Abstract
Systemic lupus erythematosus (SLE) is a condition in which autoimmune inflammation affects nearly every organ in the human body; it is characterized by a relapsing-remitting pattern. Systemic inflammation and tissue damage can arise from autoantibodies, the creation of immune complexes, and the deposition of autoantibodies, all defined as autoimmune diseases. Women of reproductive age are at a high risk of developing lupus, a chronic systemic condition. Among women between the ages of 15 and 44 years, the female-to-male ratio for the occurrence of lupus is as high as 13:1, while it is only 2:1 in children and in the elderly. In addition to accelerated atherosclerosis, SLE is associated with an increased risk of cardiovascular (CV) events such as coronary artery disease (CAD), peripheral artery disease (PAD), and cerebrovascular accident (CVA). Several SLE-specific processes, including impaired immunological regulation, impaired endothelial cell (EC) function, impaired vascular repair, hyperleptinemia, and traditional risk factors, contribute to early atherosclerosis in the disease. CAD can occur at any stage of the disease's progression, with younger individuals being much more at risk than their age-matched counterparts. This review article aims to provide a unique insight into the relationship between SLE and cardiovascular disease (CVD) by discussing the pathophysiological role of CVD in SLE, outlining screening criteria, and highlighting the treatment options for CVD in connection with SLE.
Keywords: autoimmune heart disease, glucocorticoid-induced cardiomyopathy, pathogenesis of systemic lupus erythematosus, cvd & sle, cardiovascular disease, peripheral arterial diseases, women and heart disease, atherosclerotic cardiovascular disease, systemic lupus erythematosus disease, systemic lupus erythromatosus
Introduction and background
Systemic lupus erythematosus (SLE) is a disease with a relapsing-remitting autoimmune course that may affect practically any organ in the body. It is characterized by the generation of autoantibodies, the formation of immune complexes, and the deposition of autoantibodies, resulting in systemic inflammation and tissue damage [1]. Throughout the Middle Ages, the Latin name lupus (meaning "wolf") was used to describe a variety of disorders that resulted in ulcerous lesions on the lower limbs. The name "lupus érythémateux" was used by French dermatologist Cazenave in the middle of the 18th century. The major turning point in the history of lupus occurred in the early 19th century when an understanding of the difference between cutaneous lupus and lupus vulgaris in the modern sense started to emerge progressively. Early 19th-century work by Kaposi, Sequiera and Balean, and Osler contributed to the discovery of the disease's systemic nature. DNA was later identified as the primary target of antinuclear antibodies, and interferons (IFNs) have played a crucial role in modern research [2]. SLE has a prevalence of 9-241 cases per 100,000 people per year, and an incidence rate of 0.3-23.2 cases per 100,000 people per year, according to research done throughout the world over the previous 15 years [3].
Women of reproductive age have a strong predisposition to develop lupus. Among women between the ages of 15 and 44 years, the female-to-male ratio for the occurrence of lupus is as high as 13:1, while it is only 2:1 in children and in the elderly [4-6]. While it affects people of many races, it is more common among non-Caucasians. SLE is quite rare in Africa while being more common in Europe and the United States, especially among individuals of African origin [7,8]. It is thought that genetic factors interact with environmental exposures throughout the lifespan of an individual to influence susceptibility to develop SLE. The most substantial epidemiologic evidence for the increased risk of SLE is associated with exposure to crystalline silica, current cigarette smoking, use of oral contraceptives, and postmenopausal hormone replacement therapy, while there is an inverse association with alcohol use [9]. New research suggests a link between SLE risk and exposure to solvents, household and agricultural pesticides, heavy metals, and air pollution [9]. Ultraviolet light, vitamin D deficiency, certain infections, and vaccinations have also been hypothesized to be related to SLE risk [9]. Mechanisms that link environmental exposures with SLE include epigenetic modifications, increased oxidative stress, systemic inflammation, inflammatory cytokine upregulation, and hormonal effects [9].
There are many components to the SLE's complex pathogenesis: autoantibodies and immunocomplexes, involvement of the complement system, dysregulation of many cytokines including type I IFNs, and disturbance of the clearance of nucleic acids following cell death are only some of them [10]. Immunomodulators and immunosuppression can alter SLE's natural course [10]. In addition, SLE and treatment-related consequences such as accelerated coronary artery disease (CAD) and higher infection risk contribute significantly to both morbidity and mortality [10]. The 11-50% monozygotic twin concordance and significant risk observed within families reflect a genetic element [11]. Many genes, including HLA, IRF5, ITGAM, STAT4, BLK, and CTLA4, among others, have been associated with a propensity for lupus [11,12]. SLE diagnosis can be difficult, and while numerous categorization criteria have been proposed, their clinical value is still up for debate [13]. The management of SLE is determined based on organ system involvement, and despite the efficacy of various medications in the treatment of SLE, the disease still leads to significant morbidity and mortality among patients [13]. SLE is linked to an increased risk of accelerated atherosclerosis and cardiovascular (CV) events such as CAD, cerebrovascular accident (CVA), and peripheral artery disease (PAD). CV events occur both early and late in the course of the disease, with younger patients being substantially more at risk compared to their age-matched peers. By discussing the pathophysiological role of cardiovascular disease (CVD) in SLE and outlining screening criteria and treatment options for CVD in connection with SLE, this review article aims to provide distinct insight into the correlation between SLE and CVD.
Review
Pathophysiology
Vascular Endothelium Damage and Cardiovascular Disease in Systemic Lupus Erythematosus
One of the earliest signs of a developing CVD is the malfunction of the endothelial cells (ECs). In most cases, vascular injury is predicted to be accompanied by a faster rate of endothelium healing. The increased atherosclerotic load in SLE patients has been linked to decreased numbers of circulating endothelial progenitor cells (EPCs) and aberrant function of cells involved in vascular repair. As a result, lupus EPCs/myeloid circulating angiogenic cells (CACs) have a reduced ability to develop into mature ECs and produce lower levels of vascular endothelial growth factor (VEGF) and hepatic growth factor (HGF) [14-18]. Taraborelli et al. conducted a case-control study between 2014 and 2016 among a sample population of 40 females (20 cases with 20 matched controls) between the ages of 26-56 years with a disease duration of <5 years [19]. In this study, patients with a history of CVD or risk factors that could impair peripheral artery tonometry were excluded. Enrolled patients were matched to healthy controls with the same exclusion criteria for sex, age, body mass index (BMI), and blood pressure. Patients and controls received a transthoracic Doppler echocardiogram and an evaluation of endothelial function by peripheral artery tonometry. The study concluded that endothelial dysfunction and arterial stiffness are common in people with early lupus who have no known risk factors for CVD. A more extensive study is needed to compare the characteristics associated with the abnormalities [19].
