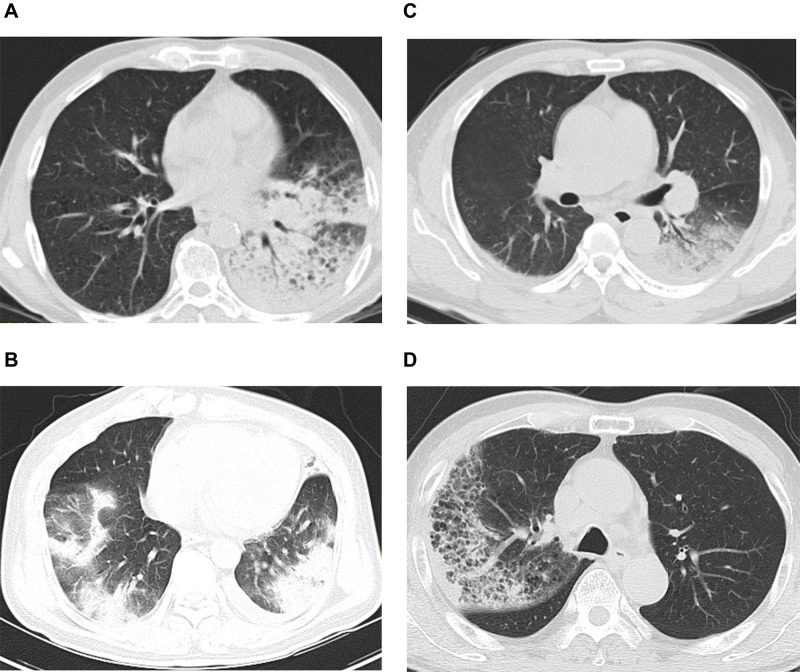
Figure 1.
(A) Chest computed tomography (CT) of Case 1: consolidation in left lower lung and a small amount of pleural effusion on the left. (B) Chest CT of Case 2: large exudative consolidation foci in both lungs, mainly in the lower lobe, and a small amount of pleural effusion on both sides. (C) Chest CT of Case 3: consolidation in both lower lungs, mainly on the left and bilateral pleural effusion. (D) Chest CT of Case 4: right lung consolidation and ground-glass shadow.
Case 2
The patient, a 54-year-old man, had a history of coronary heart disease and hypertension, and had developed fever, fatigue, and a cough 4 days previously. Two days later, his level of consciousness deteriorated, the fatigue increased, and he developed myalgia and became unable to walk. He visited the emergency department of our hospital. On examination his body temperature was 38.9°C and he appeared somnolent. The Respiratory sounds of both lungs were coarse, and the muscle strength of both legs was Grade 4. Blood tests revealed an elevated neutrophil percentage; elevated CRP, PCT, CK, myoglobin, BNP, creatinine, urea, fibroblast, D-dimer, AST, ALT, HCO3, and LDH levels; and low WBC, serum calcium, phosphorus and eGFR levels (Table 1). Urinalysis revealed: proteinuria, 2+; hematuria, 2+; erythrocyte sediment, 138/μL; urinary casts, 4.08/μL; and epithelial cells, 23.3/μL. Chest CT showed large areas of exudation and consolidation in both lungs, mainly in the lower lobes, with an air bronchogram and small bilateral pleural effusions (Figure 1B). Cerebrospinal fluid examination showed no abnormalities. The patient was treated with intravenous moxifloxacin (0.4 g daily) and meropenem (1 g 8-hourly) as antibiotic therapy in addition to a saline infusion and oral sodium bicarbonate tablets (1 g three times a day). Two days later, the patient’s condition worsened, and the patient was intubated and started on mechanical ventilation. Fiberoptic bronchoscopy with bronchoalveolar lavage was performed, and BALF was sent for mNGS. According to the mNGS results, C. psittaci (sequence number 1178) was found. When inquiring about the medical history, the family members said that the patient had been exposed to dead birds one week before the disease onset. C. psittaci pneumonia was considered, and the antibiotic therapy was switched to intravenous doxycycline (0.1 g twice a day) combined with moxifloxacin. After 2 days of doxycycline treatment, the patient’s body temperature returned to normal, and he was treated with doxycycline alone. After 14 days of treatment, reexamination showed that his CK, myoglobin, and creatinine had returned to normal levels, and he was discharged. He continued to take oral doxycycline (0.1 g twice a day) for one week after discharge. Follow-up chest CT performed in the outpatient clinic showed absorption of the lung lesions.
