Abstract
Fibrosis is defined as the excessive deposition of extracellular matrix (ECM) proteins in the interstitium. It is an essential pathological response to chronic inflammation. ECM protein deposition is initially protective and is critical for wound healing and tissue regeneration. However, pathological cardiac remodeling in excessive and continuous tissue damage with subsequent ECM deposition results in a distorted organ architecture and significantly impacts cardiac function. In this review, we summarized and discussed the histologic features of cardiac fibrosis with the signaling factors that control it. We evaluated the origin and characteristic markers of cardiac fibroblasts. We also discussed lymphatic vessels, which have become more important in recent years to improve cardiac fibrosis.
Keywords: cardiac fibrosis, pathogenesis of cardiac fibrosis, signaling of cardiac fibrosis, marker of cardiac fibroblast
1. Introduction
Heart failure (HF) is a complex syndrome resulting from structural and functional impairments of the heart [1]. From the pathological perspective, HF is characterized by interstitial fibrosis, chamber remodeling, and reduced ventricular compliance [2].
The adult mammalian heart has limited regenerative capacity. Thus, repair processes are crucial after injury. They involve inflammatory cell infiltration, the removal of necrotic cardiomyocytes, and the formation of capillary-enriched granulation tissues. Then, fibrotic scars replace granulation tissues to preserve myocardial structural and functional integrity. Fibrosis is defined as the excessive deposition of extracellular matrix (ECM) proteins, mostly collagen fibers, in the interstitium. It is an essential pathological response to chronic inflammation. ECM protein deposition is initially protective and is critical for wound healing and tissue regeneration. The ECM is also important for maintaining physiological conditions. For instance, cardiomyocyte–ECM interactions mediate cellular behaviors through cell surface receptors, which act as signal transducers for cell proliferation, migration, survival, and differentiation [3]. However, pathological cardiac remodeling in excessive and continuous tissue damage with subsequent ECM deposition results in distorted organ architecture and significantly impacts cardiac function [2,4]. Collectively, heart diseases, such as myocardial infarction (MI), cardiac hypertrophy due to pressure or volume overload, diabetic cardiomyopathy, and dilated cardiomyopathy (DCM) [2,5,6,7] are involved in cardiac fibrosis progression. Although fibrosis has pathophysiological importance in cardiovascular disease development, the development and molecular mechanisms of cardiac fibrosis still require investigation.
In this review, we summarized and discussed the histologic features of cardiac fibrosis and the signaling factors that control it. We evaluated the origin and characteristic markers of cardiac fibroblasts (CFs) under normal and pathophysiological conditions. We also discussed lymphatic vessels, which have become more important in recent years to improve cardiac fibrosis.
2. Histological Features of Cardiac Fibrosis
Microscopically, myocardial fibrosis is divided into three groups according to the fibrous deposition patterns [8,9]. Replacement or reparative fibrosis is related to cardiomyocyte loss and rearrangement by collagen fibers. A typical example is scar tissue after MI. Interstitial fibrosis is characterized by fibrous deposits surrounding the cardiac muscle bundles (the perimysium) and individual cardiomyocytes (the endomysium), and perivascular fibrosis is characterized by fibrous deposits located in the perivascular space. Interstitial and perivascular fibrosis are sequential pathological lesions. Hence, this is sometimes called reactive fibrosis. Reactive fibrosis in the absence of the massive loss of cardiomyocytes is caused by prolonged activation of fibrogenic stimuli and may represent injury processes. These generally occur in pathological conditions, such as pressure or volume overload [2] and diabetes mellitus [10]. Additionally, fibrosis in the subendocardial region is often observed in HF in endomyocardial biopsies [11]. In the progression of reactive fibrosis, ‘low-grade (smoldering) chronic inflammation’, mediated by macrophages around the perivascular spaces, might be involved [12,13,14]. These patterns usually coexist in most HF patients [15,16], thus it is practically difficult to distinguish between reactive and replacement fibrosis caused by small infarct lesions with low-flow ischemia in the advanced stages of myocardial diseases [6,7].
3. Cardiac ECM Characteristics and Homeostasis
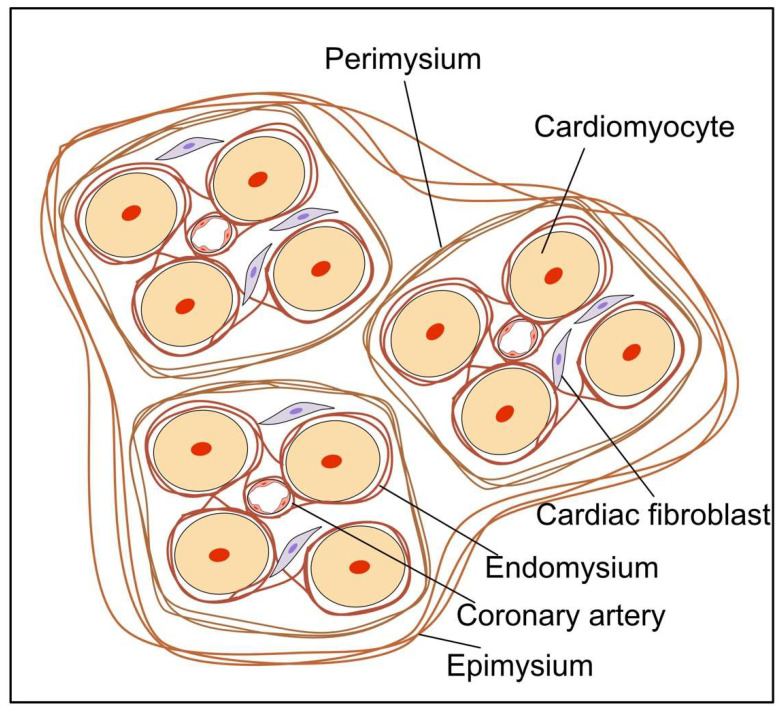
Cardiomyocytes are surrounded by an interstitial ECM network composed mainly of fibrillar collagens [2,16]. The predominant components of the adult human heart ECM are collagen type I (85%) and collagen type III (11%) [16]. Although they are present in smaller amounts, type IV and V collagen are also observed in the heart [17,18]. The ECM is a highly durable, mechanically stable fiber-containing structure that preserves cardiac configuration and function and regulates cardiac diastolic and systolic operations by transmitting contractile forces from individual cardiomyocytes. The matrix consists of an endomysium, perimysium, and epimysium arranged around the myofibrillar bundles and coronary vasculature. The endomysial collagen network interconnects individual cardiomyocytes with Z-band-integrin connections and adjacent capillaries, preventing ventricular dilatation by maintaining cardiomyocyte alignment [19,20,21,22,23]. The perimysial collagen weave segregates cardiomyocytes into bundles to provide tensile strength [24]. The epimysium is a larger collagen weave surrounding several myofibers to preserve their orientation [24] (Figure 1). In addition to collagens, the cardiac ECM contains various glycoproteins, glycosaminoglycans, proteoglycans, and soluble factors [25]. Under pathological conditions, excess ECM deposition exacerbates tissue stiffness and can interrupt cell–cell connections, preventing adequate contractions and signal transduction. Collectively, ECM components are affected by aging [26] and pathophysiological conditions including hypertensive heart disease, heart failure with preserved ejection fraction (HFpEF), and ischemic heart disease [16,27,28,29].
Figure 1.
Schematic image of the cardiac interstitial collagen network. The endomysium surrounds and interconnects individual cardiomyocytes. The perimysial weave segregates cardiomyocytes into groups. The epimysium surrounds and clusters large numbers of myofibres.
CFs and activated CFs are the main cell types responsible for maintaining ECM homeostasis. CFs synthesize collagen as a soluble procollagen with N- and C-terminal propeptide regions that prevent insoluble deposition [30]. Sequential processing steps are required in the extracellular space for procollagen to become a mature fibril collagen. A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 2, 3, and 14 cleave the N-terminal [31] (also referred to as procollagen amino-terminal proteinases (PNPs)), whereas bone morphogenic protein (BMP)-1 is the protease responsible for cleaving the C-terminal [32,33] (also referred to as procollagen carboxy-terminal proteinases (PCPs)). Additionally, PCP enhancer (PCPE)-1 and 2, enhancers of BMP-1 activity, facilitate C-terminus cleavage [34]. Collagens are further stabilized by cross-linking that occurs by lysyl oxidase (LOX)-mediated aldehyde formation in lysine or hydroxylysine residues [35] and transglutaminase 2 by the formation of ε (γ-glutamyl) lysine cross-links [36]. Collagen cross-linking via these mechanisms promotes myocardial stiffness [37,38]. CFs are also the predominant source of matrix metalloproteinases (MMPs), which are calcium-dependent zinc-containing endopeptidases responsible for matrix protein degradation. MMPs are involved in collagen deposition and pro-fibrotic signaling and are important for cardiovascular diseases [39]. MMPs are tightly regulated by an endogenous group of inhibitors known as tissue inhibitors of metalloproteinases (TIMPs) [40]. All four known TIMPs are expressed in the heart, predominantly produced by CFs but also a variety of other cell types. TIMP expression is correlated with cardiac diseases [40,41,42,43,44].
4. Cardiac Fibroblasts
CFs are an essential cell type, mainly derived from the proepicardium, endothelial cells, and neural crest cells, and reside within the myocardial and valve interstitium, epicardial, and perivascular regions [45,46]. A subset of CFs in the interventricular septum and valves are derived from the endocardium through endothelial-to-mesenchymal transition (EndoMT) [47]. CFs are essential for normal cardiac function [2,48] and mediate various physiological forces, including mechanical and electrical stimuli. CFs produce paracrine factors that significantly change the electrophysiological activity in rat cardiomyocytes [49]. CFs can interact with cardiomyocytes through gap-junctional proteins, such as connexins (e.g., Cx40, Cx43, and Cx45) [50,51,52,53]. Additionally, CFs are widely accepted as the main regulator of the heart’s response to various pathological injuries, such as ECM production, matrix degradation, inflammatory cell recruitment, and scar formation [27]. After an acute myocardial injury, the expression of various proinflammatory cytokines is upregulated in the initial inflammatory response in CFs, resulting in subsequent inflammatory cell infiltration and cytokine expression in the heart [54]. In this setting, mechanical stress and inflammation stimulate CF activation, which shows morphological characteristics of both fibroblasts and smooth muscle cells with the expression of α smooth muscle actin (αSMA) [55,56]. Activated CFs secrete elevated levels of collagen and other ECM proteins. This reaction maintains the heart’s structural integrity and pressure-generating capacity. In the advanced phases of fibrotic scar formation, the tensile strength of collagen increases within the injury site [2]. Excess deposition of ECM proteins by CFs reduces ventricular compliance and worsens HF. In addition, excess ECM deposition and fibroblast proliferation disturb the mechano-electric coupling of cardiomyocytes, thereby increasing the risk of arrhythmia and mortality [16]. Furthermore, inflammation and fibrosis within perivascular regions decrease the tissue availability of oxygen and nutrients and perpetuate the pathological response.
5. CF Activation and Activation Reversal
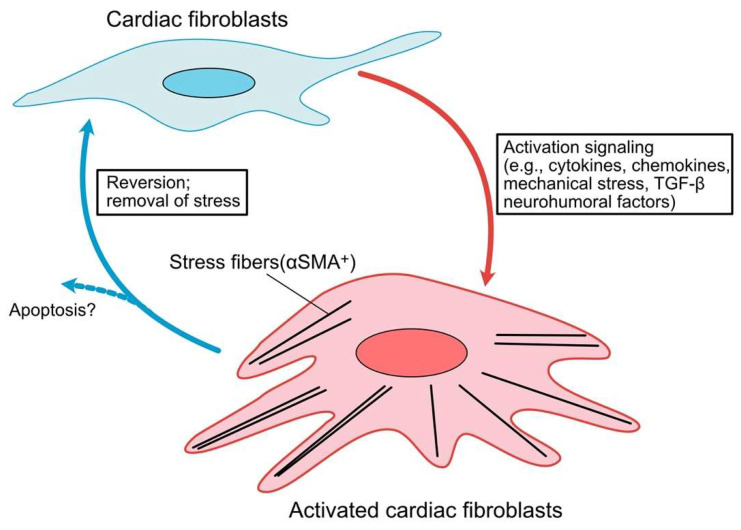
In response to cardiac injury, activated CFs release cytokines, chemokines, and neurohumoral factors [57,58]. Injured tissues secrete several inflammatory molecules, including cardiomyocytes, endothelial cells, fibroblasts, and inflammatory cells. They stimulate CF activation through diverse signaling pathways [48,59]. Pro-inflammatory cytokines (e.g., interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor (TNF)-α), chemokines (e.g., C-X-C motif ligand (CXCL) 1, 2, 5, and 8) [60], mechanical stress through integrins [59] and mechanosensitive ion channels [61] mediated by several kinase signaling pathways, including focal adhesion kinase (FAK) [62], Rho-associated protein kinase (ROCK) [63] and mitogen-activated protein kinase (MAPK) [64], growth factors, and neurohormonal factors have been shown to induce the activation of fibroblasts [29,65]. These activated CFs undergo apoptosis following scar formation [66]. However, recent studies using CFs derived from human HF patients indicate that inhibiting transforming growth factor (TGF)-β signaling reverts activated fibroblasts to resting fibroblasts, as determined by the reduction in αSMA expression [67]. Another study using an angiotensin II (Ang II) and phenylephrine infusion mouse model supported this observation. Two weeks after the cessation of drug infusion, gene expression reverted to resting fibroblast profiles [68]. Given recent findings that fibroblasts have plasticity in the gene expression profiles between resting and activated states and their significant heterogeneity revealed by single-cell RNA-seq analysis [69,70], there may be a threshold for whether activated fibroblasts undergo apoptosis or reversion to a resting state (Figure 2). Further studies are needed to answer these fundamental questions.