Several soluble adhesion molecules, such as those released following EC injury and considered to be indicators of endothelial dysfunction, are upregulated in SLE and linked to higher coronary calcium scores [20]. Rho et al. conducted a study that evaluated the levels of cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-1 alpha (IL-1α), VEGF, and inflammatory enzymes such as myeloperoxidase (MPO), matrix metalloproteinases (MMP)-9, acute-phase reactants such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and serum amyloid A (SAA), and adhesion molecules like vascular cell adhesion molecule (VCAM), intercellular adhesion molecule (ICAM), and E-selectin in 109 patients with SLE and 78 healthy controls. In this study, in individuals with SLE, the connection between inflammatory indicators and calcified plaque revealed by electron beam CT was investigated. The study concluded that in SLE, concentrations of adhesion molecules and TNF-α are linked to CAD regardless of the Framingham Risk Score [20]. According to Nhek et al., SLE sera can activate platelets, leading to EC activation and proinflammatory mediators in an IL-1-dependent way [21]. In SLE, there is a significant imbalance between EC damage and healing [15]. As a result, people with SLE have defective ECs and poor repair of damaged ECs, encouraging vascular plaque formation.
Dysregulation of the Innate Immune Response
Due to the immensely essential function of type I IFNs in SLE, these cytokines have been studied extensively as a contributing factor to the development of lupus-related CVD. Endothelial function is impaired in lupus patients with a strong type I IFN signature (Table 1) [16]. Lee et al. conducted a study in 70 SLE patients and 31 healthy controls. EPCs in the blood of SLE patients and healthy controls were counted using a colony-forming assay. Real-time polymerase chain reaction (PCR) assessment of the IFN-I-inducible gene MX1 was used to determine serum IFN-I levels. Peripheral artery plethysmography was used to assess endothelial function. When compared to controls, SLE patients had significantly lower levels of EPC colony-forming units [median: 5.7/ml peripheral blood (interquartile range: 1.9-12.8) versus 28.5/ml peripheral blood (14.7-47.3); p=0.0001], and the loss of EPCs was more pronounced in patients with elevated concentrations of IFN-I. These findings support the unique idea that endothelial dysfunction and higher CV risk are connected to EPC depletion produced by elevated IFN-I in SLE [16]. Increased serum IFN activity has been linked to a reduction in endothelial function, although serum levels of high-sensitivity CRP, adhesion molecules, and lupus disease activity have not. This shows that elevated type I IFN signaling may play a key role in increasing CV risk in SLE (Table 1) [22]. Tydén et al. recently showed that in SLE, activation of the type I IFN system could impair endothelial function, even in individuals with mild disease activity. The loss of nitric oxide production and decreased endothelial nitric oxide synthase (eNOS) activity are crucial in developing endothelial dysfunction [23]. According to a study published recently by King et al., type I IFNs have been shown to play a pathogenic function in myocardial infarction (MI) [24]. Ischemic cell death and ingestion of cell debris by macrophages in the heart drive a catastrophic response to MI by activating interferon regulatory factor 3 (IRF3) and type I IFN production via the cyclic GMP-AMP synthase-stimulator of IFN genes [24]. Furthermore, following an MI, animals who were given a type I IFN receptor-neutralizing antibody had their IFN response abated, which improved left ventricular dysfunction and survival [24].
Table 1. Summary of studies about the pathogenesis of CVD in SLE.
SLE: systemic lupus erythematosus; CVD: cardiovascular disease; PCR: polymerase chain reaction; IFN: interferons; FMD: flow-mediated dilatation; CIMT: carotid intima-media thickness; EPC: endothelial progenitor cells: CVRFs: cardiovascular risk factors
| Reference | Design | Cases | Controls | Population | Variables | Findings |
| Somers et al. (2012) [22] | Cohort study | 95 SLE patients | 38 | <55 years, from Michigan Lupus Cohort | Real-time PCR was used to measure serum type I IFN activity. The impact of type I interferons on FMD, CIMT, and cardiac calcification was studied. Patients with CVD were not allowed to participate in the study | Type I IFNs are independently associated with atherosclerosis development in lupus patients without a history of overt CVD |
| Baragetti et al. (2017) [29] | Prospective study (2012-2017) | 40 SLE patients | 50 healthy age-matched controls | Mean age: 42 ± 9 years | Clinical history and details on the principal CVRFs were obtained at baseline and after five years. Carotid Doppler ultrasonography was employed to quantify the atherosclerotic burden at baseline and follow-up. The association between basal circulating T cell subsets and atherosclerosis development was evaluated | During the 5-year follow-up, 32% of SLE patients developed carotid atherosclerosis compared to 4% of controls. Increased levels of CD4+CCR5+ T cells were independently associated with the development of carotid atherosclerosis in SLE patients |
| Lee et al. (2007) [16] | 70 SLE patients | 31 healthy controls | University of Florida Center for Autoimmune Diseases; 27-45 years, females | EPCs in the blood of SLE patients and healthy controls were counted using a colony-forming assay. A real-time PCR quantified serum IFN-I levels. Endothelial function was determined by peripheral arterial plethysmography | When compared to controls, SLE patients had significantly fewer EPC colony-forming units. The loss of EPCs was more pronounced in patients with high IFN-I levels. In patients with SLE, high IFN-I levels were linked to poor endothelial function |
Low-density granulocytes (LDGs), a subset of proinflammatory neutrophils seen in lupus patients, have been postulated to play pathogenic roles in lupus CVD through a variety of mechanisms, including their increased tendency to form neutrophil extracellular traps (NETs) [25]. Carlucci et al. found that SLE patients with overall mild-moderate disease activity have a substantial increase in aortic wall inflammation compared to healthy controls, as measured by 18F-fluorodeoxyglucose (18F-FDG) PET/CT scan. Noncalcified coronary plaque burden (NCB) and endothelial dysfunction were significantly higher in this SLE population [26]. The amount of LDGs was independently correlated with NCB when examining the correlations of lupus-related variables to these vascular abnormalities. Furthermore, an LDG gene profile established by RNA sequencing was linked to high vascular inflammation and high NCB in SLE [26]. An abnormality in neutrophil biology has been linked to an increased risk of early vascular disease in SLE.
Dysregulation of Adaptive Immune Response
In SLE patients, abnormal T cell subsets are a pivotal contributor to endothelial dysfunction and CVD. Tregs are anti-atherosclerotic T cells that suppress atherogenic T cell subsets and inflammation. In SLE, a Treg/Th17 imbalance is frequent, leading to atherosclerosis [27]. Plasmacytoid dendritic cells cause CD4+ CXC chemokine receptor 3 (CXCR3) cells to expand and migrate from the circulation into the artery wall in individuals with SLE, where they may have proatherogenic activities [28]. Baragetti et al. published a five-year prospective study in 2017, which showed that increased numbers of CD4+CC chemokine receptor (CCR)5+ T cells were linked to the development of carotid atherosclerosis in SLE patients (Table 1) [29]. According to these data, increased CD4+CCR5+ T cells in SLE may contribute to atherogenesis [29]. Smith et al. published a study in 2016, which proved that in SLE patients with asymptomatic atherosclerotic plaques, invariant natural killer T (iNKT) cells may produce an atheroprotective effect [30]. Healthy iNKT cells differentiated macrophages toward an anti-inflammatory M2 phenotype in the presence of healthy monocytes and serum from SLE patients with asymptomatic plaque. In contrast, SLE patients with clinical CVD had unresponsive iNKT cells and increased proinflammatory monocytes. Furthermore, the authors found that the anti-inflammatory iNKT cell phenotype was linked to dyslipidemia and was fueled by changes in monocyte phospholipid expression and CD1d-mediated iNKT-monocyte cross-talk [30].