Case 3
The patient, a 46-year-old man with a history of hypertension, developed fever 17 days prior to presentation, with a highest body temperature of 40°C, accompanied by fatigue, myalgia in both legs, and anorexia. He took over-the-counter antipyretics but the fever recurred. A week before presentation, he developed a cough, expectoration, shortness of breath, and diarrhea. He visited another hospital and was admitted for treatment. The blood test results were as follows: WBC, 8.9×109/L; neutrophil percentage, 82.1%; CRP, 159.66 mg/L; PCT, 2.87 ng/mL; fibroblasts, 6.85 g/L; and D-dimer, 2.31 mg/L. Chest CT revealed consolidation in both lower lungs. Intravenous moxifloxacin (0.4 g daily) and piperacillin/tazobactam (4.5 g 8-hourly) were given as antibiotic therapy. After one week of treatment, the patient still had recurrent fever and shortness of breath, and so he was transferred to our hospital. A physical examination on admission revealed the following: body temperature, 37.7°C; blood pressure, 165/81 mmHg; respiratory rate, 45 bpm; and wet rales in both lower lungs. Blood tests revealed an elevated neutrophil percentage; elevated CRP, PCT, CK, myoglobin, BNP, creatinine, urea, fibroblast, D-dimer, AST, ALT and LDH levels; and low serum calcium, phosphorus and eGFR levels (Table 1). Urinalysis revealed: proteinuria, 1+;hematuria, 2+; erythrocyte sediment, 118/μL; urinary casts, 3.58/μL; and epithelial cells, 21.6/μL. Chest CT showed consolidation in both lower lungs, mainly on the left side, and bilateral pleural effusion (Figure 1C). After admission, the patient was given intravenous meropenem (1 g,8-hourly) and moxifloxacin (0.4 g daily) as antibiotic therapy, and intravenous saline and oral sodium bicarbonate tablets (1 g three times a day). On day 2 of admission, his oxygenation index dropped to 150 mmHg, and he was intubated and started on mechanical ventilation. Fiberoptic bronchoscopy with bronchoalveolar lavage was performed, and BALF was sent for mNGS. The mNGS revealed C. psittaci (sequence number, 1563). When asked about his history of exposure to birds, family members reported that he had been exposed to live geese several times during the 2 weeks before disease onset. He was diagnosed with C. psittaci pneumonia, and the antibiotic therapy was switched to intravenous doxycycline (0.1 g twice a day) combined with moxifloxacin. Three days later, his body temperature returned to normal. After 2 weeks of treatment, his CK, myoglobin, and creatinine levels had returned to normal, and he was discharged. He continued to take oral doxycycline (0.1 g twice a day) for one week after discharge. Follow-up chest CT showed absorption of the lung lesions.