Figure 2.
Progression and characterization of a cardiac fibroblast to myofibroblast conversion. In response to cardiac injury, cytokines, chemokines and neurohumoral factors, resident cardiac fibroblasts (CFs) become activated with increasing expression of α smooth muscle actin (α-SMA). These activated CFs may undergo apoptosis following the scar formation. Activated CFs are capable of de-differentiating upon removal of stress stimuli.
6. CF Markers
Specific CF and activated CF biomarkers are difficult to determine. Recent single-cell analysis in the adult human heart has revealed significant heterogeneity in CFs [71]. Collectively, in fibroblasts from different tissues, there is also significant heterogeneity in gene expression [72]. Thus, depending on the pathophysiological situation and anatomical site of the heart, it is necessary to combine several markers. The markers often used to identify fibroblasts are periostin, CD90 (or Thy1), discoidin domain receptor 2 (DDR2), platelet-derived growth factor receptor α (PDGFRα), transcription factor (Tcf) 21, fibroblast-specific protein (FSP) 1, stem cell antigen (Sca)-1, fibronectin, vimentin, and collagen types I and III. αSMA is the most commonly used marker for activated fibroblasts. Tenascin-C (TN-C) is highly expressed in activated CFs and is a potential marker for confirming their phenotype [6,7]. Although these are still under investigation, recent single-cell analyses have revealed several candidate markers that are expressed in cardiac valve interstitial fibroblasts, including wif1 and the cartilage oligomeric matrix protein (COMP) [70]. Additionally, dermatopontin (Dpt)-CreERT2 mice efficiently mark universal fibroblasts [73] (Table 1).
Table 1.
Marker proteins:DDR2, discoidin domain receptor 2; PDGFRα, platelet derived growth factor receptor α; TCF21, Transcription factor 21; FSP1, fibroblast specific protein 1; Sca-1, stem cell antigen-1; αSMA, α smooth muscle actin; TNC, tenascin-C. Modified from [48,55,73].
| Markers/Mouse Lines | Functions | Expression in CFs | Expression in Other Cells in the Heart | References |
|---|---|---|---|---|
| Periostin | ECM protein | activated CFs | Epicardium | [55,68,73] |
| CD90 | Cell adhesion and cell communication | Resting and activated CFs | Pericytes, VSMC, ECs, immune cells | [73,74] |
| DDR2 | A membrane collagen-binding tyrosine kinase receptor | Resting CFs | Epicardium | [48,75,76] |
| PDGFRα | Tyrosine kinase receptor | CFs during development and after injury | Cardiac progenitor cells | [47,68,77,78] |
| TCF21 | Involved in epithelial-mesenchymal transition | Resting CFs | Epicardium | [79,80] |
| FSP1 | Calcium binding and promote filament depolymerization | Resting and activated CFs | Pericytes, VSMC, ECs, immune cells | [81,82] |
| Sca-1 | Stem cell antigen | Resting and activated CFs | Cardiac progenitor cells | [83,84] |
| Fibronectin | ECM protein | Resting and activated CFs | ECs | [55,73,85] |
| Vimentin | Intermediate filament protein | Resting and activated CFs | Pericytes, VSMC, ECs | [86,87] |
| collagen types I and III | ECM protein | Resting and activated CFs | Pericytes, VSMC, ECs, cardiomyocytes | [55,88] |
| αSMA | Isoforms of actin filament | activated CFs | Pericytes, VSMC, cardiomyocytes, Epicardium | [2,29,89] |
| TNC | ECM protein | activated CFs | Pericytes, VSMC, | [6,7,90] |
7. Origin of CFs in the Development of Cardiac Disease
The embryonic proepicardium, which can give rise to various tissues, including pericytes, smooth muscle cells, cardiomyocytes, and endothelial cells [91], has been identified as the major cell source of CFs in numerous developmental models [45,46]. Endothelial cells are another source of CFs. Cardiac valves are composed of fibroblast-like valve interstitial cells (VICs) and valve endothelial cells (VECs) encompassing VICs. VICs are produced by the EndoMT of VECs [92,93,94]. Additionally, some CFs in the interventricular septum are derived from endothelial cells through EndoMT, as confirmed using genetic lineage tracing with Tie2-Cre mice [47]. Neural crest cells contribute to CFs within the atrium and VICs in semilunar valves [95,96,97]. Although the origin of fibroblasts in cardiac disease models has been controversial and is still under investigation, accumulating evidence suggests that the EndoMT, recruitment of circulating fibroblast progenitors, and expansion of resident fibroblasts may contribute to cardiac fibrosis after cardiac injury [68,98,99,100,101,102].
8. Signaling Pathways in Cardiac Fibrosis
Numerous signaling pathways have been implicated in CF activation and pathological remodeling progression. Modulating these signaling pathways as novel therapeutic targets is of great interest. Therefore, we summarized the mediators and signaling pathways that influence CF function after cardiac injury.
8.1. TGF-β Signaling
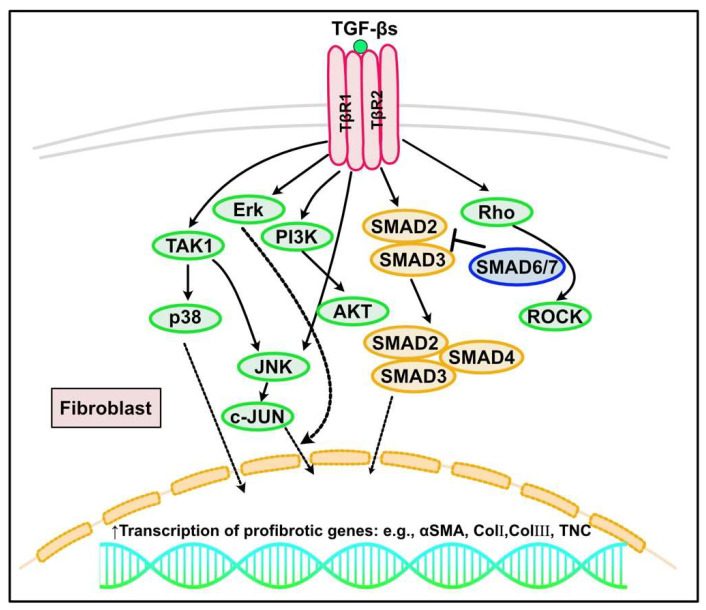
TGF-β is one of the most vigorously researched fibrotic factors. It is a pleiotropic peptide with diverse effects. Its effects on cellular behavior are dependent on cell type and environmental and cellular conditions, and are regulated in a highly context-dependent manner [103]. TGF-β mediates various biological processes, such as embryonic development, tumor growth, cell proliferation, and apoptosis [104,105,106]. TGF-β is also a central player in hypertrophic and fibrotic remodeling of the heart, mediating cardiomyocyte growth, CF activation, inflammation, and ECM deposition [107,108,109]. TGF-β includes three isoforms (TGF-β1, TGF-β2, and TGF-β3) in mammals, encoded by three different genes [104,110]. Among the three isoforms, TGF-β1 is predominant. It is crucial in pathological fibrosis and is produced by various cells, including immune cells, endothelial cells, cardiomyocytes, and activated fibroblasts [111,112]. TGF-β1 is initially secreted as an inactive complex with latent TGF-β-binding proteins and TGF-β pro-peptides. This complex is cleaved and activated during an integrin-mediated process [113,114,115]. TGF-β can induce signal transduction via canonical (SMAD-dependent) and non-canonical (SMAD-independent) pathways. In the canonical pathway, TGF-β1 binds to and causes heterodimerization of TGF-β receptor type 1 (TβRI, also known as activin-like kinase (ALK) 5) and type II (TβRII), leading to SMAD2 and SMAD3 phosphorylation. Consequently, a complex with SMAD4 forms and translocates into the nucleus, acting as a transcriptional factor to regulate the expression of target genes [28,48,59,116]. Intriguingly, recent reports have suggested the distinct roles of SMAD2 and SMAD3 in mediating TGF-β signaling [117,118,119]. SMAD6 and SMAD7 are inhibitory SMADs. They can interact with TβRI and competitively inhibit SMAD2 and SMAD3 [116,120]. In addition to the SMAD-dependent canonical pathways, TGF-β1 can induce SMAD-independent non-canonical signaling that involves several mitogen-activated protein kinases, including extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), TGF-β-activated kinase 1 (TAK1), Rho family of small GTPases, and p38 MAPK pathways [121,122]. In fibrosis regulation, TGF-β can transform fibroblasts into activated CFs and promote ECM synthesis and deposition [104], which involves SMAD3 signaling [123,124,125,126] (Figure 3). TGF-β also inhibits ECM degradation by regulating plasminogen activator inhibitor (PAI)-1 and TIMP expression levels [127]. Conversely, TGF-β can induce MMPs (e.g., MMP1, 2, and 3), suggesting that over-activation of TGF-β signaling may lead to aortic wall vulnerability in Marfan syndrome [128]. Additionally, non-canonical TGF-β signaling can induce fibrosis [121,129]. In human activated CFs, RNA-binding proteins, such as pumilio RNA binding family member 2 (PUM2) and KH domain-containing RNA binding (QKI), work as hub proteins of the canonical TGF-β1–SMAD and TGF-β1–MAPK pathway, and the non-canonical IL-11-mediated pathway, which regulates fibrogenic gene expression [130]. Vast cumulative evidence points to the role of non-coding RNAs and microRNAs in cardiac fibrosis [131,132,133,134,135]. Metabolic dysregulation, including glucose metabolism, is also involved in fibrosis progression through the regulation of TGF-β-mediated hypoxia-inducible factor (HIF)-1α and/or the renin-angiotensin system [136,137,138,139,140].
Figure 3.
TGF-β signaling in cardiac fibrosis. TGF-β could induce the signal transduction via the canonical (SMAD-dependent) and non-canonical (SMAD-independent) pathways. In the canonical pathway, TGF-β1 binds to and causes heterodimerization of TGF-β receptor type 1 (TβRI, also known as activin-like kinase (ALK) 5) and the type II receptor (TβRII), leading to the phosphorylation of SMAD2/SMAD3, which subsequently form a complex with SMAD4 and translocate into the nucleus, acting as a transcriptional factor to regulate the fibrotic gene expression (e.g., αSMA, collagen I, III or TNC). SMAD6/7 are inhibitory SMADs to inhibit transcription of SMAD2 and SMAD3. In canonical pathways, TGF-β1 can also induce SMAD-independent noncanonical signaling that involves several mitogen-activated protein kinases, including extracellular signal-regulated kinase (Erk), c-Jun-N-terminal kinase (JNK), TGF-β-activated kinase 1 (TAK1), Rho family of small GTPase, and p38 MAPK pathways.
8.2. Renin-Angiotensin-Aldosterone System
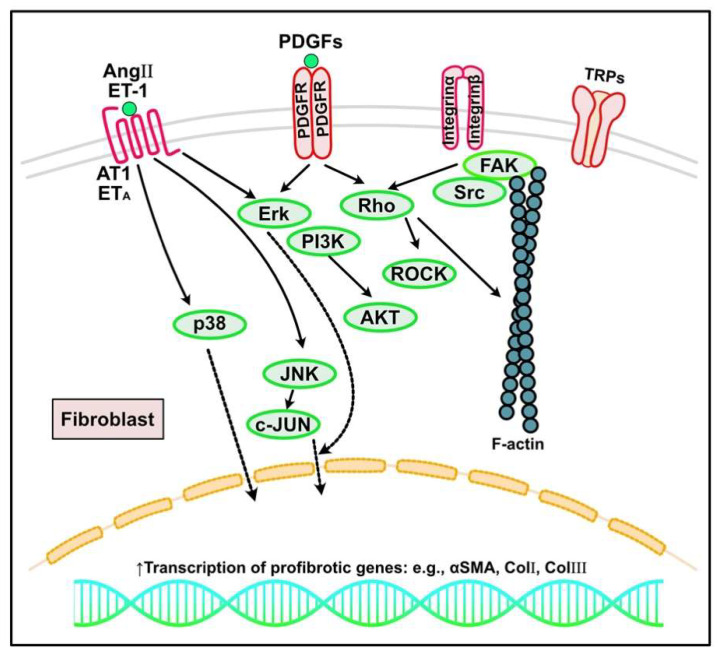
The renin-angiotensin-aldosterone system, in which Ang II is considered the most predominant isoform, promotes many pathophysiological functions, including cardiac fibrosis [141,142,143]. Systemically or locally produced Ang II acts through two specific receptors: angiotensin type (AT) 1 and AT2. Ang II through AT1 is involved in various biological processes, including CF proliferation, migration, and CF activation, with the induction of ECM protein synthesis and apoptosis [2]. In contrast, AT2 has a cardioprotective role, acting as a negative regulator of Ang II-mediated fibrogenic responses. AT2 inhibits AT1 action by suppressing CF proliferation and matrix synthesis [144]. The effects of Ang II through AT1 on CF activation are mediated through the activation of p38 MAPK, protein kinase C (PKC), and ERK cascades [145,146] (Figure 4). Ang II also interacts with TGF-β signaling in cardiomyocytes and CFs to induce cardiac hypertrophy and fibrosis. Various mediators regulate CF responses to Ang II through AT receptor expression. For instance, pro-inflammatory mediators (e.g., NF-κβ, IL-1β, IL-6, and TNF-α) make fibroblasts more responsive to Ang II by inducing AT1 synthesis [2,147].