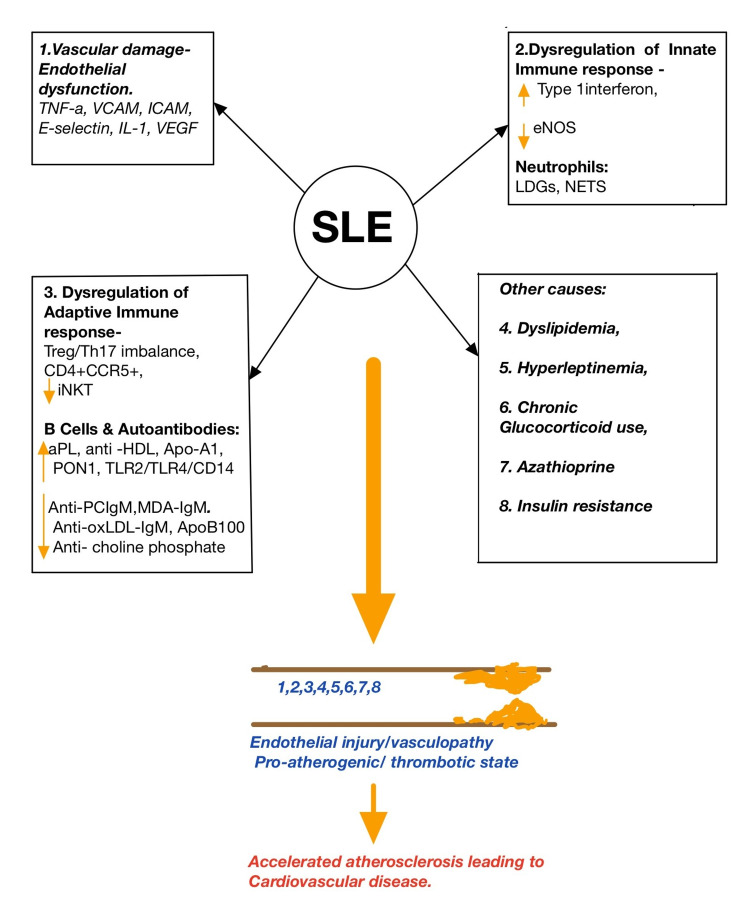
B cells primarily influence atherosclerosis by producing autoantibodies. B1 cells create harmful IgG antibodies, whereas B2 cells produce beneficial natural IgM and IgA antibodies. Overactive B cells' tendency to produce pathogenic IgG antibodies is also a risk factor for lupus-related atherosclerosis [31]. In particular, antiphospholipid antibodies (aPL) are independent indicators of atherosclerotic plaque development in SLE patients [32,33]. Atherosclerosis develops in SLE individuals with anti-high-density lipoprotein (anti-HDL)-IgG antibodies, allowing low-density lipoprotein (LDL) to enter ECs [34]. According to Kurien et al., anti-oxLDL antibodies are increased by SLE RNP and anti-Ro/LaRNP antibodies [34]. Anti-HDL antibody, anti-ApoA1 antibody, and anti-paraoxonase 1 (PON1) antibody are likely to share a common atherogenic pathway in that they disrupt the balance of PON1/MPO, which increases oxidative lipid modification and interferes with HDL's anti-inflammatory activity, all of which contribute to atherosclerosis [35-37]. Furthermore, anti-ApoA1-IgG induces atherosclerosis in a TLR2/TLR4/CD14-dependent manner through two pathways: it guides the expression of inflammatory factors by activating transcriptional nuclear factor-kappa B (NF-κB); it provides an alternative (or concomitant) signal to phosphoinositide 3-kinase (PI3K) in an Src-dependent pathway, activating L-type Ca2+ channels and potassium/calcium exchangers, resulting in the depolarization of myocardial plasma membrane [38]. Anti-oxLDL-IgM, anti-ApoB100 antibodies, anti-choline phosphate (PC) antibodies, and anti-malondialdehyde (MDA) antibodies are all possible protective autoantibodies in SLE patients. The first three have a synergistic impact in that they lower oxLDL levels, oxLDL uptake, and foam cell formation [39-41]. Anti-PC-IgM boosts Tregs, lowers IL-17 and TNF-alpha in SLE and atherosclerosis, and renders dendritic cells (DCs) immature [42]. Anti-PC-IgM and anti-MDA-IgM used together have a double preventative effect on atherosclerosis [43]. On the other hand, SLE patients were shown to have a low level of protective autoantibodies [41]. By increasing pathogenic autoantibodies and blocking possible preventive autoantibodies, SLE raises CVD risk. Figure 1 presents a summary of endothelial damage in SLE leading to CVD.
Figure 1. Summary of endothelial damage in SLE leading to CVD.
SLE: systemic lupus erythematosus; CVD: cardiovascular disease; TNF-α: tumor necrosis factor-alpha; VCAM: vascular cell adhesion molecule; ICAM: intercellular adhesion molecule; IL-1: interleukin-1; VEGF: vascular endothelial growth factor; eNOS: endothelial nitric oxide synthase; LDGs: low-density granulocytes; NETs: neutrophil extracellular traps; Treg: regulatory T cells; Th-17: T helper 17 cells; CD4+: cluster of differentiation 4+; CCR5+: cysteine-cysteine chemokine receptor 5+; iNKT: invariant natural killer T; aPL: antiphospholipid antibodies; anti-HDL: anti-high-density lipoprotein antibodies; Apo-A1: apolipoprotein A-I; PON1: paraoxonase 1; TLR: toll-like receptors; anti-PCIgM: IgM antibodies against phosphorylcholine; MDA: malondialdehyde; anti-oxLDL: anti-oxidized LDL; Apo-B100: apolipoprotein B-100
Other Causes of CVD in SLE
Dyslipidemia is a predisposing factor for atherosclerosis and a well-established risk factor for CVD. Dyslipidemia is defined as an increase in total cholesterol, LDL, triglycerides, and apolipoprotein B and a decrease in HDL in SLE [44]. Patients with SLE have higher amounts of oxidized and malfunctioning HDL and reduced cholesterol efflux ability and atherosclerosis [45-47]. HDL protects the arteries by boosting the activation of transcription factor 3 (ATF3), which leads to a reduction in toll-like receptor (TLR)-induced inflammatory reactions [48]. In contrast, according to recent research, oxidized lupus HDL enhances proinflammatory responses in macrophages. Indeed, in patients with SLE, HDL stimulates NF-κB, enhances the generation of inflammatory cytokines, and fails to prevent TLR-induced inflammation [49]. The inability of lupus HDL to suppress inflammatory responses is due to a reduced capacity to stimulate ATF3 production and nuclear translocation, which is triggered by oxidized LDL receptor signaling. Indeed, systemic administration of an HDL mimic to lupus-prone animals resulted in considerable ATF3 activation and reductions in proinflammatory cytokine levels, indicating therapeutic potential [49]. In recent studies, impaired cholesterol export capability was strongly linked with vascular inflammation and NCB in multivariate analysis, suggesting that improving HDL function may have considerable cardioprotective implications in SLE [26].