Case 4
The patient, a 56-year-old man developed fever one week prior to presentation, with a peak body temperature of 39.7°C, accompanied by fatigue, myalgia, anorexia, cough, and expectoration. A physical examination on admission revealed a body temperature of 38°C and coarse respiratory sounds in both lungs. Blood tests revealed an elevated neutrophil percentage, and elevated CRP, PCT, CK, myoglobin, BNP, creatinine, urea, fibroblast, D-dimer, AST, ALT and LDH levels; and low serum calcium, phosphorus and eGFR levels (Table 1). Urinalysis revealed: proteinuria, 2+;hematuria, 3+; erythrocyte sediment, 147/μL; urinary casts, 4.88/μL; and epithelial cells, 25.6/μL. Chest CT showed consolidation and ground-glass opacity in the right lung (Figure 1D). After admission, the patient was treated with mechanical ventilation through tracheal intubation, intravenous piperacillin/tazobactam (4.5 g 8-hourly) and levofloxacin (0.5 g daily) as antibiotic therapy, intravenous saline infusion, and oral sodium bicarbonate tablets (1 g three times a day). On day 2, fiberoptic bronchoscopy with bronchoalveolar lavage was performed. BALF was sent for mNGS, which found C. psittaci (sequence number 3102). When asked about his history of exposure to birds, family members reported that the patient had been exposed to live chickens one week before the disease onset. He was diagnosed with C. psittaci pneumonia, and the antibiotics were switched to intravenous doxycycline (0.1 g twice a day) and levofloxacin. After 2 weeks of treatment, his CK, myoglobin, and creatinine levels returned to normal, and he was discharged. He continued to take oral doxycycline (0.1 g twice a day) for one week after discharge. Follow-up chest CT showed absorption of the lung lesions.
Discussion
C. psittaci pneumonia accounts for about 1% of cases of community-acquired pneumonia, among which about 30% of cases are severe.9,10 Contact with birds or poultry is the main risk factor for C. psittaci pneumonia.11 In this study, all four patients had a history of contact with birds. The incubation period in these patients after contact with birds was 1 to 2 weeks, which is consistent with a report by Hogerwerf et al.12 We recommend that newly admitted patients with severe pneumonia should be routinely questioned about a history of contact with birds in the past month. In this study, all four patients were male. According to Raeven et al,13 males account for the majority of cases of C. psittaci pneumonia. It is unclear whether the male preponderance is related to males being more frequently engaging in breeding and slaughtering birds. This remains to be confirmed by larger studies. Skin eruptions sometimes occur in patients affected with Mycoplasma pneumoniae respiratory infections.14 No rash was found in the 4 patients with Chlamydia psittacosis pneumonia.
Bacterial atypical pneumonia induced rhabdomyolysis is more common. Causative bacteria include Francisella tularensis, Coxiella burnetii, Chlamydia pneumoniae and C. psittaci.3 Simoni et al3 reported 42 previously healthy individuals with atypical pneumonia complicated with myositis, of which 27 cases were caused by Legionella species, 6 cases by other bacteria, 9 cases by Mycoplasma pneumoniae, and one case was caused by Chlamydia psittaci. Of the 42 patients, 37 were diagnosed with rhabdomyolysis-associated AKI. Rhabdomyolysis is the destruction and necrosis of muscle tissue and the release of the cell contents into the blood.4 Infection is a common cause of rhabdomyolysis. The main mechanisms include tissue hypoxia, direct invasion of muscles by bacteria, decrease in glycolytic enzyme activity, lysosomal enzyme activation, and endotoxin release.15 Muscle biopsies performed in three patients with Mycoplasma infection and three patients with Legionella infection revealed inflammatory muscle lesions in all cases,16–19 with none of the patients testing positive for immune deposits. Diabetes is a risk factor for rhabdomyolysis.4 The blood glucose and HbA1c levels in Case 1 were markedly elevated on admission, indicating that the patient had longstanding poor blood glucose control, and he had been in a state of hyperglycemia for an extended period. Hyperglycemia and hypertonic states are more likely to cause osmotic swelling of skeletal muscle cells, calcium overload, and increased oxidative stress due to damage to mitochondria and the sarcoplasmic reticulum, resulting in skeletal muscle injury.4,20 C. psittaci pneumonia complicated by rhabdomyolysis is mainly characterized by fever, fatigue, myalgia, and tea-colored urine. In this study, all patients had fatigue, tea-colored urine, and myalgia. The myalgia was mainly in the proximal muscle groups such as those in the thighs, and lower back.4 Rhabdomyolysis can be diagnosed when the blood CK level is >1000 U/L.21,22 All 4 patients in this study met this criterion. Serum myoglobin was also increased in all four patients. When the myoglobin concentration exceeds the threshold of renal metabolism, a large amount of myoglobin will be excreted, resulting in tea-colored urine. All four patients had different degrees of hypocalcemia, which was related to the destruction of muscle cell membranes and the influx of calcium ions into cells caused by adenosine triphosphate consumption.23 AST, LDH, fibroblasts, and D-dimer increased to different degrees in all patients. Urinalysis showed positive results for occult blood, protein, urinary casts, and dead epithelial cells.