Figure 4.
Other signaling pathways regulating cardiac fibrosis. In response to increased mechanical stress and cardiac injury, inflammatory signaling, growth factors (e.g., platelet-derived growth factors (PDGFs)), neurohumoral pathways (e.g., angiotensin (Ang) II, endothelin (ET)-1), and mechanosensitive pathways mediated by integrins and ion channels such as transient receptor potential cation channels (TRPs) can activate fibroblasts into myofibroblasts, leading to excess extracellular matrix protein deposition and cardiac fibrosis. AT1, angiotensin type 1 receptor; PDGFR, PDGF receptor; ERK, extracellular signal regulated kinase; PI3K, phosphoinositide 3-kinase; JNK, c-JUN N-terminal kinase; αSMA, α-smooth muscle actin; ROCK, Rho-associated protein kinases; FAK, focal adhesion kinase.
8.3. Endothelin (ET)
ET was first identified as a potent vasoconstrictor peptide. It is now widely accepted as a multifunctional peptide involved in development, tumor growth, immune regulation, and cardiac fibrosis [148,149,150,151,152,153,154,155,156]. ET-1, the predominant isoform in humans, is thought to be secreted mainly by endothelial cells, but can also be produced by every cell type [157]. G protein-coupled receptors (GPCRs) ETA and ETB are two recognized ET-1 receptors. Although ET-1 acts mainly through ETA to promote vasoconstriction, inflammation, and cell proliferation, the ETB receptor is considered a physiological antagonist [157]. ET-1 exerts fibrogenic effects, acting as a downstream molecule of cytokines and neurohumoral mediators, thus linking inflammation and cardiac fibrosis [157,158] (Figure 4). Ang II and TGF-β induce ET-1 expression [159,160] and ET-1 upregulation is consistently confirmed in many fibrosis-associated cardiac pathologies, including MI, HF, and hypertensive heart disease [157,161]. Both genetic models and pharmacologic inhibition studies suggest the fibrogenic effects of ET-1 in myocardial disease. Cardiac ET-1 overexpression in mice induces myocardial fibrosis associated with both systolic and diastolic dysfunction [162]. Moreover, endothelium-specific loss of ET-1 attenuated fibrosis in Ang II-infused mice [163]. ET-1 inhibition improved cardiac fibrosis [164]. The anti-fibrotic effects of the ET-1 blockade may have therapeutic implications. ETA and dual ETA/ETB antagonists reduce myocardial remodeling by suppressing collagen deposition and attenuating cardiac fibrosis in animal models of aldosterone treatment that recapitulate the features of human HFpEF [165,166]. Although its effectiveness in animal experiments has been shown, clinical trials using ETA antagonists have not been beneficial for patients with heart failure with reduced ejection fraction (HFrEF) and HFpEF [157].
8.4. Platelet-Derived Growth Factors (PDGF)
PDGFs have many pathophysiological roles in embryonic development, tumor progression, vascular diseases, and fibrosis. PDGFs form homo- or heterodimers and act through two receptor tyrosine kinases (PDGFR-α and PDGFR-β). They have common domain structures, including five extracellular immunoglobulin loops and a split intracellular tyrosine kinase domain. In fibrogenic conditions, PDGF signaling, which in part interacts with TGF-β signaling, causes cell proliferation with an activated phenotype, resulting in excessive ECM production and deposition [167] (Figure 4). PDGF-A, PDGF-C, and PDGF-D are implicated as potential fibrogenic PDGFs in the myocardium through direct actions and, in part, through TGF-β [168,169]. PDGF-AA stimulates CF proliferation in vitro [170]. PDGF-A or PDGF-D overexpression can cause cardiac fibrosis due to excess fibroblast activation [171,172]. Although controversial, PDGF-B is also a potent fibrogenic PDGF [172,173]. PDGFR-α activation is consistently involved in myocardial fibrosis. Treatment with a neutralizing antibody against PDGFR-α and PDGFR-β attenuated collagen deposition [173]. Additionally, a broader PDGFR blockade through the kinase inhibitor imatinib reduces cardiac fibrosis in mouse myocarditis, MI, and isoproterenol infusion models [174,175,176]. PDGFR-β activation potentially occurs through integrin β1 and small proline-rich repeat 3 to augment fibroblast proliferation and matrix synthesis in a cardiac pressure overload mouse model [177]. PDGFR-β signaling neutralization in infarcted hearts has reduced collagen deposition with significantly increased microvascular density and reduced vascular mural cell-coated vasculature, suggesting the importance of PDGFR-β actions on vascular mural cell recruitment and differentiation [167,169,178]. PDGFs have also been shown to be involved in the cardiac fibrotic response in an Ang II-treated mouse model [179]. Ang II-induced cardiac fibrosis is reduced in Krüppel-like zinc-finger transcription factor (klf) 5 heterozygous knockout mice, correlating with reduced cardiac expression of PDGF-A with direct binding of KLF5 to the PDGF-A promoter region [180]. Together, these results implicate PDGFR-α stimulation as a predominant fibrogenic process in the heart, whereas PDGFR-β stimulation also affects vascular mural cells.
8.5. Wnt Signaling
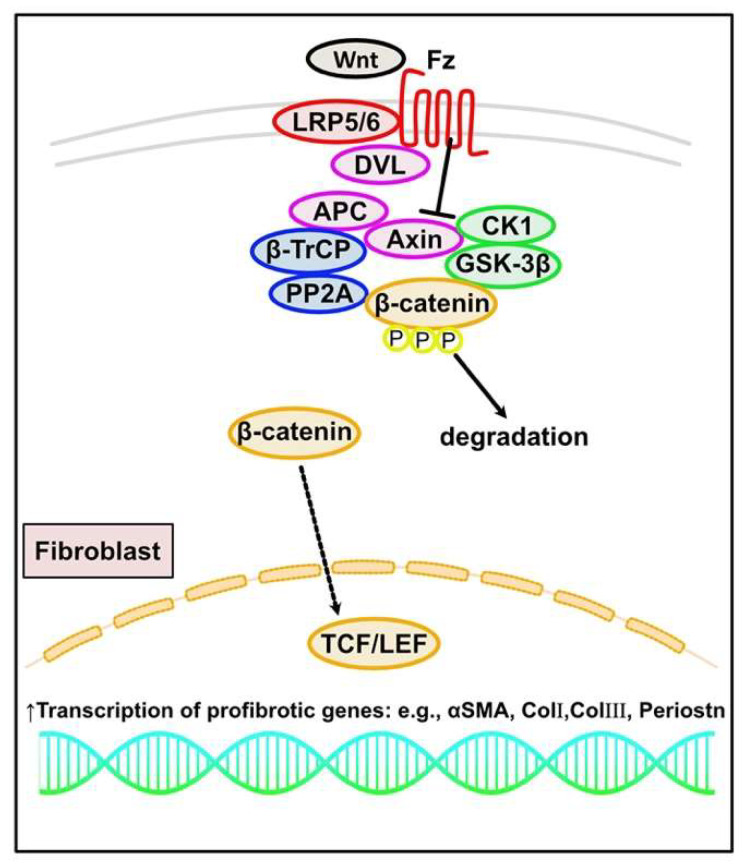
The Wnt signaling pathway has diverse roles in many biological processes, including carcinogenesis, embryonic development, immune maintenance, and fibrosis [181,182,183]. Several reports have indicated essential roles for Wnt signaling in cardiac fibrosis progression, mainly through the TGF-β pathway. The canonical Wnt/β-catenin pathway is predominantly involved in cardiac fibrosis progression, interacting with SMAD-dependent canonical TGF-β signaling [184,185]. In the absence of Wnt ligands, cytosolic β-catenin is degraded by the destruction complex, which includes tumor suppressors Axin, adenomatous polyposis coli (APC), the serine/threonine kinases, glycogen synthase kinase (GSK)-3β, casein kinase (CK) 1, protein phosphatase 2A (PP2A), and the E3-ubiquitin ligase β-transducin repeat-containing protein (β-TrCP) [186]. After a Wnt ligand binds to the seven-pass transmembrane receptor Frizzled (Fz) and the single-pass low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6) coreceptor, the Wnt–Fz–LRP5/6 complex recruits Dishevelled (DVL) and Axin through the intracellular domains of Fz and LRP5/6, resulting in β-catenin phosphorylation inhibition and β-catenin stabilization. Increased cytoplasmic and nuclear β-catenin levels promote interaction with the T cell factor/lymphoid enhancer factor (TCF/LEF) to regulate Wnt-responsive genes [187]. In CFs from MI mice and human, phosphorylated GSK-3β negatively regulates TGF-β signaling by directly interacting with SMAD3 and through β-catenin signaling. Moreover, GSK-3β deletion or inhibition in in vivo models leads to hyperactivation of TGF-β-SMAD3 signaling and cardiac fibrosis [188,189]. Secreted Fz-related proteins (sFRPs), which are endogenous modulators of Wnt signaling, have emerged as key regulators of the fibrotic response. sFRP1 inhibits Wnt ligands. sFRP1 null mice show cardiac dilation with increased expression of canonical Wnts, β-catenin, and Wnt target genes, such as Lef1 and Wisp1, leading to increased α-SMA expression and collagen production [190,191] (Figure 5). sFRP2 is a Wnt signaling inhibitor. sFRP2 null mice exhibit reduced collagen deposition and improved cardiac function after MI [192]. sFRP2 binds BMP1 through its Fz domain, enhancing the interaction between BMP1 and procollagen, thereby promoting procollagen processing and collagen deposition in the ECM [191,193]. A pressure overload model with trans-aortic constriction in mice observed increased β-catenin signaling in CFs. Fibroblast-specific loss of β-catenin led to improved cardiac function with reduced interstitial fibrosis and decreased ECM expression by directly regulating the expression of ECM-related genes, such as Col3a1 and Postn [194]. In contrast, injecting the sFRP2 protein into the infarcted left ventricle in a rat MI model inhibited cardiac fibrosis and improved cardiac function [193]. These controversial results may be caused by the use of different animal models and sFRP2 concentrations. Additional studies are needed to elucidate the action mechanisms of sFRP2 in cardiac fibrosis.
Figure 5.
Canonical WNT/β-catenin signaling in cardiac fibrosis. In the absence of Wnt ligands, cytosolic β-catenin is degraded by the destruction complex, which includes Axin and adenomatous polyposis coli (APC), glycogen synthase kinase (GSK)-3β and casein kinase (CK)1, protein phosphatase 2A (PP2A), and β-transducin repeat-containing protein (β-TrCP). After a Wnt ligand binds to the receptor Frizzled (Fz) and the receptor-related protein 5 or 6 (LRP5/6) coreceptor, the Wnt–Fz–LRP5/6 complex recruits Disheveled (DVL) and Axin through the intracellular domains of Fz and LRP5/6, resulting in β-catenin stabilization. The increased nuclear levels of β-catenin promote interaction with T cell factor/lymphoid enhancer factor (TCF/LEF) transcription factor to regulate Wnt-responsive fibrotic genes.