Insulin resistance has been linked to CVD in SLE patients. Recent research has measured insulin sensitivity in SLE patients with a meal-tolerance test. Despite adequate glucose tolerance, intact ß-cell function, and skeletal muscle glucose transporter 4 (GLUT4) translocation, SLE patients had a bi-hormone metabolic anomaly defined by enhanced insulin resistance and hyperglucagonemia. As a result, the authors believe that improving insulin sensitivity may involve more than just addressing insulin resistance [50].
Hyperleptinemia is linked to the progression of atherosclerosis by promoting inflammation through the release of proinflammatory cytokines such as IL-6, TNF-α, IL-17, and other cytokines [51]. In a cross-sectional study published in 2017, Versini et al. stated that there is a lack of data on the link between obesity and SLE. Although studies disagree on the connection between leptin and SLE, a rise in serum leptin levels may enhance systemic inflammation in SLE patients [52]. In contrast, investigations conducted in Egypt in 2018 found that SLE patients have significantly more amount of serum leptin [53].
In general, examining drugs and CVD risk is complex since there might be much confounding by indication. Patients with more severe SLE are more likely to be given glucocorticoids and other immunosuppressive drugs, whereas those with lesser illness may be given hydroxychloroquine alone [54]. Although rising glucocorticoid dosage is closely connected with more significant disease activity, the association between glucocorticoid usage and CVD risk among SLE patients is challenging to understand completely. While glucocorticoids reduce systemic inflammation, which may minimize atherogenesis, their usage is linked to an increase in several traditional risk variables, including total cholesterol, blood glucose, BMI, and systolic blood pressure [54]. More prolonged glucocorticoid usage was found to be independently related to incident CVD events, albeit the relative risk was not assessed in that study [55]. Nikpour et al. also reported that using glucocorticoids doubly increased the risk of MI, angina, and sudden cardiac death [RR: 2.01, 95% confidence interval (CI): 1.19-3.41] [56]. However, these effects have not been repeated in other studies. The difficulty of distinguishing disease activity from glucocorticoid usage, as well as the numerous ways in which glucocorticoid use has been assessed, such as duration of use and maximum, current, or total dosage, may contribute to the disparity among studies [56-59]. Two studies have found that azathioprine usage is an independent risk factor for CVD among SLE patients. One study published by Burgos et al. in 2009 predicted a three-fold higher risk of PAD [60]. And another study published by Haque et al. indicated a three-fold increased risk of MI and angina [61]. Table 1 presents a summary of studies about the pathogenesis of CVD in SLE.
Clinical implications
Evidence of an Increased CVD Risk in SLE Patients
Compared to healthy persons of the same age and gender, patients with SLE have a higher chance of having ischemic heart disease or stroke, referred to as CVD [62]. A population data-based study published by Aviña et al. in 2017, including all British Columbia and other Canadian residents, studied all patients with incident SLE with a healthy group from the general population as a matched cohort. Among 4,863 individuals with SLE (86% female, mean age: 48.9 years), the incidence rates (IRs) of MI, stroke, and CVD were 6.4, 4.4, and 9.9 events per 1,000 person-years, respectively, vs. 2.8, 2.3, and 4.7 events per 1,000 person-years in the comparison cohort. Compared with non-SLE individuals, the fully adjusted multivariable hazard ratios (HRs) among SLE patients were 2.61 (95% CI: 2.12-3.20) for MI, 2.14 (95% CI: 1.64-2.79) for stroke, and 2.28 (95% CI: 1.90-2.73) for CVD. The age, sex, and entry time-matched HRs for MI, stroke, and CVD were highest during the first year after SLE diagnosis: 5.63 (95% CI: 4.02-7.87), 6.47 (95% CI: 4.42-9.47), and 6.28 (95% CI: 4.83-8.17), respectively. Hence, CV events are more common in patients with SLE, especially in the first year after diagnosis. In this patient population, increased surveillance in tracking these potentially fatal outcomes and their modifiable risk factors is necessary (Table 2) [62].
Table 2. Summary of studies about the evidence for CVD risk in SLE.
SLE: systemic lupus erythematosus; CVD: cardiovascular disease; MI: myocardial infarction; IR: incidence rate
| Reference | Design | Cases | Controls | Population | Variables | Findings |
| Bernatsky et al. (2006) [63] | Cohort study (1958-2001) | 9,547 cases with SLE | -- | >16 years from 7 countries (Canada, US, England, Scotland, Iceland, Sweden, South Korea) | Examined the mortality in SLE patients | 1,255 fatalities, 313 of which were caused by CVD |
| Aviña-Zubieta et al. (2017) [62] | Cohort study (1996-2010) | 4,863 cases with SLE (86% females) | 10 healthy matched controls per case | Mean age: 48.9 years, from British Columbia and other parts of Canada | IRs of MI, stroke, and CVD were observed and compared | IRs of MI, stroke, and CVD were 6.4, 4.4, and 9.9 events per 1,000 person-years, vs. 2.8, 2.3, and 4.7 events per 1,000 person-years in the comparison cohort |
| Manzi et al. (1997) [64] | Cohort study (1980-1993) | 498 women with SLE | 2,208 women of similar age participating in the Framingham Offspring Study | University of Pittsburgh Medical Center | Risk factors associated with CV events occurring in SLE patients were determined | 33 first events (11 MI, 10 angina pectoris, and 12 both angina pectoris and MI). Women with lupus in the 35-44-year age group were over 50 times more likely to have an MI vs. the control group |
A major international cohort study published in 2006 by Bernatsky et al. involving 9,547 individuals with SLE found 1,255 fatalities, 313 of them caused by CVD (Table 2) [63]. SLE raises the risk of CVD by 50 times in women between the ages of 35 and 44 years. However, the increased risk is more negligible at other ages (7-10 times) (Table 2) [64]. A recent meta-analysis discovered that the prevalence of asymptomatic atherosclerotic disease was higher in SLE patients than controls, with higher carotid intima-media thickness (CIMT) scores and a 2.5-fold increase in the prevalence of carotid plaques, both surrogate markers of subclinical atherosclerosis [65]. Aortic stiffness, a key predictor of early vascular aging measured by aortic pulse wave velocity (aPWV), was shown to be affected to a similar extent in SLE as it is in hypertensive patients, indicating that SLE has a similar impact on early vascular aging [66]. Increased PWV and proximal aorta arterial stiffness were seen in children and adolescents with active SLE, implying that inflammation could factor in the increased arterial stiffness seen in young patients [67]. According to epidemiologic research to date, traditional CVD risk variables, such as hyperlipidemia, cigarette smoking, advanced age, hypertension, male sex, and high CRP, appear to be related to increased CVD risk among SLE patients. However, since previous studies did not look at all of these risk variables in the same populations simultaneously, comparisons of the relative risk associated with each are impossible [68]. According to one study, patients still had a 7-10-fold more significant risk of CVD after controlling for Framingham risk factors [69].