AKI is the most serious complication of rhabdomyolysis, and occurs in approximately 7–10% of cases.4 Once AKI occurs, the mortality rate increases by 80%.4 Active application of crystalloid fluids in the early stage can effectively prevent and treat AKI caused by rhabdomyolysis. McMahon scoring can predict the risk of death caused by renal replacement therapy or renal failure in patients with rhabdomyolysis. The main variables are age, sex, potential etiology, and laboratory test results (blood calcium, CK, phosphate, and HCO3 levels). A McMahon score ≥6 points indicates a high risk of AKI, requiring dialysis, and renal protective treatment should be provided.24,25 In this study, three patients had a McMahon score >6 points, and the two surviving patients received renal protection therapy. Case 1, who died, had a McMahon score of 9 points on admission. After admission, no standardized renal protection treatment was given, and the patient rapidly progressed to renal failure and anuria, and finally CRRT was required. However, the mortality rate of patients with acute renal injury who needed dialysis is as high as 50–80%.15 For patients with a McMahon score >6 points, rehydration should be started as soon as possible within 6 h after the diagnosis of rhabdomyolysis.26,27 Fluids should be administered at a rate that maintains a urine output of 300 mL/h or more for at least the first 24 hours. Studies have shown that the development of AKI is directly proportional to the timing of fluid administration, and that the longer it takes for hydration to be initiated, the greater the likelihood of AKI. Prompt intravenous fluid administration is associated with better outcomes.28 Initial fluid resuscitation may be given at a rate of 1 to 2 L/hour. Normally, hydration is maintained until the resolution of rhabdomyolysis or until plasma CK levels decrease to <5000 U/L. AKI is less likely to occur if the peak CK levels are under 10,000 U/L.29 Saline should be used as the main form of rehydration,30 and sodium bicarbonate can be taken orally to alkalize urine simultaneously.31 Some patients with rhabdomyolysis have been shown benefit from bicarbonate therapy. The principles of urine alkalinization are based on studies in animal models and include the following: Tamm-Horsfall protein-myoglobin complex precipitation is increased in acidic urine, hence alkalinization prevents intratubular pigment cast formation; redox cycling of myoglobin and lipid peroxidation is inhibited by alkaline urine, thus decreasing tubular damage; and myoglobin-induced vasoconstriction is observed only in acidic medium.32 There is no specific therapy once the patient develops AKI. Initiation of dialysis may be necessary for control of volume overload and correction of intractable and severe metabolic abnormalities. Daily hemodialysis or continuous hemofiltration may be required initially to remove urea and potassium that are released from damaged muscles.15 In most cases, acute renal injury is completely reversible, and most patients’ renal function can return to normal within a few months. Use of the McMahon score immediately on admission, enables early initiation of renal protection or CRRT, which may improve the prognosis.