9. Matricellular Proteins That Regulate Cardiac Fibrosis
9.1. Tenascin-C
Matricellular proteins are a family of specialized ECM molecules that are upregulated at high levels during tissue remodeling and have many biological roles [195,196]. TN-C is a typical matricellular protein and large ECM glycoprotein that is dramatically upregulated during development and in pathological tissues and interacts with other biological signaling pathways. In the heart, TN-C is expressed in a spatiotemporally limited fashion during embryonic development, although TN-C knockout or overexpressing mice exhibit grossly normal heart morphogenesis [197]. TN-C is sparsely detected in normal mice and human adult hearts but is re-upregulated around injured tissues under pathological conditions, including MI, myocarditis, DCM, Kawasaki disease, and cardiac fibrosis [6,7,198]. In the Ang II infusion heart model, TN-C was produced by perivascular interstitial cells and deposited at fibrotic lesions around the macrophage infiltration site [179]. The induction of TN-C by ANG II is partially mediated by the ET1/ETA pathway, which could be suppressed by atrial natriuretic peptides [199]. Upregulated TN-C around the injured area stimulates macrophages via the integrin αvβ3/FAK/Src/nuclear factor-κB axis, facilitating the expression of proinflammatory cytokines, such as IL-6, which stimulate fibroblasts to synthesize excess collagen, leading to fibrosis. Additionally, proinflammatory cytokines produced by macrophages stimulate TN-C synthesis in CFs, leading to additional fibrosis in a positive feedback loop [200]. TN-C may also promote fibrosis by stimulating fibroblasts via integrin αvβ1/TGF-β/SMAD2 [201] and PDGF-AB/PDGFR [179]. TN-C deletion attenuates inflammation and fibrosis [6,7]. Similar TN-C proinflammatory and profibrotic roles have been reported in the transverse aortic constriction (TAC) model [202]. Locally produced TN-Cs may amplify monocyte/macrophage recruitment by increasing RhoA/ROCK signaling-dependent motility, thereby promoting a shift toward an inflammatory phenotype and upregulating chemokine expression in CFs. Similar to the Ang II-induced model, TN-C deletion attenuated inflammatory and fibrotic changes as well as hypertrophy and contractile dysfunction in TAC heart models [203,204].
9.2. Connective Tissue Growth Factor (CTGF)
CTGF, also known as CCN2, is a matricellular protein expressed in CFs and cardiomyocytes regulating diverse cellular functions, including cell adhesion, matrix production, structural remodeling, angiogenesis, and cell proliferation and differentiation. Mechanical stresses, numerous cytokines, neurohormonal factors, and growth factors, including TGF-β, regulate CTGF gene expression. CTGF mediates the downstream pro-fibrotic actions of TGF-β in the heart [205,206]. Both TGF-β and CTGF were expressed in CFs in MI models [190]. Increased CTGF causes ECM protein upregulation, including fibronectin and collagen I and III, following MI in an animal model [207]. CTGF is strongly upregulated in human HF and animal models associated with cardiac fibrosis [208,209,210]. Although CTGF is considered a pro-fibrotic factor, its role in cardiac diseases is controversial [211,212]. Further studies are needed to dissect these points for clinical applications.
9.3. Periostin, Osteopontin (OPN), and Secreted Protein Acidic and Rich in Cysteine (SPARC)
Periostin is also involved in cardiac fibrosis [213,214]. It is produced in activated fibroblasts through mechanical stress, chemokines, changes in matrix composition, and the canonical TGF-β pathway [215,216,217]. LOX and TN-C-associated periostin acts through integrin αvβ1, β3, and β5 to activate p38 [218], FAK, and PI3K/AKT [219,220], which control fibrogenic gene expression, resulting in cardiac fibrosis [221,222].
OPN, a glycoprotein with an arginine-glycine-aspartic acid (RGD) sequence, interacts with integrins (αvβ3) and the CD44 receptor in an RGD-dependent manner [2,223]. OPN mediates diverse biological functions, including cell adhesion, chemotaxis, and signaling. OPN may regulate TGF-β, MMPs, and LOX to exert cardiac fibrogenic effects [214,224,225,226,227].
The matricellular protein SPARC regulates post-synthetic collagen processing to facilitate the formation and assembly of mature cross-linked, insoluble structural collagen fibrils [2]. The absence of SPARC causes alterations in ECM fibrillar collagen and cardiac function [228]. SPARC also induces TGF-β signaling and activates ADAMTS1. ADAMTS1 promotes collagen fiber assembly, stabilization, and release in the heart, leading to cardiac fibrosis [229].
10. Cardiac Lymphatics
The heart has an extensive lymphatic vessel network comprising a heterogeneous origin and signaling that regulates its development [230,231,232,233,234,235]. In a mouse MI model [236], cardiac lymphatic vessel impairment leads to interstitial fluid accumulation and myocardial fibrosis progression due to increased interstitial fluid pressure that might induce CF activation through mechanical stress within the ECM [237]. Additionally, lymphatic vessel dysfunction and lymphangiogenesis inhibition delay the resolution of inflammation [238]. Thus, stimulating cardiac lymphangiogenesis with growth factors (e.g., VEGF-C) in MI model mice reduces fibrosis and preserves cardiac function [231,232,237,239]. Cardiac edema induced by lymphatic ligation also increases collagen mRNA expression and deposition, enhancing cardiac fibrosis in rabbits. This confirms the importance of lymphatic vessels in cardiac fibrosis progression [240]. Recent reports suggest that lymphangiocrine signals from lymphatic vessels are another potential therapeutic target to promote cardiac growth and repair [241]. Thus, lymphatic vessels may regulate cardiac fibrosis through lymphangiocrine signaling.
11. Future Directions and Conclusions
Cardiac fibrosis influences HF progression and represents a major unmet public health requirement. Accumulating evidence points to the pathophysiologic heterogeneity of cardiac fibrosis and the complexity of CFs. Thus, it is very complicated to develop anti-fibrotic strategies for cardiac disease. Considering the complexity of CFs in health and disease, it is critical to identify the detailed relationships between distinct transcriptomic or proteomic profiles and functional properties. Collectively, it is important to investigate when and what cell types in cardiac fibrosis progression should be examined. Developing more specific in vivo approaches and identifying more specific targets is urgently required, and improving the understanding of cardiac fibrosis pathophysiology will lead to better-precision medicine-based treatment.
Author Contributions
K.M. and K.I.-Y. wrote and edited the manuscript. Both authors approved the manuscript for submission and agreed to be accountable for its contents. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan [20K17072 to K.M., JP19H03442 to K.I.-Y.], Japan Foundation for Applied Enzymology [VBIC to K.M.], Miyata Foundation Bounty for Pediatric Cardiovascular Research [to K.M.], Senshin Medical Research Foundation [to K.M.], and Japan Heart Foundation Research Grant on Dilated Cardiomyopathy [to K.I.-Y.].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rossignol P., Hernandez A.F., Solomon S.D., Zannad F. Heart failure drug treatment. Lancet. 2019;393:1034–1044. doi: 10.1016/S0140-6736(18)31808-7. [DOI] [PubMed] [Google Scholar]
- 2.Frangogiannis N.G. Cardiac fibrosis. Cardiovasc. Res. 2020;117:1450–1488. doi: 10.1093/cvr/cvaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sainio A., Järveläinen H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell Signal. 2020;66:109487. doi: 10.1016/j.cellsig.2019.109487. [DOI] [PubMed] [Google Scholar]
- 4.Li L., Zhao Q., Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018;68:490–506. doi: 10.1016/j.matbio.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Krejci J., Mlejnek D., Sochorova D., Nemec P. Inflammatory Cardiomyopathy: A Current View on the Pathophysiology, Diagnosis, and Treatment. Biomed. Res. Int. 2016;2016:4087632. doi: 10.1155/2016/4087632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imanaka-Yoshida K. Tenascin-C in Heart Diseases—The Role of Inflammation. Int. J. Mol. Sci. 2021;22:5828. doi: 10.3390/ijms22115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imanaka-Yoshida K., Tawara I., Yoshida T. Tenascin-C in cardiac disease: A sophisticated controller of inflammation, repair, and fibrosis. Am. J. Physiol.-Cell Physiol. 2020;319:C781–C796. doi: 10.1152/ajpcell.00353.2020. [DOI] [PubMed] [Google Scholar]
- 8.Eijgenraam T.R., Silljé H.H.W., de Boer R.A. Current understanding of fibrosis in genetic cardiomyopathies. Trends Cardiovasc. Med. 2019;30:353–361. doi: 10.1016/j.tcm.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Frangogiannis N.G. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ. Res. 2019;125:117–146. doi: 10.1161/CIRCRESAHA.119.311148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regan T.J., Lyons M.M., Ahmed S.S., Levinson G.E., Oldewurtel H.A., Ahmad M.R., Haider B. Evidence for Cardiomyopathy in Familial Diabetes Mellitus. J. Clin. Investig. 1977;60:885–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treibel T.A., López B., González A., Menacho K., Schofield R.S., Ravassa S., Fontana M., White S.K., DiSalvo C., Roberts N., et al. Reappraising myocardial fibrosis in severe aortic stenosis: An invasive and non-invasive study in 133 patients. Eur. Heart J. 2017;39:699–709. doi: 10.1093/eurheartj/ehx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manabe I. Chronic Inflammation Links Cardiovascular, Metabolic and Renal Diseases. Circ. J. 2011;75:2739–2748. doi: 10.1253/circj.CJ-11-1184. [DOI] [PubMed] [Google Scholar]
- 13.Oishi Y., Manabe I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Dis. 2016;2:16018. doi: 10.1038/npjamd.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linthout S.V., Elsanhoury A., Klein O., Sosnowski M., Miteva K., Lassner D., Abou-El-Enein M., Pieske B., Kühl U., Tschöpe C. Telbivudine in chronic lymphocytic myocarditis and human parvovirus B19 transcriptional activity. ESC Hear. Fail. 2018;5:818–829. doi: 10.1002/ehf2.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galati G., Leone O., Pasquale F., Olivotto I., Biagini E., Grigioni F., Pilato E., Lorenzini M., Corti B., Foà A., et al. Histological and Histometric Characterization of Myocardial Fibrosis in End-Stage Hypertrophic Cardiomyopathy. Circ. Hear. Fail. 2016;9:e003090. doi: 10.1161/CIRCHEARTFAILURE.116.003090. [DOI] [PubMed] [Google Scholar]
- 16.Hinderer S., Schenke-Layland K. Cardiac fibrosis–A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019;146:77–82. doi: 10.1016/j.addr.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 17.De Jong S., van Veen T.A.B., de Bakker J.M.T., Vos M.A., van Rijen H.V.M. Biomarkers of Myocardial Fibrosis. J. Cardiovasc. Pharm. 2011;57:522–535. doi: 10.1097/FJC.0b013e31821823d9. [DOI] [PubMed] [Google Scholar]
- 18.Yokota T., McCourt J., Ma F., Ren S., Li S., Kim T.-H., Kurmangaliyev Y.Z., Nasiri R., Ahadian S., Nguyen T., et al. Type V Collagen in Scar Tissue Regulates the Size of Scar after Heart Injury. Cell. 2020;182:545–562.e23. doi: 10.1016/j.cell.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson T.F., Factor S.M., Capasso J.M., Wittenberg B.A., Blumenfeld O.O., Seifter S. Morphology, composition, and function of struts between cardiac myocytes of rat and hamster. Cell Tissue Res. 1987;249:247–255. doi: 10.1007/BF00215507. [DOI] [PubMed] [Google Scholar]
- 20.Caulfield J.B., Norton P., Weaver R.D. Cardiac dilatation associated with collagen alterations. Mol. Cell Biochem. 1992;116:171–179. doi: 10.1007/BF00299396. [DOI] [PubMed] [Google Scholar]
- 21.Imanaka-Yoshida K., Enomoto-Iwamoto M., Yoshida T., Sakakura T. Vinculin, talin, integrin α6β1 and laminin can serve as components of attachment complex mediating contraction force transmission from cardiomyocytes to extracellular matrix. Cell Motil. Cytoskel. 1999;42:1–11. doi: 10.1002/(SICI)1097-0169(1999)42:1<1::AID-CM1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Imanaka-Yoshida K., Danowski B.A., Sanger J.M., Sanger J.W. Living adult rat cardiomyocytes in culture: Evidence for dissociation of costameric distribution of vinculin from costameric distributions of attachments. Cell Motil. Cytoskel. 1996;33:263–275. doi: 10.1002/(SICI)1097-0169(1996)33:4<263::AID-CM3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Danowski B.A., Imanaka-Yoshida K., Sanger J.M., Sanger J.W. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J. Cell Biol. 1992;118:1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber K.T., Sun Y., Bhattacharya S.K., Ahokas R.A., Gerling I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 25.Rienks M., Papageorgiou A.-P., Frangogiannis N.G., Heymans S. Myocardial Extracellular Matrix. Circ. Res. 2014;114:872–888. doi: 10.1161/CIRCRESAHA.114.302533. [DOI] [PubMed] [Google Scholar]
