Abstract
Temporal variations in the renal toxicity of aminoglycosides have been reported for experimental animals as well as for humans. In fact, maximal renal toxicity of aminoglycosides was observed when the drug was given during the rest period, while a lower toxicity was observed when the drug was injected during the activity period. The aim of the present study was to evaluate temporal variations in the effectiveness and renal toxicity of gentamicin in an experimental model of pyelonephritis in rats. The experiments were carried out with female Sprague-Dawley rats (185 to 250 g). They had free access to food and water throughout the study and were maintained on a 14-h light–10-h dark cycle. Animals were divided into four groups corresponding to the respective time of induction of pyelonephritis and treatment: 0700, 1300, 1900, and 0100 h. Pyelonephritis was induced by a direct inoculation of Escherichia coli (107 to 108 CFU) in the left kidney. Animals were treated for 3 and 7 days with a single daily dose of gentamicin (20 and 40 mg/kg of body weight, respectively) or saline (NaCl, 0.9%) at either 0700, 1300, 1900, or 0100 h. Animals treated at 0100 h for 3 days with gentamicin (20 mg/kg) showed a significantly lower number of bacteria in their kidneys than did all other groups (P < 0.01). After 7 days of treatment, the efficacy, evaluated by the log CFU per gram of tissue and by the percentage of sterilized kidneys, was also higher when gentamicin was administered at 0100 h. The β-galactosidase and the N-acetyl-β-d-glucosaminidase activities were significantly higher in urine of rats given gentamicin at 1300 h than in urine of rats treated at another time of day (P < 0.05). Gentamicin injected at 1300 h induced a significantly greater increase of [3H]thymidine incorporation into DNA of renal cortex (P < 0.01), a significantly greater inhibition of sphingomyelinase activity (P < 0.05), and significantly more histopathological lesions than the same dose injected at another time of the day. Creatinine and blood urea nitrogen levels in serum were significantly higher (P < 0.05) and the creatinine clearance was significantly lower (P < 0.05) when gentamicin was injected at 1300 h than when it was injected at another time of day. Our data suggest temporal variations in both the toxicity and the effectiveness of gentamicin, the drug being more effective and less toxic when injected during the activity period of the animals.
Although antibiotics have been used in therapeutics for more than 60 years, no rational and standardized approaches have been defined for the treatment of urinary tract infections (12, 27, 47). In Canada and the United States, patients consult physicians more than 6 million times a year for this type of infection, which is one of the most commonly observed in clinical practice (48). Approximately 250,000 patients suffer from acute pyelonephritis, and hospitalization is often needed (12, 41).
Aminoglycosides are widely used in the treatment of severe gram-negative bacterial infections. Unfortunately, the clinical use of aminoglycosides is limited by their potential ototoxicity and nephrotoxicity (25). Nevertheless, their concentration-dependent bactericidal action, their antibacterial synergism with β-lactam antibiotics, their postantibiotic effect, their low cost, and the better understanding of risk factors associated with the use of these agents are responsible for the maintenance of their clinical use.
In the last 10 years, new approaches to the management of renal pathologies have been evaluated, but the treatment of pyelonephritis has not changed significantly (11, 12). However, several approaches to reducing the incidence of renal toxicity associated with aminoglycoside treatment have been studied. For instance, animal studies showed that the coadministration of agents such as poly-l-aspartic acid (8), daptomycin (9), ceftriaxone (10), and fleroxacin (7) with aminoglycosides significantly reduced the nephrotoxicity of aminoglycosides. However, the clinical application of these approaches remains uncertain.
The once-daily administration of aminoglycosides is actually the most attractive alternative for reducing the renal toxicity of these agents in patients. Animal studies indicated that extending the frequency of injection was as effective as and less toxic than more frequent administration of aminoglycosides (18, 21). Several meta-analyses of randomized human clinical trials compared the clinical efficacy, the bacteriological cure, and the incidence of nephrotoxicity of the same total daily dose of aminoglycosides administered once daily with the effects of multiple doses injected during the day (1–3, 20, 22, 23, 36). These analyses suggested that the once-daily dosing with aminoglycosides is at least as effective and safe as the multiple-dose regimen of aminoglycosides.
Unfortunately, these studies did not take into account the time of injection of these antibiotics throughout the day. In fact, we previously reported for rats temporal variations in the renal toxicity of aminoglycosides (30, 33), and these data were in agreement with those obtained by other investigators (15, 37, 39, 40, 51, 52). Peak renal toxicity was observed when aminoglycosides were injected in the middle of the rest period of the experimental animals, while lower toxicity was found when they were treated in the middle of the activity period. These data are also in agreement with those of Prins et al. (44), recently obtained for patients. In fact, they reported that nephrotoxicity occurred more frequently when aminoglycosides were administered to patients between midnight and 0730 h than when administered at any other hour of the day.
The temporal variations in the effectiveness of aminoglycosides have never been investigated for animals. Thus, the objective of this study was to investigate the circadian variations in the toxicity and the efficacy of gentamicin in an experimental model of pyelonephritis in rats.
MATERIALS AND METHODS
Animals and treatment.
Female Sprague-Dawley rats (Charles River Breeding Laboratories, Inc., Montréal, Québec, Canada) aged 58 to 82 days and weighing 185 to 250 g were used in this study. They had free access to food and water throughout the experiment. They were maintained on a 14-h-light (rest period) and a 10-h-dark (active period) schedule with the light on at 0600 h, for 1 week before induction of pyelonephritis. Animals were divided into four groups on the basis of the time of day that infection was induced and treatment was given.
Pyelonephritis model.