Tetracyclines, macrolides, and quinolones can be used to treat C. psittaci pneumonia.33 Tetracyclines such as doxycycline, tetracycline, vibramycin, and minocycline are the antibiotics of choice for treating psittacosis pneumonia.1 Doxycycline is recommended for severely ill patients.34 Because there was no vibramycin in our hospital, after three severely ill patients were diagnosed, we adopted doxycycline combined with quinolones and achieved good results. The recommended duration of antibiotic therapy for psittacosis pneumonia is 14 to 21 days.1 In this study, the patient who died did not receive any antibiotics, including quinolones, before diagnosis, while the three surviving patients were administered β-lactams combined with quinolones as antibiotic therapy before diagnosis. We recommend that physicians routinely use β-amides combined with quinolones as empiric antibiotic treatment in severely ill patients34 pending diagnosis.
Conclusion
Psittacosis with rhabdomyolysis is rare, with fever, fatigue, myalgia and tea-colored urine as the main clinical manifestations, and a significant increase in the serum CK and myoglobin levels. Early diagnosis is very important. The McMahon score should be applied in the early stage to assess the risk of AKI and the need for CRRT, and CRRT should be initiated early. In severely ill patients, early use of empiric antibiotic regimens, including quinolones, may improve the prognosis.
Funding Statement
This work was supported by Guangdong Medical Science and Technology Research Project Foundation (No.C2019032), and Zhongshan Medical Research Project (No.2021J096).
Data Sharing Statement
Not applicable.
Ethics Approval and Informed Consent
The Ethics Committees of Zhongshan People’s Hospital (K2021-137) approved this study. Informed consents were obtained from patients and guardians.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or Critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Balsamo G, Maxted AM, Midla JW, et al. Compendium of Measures to Control Chlamydia psittaci Infection Among Humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2017. Journal of Avian Medicine and Surgery. 2017;31(3):262–282. doi: 10.1647/217-265 [DOI] [PubMed] [Google Scholar]
- 2.Lamoth F, Greub G. Fastidious intracellular bacteria as causal agents of community-acquired pneumonia. Expert Rev Anti Infect Ther. 2010;8(7):775–790. [DOI] [PubMed] [Google Scholar]
- 3.Simoni C, Camozzi P, Faré PB, et al. Myositis and acute kidney injury in bacterial atypical pneumonia: systematic literature review. J Infect Public Health. 2020;13(12):2020–2024. [DOI] [PubMed] [Google Scholar]
- 4.Cabral B, Edding SN, Portocarrero JP, et al. Rhabdomyolysis. Dis Mon. 2020;66(8):101015. [DOI] [PubMed] [Google Scholar]
- 5.Matsushima H, Takayanagi N, Ubukata M, et al. [A case of fulminant psittacosis with rhabdomyolysis]. Nihon Kokyuki Gakkai Zasshi. 2002;40(7):612–616. Japanese. [PubMed] [Google Scholar]
- 6.Kawamura S, Ikematsu H, Ogimoto H. [Two cases of psittacosis accompanied with rhabdomyolysis]. Kansenshogaku Zasshi. 1990;64(9):1239–1243. Japanese. [DOI] [PubMed] [Google Scholar]
- 7.Qi YF, Huang JL, Chen JH, et al. [Chlamydia psittaci pneumonia complicated by rhabdomyolysis: a case report and literature review]. Chinese Journal of Tuberculosis and Respiratory Diseases. 2021;44(9):806–811. Chinese. [DOI] [PubMed] [Google Scholar]
- 8.Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl 2):S231–S240. [DOI] [PubMed] [Google Scholar]
- 9.Hogerwerf L, Gier B, Baan B, et al. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knittler MR, Berndt A, Bocker S, et al. Chlamydia psittaci: new insights into genomic diversity, clinical pathology, host-pathogen interaction and anti-bacterial immunity. Int J Med Microbiol. 2014;304(7):877–893. [DOI] [PubMed] [Google Scholar]