- 26.Levi N., Papismadov N., Solomonov I., Sagi I., Krizhanovsky V. The ECM path of senescence in aging: Components and modifiers. FEBS J. 2020;287:2636–2646. doi: 10.1111/febs.15282. [DOI] [PubMed] [Google Scholar]
- 27.Liu M., de Abad B.L.J., Cheng K. Cardiac fibrosis: Myofibroblast-mediated pathological regulation and drug delivery strategies. Adv. Drug Deliv. Rev. 2021;173:504–519. doi: 10.1016/j.addr.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangogiannis N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2018;65:70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Kurose H. Cardiac Fibrosis and Fibroblasts. Cells. 2021;10:1716. doi: 10.3390/cells10071716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onursal C., Dick E., Angelidis I., Schiller H.B., Staab-Weijnitz C.A. Collagen Biosynthesis, Processing, and Maturation in Lung Ageing. Front. Med. 2021;8:593874. doi: 10.3389/fmed.2021.593874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leduc C., Dupont L., Joannes L., Monseur C., Baiwir D., Mazzucchelli G., Deroanne C., Colige A., Bekhouche M. In vivo N-Terminomics Highlights Novel Functions of ADAMTS2 and ADAMTS14 in Skin Collagen Matrix Building. Front. Mol. Biosci. 2021;8:643178. doi: 10.3389/fmolb.2021.643178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessler E., Takahara K., Biniaminov L., Brusel M., Greenspan D.S. Bone Morphogenetic Protein-1: The Type I Procollagen C-Proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 33.Li S.W., Sieron A.L., Fertala A., Hojima Y., Arnold W.V., Prockop D.J. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein. Proc. Natl. Acad. Sci. USA. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagoutte P., Bettler E., Goff S.V.-L., Moali C. Procollagen C-proteinase enhancer-1 (PCPE-1), a potential biomarker and therapeutic target for fibrosis. Matrix Biol. Plus. 2021;11:100062. doi: 10.1016/j.mbplus.2021.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronoff M.R., Hiebert P., Hentzen N.B., Werner S., Wennemers H. Imaging and targeting LOX-mediated tissue remodeling with a reactive collagen peptide. Nat. Chem. Biol. 2021;17:865–871. doi: 10.1038/s41589-021-00830-6. [DOI] [PubMed] [Google Scholar]
- 36.Fortunati D., Chau D.Y.S., Wang Z., Collighan R.J., Griffin M. Cross-linking of collagen I by tissue transglutaminase provides a promising biomaterial for promoting bone healing. Amino Acids. 2014;46:1751–1761. doi: 10.1007/s00726-014-1732-0. [DOI] [PubMed] [Google Scholar]
- 37.Herum K.M., Lunde I.G., Skrbic B., Louch W.E., Hasic A., Boye S., Unger A., Brorson S.-H., Sjaastad I., Tønnessen T., et al. Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart. Cardiovasc. Res. 2015;106:217–226. doi: 10.1093/cvr/cvv002. [DOI] [PubMed] [Google Scholar]
- 38.González A., López B., Ravassa S., José G.S., Díez J. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim. Et Biophys. Acta Mol. Cell Res. 2019;1866:1421–1432. doi: 10.1016/j.bbamcr.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Sygitowicz G., Maciejak-Jastrzębska A., Sitkiewicz D. A Review of the Molecular Mechanisms Underlying Cardiac Fibrosis and Atrial Fibrillation. J. Clin. Med. 2021;10:4430. doi: 10.3390/jcm10194430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raeeszadeh-Sarmazdeh M., Do L.D., Hritz B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells. 2020;9:1313. doi: 10.3390/cells9051313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobusiak-Prokopowicz M., Kaaz K., Marciniak D., Karolko B., Mysiak A. Relationships between Circulating Matrix Metalloproteinases, Tissue Inhibitor TIMP-2, and Renal Function in Patients with Myocarditis. Kidney Blood Press. Res. 2021;46:749–757. doi: 10.1159/000519594. [DOI] [PubMed] [Google Scholar]
- 42.Kobusiak-Prokopowicz M., Krzysztofik J., Kaaz K., Jolda-Mydlowska B., Mysiak A. MMP-2 and TIMP-2 in Patients with Heart Failure and Chronic Kidney Disease. Open Med.-Wars. 2018;13:237–246. doi: 10.1515/med-2018-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polina E.R., Araújo R.R.C.V., Sbruzzi R.C., Biolo A., Rohde L.E., Clausell N., dos Santos K.G. Relationship of polymorphisms in the tissue inhibitor of metalloproteinase (TIMP)-1 and -2 genes with chronic heart failure. Sci. Rep. 2018;8:9446. doi: 10.1038/s41598-018-27857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doxakis A., Polyanthi K., Androniki T., Savvas P., Eleni Z., Roubini L., Nikolaos R. Targeting metalloproteinases in cardiac remodeling. J. Cardiovasc. Med. Cardiol. 2019;6:51–60. doi: 10.17352/2455-2976.000092. [DOI] [Google Scholar]
- 45.Fu X., Liu Q., Li C., Li Y., Wang L. Cardiac Fibrosis and Cardiac Fibroblast Lineage-Tracing: Recent Advances. Front. Physiol. 2020;11:416. doi: 10.3389/fphys.2020.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore-Morris T., Cattaneo P., Puceat M., Evans S.M. Origins of cardiac fibroblasts. J. Mol. Cell. Cardiol. 2016;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore-Morris T., Guimarães-Camboa N., Banerjee I., Zambon A.C., Kisseleva T., Velayoudon A., Stallcup W.B., Gu Y., Dalton N.D., Cedenilla M., et al. Resident fibroblast lineages mediate pressure overload–induced cardiac fibrosis. J. Clin. Investig. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Travers J.G., Kamal F.A., Robbins J., Yutzey K.E., Blaxall B.C. Cardiac Fibrosis. Circ. Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pedrotty D.M., Klinger R.Y., Kirkton R.D., Bursac N. Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes. Cardiovasc. Res. 2009;83:688–697. doi: 10.1093/cvr/cvp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohl P., Gourdie R.G. Fibroblast–myocyte electrotonic coupling: Does it occur in native cardiac tissue? J. Mol. Cell. Cardiol. 2014;70:37–46. doi: 10.1016/j.yjmcc.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ongstad E., Kohl P. Fibroblast–myocyte coupling in the heart: Potential relevance for therapeutic interventions. J. Mol. Cell. Cardiol. 2016;91:238–246. doi: 10.1016/j.yjmcc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talman V., Ruskoaho H. Cardiac fibrosis in myocardial infarction—From repair and remodeling to regeneration. Cell Tissue Res. 2016;365:563–581. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson R.D., Camelliti P. Role of Non-Myocyte Gap Junctions and Connexin Hemichannels in Cardiovascular Health and Disease: Novel Therapeutic Targets? Int. J. Mol. Sci. 2018;19:866. doi: 10.3390/ijms19030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawaguchi M., Takahashi M., Hata T., Kashima Y., Usui F., Morimoto H., Izawa A., Takahashi Y., Masumoto J., Koyama J., et al. Inflammasome Activation of Cardiac Fibroblasts Is Essential for Myocardial Ischemia/Reperfusion Injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 55.Ivey M.J., Tallquist M.D. Defining the Cardiac Fibroblast. Circ. J. 2016;80:2269–2276. doi: 10.1253/circj.CJ-16-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tallquist M.D., Molkentin J.D. Redefining the identity of cardiac fibroblasts. Nat. Rev. Cardiol. 2017;14:484–491. doi: 10.1038/nrcardio.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schafer S., Viswanathan S., Widjaja A.A., Lim W.-W., Moreno-Moral A., DeLaughter D.M., Ng B., Patone G., Chow K., Khin E., et al. IL-11 is a crucial determinant of cardiovascular fibrosis. Nature. 2017;552:110–115. doi: 10.1038/nature24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh S., Torzewski M. Fibroblasts and Their Pathological Functions in the Fibrosis of Aortic Valve Sclerosis and Atherosclerosis. Biomolecules. 2019;9:472. doi: 10.3390/biom9090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.López B., Ravassa S., Moreno M.U., José G.S., Beaumont J., González A., Díez J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021;18:479–498. doi: 10.1038/s41569-020-00504-1. [DOI] [PubMed] [Google Scholar]
- 60.Ma Y., Iyer R.P., Jung M., Czubryt M.P., Lindsey M.L. Cardiac Fibroblast Activation Post-Myocardial Infarction: Current Knowledge Gaps. Trends Pharm. Sci. 2017;38:448–458. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falcón D., Galeano-Otero I., Calderón-Sánchez E., Toro R.D., Martín-Bórnez M., Rosado J.A., Hmadcha A., Smani T. TRP Channels: Current Perspectives in the Adverse Cardiac Remodeling. Front. Physiol. 2019;10:159. doi: 10.3389/fphys.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leask A. Integrin β1: A Mechanosignaling Sensor Essential for Connective Tissue Deposition by Fibroblasts. Adv. Wound Care. 2013;2:160–166. doi: 10.1089/wound.2012.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu T., Liao J.K. Rho Kinases and Cardiac Remodeling. Circ. J. 2016;80:1491–1498. doi: 10.1253/circj.CJ-16-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Chen H., Seth A., McCulloch C.A. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am. J. Physiol.-Heart C. 2003;285:H1871–H1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- 65.Humeres C., Frangogiannis N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic Transl. Sci. 2019;4:449–467. doi: 10.1016/j.jacbts.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am. J. Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 67.Nagaraju C.K., Robinson E.L., Abdesselem M., Trenson S., Dries E., Gilbert G., Janssens S., Cleemput J.V., Rega F., Meyns B., et al. Myofibroblast Phenotype and Reversibility of Fibrosis in Patients With End-Stage Heart Failure. J. Am. Coll. Cardiol. 2019;73:2267–2282. doi: 10.1016/j.jacc.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 68.Kanisicak O., Khalil H., Ivey M.J., Karch J., Maliken B.D., Correll R.N., Brody M.J., Lin S.-C.J., Aronow B.J., Tallquist M.D., et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016;7:12260. doi: 10.1038/ncomms12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng C.-C., Hu Y.-F., Zhu D.-H., Cheng Q., Gu J.-J., Feng Q.-L., Zhang L.-X., Xu Y.-P., Wang D., Rong Z., et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat. Commun. 2021;12:3709. doi: 10.1038/s41467-021-24110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muhl L., Genové G., Leptidis S., Liu J., He L., Mocci G., Sun Y., Gustafsson S., Buyandelger B., Chivukula I.V., et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun. 2020;11:3953. doi: 10.1038/s41467-020-17740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Litviňuková M., Talavera-López C., Maatz H., Reichart D., Worth C.L., Lindberg E.L., Kanda M., Polanski K., Heinig M., Lee M., et al. Cells of the adult human heart. Nature. 2020;588:466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buechler M.B., Pradhan R.N., Krishnamurty A.T., Cox C., Calviello A.K., Wang A.W., Yang Y.A., Tam L., Caothien R., Roose-Girma M., et al. Cross-tissue organization of the fibroblast lineage. Nature. 2021;593:575–579. doi: 10.1038/s41586-021-03549-5. [DOI] [PubMed] [Google Scholar]
- 73.Umbarkar P., Ejantkar S., Tousif S., Lal H. Mechanisms of Fibroblast Activation and Myocardial Fibrosis: Lessons Learned from FB-Specific Conditional Mouse Models. Cells. 2021;10:2412. doi: 10.3390/cells10092412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudon-David F., Bouzeghrane F., Couture P., Thibault G. Thy-1 expression by cardiac fibroblasts: Lack of association with myofibroblast contractile markers. J. Mol. Cell. Cardiol. 2007;42:991–1000. doi: 10.1016/j.yjmcc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 75.DeLeon-Pennell K.Y. May the fibrosis be with you: Is discoidin domain receptor 2 the receptor we have been looking for? J. Mol. Cell. Cardiol. 2016;91:201–203. doi: 10.1016/j.yjmcc.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Cowling R.T., Yeo S.J., Kim I.J., Park J.I., Gu Y., Dalton N.D., Peterson K.L., Greenberg B.H. Discoidin domain receptor 2 germline gene deletion leads to altered heart structure and function in the mouse. Am. J. Physiol.-Heart C. 2014;307:H773–H781. doi: 10.1152/ajpheart.00142.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith C.L., Baek S.T., Sung C.Y., Tallquist M.D. Epicardial-Derived Cell Epithelial-to-Mesenchymal Transition and Fate Specification Require PDGF Receptor Signaling. Circ. Res. 2011;108:e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chong J.J.H., Reinecke H., Iwata M., Torok-Storb B., Stempien-Otero A., Murry C.E. Progenitor Cells Identified by PDGFR-Alpha Expression in the Developing and Diseased Human Heart. Stem Cells Dev. 2013;22:1932–1943. doi: 10.1089/scd.2012.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acharya A., Baek S.T., Huang G., Eskiocak B., Goetsch S., Sung C.Y., Banfi S., Sauer M.F., Olsen G.S., Duffield J.S., et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Acharya A., Baek S.T., Banfi S., Eskiocak B., Tallquist M.D. Efficient inducible Cre-mediated recombination in Tcf21cell lineages in the heart and kidney. Genesis. 2011;49:870–877. doi: 10.1002/dvg.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Österreicher C.H., Penz-Österreicher M., Grivennikov S.I., Guma M., Koltsova E.K., Datz C., Sasik R., Hardiman G., Karin M., Brenner D.A. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl. Acad. Sci. USA. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strutz F., Okada H., Lo C.W., Danoff T., Carone R.L., Tomaszewski J.E., Neilson E.G. Identification and characterization of a fibroblast marker. FSPJ Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valente M., Nascimento D.S., Cumano A., do Pinto Ó.P. Sca-1+ Cardiac Progenitor Cells and Heart-Making: A Critical Synopsis. Stem Cells Dev. 2014;23:2263–2273. doi: 10.1089/scd.2014.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McQualter J.L., Brouard N., Williams B., Baird B.N., Sims-Lucas S., Yuen K., Nilsson S.K., Simmons P.J., Bertoncello I. Endogenous Fibroblastic Progenitor Cells in the Adult Mouse Lung Are Highly Enriched in the Sca-1 Positive Cell Fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 85.Bagchi R.A., Lin J., Wang R., Czubryt M.P. Regulation of fibronectin gene expression in cardiac fibroblasts by scleraxis. Cell Tissue Res. 2016;366:381–391. doi: 10.1007/s00441-016-2439-1. [DOI] [PubMed] [Google Scholar]