Cardiovascular Disease Screening and Assessment in Systemic Lupus Erythematosus
Recently, Mavrogeni et al. showed that CV MRI could detect silent cardiac illness overlooked by echocardiography. Indeed, 27.5% of SLE patients with regular echocardiograms but silent/past myocarditis, MI, or vasculitis had abnormalities discovered using CV MRI [70]. According to a recent study published by Seguro et al., SLE is linked to increased visceral adipose tissue and altered adiposity distribution [71]. Aortic perivascular adipose tissue density was also linked to aortic calcification in women with SLE, implying that adipose tissue malfunction may play an important role in CVD [72].
In the early stages of acute MI, cardiac troponin T (cTnT) has been postulated to measure myocyte necrosis and damage [73]. In the general population with a low CVD risk, high-sensitivity cTnT has shown promise in predicting CVD [74]. Divard et al. have observed that levels of high-sensitivity cTnT were independently related with subclinical atherosclerosis in asymptomatic SLE patients regarded as low risk for CVD based on conventional risk factors in a recent cross-sectional controlled study [75].
LDGs, as previously stated, have a pathogenic role in lupus CVD [26]. In an uncorrected linear regression analysis, LDG levels were substantially linked with NCB severity and reduced cholesterol efflux capability in SLE. Furthermore, the authors discovered that a neutrophil gene signature was related to vascular pathology in SLE patients. Indeed, some of the genes found to be elevated in high-NCB SLE groups were also shown to be increased in LDGs when compared to normal-density neutrophils. These data indicate that LDG levels in SLE patients might be used to predict CVD risk [26].
Reduced overall antioxidant capacity in SLE patients without typical CV risk factors is linked to subclinical coronary microvascular dysfunction [76]. PON1, an antioxidant enzyme that binds to HDL and protects LDL from oxidation, is lower in those with SLE, and it is linked to vascular damage [77,78]. Anti-PON1 and anti-HDL antibodies were studied as biomarkers for lupus CVD in recent research. It was discovered that anti-HDL antibodies were linked to a greater risk of CVD, while anti-PON1 antibodies were coupled to CIMT in SLE patients [36]. As a result, such antibodies might be used as early indicators for SLE-related premature atherosclerosis [36]. According to a recent study, biomarkers reflecting receptor-activated apoptosis and tissue degradation, such as Fas, TNF receptor 1, TNF-related apoptosis-inducing ligand receptor 2, MMP-1, and MMP-7, are significantly higher in SLE patients with CVD than those without CVD [79].
Management of CVD in SLE
SLE is a distinct CVD risk factor caused by traditional and illness-related risk factors, such as chronic condition activity, lupus nephritis (LN), aPL, and glucocorticoid usage [80]. The use of statins should be based on other conventional risk factors and cholesterol levels. Although the actual risk in people with SLE is overestimated, calculating the 10-year CVD risk using, for example, the systematic coronary risk evaluation (SCORE) is recommended [81]. Blood pressure should be kept at 140/90 mmHg; thus, this should be the general target for SLE patients [82]. Patients with blood pressure higher than 130/80 mmHg who have clinical CVD or a highly predicted CVD risk (>10%) should be treated to reach a goal of <130/80 mmHg [83,84]. SLE was independently associated with a high indicator of in-hospital mortality after percutaneous coronary angioplasty (PCI) and was linked to overall mortality, repeat revascularization, and major adverse CV events in a study based on Taiwan's National Health Insurance Research Database. The study showed the inherent risks of SLE in PCI patients and emphasized the need for better treatment and secondary preventive initiatives for these high-risk individuals [85].
Specific Treatment of SLE
Antimalarial's impact on CV risk in SLE has been analyzed in five studies: one meta-analysis, one cohort study, two case-control studies, and one cross-sectional study. In a meta-analysis, Ruiz-Irastorza et al. showed that hydroxychloroquine is effective in the primary prevention of thrombotic events in patients with SLE and cardiovascular risk factors (CVRFs) [86]. Antimalarials were also linked to a decreased incidence of thrombotic events in individual studies [87-89]. Penn et al. reviewed the medical records of 149 non-diabetic SLE patients, half of whom had received antimalarial therapy [90]. Over 16 years, the researchers measured glucose levels (mg/dL), homeostasis model assessment-estimated insulin resistance (HOMA-IR), and LDL. Compared to the control group, the antimalarial group had reduced fasting glucose levels (87.1 mg/dL against 91.5 mg/dL) and insulin resistance (HOMA-IR: 194 vs. 179). LDL levels were also lower in the treated group compared to the untreated group (LDL of 102 mg/dL vs. 118 mg/dL in the treated and untreated groups, respectively). Ultimately, hydroxychloroquine appears to be beneficial for the primary prevention of thrombotic events in patients with SLE and CVRFs, with a degree of scientific evidence and a grade of recommendation of SIGN 1+ B. Also, with a level of scientific evidence 1 for hydroxychloroquine, it appears to have a favorable effect on glucose levels, insulin resistance, and LDL levels. Still, the degree of SIGN recommendation could not be determined [90].
Limitations
This study does not address the traditional risk factors like smoking, obesity, age, hypertension, diabetes mellitus, menopause, and family history of CAD independently leading to CVD, as we focus specifically on SLE leading to CVD.