The bacterial strain used to induce pyelonephritis was Escherichia coli Yale EY-9 (furnished by V. Andreoli, Yale University). Before the induction of pyelonephritis, animals were anesthetized with sodium pentobarbital (Nembutal sodium [Abbott Laboratories]) at 50 mg/kg of body weight, intraperitoneally (i.p.). The side of the animals was shaved and asepticized, and a small incision was made at the level of the kidney. The left kidney was exposed, and 0.05 ml of an inoculum containing 107 to 108 bacteria was injected through the upper and lower poles of the kidney. This technique, described previously by Kaye (26), produces a constant and severe pyelonephritis in the left kidney with extensive inflammation and abscess formation induced by the direct inoculation of E. coli and a less severe pyelonephritis in the right kidney due to the reflux of infected urine. Pyelonephritis was induced at either 0700, 1300, 1900, or 0100 h, and the treatment was initiated exactly 24 h after the time of inoculation. The MIC of gentamicin against the E. coli strain was 0.5 μg/ml.
Effectiveness study.
Infected animals were treated with a single daily injection of gentamicin (20 and 40 mg/kg, i.p.) for 3 and 7 days at the same time of day at which the infection was previously induced: 0700, 1300, 1900, or 0100 h. Saline (NaCl [0.9%]) was injected into control animals in a volume of 0.2 ml at the same hour of the day as that for the aminoglycoside. Twenty-four hours after the last injection, a minimum of 10 rats per group were anesthetized as described above, blood was sampled by cardiac puncture, and animals were killed by decapitation. At the time of sacrifice, a midline abdominal incision was made and both kidneys were aseptically removed, decapsulated, weighed, and homogenized in 3 ml of sterile saline at 4°C. Appropriate dilutions of homogenized kidneys were made, and 10-μl samples were placed in triplicate on MacConkey agar. The number of CFU of E. coli in the kidneys was determined after an incubation of 18 h at 37°C (the CFU per milliliter of homogenate were transformed into CFU per gram of tissue). The bacterial enumeration was done at the dilution that allowed us to detect between 30 and 300 CFU/g of kidney. The limit of detection was 30 CFU/ g of kidney. Kidneys were considered sterile when no CFU were detected on the agar. The efficacy of gentamicin was assessed by comparing the number of CFU measured in the infected and treated rats with that of animals injected with saline at the same time. The efficacy of gentamicin was also evaluated by comparing the percentages of sterile kidneys in the infected and treated animals at the four times of day.
Nephrotoxicity study.
Groups of rats were infected as described above at 0700, 1300, 1900, and 0100 h. Exactly 24 h after the induction of the infection, groups of at least six infected rats were treated with a single daily i.p. injection of gentamicin (40 mg/kg) or saline for 7 days at the same time at which the infection was induced. During treatment, animals were placed individually in metabolic cages to collect urine over a 24-h period for the measurement of specific enzyme activities as markers of toxicity. Urine was collected under mineral oil immediately after the first gentamicin injection (day 1) and 24 h before sacrifice (day 7), and the volume was noted. All samples were centrifuged (1,340 × g) for 15 min, and the enzymuria was assessed immediately after centrifugation. At the time of sacrifice, a midline abdominal incision was made and the right kidney of each animal was rapidly removed and bisected. The blood was centrifuged, and the serum collected was quickly frozen at −20°C for the determination of creatinine and blood urea nitrogen (BUN) levels. The cortex of the kidneys was dissected, and a piece of tissue was quickly frozen in dry ice for further determination of sphingomyelinase activity and the [3H]thymidine/DNA ratio. Another part of the cortical tissue was cut into small blocks (approximately 1 mm3) in a drop of 0.5% glutaraldehyde–0.1 M phosphate buffer (pH 7.4) and left overnight in the same fixative at 4°C.
Enzymuria.
The activities of β-galactosidase, N-acetyl-β-d-glucosaminidase, and γ-glutamyltransferase were measured as an index of tubular damages. The activities of β-galactosidase and N-acetyl-β-d-glucosaminidase, lysosomal enzymes, were measured by the method of Maruhn (35). The activity of γ-glutamyl transpeptidase, an enzyme of the brush border membrane, was evaluated by the methodology of Persijn and van der Slik (42).
Biochemical analysis.
The cellular regeneration in the renal cortex was evaluated by measuring the radioactivity of [3H]thymidine incorporated into DNA as described by Laurent et al. (29) on purified DNA obtained from the cortical tissue of the right kidney. Sphingomyelinase (EC 3.1.4.12) activity was assayed in the renal cortex as described previously (28). Serum creatinine and BUN levels were evaluated with a Hitachi 737 apparatus.
Histology.
Cubes previously fixed overnight were washed with phosphate buffer (0.1 M, pH 7.4), fixed in 1% osmium tetroxide for 60 min at room temperature, dehydrated in ascending grades of alcohol, and embedded in Araldite 502 epoxy resin. Thick sections (1 μm) were cut with an Ultracut E ultramicrotome (Leica Canada, Inc., Québec, Québec, Canada), stained with hot toluidine blue, and examined with a blind code to identify gross lesions. Microscopic renal lesions were scored on plastic sections at a magnification of ×400. Each slide was coded so that identification of the groups was not possible for the observer. Slices came from three different pieces of renal cortex for each rat, and five rats per group were used. The following lesions in the renal cortex were recorded: isolated cell necrosis, tubular necrosis (proximal tubule with more than 50% necrotic cells), tubular desquamation (proximal tubule with 100% necrotic cells), the number of proximal tubules with metachromatic material in their lumens, and the number of interstitial cells (no specific identification of cell type was made). The total number of proximal tubules was also measured on each slice. The number of isolated necrotic cells, the number of necrotic proximal tubules, the number of desquamated proximal tubules, and the number of proximal tubules with metachromatic material in their lumens were recorded as the percentages of the total number of proximal tubules on each respective slice and assigned a score as follows: 0 to 9%, 1; 10 to 19%, 2; 20 to 29%, 3; etc. The score for the interstitial cells was obtained by dividing the total number of interstitial cells (excluding endothelial capillary cells) by the total number of proximal tubules on each respective slice. The lesion scores were summed up to produce a single toxicity score for each animal.
