Abstract
BACKGROUND AND PURPOSE:
Rescue therapies are increasingly used in the setting of endovascular therapy for large-vessel occlusion strokes. Among these, cangrelor, a new P2Y12 inhibitor, offers promising pharmacologic properties to join the reperfusion strategies in acute stroke. We assessed the safety and efficacy profiles of cangrelor combined with endovascular therapy in patients with large-vessel-occlusion stroke.
MATERIALS AND METHODS:
We performed a retrospective patient data analysis in the ongoing prospective multicenter observational Endovascular Treatment in Ischemic Stroke Registry in France from July 2018 to December 2020 and conducted a systematic review and meta-analysis using several data bases. Indications for cangrelor administration were rescue strategy in case of refractory intracranial occlusion with or without intracranial rescue stent placement, and cervical carotid artery stent placement in case of cervical occlusion (tandem occlusion or isolated cervical carotid occlusion).
RESULTS:
In the clinical registry, 44 patients were included (median initial NIHSS score, 12; prior intravenous thrombolysis, 29.5%). Intracranial stent placement was performed in 54.5% (n = 24/44), and cervical stent placement, in 27.3% (n = 12/44). Adjunctive aspirin and heparin were administered in 75% (n = 33/44) and 40.9% (n = 18/44), respectively. Rates of symptomatic intracerebral hemorrhage, parenchymal hematoma, and 90-day mortality were 9.5% (n = 4/42), 9.5% (n = 4/42), and 24.4% (n = 10/41). Favorable outcome (90-day mRS, 0–2) was reached in 51.2% (n = 21/41), and successful reperfusion, in 90.9% (n = 40/44). The literature search identified 6 studies involving a total of 171 subjects. In the meta-analysis, including our series data, symptomatic intracerebral hemorrhage occurred in 8.6% of patients (95% CI, 5.0%–14.3%) and favorable outcome was reached in 47.6% of patients (95% CI, 27.4%–68.7%). The 90-day mortality rate was 22.6% (95% CI, 13.6%–35.2%). Day 1 artery patency was observed in 89.7% (95% CI, 81.4%–94.6%).
CONCLUSIONS:
Cangrelor offers promising safety and efficacy profiles, especially considering the complex endovascular reperfusion procedures in which it is usually applied. Further large prospective data are required to confirm these findings.
Endovascular therapy (EVT), with or without intravenous thrombolysis, is the standard of care for large-vessel-occlusion stroke (LVOS).1 Despite continuous improvement, successful reperfusion rates still vary around 80%, and only half of treated patients reach functional independence. Rescue approaches are increasingly considered in complex reperfusion strategy, such as refractory intracranial occlusions, early reocclusions, tandem occlusions with or without acute stent placement,2-7 or even as combined treatment during EVT in selected patients (REperfusion With P2Y12 Inhibitors in Addition to mEchanical thRombectomy for perFUsion Imaging Selected Acute Stroke patiEnts [REPERFUSE] trial, NCT04667078). In the literature, adjuvant pharmacologic agents are mostly considered, and most published data concern glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors. The literature regarding cangrelor in the treatment of LVOS is scarce.8-16 Cangrelor is a new P2Y12 inhibitor, inducing an immediate platelet inhibition, with a rapid platelet function recovery after treatment interruption if necessary and an easy transition to oral dual-antiplatelet therapy.17 These characteristics might be of interest in comparison with GP IIb/IIIa inhibitors, having a delayed onset of action and longer half-life with persisting efficacy (potentially harmful in case of intracranial hemorrhage). We, therefore, analyzed data from our national registry and conducted a systematic review and meta-analysis to assess the safety and efficacy profiles of cangrelor use in patients with LVOS treated by EVT.
MATERIALS AND METHODS
The data used in this study are available from the corresponding author on reasonable request.
Study Population
We performed a retrospective analysis of the Endovascular Treatment of Ischemic Stroke (ETIS) Registry from July 2018 to December 2020 (Endovascular Treatment in Ischemic Stroke Follow-up Evaluation; NCT03776877). ETIS is an ongoing prospective, multicenter, observational study that includes all consecutive patients undergoing EVT for LVOS in 22 comprehensive stroke centers in France. The local institutional review boards approved the data collection and analysis. All data in the ETIS registry were collected, stored, and accessed locally following the recommendations of the “Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de santé.” Patients treated with EVT combined with perioperative cangrelor administration were analyzed in the present study. Indications for cangrelor infusion were rescue strategy in case of refractory intracranial occlusion with or without rescue stent placement and cervical carotid artery stent placement in case of severe lesions (tandem occlusion or isolated cervical occlusion). Acute stroke due to the thrombosis of an underlying pre-existing previously deployed stent, or iatrogenic intracranial occlusion after EVT (aneurysm or arteriovenous shunt embolization), or both were excluded.