Conclusions
Based on the studies discussed in this article, SLE is linked to an elevated risk of accelerated atherosclerosis and CV events, including CAD, stroke, and vascular disease. CV events can occur both early and late in the course of the illness, with younger patients being substantially more at risk than their age-matched peers. In summary, the clinical implication of this article is to establish a strong link between several SLE-specific processes, including impaired immunological regulation, impaired EC function, impaired vascular repair, hyperleptinemia, and traditional risk factors that contribute to the development of early atherosclerosis in the disease. We believe this article can serve as a tool to overcome these challenges by providing a unique approach to the connection between the two entities, i.e., SLE and CVD, by highlighting the pathogenesis, clinical evidence, screening, and management options. We explicitly delineated the challenges faced by physicians while tackling the various association between SLE and CVD by enumerating the different mechanisms involved, specific treatment-related issues, and management of the disease altogether in this article. Despite considerable advances in understanding the mechanisms of such intricate interactions, more research is needed to evaluate the therapeutic value of treating those components in preventing SLE-related CVD. Also, in spite of the advances in treatment, standardized mortality rates in SLE remain three times higher than in the general population. The risk of mortality is significantly increased due to CVD, which is one of the main factors we covered in this article, others being renal involvement and infections. Continued efforts to perform research on alternative processes that contribute to accelerated CVD in SLE and more studies on screening modalities are required to identify better molecular possibilities, facilitate early diagnosis and primary prevention for therapeutic targeting, as well as an emphasis on secondary preventive initiatives for these high-risk individuals, in the long run, to improve the CV outcomes.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Definitions of and contributions to cardiovascular disease in systemic lupus erythematosus. Gustafsson JT, Svenungsson E. Autoimmunity. 2014;47:67–76. doi: 10.3109/08916934.2013.856005. [DOI] [PubMed] [Google Scholar]
- 2.The history of lupus throughout the ages [Epub ahead of print] Felten R, Lipsker D, Sibilia J, Chasset F, Arnaud L. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.04.150. [DOI] [PubMed] [Google Scholar]
- 3.The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. Ann Rheum Dis. 2016;75:136–141. doi: 10.1136/annrheumdis-2014-206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology of systemic lupus erythematosus. Petri M. Best Pract Res Clin Rheumatol. 2002;16:847–858. doi: 10.1053/berh.2002.0259. [DOI] [PubMed] [Google Scholar]
- 5.The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth. Johnson AE, Gordon C, Palmer RG, Bacon PA. Arthritis Rheum. 1995;38:551–558. doi: 10.1002/art.1780380415. [DOI] [PubMed] [Google Scholar]
- 6.Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Danchenko N, Satia JA, Anthony MS. Lupus. 2006;15:308–318. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 7.Frequency of lupus in people of African origin. Symmons DP. Lupus. 1995;4:176–178. doi: 10.1177/096120339500400303. [DOI] [PubMed] [Google Scholar]
- 8.Understanding the epidemiology and progression of systemic lupus erythematosus. Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Semin Arthritis Rheum. 2010;39:257–268. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Understanding the role of environmental factors in the development of systemic lupus erythematosus. Parks CG, de Souza Espindola Santos A, Barbhaiya M, Costenbader KH. Best Pract Res Clin Rheumatol. 2017;31:306–320. doi: 10.1016/j.berh.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Systemic lupus erythematosus: diagnosis and clinical management. Fava A, Petri M. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessons learned from twins in autoimmune and chronic inflammatory diseases. Generali E, Ceribelli A, Stazi MA, Selmi C. J Autoimmun. 2017;83:51–61. doi: 10.1016/j.jaut.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Immunogenetics of systemic lupus erythematosus: a comprehensive review. Ghodke-Puranik Y, Niewold TB. J Autoimmun. 2015;64:125–136. doi: 10.1016/j.jaut.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justiz Vaillant AA, Goyal A, Bansal P, Varacallo M. Treasure Island, FL: StatPearls; 2021. Systemic Lupus Erythematosus. [PubMed] [Google Scholar]
- 14.Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Denny MF, Thacker S, Mehta H, et al. Blood. 2007;110:2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Rajagopalan S, Somers EC, Brook RD, et al. Blood. 2004;103:3677–3683. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 16.Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Lee PY, Li Y, Richards HB, et al. Arthritis Rheum. 2007;56:3759–3769. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- 17.Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Moonen JR, de Leeuw K, van Seijen XJ, Kallenberg CG, van Luyn MJ, Bijl M, Harmsen MC. Arthritis Res Ther. 2007;9:0. doi: 10.1186/ar2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Westerweel PE, Luijten RK, Hoefer IE, Koomans HA, Derksen RH, Verhaar MC. Ann Rheum Dis. 2007;66:865–870. doi: 10.1136/ard.2006.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endothelial dysfunction in early systemic lupus erythematosus patients and controls without previous cardiovascular events. Taraborelli M, Sciatti E, Bonadei I, et al. Arthritis Care Res (Hoboken) 2018;70:1277–1283. doi: 10.1002/acr.23495. [DOI] [PubMed] [Google Scholar]
- 20.Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. Rho YH, Chung CP, Oeser A, et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2574747/ J Rheumatol. 2008;35:1789–1794. [PMC free article] [PubMed] [Google Scholar]
- 21.Activated platelets induce endothelial cell activation via an interleukin-1β pathway in systemic lupus erythematosus. Nhek S, Clancy R, Lee KA, et al. Arterioscler Thromb Vasc Biol. 2017;37:707–716. doi: 10.1161/ATVBAHA.116.308126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. Somers EC, Zhao W, Lewis EE, et al. PLoS One. 2012;7:0. doi: 10.1371/journal.pone.0037000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endothelial dysfunction is associated with activation of the type I interferon system and platelets in patients with systemic lupus erythematosus. Tydén H, Lood C, Gullstrand B, et al. RMD Open. 2017;3:0. doi: 10.1136/rmdopen-2017-000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IRF3 and type I interferons fuel a fatal response to myocardial infarction. King KR, Aguirre AD, Ye YX, et al. Nat Med. 2017;23:1481–1487. doi: 10.1038/nm.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupus neutrophils: 'NET' gain in understanding lupus pathogenesis. Knight JS, Kaplan MJ. Curr Opin Rheumatol. 2012;24:441–450. doi: 10.1097/BOR.0b013e3283546703. [DOI] [PubMed] [Google Scholar]