Statistics.
All statistical analyses were performed with the Stat-View SE + Graphics 1988 program (Abacus Concepts, Inc., Berkeley, Calif.). Analysis of variance by a least-squares method was used to determine the statistical significance of the difference between groups. A p value smaller than 0.05 (P < 0.05) was considered significant. Group comparisons were performed by Fisher’s protected least significant difference test.
RESULTS
Effectiveness study.
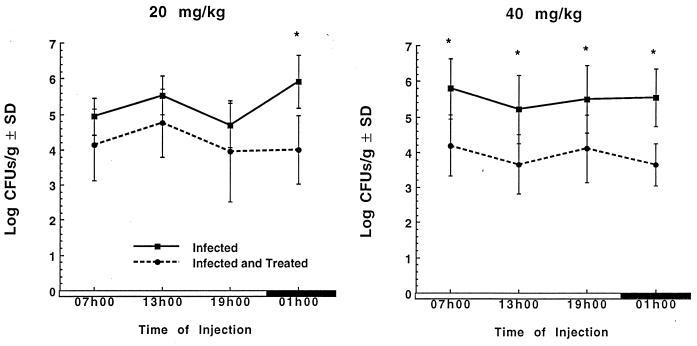
Figure 1 shows the effectiveness of gentamicin (20 and 40 mg/kg) given once daily to infected rats for 3 days. In infected animals treated with gentamicin (20 mg/kg), the log CFU per gram was significantly lower than that for the infected controls only when gentamicin was administered at 0100 h (P < 0.05). When gentamicin was injected at a dose of 40 mg/kg, a significant reduction (P < 0.05) in the log CFU per gram of tissue compared with that for control infected rats was observed at all injection times, but the reduction in the bacterial counts was slightly greater (not significant) when gentamicin was injected at 0100 h than at other times of day.
FIG. 1.
Log CFU per gram of kidney (± standard deviations [SD]) of animals infected with E. coli and treated with either saline (infected) or a single daily injection of gentamicin (infected and treated) at doses of 20 and 40 mg/kg for 3 days at either 0700, 1300, 1900, or 0100 h. Treatment was initiated 24 h after the induction of infection. ∗, significantly different from time-matched infected and treated animals (P < 0.05). Open boxes, light period; closed boxes, dark period.
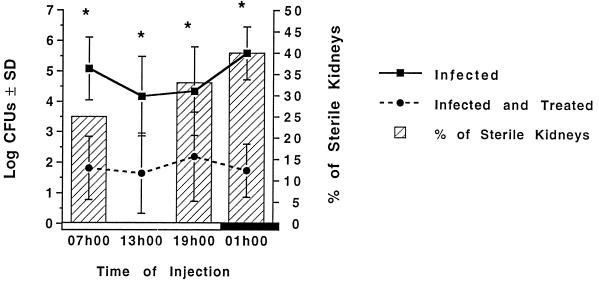
Figure 2 shows the effectiveness of a once-daily gentamicin (40 mg/kg) injection of infected rats for 7 days. Gentamicin produced a significant reduction (P < 0.05) of the log CFU per gram of tissue in all treated groups (Fig. 2, dotted line) in comparison to that of their time-matched controls (solid line). Figure 2 shows also that gentamicin injected at 0100 h sterilized 40% of infected kidneys, while the percentage of sterile kidneys was 25, 0, and 33% in animals treated at 0700, 1300, and 1900 h, respectively.
FIG. 2.
Log CFU per gram of kidney (± standard deviations [SD]) and percentages of sterile kidneys (treated animals only) of animals infected with E. coli and treated with either saline (infected) or a single daily injection of gentamicin (infected and treated) at doses of 40 mg/kg for 7 days at either 0700, 1300, 1900, or 0100 h. ∗, significantly different from time-matched infected and treated animals (P < 0.05). Open box, light period; closed box, dark period.
Nephrotoxicity study.
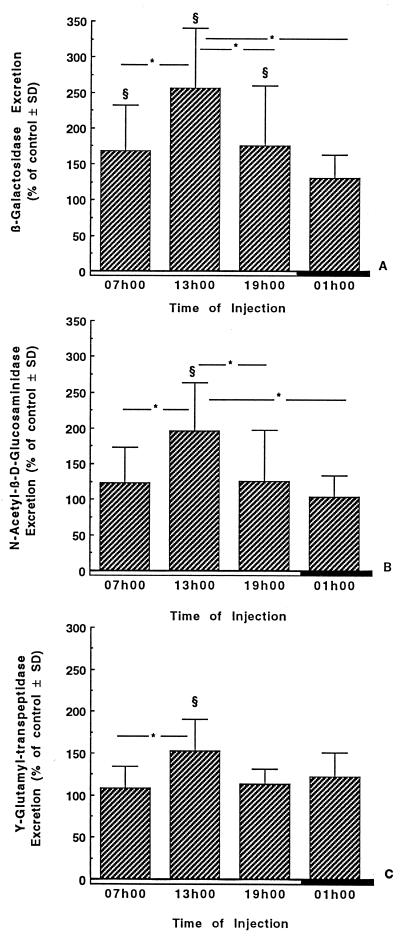
Figure 3 shows excretion of β-galactosidase, N-acetyl-β-d-glucosaminidase, and γ-glutamyltransferase in the 24-h urine of infected rats treated for 7 days with gentamicin (40 mg/kg/day) at 0700, 1300, 1900, and 0100 h. The excretion of each enzyme was significantly higher in urine of animals injected at 1300 than in urine of animals injected at any other time of day. Similar effects were observed on the first day of treatment, but the level was much greater on day 7.