- 86.Camelliti P., Borg T.K., Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 87.Blankesteijn W.M. Has the search for a marker of activated fibroblasts finally come to an end? J. Mol. Cell. Cardiol. 2015;88:120–123. doi: 10.1016/j.yjmcc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Carver W., Nagpal M.L., Nachtigal M., Borg T.K., Terracio L. Collagen expression in mechanically stimulated cardiac fibroblasts. Circ. Res. 1991;69:116–122. doi: 10.1161/01.RES.69.1.116. [DOI] [PubMed] [Google Scholar]
- 89.Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: A new probe for smooth muscle differentiation. J. Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Imanaka-Yoshida K., Yoshida T., Miyagawa-Tomita S. Tenascin-C in Development and Disease of Blood Vessels. Anat. Rec. 2014;297:1747–1757. doi: 10.1002/ar.22985. [DOI] [PubMed] [Google Scholar]
- 91.Quijada P., Trembley M.A., Small E.M. The Role of the Epicardium During Heart Development and Repair. Circ. Res. 2020;126:377–394. doi: 10.1161/CIRCRESAHA.119.315857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rutkovskiy A., Malashicheva A., Sullivan G., Bogdanova M., Kostareva A., Stensløkken K., Fiane A., Vaage J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Hear. Assoc. Cardiovasc. 2017;6:e006339. doi: 10.1161/JAHA.117.006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wessels A., van den Hoff M.J.B., Adamo R.F., Phelps A.L., Lockhart M.M., Sauls K., Briggs L.E., Norris R.A., van Wijk B., Perez-Pomares J.M., et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012;366:111–124. doi: 10.1016/j.ydbio.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Combs M.D., Yutzey K.E. Heart Valve Development. Circ. Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang X., Rowitch D.H., Soriano P., McMahon A.P., Sucov H.M. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 96.Kirby M., Gale T., Stewart D. Neural crest cells contribute to normal aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 97.Waldo K., Miyagawa-Tomita S., Kumiski D., Kirby M.L. Cardiac Neural Crest Cells Provide New Insight into Septation of the Cardiac Outflow Tract: Aortic Sac to Ventricular Septal Closure. Dev. Biol. 1998;196:129–144. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- 98.Zeisberg E.M., Tarnavski O., Zeisberg M., Dorfman A.L., McMullen J.R., Gustafsson E., Chandraker A., Yuan X., Pu W.T., Roberts A.B., et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 99.Möllmann H., Nef H.M., Kostin S., von Kalle C., Pilz I., Weber M., Schaper J., Hamm C.W., Elsässer A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc. Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 100.Van Amerongen M., Bou-Gharios G., Popa E., van Ark J., Petersen A., van Dam G., van Luyn M., Harmsen M. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J. Pathol. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 101.Aisagbonhi O., Rai M., Ryzhov S., Atria N., Feoktistov I., Hatzopoulos A.K. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis. Model. Mech. 2011;4:469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y., Lui K.O., Zhou B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018;15:445–456. doi: 10.1038/s41569-018-0023-y. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y., Alexander P.B., Wang X.-F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. CSH Perspect. Biol. 2017;9:a022145. doi: 10.1101/cshperspect.a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tzavlaki K., Moustakas A. TGF-β Signaling. Biomolecules. 2020;10:487. doi: 10.3390/biom10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zinski J., Tajer B., Mullins M.C. TGF-β Family Signaling in Early Vertebrate Development. CSH Perspect. Biol. 2018;10:a033274. doi: 10.1101/cshperspect.a033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Batlle E., Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lucas J.A., Zhang Y., Li P., Gong K., Miller A.P., Hassan E., Hage F., Xing D., Wells B., Oparil S., et al. Inhibition of transforming growth factor-β signaling induces left ventricular dilation and dysfunction in the pressure-overloaded heart. Am. J. Physiol.-Heart C. 2010;298:H424–H432. doi: 10.1152/ajpheart.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu C., Lim S.T., Teo M.H.Y., Tan M.S.Y., Kulkarni M.D., Qiu B., Li A., Lal S., dos Remedios C.G., Tan N.S., et al. Collaborative Regulation of LRG1 by TGF-β1 and PPAR-β/δ Modulates Chronic Pressure Overload–Induced Cardiac Fibrosis. Circ. Hear. Fail. 2019;12:e005962. doi: 10.1161/CIRCHEARTFAILURE.119.005962. [DOI] [PubMed] [Google Scholar]
- 109.Liu G., Ma C., Yang H., Zhang P.-Y. Transforming growth factor β and its role in heart disease. Exp. Ther. Med. 2017;13:2123–2128. doi: 10.3892/etm.2017.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walton K.L., Johnson K.E., Harrison C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharm. 2017;8:461. doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanna A., Frangogiannis N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019;6:140. doi: 10.3389/fcvm.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nicin L., Wagner J.U.G., Luxán G., Dimmeler S. Fibroblast-mediated intercellular crosstalk in the healthy and diseased heart. FEBS Lett. 2021 doi: 10.1002/1873-3468.14234. [DOI] [PubMed] [Google Scholar]
- 113.Robertson I.B., Horiguchi M., Zilberberg L., Dabovic B., Hadjiolova K., Rifkin D.B. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robertson I.B., Rifkin D.B. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. CSH Perspect. Biol. 2016;8:a021907. doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown N.F., Marshall J.F. Integrin-Mediated TGFβ Activation Modulates the Tumour Microenvironment. Cancers. 2019;11:1221. doi: 10.3390/cancers11091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chung J.Y.-F., Chan M.K.-K., Li J.S.-F., Chan A.S.-W., Tang P.C.-T., Leung K.-T., To K.-F., Lan H.-Y., Tang P.M.-K. TGF-β Signaling: From Tissue Fibrosis to Tumor Microenvironment. Int. J. Mol. Sci. 2021;22:7575. doi: 10.3390/ijms22147575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang S., Chen B., Su Y., Alex L., Humeres C., Shinde A.V., Conway S.J., Frangogiannis N.G. Distinct roles of myofibroblast-specific Smad2 and Smad3 signaling in repair and remodeling of the infarcted heart. J. Mol. Cell. Cardiol. 2019;132:84–97. doi: 10.1016/j.yjmcc.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aragón E., Wang Q., Zou Y., Morgani S.M., Ruiz L., Kaczmarska Z., Su J., Torner C., Tian L., Hu J., et al. Structural basis for distinct roles of SMAD2 and SMAD3 in FOXH1 pioneer-directed TGF-β signaling. Gene Dev. 2019;33:1506–1524. doi: 10.1101/gad.330837.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu L., Liu X., Ren X., Tian Y., Chen Z., Xu X., Du Y., Jiang C., Fang Y., Liu Z., et al. Smad2 and Smad3 have differential sensitivity in relaying TGFβ signaling and inversely regulate early lineage specification. Sci. Rep. 2016;6:21602. doi: 10.1038/srep21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miyazawa K., Miyazono K. Regulation of TGF-β Family Signaling by Inhibitory Smads. Cold Spring Harb. Perspect. Biol. 2016;9:a022095. doi: 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y.E. Non-Smad Signaling Pathways of the TGF-β Family. CSH Perspect. Biol. 2017;9:a022129. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frangogiannis N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020;217:e20190103. doi: 10.1084/jem.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bujak M., Frangogiannis N.G. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bujak M., Ren G., Kweon H.J., Dobaczewski M., Reddy A., Taffet G., Wang X.-F., Frangogiannis N.G. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 125.Dobaczewski M., Bujak M., Li N., Gonzalez-Quesada C., Mendoza L.H., Wang X.-F., Frangogiannis N.G. Smad3 Signaling Critically Regulates Fibroblast Phenotype and Function in Healing Myocardial Infarction. Circ. Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khalil H., Kanisicak O., Prasad V., Correll R.N., Fu X., Schips T., Vagnozzi R.J., Liu R., Huynh T., Lee S.-J., et al. Fibroblast-specific TGF-β–Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017;127:3770–3783. doi: 10.1172/JCI94753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schiller M., Javelaud D., Mauviel A. TGF-β-induced SMAD signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 128.Benke K., Ágg B., Szilveszter B., Tarr F., Nagy Z.B., Pólos M., Daróczi L., Merkely B., Szabolcs Z. The role of transforming growth factor-beta in Marfan syndrome. Cardiol. J. 2013;20:227–234. doi: 10.5603/CJ.2013.0066. [DOI] [PubMed] [Google Scholar]
- 129.Finnson K.W., Almadani Y., Philip A. Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: Mechanisms and targets. Semin. Cell Dev. Biol. 2020;101:115–122. doi: 10.1016/j.semcdb.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 130.Chothani S., Schäfer S., Adami E., Viswanathan S., Widjaja A.A., Langley S.R., Tan J., Wang M., Quaife N.M., Pua C.J., et al. Widespread Translational Control of Fibrosis in the Human Heart by RNA-Binding Proteins. Circulation. 2019;140:937–951. doi: 10.1161/CIRCULATIONAHA.119.039596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Piccoli M.-T., Bär C., Thum T. Non-coding RNAs as modulators of the cardiac fibroblast phenotype. J. Mol. Cell. Cardiol. 2016;92:75–81. doi: 10.1016/j.yjmcc.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 132.Micheletti R., Plaisance I., Abraham B.J., Sarre A., Ting C.-C., Alexanian M., Maric D., Maison D., Nemir M., Young R.A., et al. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci. Transl. Med. 2017;9:eaai9118. doi: 10.1126/scitranslmed.aai9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yousefi F., Shabaninejad Z., Vakili S., Derakhshan M., Movahedpour A., Dabiri H., Ghasemi Y., Mahjoubin-Tehran M., Nikoozadeh A., Savardashtaki A., et al. TGF-β and WNT signaling pathways in cardiac fibrosis: Non-coding RNAs come into focus. Cell Commun. Signal. 2020;18:87. doi: 10.1186/s12964-020-00555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hobuß L., Bär C., Thum T. Long Non-coding RNAs: At the Heart of Cardiac Dysfunction? Front. Physiol. 2019;10:30. doi: 10.3389/fphys.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao G. Significance of non-coding circular RNAs and micro RNAs in the pathogenesis of cardiovascular diseases. J. Med. Genet. 2018;55:713–720. doi: 10.1136/jmedgenet-2018-105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao X., Kwan J.Y.Y., Yip K., Liu P.P., Liu F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov. 2020;19:57–75. doi: 10.1038/s41573-019-0040-5. [DOI] [PubMed] [Google Scholar]
- 137.Gibb A.A., Lazaropoulos M.P., Elrod J.W. Myofibroblasts and Fibrosis. Circ. Res. 2020;127:427–447. doi: 10.1161/CIRCRESAHA.120.316958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cheng X., Gao W., Dang Y., Liu X., Li Y., Peng X., Ye X. Both ERK/MAPK and TGF-Beta/Smad Signaling Pathways Play a Role in the Kidney Fibrosis of Diabetic Mice Accelerated by Blood Glucose Fluctuation. J. Diabetes Res. 2013;2013:463740. doi: 10.1155/2013/463740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wenzl F.A., Ambrosini S., Mohammed S.A., Kraler S., Lüscher T.F., Costantino S., Paneni F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021;8:742178. doi: 10.3389/fcvm.2021.742178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jia G., Hill M.A., Sowers J.R. Diabetic Cardiomyopathy. Circ. Res. 2018;122:624–638. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schnee J.M., Hsueh W.A. Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc. Res. 2000;46:264–268. doi: 10.1016/S0008-6363(00)00044-4. [DOI] [PubMed] [Google Scholar]