- 26.Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. Carlucci PM, Purmalek MM, Dey AK, et al. JCI Insight. 2018;3:276. doi: 10.1172/jci.insight.99276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dysregulated CD4+ T cells from SLE-susceptible mice are sufficient to accelerate atherosclerosis in LDLr-/- mice. Wilhelm AJ, Rhoads JP, Wade NS, Major AS. Ann Rheum Dis. 2015;74:778–785. doi: 10.1136/annrheumdis-2013-203759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CD4+CXCR3+ T cells and plasmacytoid dendritic cells drive accelerated atherosclerosis associated with systemic lupus erythematosus. Clement M, Charles N, Escoubet B, et al. J Autoimmun. 2015;63:59–67. doi: 10.1016/j.jaut.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Disease trends over time and CD4+CCR5+ T-cells expansion predict carotid atherosclerosis development in patients with systemic lupus erythematosus. Baragetti A, Ramirez GA, Magnoni M, et al. Nutr Metab Cardiovasc Dis. 2018;28:53–63. doi: 10.1016/j.numecd.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Cross-talk between iNKT cells and monocytes triggers an atheroprotective immune response in SLE patients with asymptomatic plaque. Smith E, Croca S, Waddington KE, et al. Sci Immunol. 2016;1:4081. doi: 10.1126/sciimmunol.aah4081. [DOI] [PubMed] [Google Scholar]
- 31.B-cell activating factor and related genetic variants in lupus related atherosclerosis. Theodorou E, Nezos A, Antypa E, et al. J Autoimmun. 2018;92:87–92. doi: 10.1016/j.jaut.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Progression of subclinical atherosclerosis in systemic lupus erythematosus versus rheumatoid arthritis: the impact of low disease activity. Kravvariti E, Konstantonis G, Sfikakis PP, Tektonidou MG. Rheumatology (Oxford) 2018;57:2158–2166. doi: 10.1093/rheumatology/key233. [DOI] [PubMed] [Google Scholar]
- 33.Gene profiling reveals specific molecular pathways in the pathogenesis of atherosclerosis and cardiovascular disease in antiphospholipid syndrome, systemic lupus erythematosus and antiphospholipid syndrome with lupus. Perez-Sanchez C, Barbarroja N, Messineo S, et al. Ann Rheum Dis. 2015;74:1441–1449. doi: 10.1136/annrheumdis-2013-204600. [DOI] [PubMed] [Google Scholar]
- 34.Anti-Ro and concomitant anti-La autoantibodies strongly associated with anti-oxLDL or anti-phospholipid antibody in systemic lupus erythematosus. Kurien BT, Fesmire J, Anderson CJ, Scofield RH. J Clin Rheumatol. 2016;22:418–425. doi: 10.1097/RHU.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 35.Reduced paraoxonase1 activity is a risk for atherosclerosis in patients with systemic lupus erythematosus. Kiss E, Seres I, Tarr T, Kocsis Z, Szegedi G, Paragh G. Ann N Y Acad Sci. 2007;1108:83–91. doi: 10.1196/annals.1422.009. [DOI] [PubMed] [Google Scholar]
- 36.Serum levels of anti-PON1 and anti-HDL antibodies as potential biomarkers of premature atherosclerosis in systemic lupus erythematosus. López P, Rodríguez-Carrio J, Martínez-Zapico A, et al. Thromb Haemost. 2017;117:2194–2206. doi: 10.1160/TH17-03-0221. [DOI] [PubMed] [Google Scholar]
- 37.Antibodies toward high-density lipoprotein components inhibit paraoxonase activity in patients with systemic lupus erythematosus. Batuca JR, Ames PR, Isenberg DA, Alves JD. Ann N Y Acad Sci. 2007;1108:137–146. doi: 10.1196/annals.1422.016. [DOI] [PubMed] [Google Scholar]
- 38.ApoA1 and ApoA1-specific self-antibodies in cardiovascular disease. Chistiakov DA, Orekhov AN, Bobryshev YV. Lab Invest. 2016;96:708–718. doi: 10.1038/labinvest.2016.56. [DOI] [PubMed] [Google Scholar]
- 39.Natural antibodies against phosphorylcholine in cardiovascular disease. de Faire U, Frostegård J. Ann N Y Acad Sci. 2009;1173:292–300. doi: 10.1111/j.1749-6632.2009.04748.x. [DOI] [PubMed] [Google Scholar]
- 40.Phosphorylcholine-targeting immunization reduces atherosclerosis. Caligiuri G, Khallou-Laschet J, Vandaele M, et al. J Am Coll Cardiol. 2007;50:540–546. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 41.Decreased levels of autoantibodies against apolipoprotein B-100 antigens are associated with cardiovascular disease in systemic lupus erythematosus. Svenungsson E, Engelbertsen D, Wigren M, et al. Clin Exp Immunol. 2015;181:417–426. doi: 10.1111/cei.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.IgM antibodies against phosphorylcholine promote polarization of T regulatory cells from patients with atherosclerotic plaques, systemic lupus erythematosus and healthy donors. Sun J, Lundström SL, Zhang B, et al. Atherosclerosis. 2018;268:36–48. doi: 10.1016/j.atherosclerosis.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 43.IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: Regulation and underlying mechanisms. Rahman M, Sing S, Golabkesh Z, et al. Clin Immunol. 2016;166:27–37. doi: 10.1016/j.clim.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Dyslipidemia in systemic lupus erythematosus. Szabó MZ, Szodoray P, Kiss E. Immunol Res. 2017;65:543–550. doi: 10.1007/s12026-016-8892-9. [DOI] [PubMed] [Google Scholar]
- 45.Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Smith CK, Vivekanandan-Giri A, Tang C, et al. Arthritis Rheumatol. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ronda N, Favari E, Borghi MO, et al. Ann Rheum Dis. 2014;73:609–615. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 47.Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. McMahon M, Grossman J, FitzGerald J, et al. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 48.High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. De Nardo D, Labzin LI, Kono H, et al. Nat Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupus high-density lipoprotein induces proinflammatory responses in macrophages by binding lectin-like oxidised low-density lipoprotein receptor 1 and failing to promote activating transcription factor 3 activity. Smith CK, Seto NL, Vivekanandan-Giri A, et al. Ann Rheum Dis. 2017;76:602–611. doi: 10.1136/annrheumdis-2016-209683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Increased insulin resistance and glucagon levels in mild/inactive systemic lupus erythematosus patients despite normal glucose tolerance. Miyake CN, Gualano B, Dantas WS, et al. Arthritis Care Res (Hoboken) 2018;70:114–124. doi: 10.1002/acr.23237. [DOI] [PubMed] [Google Scholar]
- 51.Effect of leptin on chronic inflammatory disorders: insights to therapeutic target to prevent further cardiovascular complication. Dessie G, Ayelign B, Akalu Y, Shibabaw T, Molla MD. Diabetes Metab Syndr Obes. 2021;14:3307–3322. doi: 10.2147/DMSO.S321311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smoking and obesity in systemic lupus erythematosus: a cross-sectional study. Versini M, Tiosano S, Comaneshter D, Shoenfeld Y, Cohen AD, Amital H. Eur J Clin Invest. 2017;47:422–427. doi: 10.1111/eci.12757. [DOI] [PubMed] [Google Scholar]
- 53.Serum leptin in systemic lupus erythematosus patients: its correlation with disease activity and some disease parameters. Mohammed SF, Abdalla MA, Ismaeil WM, Sheta MM. Egypt Rheumatol. 2018;40:23–27. [Google Scholar]
- 54.Recent corticosteroid use and recent disease activity: independent determinants of coronary heart disease risk factors in systemic lupus erythematosus? Karp I, Abrahamowicz M, Fortin PR, Pilote L, Neville C, Pineau CA, Esdaile JM. Arthritis Rheum. 2008;59:169–175. doi: 10.1002/art.23352. [DOI] [PubMed] [Google Scholar]