FIG. 3.
Twenty-four-hour urinary excretion of β-galactosidase (A), N-acetyl-β-d-glucosaminidase (B), and γ-glutamyl transpeptidase (C) in infected rats treated for 7 days with saline or gentamicin. Gentamicin was given as a single daily dose of 40 mg/kg/day i.p. at either 0700, 1300, 1900, or 0100 h. The enzymuria was expressed as a percentage of control animals ± standard deviation (SD). §, significantly different from the respective time-matched controls (P < 0.01); ∗, statistical differences (P < 0.05) between the groups under the extremities of the line. Open boxes, light period; closed boxes, dark period.
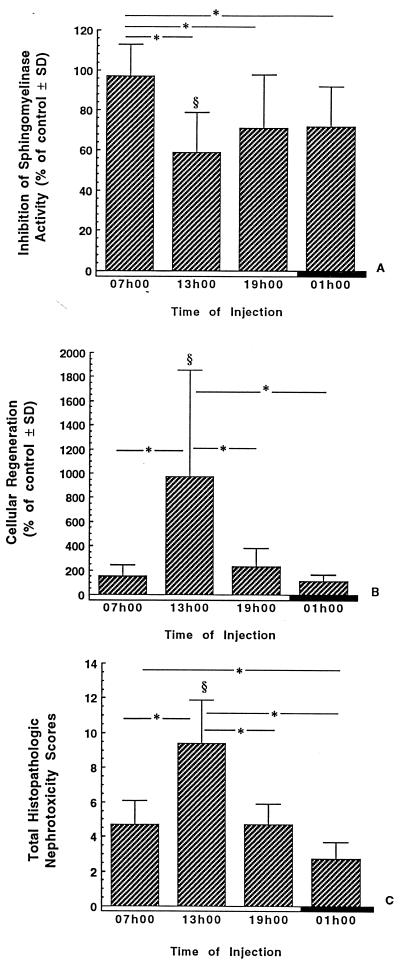
The inhibition of sphingomyelinase activity, the cellular regeneration, and the histopathological scores measured in the renal cortex of rats treated for 7 days are presented in Fig. 4. The inhibition of sphingomyelinase activity was significantly more severe when gentamicin was injected at 1300, 1900, and 0100 h than when animals were injected with gentamicin at 0700 h (P < 0.01). In comparison to their time-matched controls, the animals treated at 1300 h were the only ones to show a significant inhibition of the sphingomyelinase activity (P < 0.01). The cellular regeneration was significantly higher in the renal cortex of animals treated with gentamicin at 1300 than in their time-matched controls (P < 0.01) and in other treated groups (P < 0.01). The histopathological nephrotoxicity scores were also significantly higher for animals treated with gentamicin at 1300 h than for their time-matched controls (P < 0.01) and for the other groups treated at the other times of day (P < 0.01).
FIG. 4.
Inhibition of sphingomyelinase activity (A), cellular regeneration (B), and total histopathologic nephrotoxicity scores (C) in renal cortices of infected rats treated for 7 days with gentamicin. Gentamicin was given once daily at either 0700, 1300, 1900, or 0100 h at a dose of 40 mg/kg/day. The data are presented as the means ± standard deviations (SD) and expressed as the percentages of the values measured for control animals. §, significantly different from the respective time-matched controls (P < 0.01); ∗, statistical differences (P < 0.01) between the groups under the extremities of the line. Open boxes, light period; closed boxes, dark period.
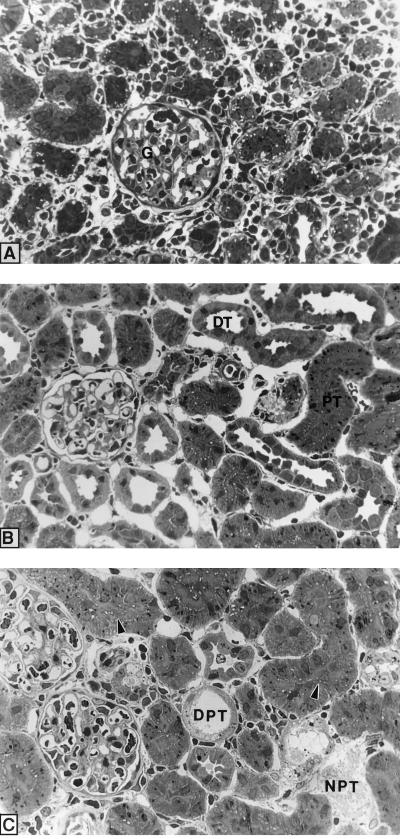
Figure 5 shows plastic sections of the left renal cortex of infected nontreated animals (controls) (A) and the renal cortex of infected animals treated with gentamicin for 7 days at 0100 (B) or 1300 (C) h. The renal cortex of infected control animals shows typical signs of pyelonephritis such as the presence of intensive peritubular infiltration with inflammatory cells and the loss of the structural integrity of the tissue. The peritubular cell infiltration was less severe in gentamicin-treated infected rats. In infected animals treated with gentamicin at 1300 h, the renal cortex still shows peritubular cell infiltration as well as signs of gentamicin toxicity such as desquamated and necrotic proximal tubular cells (Fig. 5C) while these changes were not observed for animals treated with gentamicin at 0100 h (Fig. 5B). However, large lysosomes can be observed in proximal tubular cells in the latter group (Fig. 5B). These observations are consistent with the data for the histopathological quantification of the cellular toxicity induced by gentamicin presented in Fig. 4.
FIG. 5.
Plastic sections of the right renal cortex of infected control animals (A) and infected animals treated for 7 days with gentamicin at either 0100 h (B) or 1300 h (C). G, glomerulus; PT, proximal tubule; DT, distal tubule; NPT, necrotic proximal tubule; DPT, desquamated proximal tubule. Arrowheads, metachromatic material in tubular lumen. Magnification, ca. ×300.