- 142.Jia G., Aroor A.R., Hill M.A., Sowers J.R. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension. 2018;72:537–548. doi: 10.1161/HYPERTENSIONAHA.118.11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bakogiannis C., Theofilogiannakos E., Papadopoulos C., Lazaridis C., Bikakis I., Tzikas S., Vassilikos V. A translational approach to the renin-angiotensin-aldosterone system in heart failure. Ann. Res. Hosp. 2019;3:1–11. doi: 10.21037/arh.2019.05.01. [DOI] [Google Scholar]
- 144.Ocaranza M.P., Riquelme J.A., García L., Jalil J.E., Chiong M., Santos R.A.S., Lavandero S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kawai T., Forrester S.J., O’Brien S., Baggett A., Rizzo V., Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharm. Res. 2017;125:4–13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Satou R., Penrose H., Navar L.G. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr. Hypertens Rep. 2018;20:100. doi: 10.1007/s11906-018-0900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sato T., Kurihara Y., Asai R., Kawamura Y., Tonami K., Uchijima Y., Heude E., Ekker M., Levi G., Kurihara H. An endothelin-1 switch specifies maxillomandibular identity. Proc. Natl. Acad. Sci. USA. 2008;105:18806–18811. doi: 10.1073/pnas.0807345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kitazawa T., Takechi M., Hirasawa T., Adachi N., Narboux-Nême N., Kume H., Maeda K., Hirai T., Miyagawa-Tomita S., Kurihara Y., et al. Developmental genetic bases behind the independent origin of the tympanic membrane in mammals and diapsids. Nat. Commun. 2015;6:6853. doi: 10.1038/ncomms7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kurihara Y., Kurihara H., Suzuki H., Kodama T., Maemura K., Nagai R., Oda H., Kuwaki T., Cao W.-H., Kamada N., et al. Elevated blood pressure and craniofaclal abnormalities in mice deficient in endothelin. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 151.Maeda K., Asai R., Maruyama K., Kurihara Y., Nakanishi T., Kurihara H., Miyagawa-Tomita S. Postotic and preotic cranial neural crest cells differently contribute to thyroid development. Dev. Biol. 2015;409:72–83. doi: 10.1016/j.ydbio.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 152.Arima Y., Miyagawa-Tomita S., Maeda K., Asai R., Seya D., Minoux M., Rijli F.M., Nishiyama K., Kim K.-S., Uchijima Y., et al. Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signalling. Nat. Commun. 2012;3:1267. doi: 10.1038/ncomms2258. [DOI] [PubMed] [Google Scholar]
- 153.Elisa T., Antonio P., Giuseppe P., Alessandro B., Giuseppe A., Federico C., Marzia D., Ruggero B., Giacomo M., Andrea O., et al. Endothelin Receptors Expressed by Immune Cells Are Involved in Modulation of Inflammation and in Fibrosis: Relevance to the Pathogenesis of Systemic Sclerosis. J. Immunol. Res. 2015;2015:147616. doi: 10.1155/2015/147616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dhaun N., Webb D.J. Endothelins in cardiovascular biology and therapeutics. Nat. Rev. Cardiol. 2019;16:491–502. doi: 10.1038/s41569-019-0176-3. [DOI] [PubMed] [Google Scholar]
- 155.Wang X., Guo Z., Ding Z., Khaidakov M., Lin J., Xu Z., Sharma S.G., Jiwani S., Mehta J.L. Endothelin-1 upregulation mediates aging-related cardiac fibrosis. J. Mol. Cell. Cardiol. 2015;80:101–109. doi: 10.1016/j.yjmcc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 156.Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 157.Barton M., Yanagisawa M. Endothelin: 30 Years From Discovery to Therapy. Hypertension. 2019;74:1232–1265. doi: 10.1161/HYPERTENSIONAHA.119.12105. [DOI] [PubMed] [Google Scholar]
- 158.Leask A. Potential Therapeutic Targets for Cardiac Fibrosis. Circ. Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 159.Shi-wen X., Kennedy L., Renzoni E.A., Bou-Gharios G., du Bois R.M., Black C.M., Denton C.P., Abraham D.J., Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor β in human lung fibroblasts. Arthritis Rheum. 2007;56:4189–4194. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- 160.Liu J., Zhuang T., Pi J., Chen X., Zhang Q., Li Y., Wang H., Shen Y., Tomlinson B., Chan P., et al. Endothelial Forkhead Box Transcription Factor P1 Regulates Pathological Cardiac Remodeling Through Transforming Growth Factor-β1–Endothelin-1 Signal Pathway. Circulation. 2019;140:665–680. doi: 10.1161/CIRCULATIONAHA.119.039767. [DOI] [PubMed] [Google Scholar]
- 161.Enevoldsen F.C., Sahana J., Wehland M., Grimm D., Infanger M., Krüger M. Endothelin Receptor Antagonists: Status Quo and Future Perspectives for Targeted Therapy. J. Clin. Med. 2020;9:824. doi: 10.3390/jcm9030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mueller E.E., Momen A., Massé S., Zhou Y.-Q., Liu J., Backx P.H., Henkelman R.M., Nanthakumar K., Stewart D.J., Husain M. Electrical remodelling precedes heart failure in an endothelin-1-induced model of cardiomyopathy. Cardiovasc. Res. 2011;89:623–633. doi: 10.1093/cvr/cvq351. [DOI] [PubMed] [Google Scholar]
- 163.Adiarto S., Heiden S., Vignon-Zellweger N., Nakayama K., Yagi K., Yanagisawa M., Emoto N. ET-1 from endothelial cells is required for complete angiotensin II-induced cardiac fibrosis and hypertrophy. Life Sci. 2012;91:651–657. doi: 10.1016/j.lfs.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 164.Ammarguellat F., Larouche I., Schiffrin E.L. Myocardial Fibrosis in DOCA-Salt Hypertensive Rats. Circulation. 2001;103:319–324. doi: 10.1161/01.CIR.103.2.319. [DOI] [PubMed] [Google Scholar]
- 165.Park J.B., Schiffrin E.L. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: Role of endothelin. Am. J. Hypertens. 2002;15:164–169. doi: 10.1016/S0895-7061(01)02291-9. [DOI] [PubMed] [Google Scholar]
- 166.Valero-Munoz M., Li S., Wilson R.M., Boldbaatar B., Iglarz M., Sam F. Dual Endothelin-A/Endothelin-B Receptor Blockade and Cardiac Remodeling in Heart Failure with Preserved Ejection Fraction. Circ. Hear. Fail. 2016;9:e003381. doi: 10.1161/CIRCHEARTFAILURE.116.003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Andrae J., Gallini R., Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Gene Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tuuminen R., Nykänen A.I., Krebs R., Soronen J., Pajusola K., Keränen M.A.I., Koskinen P.K., Alitalo K., Lemström K.B. PDGF-A, -C, and -D but not PDGF-B Increase TGF-β1 and Chronic Rejection in Rat Cardiac Allografts. Arterioscler. Thromb. Vasc Biol. 2009;29:691–698. doi: 10.1161/ATVBAHA.108.178558. [DOI] [PubMed] [Google Scholar]
- 169.Kalra K., Eberhard J., Farbehi N., Chong J.J., Xaymardan M. Role of PDGF-A/B Ligands in Cardiac Repair After Myocardial Infarction. Front. Cell Dev. Biol. 2021;9:669188. doi: 10.3389/fcell.2021.669188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Simm A., Nestler M., Hoppe V. Mitogenic effect of PDGF-AA on cardiac fibroblasts. Basic Res. Cardiol. 1998;93:S040–S043. doi: 10.1007/s003950050209. [DOI] [PubMed] [Google Scholar]
- 171.Pontén A., Folestad E.B., Pietras K., Eriksson U. Platelet-Derived Growth Factor D Induces Cardiac Fibrosis and Proliferation of Vascular Smooth Muscle Cells in Heart-Specific Transgenic Mice. Circ. Res. 2005;97:1036–1045. doi: 10.1161/01.RES.0000190590.31545.d4. [DOI] [PubMed] [Google Scholar]
- 172.Gallini R., Lindblom P., Bondjers C., Betsholtz C., Andrae J. PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice. Exp. Cell Res. 2016;349:282–290. doi: 10.1016/j.yexcr.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 173.Zymek P., Bujak M., Chatila K., Cieslak A., Thakker G., Entman M.L., Frangogiannis N.G. The Role of Platelet-Derived Growth Factor Signaling in Healing Myocardial Infarcts. J. Am. Coll. Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 174.Leipner C., Grün K., Müller A., Buchdunger E., Borsi L., Kosmehl H., Berndt A., Janik T., Uecker A., Kiehntopf M., et al. Imatinib mesylate attenuates fibrosis in coxsackievirus b3-induced chronic myocarditis. Cardiovasc. Res. 2008;79:118–126. doi: 10.1093/cvr/cvn063. [DOI] [PubMed] [Google Scholar]
- 175.Liu C., Zhao W., Meng W., Zhao T., Chen Y., Ahokas R.A., Liu H., Sun Y. Platelet-derived growth factor blockade on cardiac remodeling following infarction. Mol. Cell Biochem. 2014;397:295–304. doi: 10.1007/s11010-014-2197-x. [DOI] [PubMed] [Google Scholar]
- 176.Wang L.-X., Yang X., Yue Y., Fan T., Hou J., Chen G.-X., Liang M.-Y., Wu Z.-K. Imatinib attenuates cardiac fibrosis by inhibiting platelet-derived growth factor receptors activation in isoproterenol induced model. PLoS ONE. 2017;12:e0178619. doi: 10.1371/journal.pone.0178619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saraswati S., Lietman C.D., Li B., Mathew S., Zent R., Young P.P. Small proline-rich repeat 3 is a novel coordinator of PDGFRβ and integrin β1 crosstalk to augment proliferation and matrix synthesis by cardiac fibroblasts. FASEB J. 2020;34:7885–7904. doi: 10.1096/fj.201902815R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alex L., Frangogiannis N.G. Pericytes in the infarcted heart. VASC Biol. 2019;1:H23–H31. doi: 10.1530/VB-19-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nishioka T., Suzuki M., Onishi K., Takakura N., Inada H., Yoshida T., Hiroe M., Imanaka-Yoshida K. Eplerenone Attenuates Myocardial Fibrosis in the Angiotensin II-Induced Hypertensive Mouse: Involvement of Tenascin-C Induced by Aldosterone-Mediated Inflammation. J. Cardiovasc. Pharm. 2007;49:261–268. doi: 10.1097/FJC.0b013e318033dfd4. [DOI] [PubMed] [Google Scholar]
- 180.Shindo T., Manabe I., Fukushima Y., Tobe K., Aizawa K., Miyamoto S., Kawai-Kowase K., Moriyama N., Imai Y., Kawakami H., et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat. Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 181.Tao H., Yang J.-J., Shi K.-H., Li J. Wnt signaling pathway in cardiac fibrosis: New insights and directions. Metabolis. 2016;65:30–40. doi: 10.1016/j.metabol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 182.Patel S., Alam A., Pant R., Chattopadhyay S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol. 2019;10:2872. doi: 10.3389/fimmu.2019.02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang Y., Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burgy O., Königshoff M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018;68–69:67–80. doi: 10.1016/j.matbio.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 185.Xu L., Cui W., Zhou W., Li D., Li L., Zhao P., Mo X., Zhang Z., Gao J. Activation of Wnt/β-catenin signalling is required for TGF-β/Smad2/3 signalling during myofibroblast proliferation. J. Cell Mol. Med. 2017;21:1545–1554. doi: 10.1111/jcmm.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Z., Zhao T., Zhang S., Wang J., Chen Y., Zhao H., Yang Y., Shi S., Chen Q., Liu K. The Wnt signaling pathway in tumorigenesis, pharmacological targets, and drug development for cancer therapy. Biomark Res. 2021;9:68. doi: 10.1186/s40364-021-00323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhan T., Rindtorff N., Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lal H., Ahmad F., Zhou J., Yu J.E., Vagnozzi R.J., Guo Y., Yu D., Tsai E.J., Woodgett J., Gao E., et al. Cardiac Fibroblast Glycogen Synthase Kinase-3β Regulates Ventricular Remodeling and Dysfunction in Ischemic Heart. Circulation. 2014;130:419–430. doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo Y., Gupte M., Umbarkar P., Singh A.P., Sui J.Y., Force T., Lal H. Entanglement of GSK-3β, β-catenin and TGF-β1 signaling network to regulate myocardial fibrosis. J. Mol. Cell. Cardiol. 2017;110:109–120. doi: 10.1016/j.yjmcc.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sklepkiewicz P., Shiomi T., Kaur R., Sun J., Kwon S., Mercer B., Bodine P., Schermuly R.T., George I., Schulze P.C., et al. Loss of Secreted Frizzled-Related Protein-1 Leads to Deterioration of Cardiac Function in Mice and Plays a Role in Human Cardiomyopathy. Circ. Hear. Fail. 2018;8:362–372. doi: 10.1161/CIRCHEARTFAILURE.114.001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang A., Huang Y. Role of Sfrps in cardiovascular disease. Ther. Adv. Chronic. Dis. 2020;11:2040622320901990. doi: 10.1177/2040622320901990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kobayashi K., Luo M., Zhang Y., Wilkes D.C., Ge G., Grieskamp T., Yamada C., Liu T.-C., Huang G., Basson C.T., et al. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat. Cell Biol. 2009;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.He W., Zhang L., Ni A., Zhang Z., Mirotsou M., Mao L., Pratt R.E., Dzau V.J. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc. Natl. Acad. Sci. USA. 2010;107:21110–21115. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xiang F.-L., Fang M., Yutzey K.E. Loss of β-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat. Commun. 2017;8:712. doi: 10.1038/s41467-017-00840-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bornstein P. Matricellular proteins: An overview. J. Cell Commun. Signal. 2009;3:163–165. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Murphy-Ullrich J.E., Sage E.H. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Imanaka-Yoshida K., Matsumoto K., Hara M., Sakakura T., Yoshida T. The dynamic expression of tenascin-C and tenascin-X during early heart development in the mouse. Differentiation. 2003;71:291–298. doi: 10.1046/j.1432-0436.2003.7104506.x. [DOI] [PubMed] [Google Scholar]