- 55.Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Am J Med. 1992;93:513–519. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 56.Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective proof-of-concept cohort study. Nikpour M, Urowitz MB, Ibanez D, Harvey PJ, Gladman DD. Arthritis Res Ther. 2011;13:0. doi: 10.1186/ar3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Urowitz MB, Gladman D, Ibañez D, et al. Arthritis Care Res (Hoboken) 2010;62:881–887. doi: 10.1002/acr.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clinical manifestations and coronary artery disease risk factors at diagnosis of systemic lupus erythematosus: data from an international inception cohort. Urowitz MB, Gladman D, Ibañez D, et al. Lupus. 2007;16:731–735. doi: 10.1177/0961203307081113. [DOI] [PubMed] [Google Scholar]
- 59.Predictors of cardiovascular damage in patients with systemic lupus erythematosus: data from LUMINA (LXVIII), a multiethnic US cohort. Pons-Estel GJ, González LA, Zhang J, Burgos PI, Reveille JD, Vilá LM, Alarcón GS. Rheumatology (Oxford) 2009;48:817–822. doi: 10.1093/rheumatology/kep102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peripheral vascular damage in systemic lupus erythematosus: data from LUMINA, a large multi-ethnic U.S. cohort (LXIX) Burgos PI, Vilá LM, Reveille JD, Alarcón GS. Lupus. 2009;18:1303–1308. doi: 10.1177/0961203309105877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Risk factors for clinical coronary heart disease in systemic lupus erythematosus: the lupus and atherosclerosis evaluation of risk (LASER) study. Haque S, Gordon C, Isenberg D, et al. J Rheumatol. 2010;37:322–329. doi: 10.3899/jrheum.090306. [DOI] [PubMed] [Google Scholar]
- 62.Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population‐based study. Aviña-Zubieta JA, To F, Vostretsova K, De Vera M, Sayre EC, Esdaile JM. Arthritis Care Res (Hoboken) 2017;69:849–856. doi: 10.1002/acr.23018. [DOI] [PubMed] [Google Scholar]
- 63.Mortality in systemic lupus erythematosus. Bernatsky S, Boivin JF, Joseph L, et al. Arthritis Rheum. 2006;54:2550–2557. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 64.Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Manzi S, Meilahn EN, Rairie JE, et al. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 65.Subclinical atherosclerosis in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, Ye DQ. Autoimmun Rev. 2016;15:22–37. doi: 10.1016/j.autrev.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Early vascular aging in normotensive patients with systemic lupus erythematosus: comparison with young patients having hypertension. Morreale M, Mulè G, Ferrante A, D'ignoto F, Cottone S. Angiology. 2016;67:676–682. doi: 10.1177/0003319715613917. [DOI] [PubMed] [Google Scholar]
- 67.Proximal aortic stiffness is increased in systemic lupus erythematosus activity in children and adolescents. El Gamal YM, Elmasry OA, El Hadidi IS, Soliman OK. ISRN Pediatr. 2013;2013:765253. doi: 10.1155/2013/765253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Schoenfeld SR, Kasturi S, Costenbader KH. Semin Arthritis Rheum. 2013;43:77–95. doi: 10.1016/j.semarthrit.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 70.Cardiovascular magnetic resonance detects silent heart disease missed by echocardiography in systemic lupus erythematosus. Mavrogeni S, Koutsogeorgopoulou L, Markousis-Mavrogenis G, et al. Lupus. 2018;27:564–571. doi: 10.1177/0961203317731533. [DOI] [PubMed] [Google Scholar]
- 71.Increased visceral adipose tissue and altered adiposity distribution in premenopausal lupus patients: correlation with cardiovascular risk factors. Seguro LP, Paupitz JA, Caparbo VF, Bonfa E, Pereira RM. Lupus. 2018;27:1001–1006. doi: 10.1177/0961203318758504. [DOI] [PubMed] [Google Scholar]
- 72.Association of aortic perivascular adipose tissue density with aortic calcification in women with systemic lupus erythematosus. Shields KJ, El Khoudary SR, Ahearn JM, Manzi S. Atherosclerosis. 2017;262:55–61. doi: 10.1016/j.atherosclerosis.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 73.Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Katus HA, Remppis A, Neumann FJ, et al. Circulation. 1991;83:902–912. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- 74.Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Saunders JT, Nambi V, de Lemos JA, et al. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.High-sensitivity cardiac troponin T is a biomarker for atherosclerosis in systemic lupus erythematous patients: a cross-sectional controlled study. Divard G, Abbas R, Chenevier-Gobeaux C, et al. Arthritis Res Ther. 2017;19:132. doi: 10.1186/s13075-017-1352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Association between serum total antioxidant status and coronary microvascular functions in patients with SLE. Yılmaz S, Caliskan M, Kulaksızoglu S, et al. Echocardiography. 2012;29:1218–1223. doi: 10.1111/j.1540-8175.2012.01797.x. [DOI] [PubMed] [Google Scholar]
- 77.How HDL protects LDL against atherogenic modification: paraoxonase 1 and other dramatis personae. Soran H, Schofield JD, Liu Y, Durrington PN. Curr Opin Lipidol. 2015;26:247–256. doi: 10.1097/MOL.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 78.Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Delgado Alves J, Ames PR, Donohue S, Stanyer L, Nourooz-Zadeh J, Ravirajan C, Isenberg DA. Arthritis Rheum. 2002;46:2686–2694. doi: 10.1002/art.10542. [DOI] [PubMed] [Google Scholar]
- 79.Cardiovascular disease in systemic lupus erythematosus is associated with increased levels of biomarkers reflecting receptor-activated apoptosis. Wigren M, Svenungsson E, Mattisson IY, et al. Atherosclerosis. 2018;270:1–7. doi: 10.1016/j.atherosclerosis.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 80.Cardiovascular disease in systemic lupus erythematosus: recent data on epidemiology, risk factors and prevention. Kostopoulou M, Nikolopoulos D, Parodis I, Bertsias G. Curr Vasc Pharmacol. 2020;18:549–565. doi: 10.2174/1570161118666191227101636. [DOI] [PubMed] [Google Scholar]
- 81.2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Piepoli MF, Hoes AW, Agewall S, et al. Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Impact of the new American College of Cardiology/American Heart Association definition of hypertension on atherosclerotic vascular events in systemic lupus erythematosus. Tselios K, Gladman DD, Su J, Urowitz M. Ann Rheum Dis. 2020;79:612–617. doi: 10.1136/annrheumdis-2019-216764. [DOI] [PubMed] [Google Scholar]
- 83.2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Whelton PK, Carey RM, Aronow WS, et al. J Am Coll Cardiol. 2018;71:0–248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 84.2018 ESC/ESH Guidelines for the management of arterial hypertension. Williams B, Mancia G, Spiering W, et al. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 85.Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Lai CH, Lai WW, Chiou MJ, Lin WC, Yang YJ, Li CY, Tsai LM. Ann Rheum Dis. 2016;75:1350–1356. doi: 10.1136/annrheumdis-2015-207719. [DOI] [PubMed] [Google Scholar]
- 86.Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Ann Rheum Dis. 2010;69:20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 87.Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Kaiser R, Cleveland CM, Criswell LA. Ann Rheum Dis. 2009;68:238–241. doi: 10.1136/ard.2008.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Jung H, Bobba R, Su J, et al. Arthritis Rheum. 2010;62:863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 89.Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Lupus. 2006;15:577–583. doi: 10.1177/0961203306071872. [DOI] [PubMed] [Google Scholar]
- 90.Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. Penn SK, Kao AH, Schott LL, et al. J Rheumatol. 2010;37:1136–1142. doi: 10.3899/jrheum.090994. [DOI] [PMC free article] [PubMed] [Google Scholar]