- 11.Hahn DL, Azenabor AA, Beatty WL, et al. Chlamydia pneumoniae as a respiratory pathogen. Front Biosci. 2002;7:e66–e76. [DOI] [PubMed] [Google Scholar]
- 12.Hogerwerf L, Roof I, de Jong M, et al. Animal sources for zoonotic transmission of psittacosis: a systematic review. BMC Infect Dis. 2020;20(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raeven VM, Spoorenberg SM, Boersma WG, et al. Atypical aetiology in patients hospitalised with community-acquired pneumonia is associated with age, gender and season; a data-analysis on four Dutch cohorts. BMC Infect Dis. 2016;16:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terraneo L, Lava SA, Camozzi P, et al. Unusual Eruptions Associated with Mycoplasma pneumoniae Respiratory Infections: review of the Literature. Dermatology. 2015;231(2):152–157. [DOI] [PubMed] [Google Scholar]
- 15.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis – an overview for clinicians. Crit Care. 2005;9(2):158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett MR, O’Connor HJ, Neuberger JM, et al. Acute polymyositis in an adult associated with Mycoplasma pneumoniae infection. Postgrad Med J. 1990;66(771):47–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brivet F, Pham Van T, Petitpretz P, et al. Rhabdomyolysis, acute renal failure and Legionnaires’ disease. Chest. 1984;86(6):943–944. [DOI] [PubMed] [Google Scholar]
- 18.Posner MR, Caudill MA, Brass R, et al. Legionnaires’ disease associated with rhabdomyolysis and myoglobinuria. Arch Intern Med. 1980;140(6):848–850. [PubMed] [Google Scholar]
- 19.Warner CL, Fayad PB, Heffner RR Jr. Legionella myositis. Neurology. 1991;41(5):750–752. [DOI] [PubMed] [Google Scholar]
- 20.Chen IW, Lin CW. Improvement in renal prognosis with prompt hemodialysis in hyperosmolar hyperglycemic state-related rhabdomyolysis: a case report. Medicine. 2018;97(50):e13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauret JM, Marinides G, Wang GK. Rhabdomyolysis. Am Fam Physician. 2002;65(5):907–912. [PubMed] [Google Scholar]
- 22.Stahl K, Rastelli E, Schoser B. A systematic review on the definition of rhabdomyolysis. J Neurol. 2020;267(4):877–882. [DOI] [PubMed] [Google Scholar]
- 23.Knochel JP. Mechanisms of rhabdomyolysis. Curr Opin Rheumatol. 1993;5(6):725–731. [DOI] [PubMed] [Google Scholar]
- 24.McMahon GM, Zeng X, Waikar SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson JP, Taylor A, Sudhan N, et al. Rhabdomyolysis and acute kidney injury: creatine kinase as a prognostic marker and validation of the McMahon Score in a 10-year cohort. Eur J Anaesthesiol. 2016;33(12):906–912. [DOI] [PubMed] [Google Scholar]
- 26.Petejova N, Martinek A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: a critical review. Crit Care. 2014;18(3):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: a systematic review. Ann Pharmacother. 2013;47(1):90–105. [DOI] [PubMed] [Google Scholar]
- 28.Ron D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Intern Med. 1984;144(2):277–280. [PubMed] [Google Scholar]
- 29.Shefner JM Clinical manifestations and diagnosis of rhabdomyolysis; 2021. Available from: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-rhabdomyolysis. Accessed February 7, 2022.
- 30.Cho YS, Lim H, Kim SH. Comparison of lactated Ringer’s solution and 0.9% saline in the treatment of rhabdomyolysis induced by doxylamine intoxication. Emerg Med J. 2007;24(4):276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatzizisis YS, Misirli G, Hatzitolios AI, et al. The syndrome of rhabdomyolysis: complications and treatment. Eur J Intern Med. 2008;19(8):568–574. [DOI] [PubMed] [Google Scholar]
- 32.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361(1):62–72. [DOI] [PubMed] [Google Scholar]
- 33.Kohlhoff SA, Hammerschlag MR. Treatment of Chlamydial infections: 2014 update. Expert Opin Pharmacother. 2015;16(2):205–212. [DOI] [PubMed] [Google Scholar]
- 34.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]