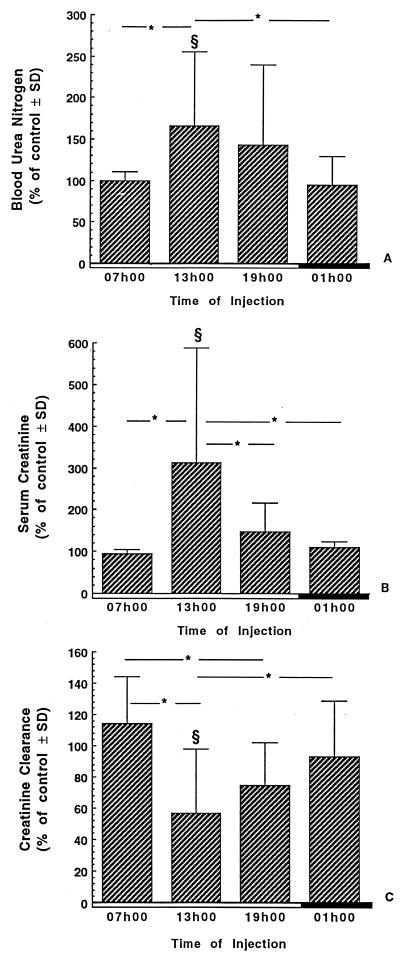
Renal function. Figure 6 shows creatinine and BUN levels in serum as well as the creatinine clearance of infected rats treated with a single daily injection of gentamicin at a dose of 40 mg/kg for 7 days. Serum BUN and creatinine levels were significantly higher in animals treated with gentamicin at 1300 h than in their time-matched controls (P < 0.05) and in rats treated at 0700 and 0100 h (P < 0.05). The creatinine clearance was significantly lower in animals treated with gentamicin at 1300 than in their time-matched controls (P < 0.05) and in rats treated at 0700 and 0100 h (P < 0.05). Furthermore, the creatinine clearance was significantly lower in animals treated with gentamicin at 1900 than in animals treated at 0700 h (P < 0.05).
FIG. 6.
BUN level (A), serum creatinine level (B), and creatinine clearance (C) of infected rats treated for 7 days with saline or gentamicin. Gentamicin was given as a single daily dose of 40 mg/kg at either 0700, 1300, 1900, or 0100 h. The data are presented as the means ± standard deviations (SD) and expressed as the percentages of the values measured for control animals. §, significantly different from the respective time-matched controls (P < 0.05); ∗, statistical differences (P < 0.05) between the groups under the extremities of the line. Open boxes, light period; closed boxes, dark period.
DISCUSSION
The objective of our study was to evaluate the effects of the time of administration on the effectiveness and the renal toxicity of gentamicin in an experimental model of pyelonephritis in rats. Our data show that both the effectiveness and the toxicity of gentamicin varied over the 24-h period and that the efficacy was best at the time when the toxicity of the drug was the lowest. Data indicated also that the presence of infection does not modify the circadian rhythm of aminoglycoside toxicity.
Temporal variations in the renal toxicity of aminoglycosides have been reported for experimental animals since 1982. Briefly, Nakano and Ogawa (37) showed for the first time that the survival rate of mice treated once daily with gentamicin was higher when the drug was injected in the middle of the activity period (0200 h) and lower when gentamicin was injected in the middle of the rest period (1400 h) of the animals. Similar results were reported by Cambar and his colleagues (13, 39) with high doses of gentamicin, dibekacin, and netilmicin. Temporal variations in the nephrotoxicity of aminoglycosides were also observed with lower doses of gentamicin (19, 40, 51), amikacin (14, 16), and isepamicin (52). In all these studies, the high acute doses of aminoglycosides produced greater toxicity when they were injected in the middle of the rest period, while toxicity was less when the antibiotics were injected in the middle of the activity period of the experimental animals. Studies done in our laboratory showed similar results, as we injected daily small doses of aminoglycosides over 7 to 10 days. In fact, our data showed that tobramycin (30, 33) and isepamicin (49) induced higher toxicity when injected at 1400 h than when the same dose was injected at 0200 h. Recently, Prins et al. showed for the first time that the incidence of nephrotoxicity was significantly higher in patients receiving aminoglycosides during their sleeping period (midnight to 0730 h) than in patients receiving drugs at other times of day (44).
The mechanisms responsible for the temporal variation in renal toxicity of aminoglycosides are still unknown. Some authors suggested that circadian rhythms in the urinary excretion of water, electrolyte, and renal perfusion (45); in membrane structure and fluidity (17); and in the number and size of autophagic vacuoles in the cytoplasm of tubular cells (43) might be responsible for the temporal variations in the renal toxicity of aminoglycosides. Temporal variations in the pharmacokinetics of aminoglycosides in serum could also be involved in the time-dependent changes in aminoglycoside nephrotoxicity. Such changes were found in the levels of these antibiotics in plasma in animals (24, 30, 38, 46, 52) and in humans (34, 38, 50). Time-dependent changes were also found in the cortical accumulation (30, 40, 51, 52) and in the kinetics of the intracortical accumulation rate (31) of different aminoglycosides.
Other experiments in our laboratory showed that the 24-h variation in the serum corticosterone levels or changes in the subcellular distribution of aminoglycosides could not explain the chrononephrotoxicity of aminoglycosides (6). However, our data showed that a time-restricted feeding schedule induced a shift in the maximal and minimal levels of gentamicin nephrotoxicity in animals injected at different times of the day (5). Furthermore, fasting abolished the 24-h variations in the nephrotoxicity of gentamicin (4). Fasting was also associated with higher levels of tobramycin in the serum and cortex (32). We thus concluded that the time of dosing of gentamicin relative to the time of feeding seems to be a more important modulator of aminoglycoside nephrotoxicity than is the light-dark cycle.