- 198.Imanaka-Yoshida K. Tenascin-C in Cardiovascular Tissue Remodeling. Circ. J. 2012;76:2513–2520. doi: 10.1253/circj.CJ-12-1033. [DOI] [PubMed] [Google Scholar]
- 199.Fujita S., Shimojo N., Terasaki F., Otsuka K., Hosotani N., Kohda Y., Tanaka T., Nishioka T., Yoshida T., Hiroe M., et al. Atrial natriuretic peptide exerts protective action against angiotensin II-induced cardiac remodeling by attenuating inflammation via endothelin-1/endothelin receptor A cascade. Heart Vessel. 2013;28:646–657. doi: 10.1007/s00380-012-0311-0. [DOI] [PubMed] [Google Scholar]
- 200.Shimojo N., Hashizume R., Kanayama K., Hara M., Suzuki Y., Nishioka T., Hiroe M., Yoshida T., Imanaka-Yoshida K. Tenascin-C May Accelerate Cardiac Fibrosis by Activating Macrophages via the Integrin αVβ3/Nuclear Factor–κB/Interleukin-6 Axis. Hypertension. 2015;66:757–766. doi: 10.1161/HYPERTENSIONAHA.115.06004. [DOI] [PubMed] [Google Scholar]
- 201.Katoh D., Kozuka Y., Noro A., Ogawa T., Imanaka-Yoshida K., Yoshida T. Tenascin-C Induces Phenotypic Changes in Fibroblasts to Myofibroblasts with High Contractility through the Integrin αvβ1/Transforming Growth Factor β/SMAD Signaling Axis in Human Breast Cancer. Am. J. Pathol. 2020;190:2123–2135. doi: 10.1016/j.ajpath.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 202.Abbadi D., Laroumanie F., Bizou M., Pozzo J., Daviaud D., Delage C., Calise D., Gaits-Iacovoni F., Dutaur M., Tortosa F., et al. Local production of tenascin-C acts as a trigger for monocyte/macrophage recruitment that provokes cardiac dysfunction. Cardiovasc. Res. 2017;114:123–137. doi: 10.1093/cvr/cvx221. [DOI] [PubMed] [Google Scholar]
- 203.Imanaka-Yoshida K. Inflammation in myocardial disease: From myocarditis to dilated cardiomyopathy. Pathol. Int. 2020;70:1–11. doi: 10.1111/pin.12868. [DOI] [PubMed] [Google Scholar]
- 204.Nishioka T., Onishi K., Shimojo N., Nagano Y., Matsusaka H., Ikeuchi M., Ide T., Tsutsui H., Hiroe M., Yoshida T., et al. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am. J. Physiol.-Heart C. 2010;298:H1072–H1078. doi: 10.1152/ajpheart.00255.2009. [DOI] [PubMed] [Google Scholar]
- 205.Daniels A., Bilsen M.V., Goldschmeding R., Vusse G.J.V.D., Nieuwenhoven F.A.V. Connective tissue growth factor and cardiac fibrosis. Acta Physiol. 2009;195:321–338. doi: 10.1111/j.1748-1716.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 206.Ungvari Z., Valcarcel-Ares M.N., Tarantini S., Yabluchanskiy A., Fülöp G.A., Kiss T., Csiszar A. Connective tissue growth factor (CTGF) in age-related vascular pathologies. Geroscience. 2017;39:491–498. doi: 10.1007/s11357-017-9995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen M.M., Lam A., Abraham J.A., Schreiner G.F., Joly A.H. CTGF Expression is Induced by TGF- β in Cardiac Fibroblasts and Cardiac Myocytes: A Potential Role in Heart Fibrosis. J. Mol. Cell. Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 208.Lopes S.M.C.D.S., Feijen A., Korving J., Korchynskyi O., Larsson J., Karlsson S., Dijke P.T., Lyons K.M., Goldschmeding R., Doevendans P., et al. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev. Dyn. 2004;231:542–550. doi: 10.1002/dvdy.20162. [DOI] [PubMed] [Google Scholar]
- 209.Koshman Y.E., Patel N., Chu M., Iyengar R., Kim T., Ersahin C., Lewis W., Heroux A., Samarel A.M. Regulation of Connective Tissue Growth Factor Gene Expression and Fibrosis in Human Heart Failure. J. Card. Fail. 2013;19:283–294. doi: 10.1016/j.cardfail.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tsoutsman T., Wang X., Garchow K., Riser B., Twigg S., Semsarian C. CCN2 plays a key role in extracellular matrix gene expression in severe hypertrophic cardiomyopathy and heart failure. J. Mol. Cell. Cardiol. 2013;62:164–178. doi: 10.1016/j.yjmcc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 211.Vainio L.E., Szabó Z., Lin R., Ulvila J., Yrjölä R., Alakoski T., Piuhola J., Koch W.J., Ruskoaho H., Fouse S.D., et al. Connective Tissue Growth Factor Inhibition Enhances Cardiac Repair and Limits Fibrosis After Myocardial Infarction. JACC Basic Transl. Sci. 2019;4:83–94. doi: 10.1016/j.jacbts.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dorn L.E., Petrosino J.M., Wright P., Accornero F. CTGF/CCN2 is an autocrine regulator of cardiac fibrosis. J. Mol. Cell. Cardiol. 2018;121:205–211. doi: 10.1016/j.yjmcc.2018.07.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shimazaki M., Nakamura K., Kii I., Kashima T., Amizuka N., Li M., Saito M., Fukuda K., Nishiyama T., Kitajima S., et al. Periostin is essential for cardiac healingafter acute myocardial infarction. J. Exp. Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kudo A., Kii I. Periostin function in communication with extracellular matrices. J. Cell Commun. Signal. 2018;12:301–308. doi: 10.1007/s12079-017-0422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Landry N.M., Cohen S., Dixon I.M.C. Periostin in cardiovascular disease and development: A tale of two distinct roles. Basic Res. Cardiol. 2017;1:113. doi: 10.1007/s00395-017-0659-5. [DOI] [PubMed] [Google Scholar]
- 216.Nanri Y., Nunomura S., Terasaki Y., Yoshihara T., Hirano Y., Yokosaki Y., Yamaguchi Y., Feghali-Bostwick C., Ajito K., Murakami S., et al. Cross-Talk between Transforming Growth Factor-β and Periostin Can Be Targeted for Pulmonary Fibrosis. Am. J. Respir. Cell Mol. 2019;62:204–216. doi: 10.1165/rcmb.2019-0245OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kii I., Nishiyama T., Li M., Matsumoto K., Saito M., Amizuka N., Kudo A. Incorporation of Tenascin-C into the Extracellular Matrix by Periostin Underlies an Extracellular Meshwork Architecture*. J. Biol. Chem. 2010;285:2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li L., Fan D., Wang C., Wang J.-Y., Cui X.-B., Wu D., Zhou Y., Wu L.-L. Angiotensin II Increases Periostin Expression via Ras/P38 MAPK/CREB and ERK1/2/TGF-Β1 Pathways in Cardiac Fibroblasts. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc. Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 219.Chen Z., Xie J., Hao H., Lin H., Wang L., Zhang Y., Chen L., Cao S., Huang X., Liao W., et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3β/cyclin D1 signalling pathway. Cardiovasc. Res. 2017;113:620–632. doi: 10.1093/cvr/cvx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li G., Jin R., Norris R.A., Zhang L., Yu S., Wu F., Markwald R.R., Nanda A., Conway S.J., Smyth S.S., et al. Periostin mediates vascular smooth muscle cell migration through the integrins ανβ3 and ανβ5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208:358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu H., Chen L., Xie J., Li R., Li G.-N., Chen Q.-H., Zhang X.-L., Kang L.-N., Xu B. Periostin expression induced by oxidative stress contributes to myocardial fibrosis in a rat model of high salt-induced hypertension. Mol. Med. Rep. 2016;14:776–782. doi: 10.3892/mmr.2016.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao S., Wu H., Xia W., Chen X., Zhu S., Zhang S., Shao Y., Ma W., Yang D., Zhang J. Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. J. Cardiol. 2014;63:373–378. doi: 10.1016/j.jjcc.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 223.Kahles F., Findeisen H.M., Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014;3:384–393. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yousefi K., Irion C.I., Takeuchi L.M., Ding W., Lambert G., Eisenberg T., Sukkar S., Granzier H.L., Methawasin M., Lee D.I., et al. Osteopontin Promotes Left Ventricular Diastolic Dysfunction Through a Mitochondrial Pathway. J. Am. Coll. Cardiol. 2019;73:2705–2718. doi: 10.1016/j.jacc.2019.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Icer M.A., Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018;59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 226.Mohamed I.A., Gadeau A.-P., Hasan A., Abdulrahman N., Mraiche F. Osteopontin: A Promising Therapeutic Target in Cardiac Fibrosis. Cells. 2019;8:1558. doi: 10.3390/cells8121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Matsui Y., Morimoto J., Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J. Biol. Chem. 2010;1:69–80. doi: 10.4331/wjbc.v1.i5.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schellings M.W.M., Vanhoutte D., Swinnen M., Cleutjens J.P., Debets J., van Leeuwen R.E.W., d’Hooge J., de Werf F.V., Carmeliet P., Pinto Y.M., et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J. Exp. Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Toba H., de Brás L.E.C., Baicu C.F., Zile M.R., Lindsey M.L., Bradshaw A.D. Increased ADAMTS1 mediates SPARC-dependent collagen deposition in the aging myocardium. Am. J. Physiol.-Endocrinal. Matab. 2016;310:E1027–E1035. doi: 10.1152/ajpendo.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Maruyama K., Miyagawa-Tomita S., Mizukami K., Matsuzaki F., Kurihara H. Isl1-expressing non-venous cell lineage contributes to cardiac lymphatic vessel development. Dev. Biol. 2019;452:134–143. doi: 10.1016/j.ydbio.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 231.Maruyama K., Naemura K., Arima Y., Uchijima Y., Nagao H., Yoshihara K., Singh M.K., Uemura A., Matsuzaki F., Yoshida Y., et al. Semaphorin3E-PlexinD1 signaling in coronary artery and lymphatic vessel development with clinical implications in myocardial recovery. Iscience. 2021;24:102305. doi: 10.1016/j.isci.2021.102305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Klotz L., Norman S., Vieira J.M., Masters M., Rohling M., Dubé K.N., Bollini S., Matsuzaki F., Carr C.A., Riley P.R. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2016;522:62. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lioux G., Liu X., Temiño S., Oxendine M., Ayala E., Ortega S., Kelly R.G., Oliver G., Torres M. A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics. A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics. Dev. Cell. 2020;52:350–363.e6. doi: 10.1016/j.devcel.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Martucciello S., Turturo M.G., Bilio M., Cioffi S., Chen L., Baldini A., Illingworth E. A dual role for Tbx1 in cardiac lymphangiogenesis through genetic interaction with Vegfr3. FASEB J. 2020;34:15062–15079. doi: 10.1096/fj.201902202R. [DOI] [PubMed] [Google Scholar]
- 235.Chen L., Mupo A., Huynh T., Cioffi S., Woods M., Jin C., McKeehan W., Thompson-Snipes L., Baldini A., Illingworth E. Tbx1 regulates Vegfr3 and is required for lymphatic vessel development. J. Cell Biol. 2010;189:417–424. doi: 10.1083/jcb.200912037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Maruyama K., Naemura K., Yoshihara K., Imanaka-Yoshida K., Kurihara H., Miyagawa-Tomita S. Surgical Protocol for Permanent Ligation of the Left Anterior Descending Coronary Artery in Mice to Generate a Model of Myocardial Infarction. Star Protoc. 2021;2:100775. doi: 10.1016/j.xpro.2021.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Henri O., Pouehe C., Houssari M., Galas L., Nicol L., Edwards-Lévy F., Henry J.-P., Dumesnil A., Boukhalfa I., Banquet S., et al. Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation. 2016;133:1484–1497. doi: 10.1161/CIRCULATIONAHA.115.020143. [DOI] [PubMed] [Google Scholar]
- 238.Brakenhielm E., Alitalo K. Cardiac lymphatics in health and disease. Nat. Rev. Cardiol. 2019;16:56–68. doi: 10.1038/s41569-018-0087-8. [DOI] [PubMed] [Google Scholar]
- 239.Vieira J.M., Norman S., del Campo C.V., Cahill T.J., Barnette D.N., Gunadasa-Rohling M., Johnson L.A., Greaves D.R., Carr C.A., Jackson D.G., et al. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J. Clin. Investig. 2018;128:3402–3412. doi: 10.1172/JCI97192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kong D., Kong X., Wang L. Effect of cardiac lymph flow obstruction on cardiac collagen synthesis and interstitial fibrosis. Physiol. Res. Acad. Sci. Bohemoslov. 2005;55:253–258. doi: 10.33549/physiolres.930727. [DOI] [PubMed] [Google Scholar]
- 241.Liu X., la Cruz E.D., Gu X., Balint L., Oxendine-Burns M., Terrones T., Ma W., Kuo H.-H., Lantz C., Bansal T., et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature. 2020;588:705–711. doi: 10.1038/s41586-020-2998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]