In this study, we showed again that gentamicin induced higher toxicity when injected in the middle of the rest period (1300 h) and that toxicity was lower when the drug was injected in the middle of the activity period (0100 h). Moreover, no temporal variations in the cortical accumulation of gentamicin were observed in the present study (data not shown), as recently demonstrated with tobramycin (33). As these data were observed for animals suffering from pyelonephritis, it can thus be concluded that the presence of infection did not modify the temporal variations in the renal toxicity of gentamicin.
Our results also demonstrate a circadian rhythm in the therapeutic efficacy of gentamicin in the treatment of experimental E. coli pyelonephritis. The effectiveness of gentamicin was evaluated by two methods: (i) the log CFU of bacteria in the renal tissue in the treated animals compared to those in their time-matched infected controls and (ii) the percentage of sterile kidneys, observed at the end of therapy. In fact, the percentage of sterile kidneys was higher when the drug was injected at 0100 h than when it was injected at 0700, 1300, and 1900 h. Thus, the effectiveness of gentamicin was highest during the activity period in animals treated with gentamicin at doses of 40 mg/kg for 7 days.
Several factors might explain the temporal variations in the effectiveness of gentamicin in this experimental model. First, the higher water and food intake, resulting in a higher urine output, might have contributed to increasing the efficacy and decreasing the toxicity of gentamicin in infected animals when injected during the activity period. In fact, it might have contributed also to increasing the clearance of bacteria from the kidney and increasing the efficacy of gentamicin during this period of the day. However, since no temporal variations were observed in the cortical accumulation of gentamicin in the present study, these drug levels could not contribute to the temporal variations in the effectiveness of the drug. It is interesting to note that Hosokawa et al. (24) also showed temporal variations in the effectiveness of a single dose of amikacin in an experimental model of i.p. infection with Pseudomonas aeruginosa. In infected mice, the 50% effective dose of amikacin was lower in the midlight phase (1300 h) at time of lower clearance than in the middark phase (0100 h), when clearance was higher.
Based on our studies and on the evidence available in the current literature, it is quite clear that the renal toxicity of aminoglycosides can be reduced by giving the drug as a single daily injection when patients are active. Even if extrapolation of experimental data to humans is always uncertain, it is reasonable to believe that the administration of gentamicin in the beginning or the middle of the day in humans may reduce renal toxicity and increase the efficacy of these antibiotics. In fact, some chronopharmacologic studies have already been applied to patients with very conclusive results (34, 38, 50). In humans, the clearance of aminoglycosides was lower during the night (in the rest period) and maximal during the activity period (day). These results perfectly correlate with those obtained for experimental animals. The prospective study of Prins et al. (44) clearly showed that the incidence of toxicity in 179 patients was significantly higher when the drug was given as a once-daily injection during the sleeping period of patients. Further investigations must be done to investigate the effect of the time of administration on aminoglycoside effectiveness in patients, but physicians should already be careful when administering aminoglycosides to patients at night. The clinical applications of these results may be eventually extended to pathologies such as pyelonephritis and pneumonia that could be treated with once-a-day aminoglycosides.
ACKNOWLEDGMENTS
This study was supported by the Kidney Foundation of Canada. M. LeBrun is the recipient of a studentship from the Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (F.C.A.R.).
REFERENCES
- 1.Ali M Z, Goetz M B. A meta-analysis of the relative efficacy and toxicity of single daily dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:796–809. doi: 10.1093/clinids/24.5.796. [DOI] [PubMed] [Google Scholar]
- 2.Bailey T C, Little J R, Littenberg B, Reichley R M, Dunagan W C. A meta-analysis of extended-interval dosing versus multiple daily dosing of aminoglycosides. Clin Infect Dis. 1997;24:786–795. doi: 10.1093/clinids/24.5.786. [DOI] [PubMed] [Google Scholar]
- 3.Barza M, Ioannidis J P, Cappelleri J C, Lau J. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ. 1996;312:338–345. doi: 10.1136/bmj.312.7027.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauchamp D, Collin P, Grenier L, LeBrun M, Couture M, Thibault L, Labrecque G, Bergeron M G. Effects of fasting on temporal variations in nephrotoxicity of gentamicin in rats. Antimicrob Agents Chemother. 1996;40:670–676. doi: 10.1128/aac.40.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchamp D, Guimont C, Grenier L, LeBrun M, Tardif D, Gourde P, Bergeron M G, Thibault L, Labrecque G. Time-restricted feeding schedules modify temporal variation of gentamicin experimental nephrotoxicity. Antimicrob Agents Chemother. 1997;41:1468–1474. doi: 10.1128/aac.41.7.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchamp D, Labrecque G, Bergeron M G. [Is it still possible to reduce the incidence of nephrotoxicity of aminoglycosides?] Pathol. Biol (Paris) 1995;43:779–787. . (In French.) [PubMed] [Google Scholar]
- 7.Beauchamp D, Laurent G, Grenier L, Gourde P, Zanen J, Heuson Stiennon J A, Bergeron M G. Attenuation of gentamicin-induced nephrotoxicity in rats by fleroxacin. Antimicrob Agents Chemother. 1997;41:1237–1245. doi: 10.1128/aac.41.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauchamp D, Laurent G, Maldague P, Abid S, Kishore B K, Tulkens P M. Protection against gentamicin-induced early renal alterations (phospholipidosis and increased DNA synthesis) by coadministration of poly-l-aspartic acid. J Pharmacol Exp Ther. 1990;255:858–866. [PubMed] [Google Scholar]
- 9.Beauchamp D, Pellerin M, Gourde P, Pettigrew M, Bergeron M G. Effects of daptomycin and vancomycin on tobramycin nephrotoxicity in rats. Antimicrob Agents Chemother. 1990;34:139–147. doi: 10.1128/aac.34.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauchamp D, Theriault G, Grenier L, Gourde P, Perron S, Bergeron Y, Fontaine L, Bergeron M G. Ceftriaxone protects against tobramycin nephrotoxicity. Antimicrob Agents Chemother. 1994;38:750–756. doi: 10.1128/aac.38.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergeron M G. Current concepts in the treatment of pyelonephritis. Intern Med. 1989;10:65–84. [Google Scholar]
- 12.Bergeron M G. Treatment of pyelonephritis in adults. Med Clin N Am. 1995;79:619–649. doi: 10.1016/s0025-7125(16)30060-8. [DOI] [PubMed] [Google Scholar]
- 13.Cambar J, Dorian C, Cal J C. [Chronobiology and renal physiopathology.] Pathol. Biol (Paris) 1987;35:977–984. . (In French.) [PubMed] [Google Scholar]
- 14.Dorian C, Bordenave C, Cambar J. Circadian and seasonal variations in amikacin-induced acute renal failure evaluated by γ-glutamyl-transferase excretion changes. Annu Rev Chronopharmacol. 1986;3:111–114. [Google Scholar]
- 15.Dorian C, Catroux P, Cambar J. [Chrononephrotoxicity of amikacin after 7-day chronic poisoning in rats.] Pathol. Biol (Paris) 1987;35:735–738. . (In French.) [PubMed] [Google Scholar]
- 16.Dorian C, Catroux P, Cambar J. [Demonstration of seasonal changes of circadian rhythms of amikacin chrononephrotoxicity in rats.] Pathol. Biol (Paris) 1987;35:731–734. . (In French.) [PubMed] [Google Scholar]
- 17.Doring R, Rensing L. Daily changes of content and synthesis of electrophoretically separated RNA fractions in rat liver. J Interdiscip Cycle Res. 1979;10:111–117. [Google Scholar]
- 18.Fantin B, Pangon B, Potel G, Vallois J M, Caron F, Bure A, Carbon C. Ceftriaxone-netilmicin combination in single-daily-dose treatment of experimental Escherichia coli endocarditis. Antimicrob Agents Chemother. 1989;33:767–770. doi: 10.1128/aac.33.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauconneau B, De Lemos E, Pariat C, Bouquet S, Courtois P, Piriou A. Chrononephrotoxicity in rat of a vancomycin and gentamicin combination. Pharmacol Toxicol. 1992;71:31–36. doi: 10.1111/j.1600-0773.1992.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferriols Lisart R, Alos Alminana M. Effectiveness and safety of once-daily aminoglycosides: a meta-analysis. Am J Health Syst Pharm. 1996;53:1141–1150. doi: 10.1093/ajhp/53.10.1141. [DOI] [PubMed] [Google Scholar]
- 21.Francioli P B, Glauser M P. Synergistic activity of ceftriaxone combined with netilmicin administered once daily for treatment of experimental streptococcal endocarditis. Antimicrob Agents Chemother. 1993;37:207–212. doi: 10.1128/aac.37.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galloe A M, Graudal N, Christensen H R, Kampmann J P. Aminoglycosides: single or multiple daily dosing? A meta-analysis on efficacy and safety. Eur J Clin Pharmacol. 1995;48:39–43. doi: 10.1007/BF00202170. [DOI] [PubMed] [Google Scholar]
- 23.Hatala R, Dinh T, Cook D J. Once-daily aminoglycoside dosing in immunocompetent adults: a meta-analysis. Ann Intern Med. 1996;124:717–725. doi: 10.7326/0003-4819-124-8-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 24.Hosokawa H, Nyu S, Nakamura K, Mifune K, Nakano S. Circadian variation in amikacin clearance and its effects on efficacy and toxicity in mice with and without immunosuppression. Chronobiol Int. 1993;10:259–270. doi: 10.1080/07420529309059708. [DOI] [PubMed] [Google Scholar]
- 25.Kahlmeter G, Dahlager J I. Aminoglycoside toxicity—a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984;13(Suppl. A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 26.Kaye D. The effect of water diuresis on spread of bacteria through the urinary tract. J Infect Dis. 1971;124:297–305. doi: 10.1093/infdis/124.3.297. [DOI] [PubMed] [Google Scholar]
- 27.Komaroff A L. Acute dysuria in women. N Engl J Med. 1984;310:368–375. doi: 10.1056/NEJM198402093100607. [DOI] [PubMed] [Google Scholar]
- 28.Laurent G, Carlier M B, Rollman B, Van Hoof F, Tulkens P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem Pharmacol. 1982;31:3861–3870. doi: 10.1016/0006-2952(82)90303-3. [DOI] [PubMed] [Google Scholar]
- 29.Laurent G, Maldague P, Carlier M B, Tulkens P M. Increased renal DNA synthesis in vivo after administration of low doses of gentamicin to rats. Antimicrob Agents Chemother. 1983;24:586–593. doi: 10.1128/aac.24.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin L, Grenier L, Bergeron Y, Simard M, Bergeron M G, Labrecque G, Beauchamp D. Temporal changes of pharmacokinetics, nephrotoxicity, and subcellular distribution of tobramycin in rats. Antimicrob Agents Chemother. 1994;38:54–60. doi: 10.1128/aac.38.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin L, Grenier L, Guimont C, LeBrun M, Labrecque G, Bergeron M G, Beauchamp D. Circadian variation in the intracortical accumulation kinetics of tobramycin in conscious rats. Chronobiol Int. 1995;12:188–194. [Google Scholar]
- 32.Lin L, Grenier L, LeBrun M, Bergeron M G, Thibault L, Labrecque G, Beauchamp D. Day-night treatment difference of tobramycin serum and intrarenal drug distribution and nephrotoxicity in rats: effects of fasting. Chronobiol Int. 1996;13:113–121. doi: 10.3109/07420529609037075. [DOI] [PubMed] [Google Scholar]
- 33.Lin L, Grenier L, Theriault G, Gourde P, Yoshiyama Y, Bergeron M G, Labrecque G, Beauchamp D. Nephrotoxicity of low doses of tobramycin in rats: effect of the time of administration. Life Sci. 1994;55:169–177. doi: 10.1016/0024-3205(94)00877-9. [DOI] [PubMed] [Google Scholar]
- 34.Lucht F, Tigaud S, Esposito G, Cougnard J, Fargier M P, Peyramond D, Bertrand J L. Chronokinetic study of netilmicin in man. Eur J Clin Pharmacol. 1990;39:199–201. doi: 10.1007/BF00280062. [DOI] [PubMed] [Google Scholar]
- 35.Maruhn D. Rapid colorimetric assay of beta-galactosidase and N-acetyl-beta-glucosaminidase in human urine. Clin Chim Acta. 1976;73:453–461. doi: 10.1016/0009-8981(76)90147-9. [DOI] [PubMed] [Google Scholar]
- 36.Munckhof W J, Grayson M L, Turnidge J D. A meta-analysis of studies on the safety and efficacy of aminoglycosides given either once daily or as divided doses. J Antimicrob Chemother. 1996;37:645–663. doi: 10.1093/jac/37.4.645. [DOI] [PubMed] [Google Scholar]
- 37.Nakano S, Ogawa N. Chronotoxicity of gentamicin in mice. IRCS Med Sci. 1982;10:592–593. [Google Scholar]
- 38.Nakano S, Song J, Ogawa N. Chronopharmacokinetics of gentamicin: comparison between man and mice. Annu Rev Chronopharmacol. 1990;7:277–280. [Google Scholar]
- 39.Pariat C, Cambar J, Courtois P. Circadian variations in the acute toxicity of three aminoglycosides: gentamicin, dibekacin, and netilmicin in mice. Annu Rev Chronopharmacol. 1984;1:381–384. [Google Scholar]
- 40.Pariat C, Courtois P, Cambar J, Piriou A, Bouquet S. Circadian variations in the renal toxicology of gentamicin in rats. Toxicol Lett. 1988;40:175–182. doi: 10.1016/0378-4274(88)90159-2. [DOI] [PubMed] [Google Scholar]
- 41.Patton J P, Nash D B, Abrutyn E. Urinary tract infection: economic considerations. Med Clin N Am. 1991;75:495–513. doi: 10.1016/s0025-7125(16)30466-7. [DOI] [PubMed] [Google Scholar]
- 42.Persijn J P, van der Slik W. A new method for the determination of gamma-glutamyltransferase in serum. J Clin Chem Clin Biochem. 1976;14:421–427. doi: 10.1515/cclm.1976.14.1-12.421. [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer U, Scheller H. A morphometric study of cellular autophagy including diurnal variations in kidney tubules of normal rats. J Cell Biol. 1975;64:608–621. doi: 10.1083/jcb.64.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prins J M, Weverling G J, van Ketel R J, Speelman P. Circadian variations in serum levels and the renal toxicity of aminoglycosides in patients. Clin Pharmacol Ther. 1997;62:106–111. doi: 10.1016/S0009-9236(97)90156-9. [DOI] [PubMed] [Google Scholar]
- 45.Roelfsema F, van der Heide D, Smeenk D. Circadian rhythms of urinary electrolyte excretion in freely moving rats. Life Sci. 1980;27:2303–2309. doi: 10.1016/0024-3205(80)90498-1. [DOI] [PubMed] [Google Scholar]
- 46.Song J, Ohdo S, Ogawa N, Nakano S. Influence of feeding schedule on chronopharmacological aspects of gentamicin in mice. Chronobiol Int. 1993;10:338–348. doi: 10.3109/07420529309064488. [DOI] [PubMed] [Google Scholar]
- 47.Stamm W E, Hooton T M. Management of urinary tract infections in adults. N Engl J Med. 1993;329:1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 48.Tolkoff Rubin N E, Rubin R H. New approaches to the treatment of urinary tract infection. Am J Med. 1987;82:270–277. [PubMed] [Google Scholar]
- 49.Yoshiyama Y, Grenier L, Gourde P, Simard M, Lin L, Morin N J, Bergeron M G, Labrecque G, Beauchamp D. Temporal variation in nephrotoxicity of low doses of isepamicin in rats. Antimicrob Agents Chemother. 1996;40:802–806. doi: 10.1128/aac.40.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshiyama Y, Kobayashi T, Ohdo S, Ogawa N, Bergeron M G, Labrecque G, Beauchamp D, Nakano N. Dosing time-dependent changes of pharmacokinetics of isepamicin in man. J Infect Chemother. 1996;2:106–109. doi: 10.1007/BF02350851. [DOI] [PubMed] [Google Scholar]
- 51.Yoshiyama Y, Kobayashi T, Tomonaga F, Nakano S. Chronotoxical study of gentamicin induced nephrotoxicity in rats. J Antibiot (Tokyo) 1992;45:806–808. doi: 10.7164/antibiotics.45.806. [DOI] [PubMed] [Google Scholar]
- 52.Yoshiyama Y, Nishikawa S, Sugiyama T, Kobayashi T, Shimada H, Tomonaga F, Ohdo S, Ogawa N, Nakano S. Influence of circadian-stage-dependent dosing schedule on nephrotoxicity and pharmacokinetics of isepamicin in rats. Antimicrob Agents Chemother. 1993;37:2042–2043. doi: 10.1128/aac.37.9.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]