Treatments
The EVT indication was determined according to the current guidelines, depending on the patient’s condition, imaging data, and timeframe. Intravenous thrombolysis (IVT) with 0.9 mg/kg of alteplase or tenecteplase, 0.25 mg/kg, was administered prior to EVT if the patient presented within 4.5 hours after stroke onset in the absence of contraindications, according to the recommendations. EVT was performed with the patient under sedation, local or general anesthesia depending on local protocol and the patient’s condition. According to the LVOS presentation and the operator’s experience, the type of EVT was left to the discretion of the operator. As mentioned above, cangrelor adjunctive pharmacologic therapy was decided on a case-by-case basis in 2 distinct situations: 1) intracranial refractory occlusion (recanalization failure using a standard endovascular approach, early reocclusion, or identified underlying arterial wall disease with a high risk of reocclusion) eventually treated with intracranial stent placement; or 2) underlying cervical artery disease such as tandem or isolated cervical occlusions due to atherosclerosis or dissection also eventually treated with stent placement. The decision for cangrelor administration was also made according to the patient’s comorbidities, imaging data (including perioperative flat panel CT), prior antithrombotic treatment, and IVT.
Cangrelor administration protocol was as follows: a 30-µg/kg intravenous bolus continued with a 4-µg/kg/min infusion. An aspirin or heparin bolus could also be administered on a case-by-case basis, according to local protocol and the operator’s decision. Postoperative mid- and long-term antithrombotic therapy, in particular dual-antiplatelet therapy, was introduced on the basis of early clinical and imaging data. According to the infarct extension, intracranial hemorrhage, and cervical and/or intracranial artery patency on postoperative imaging, a dual-antiplatelet therapy with ticagrelor and aspirin was potentially introduced during the first 24 hours after the endovascular procedure.
Outcomes
Clinical, imaging, timeline, and angiographic data were recorded prospectively by 1 experienced neuroradiologist (>10 years’ experience) in each center. The ASPECTS was assessed using anterior or posterior circulation scores, depending on the initial arterial occlusion localization. Ninety days after the acute event, functional outcome was assessed by board-certified vascular neurologists during a routinely scheduled clinical visit or by a study nurse certified in administering the mRS during a standardized telephone interview if the patient was unable to attend. Favorable outcome was defined as a 90-day mRS of 0–2. Early neurologic changes (>4-point improvement in the NIHSS during the first 24 hours) were recorded. Favorable reperfusion was defined as modified TICI 2b, 2c, or 3. Symptomatic intracranial hemorrhage (sICH) was defined as neurologic deterioration (NIHSS worsening of ≥4 points) along with intracranial hemorrhage (ICH).
Systematic Review
We conducted a literature review according to the Preferred Reporting Items for Systematic Reviews (PRISMA) (see the PRISMA checklist in the Online Supplemental Data). PubMed, EMBASE, and MEDLINE data bases were researched using the following combined key words: “cangrelor” and “thrombectomy” and/or “stroke” and/or “neurovascular” and/or “cerebral endovascular” from 2010 until December 2021. The literature search and publications were analyzed by 2 authors (G.M. and S.F.). English literature and series including >5 patients were considered. Publications reporting adjunctive perioperative use of cangrelor during endovascular treatment of LVOS were included. Studies reporting cangrelor use in the setting of cerebrovascular diseases other than EVT for acute ischemic stroke (such as intracranial aneurysm treatment, for example) were excluded. Possible redundant study populations within distinct publications were excluded. In cases of authors and/or participating centers identified in different articles, only the most recently published article was considered in our analysis. Quality assessment was performed independently by 2 authors (G.M. and S.F.) using the Cochrane Risk of Bias Tool. Any disagreements were resolved by discussion or by recourse to a third-party reviewer (B.G.).
Statistical Analysis
For the ETIS Registry analysis, categoric variables are expressed as frequencies and percentages. Continuous variables are expressed by their means and SDs, or, in the case of non-normal distribution, by median and interquartile range. For the meta-analysis, summary effects were calculated using a random-effects model by means of DerSimonian and Laird estimators with a logit transformation of raw proportions. Individual effect sizes and their sampling variances were calculated by the inverse variance method. Outcomes are presented as proportions with 95% CIs. Heterogeneity of treatment effect across studies was assessed using the Cochran Q test (a P value threshold of .05) and was quantified with the I2 statistic, with I2 > 50% suggesting substantial heterogeneity. Publication bias was estimated visually by funnel plots. The analysis was performed using the metafor package of the R statistical and computing software, Version 3.6.2 (http://www.r-project.org/).
RESULTS
Registry Data
During the study period, among 4813 EVTs performed in the participating centers, 44 patients met the inclusion/exclusion criteria. Baseline characteristics of the study population are presented in the Online Supplemental Data. The mean age was 64 (SD,14) years, and 38.6% were women. A history of high blood pressure, previous stroke, and ischemic heart disease was observed in 59.5%, 14.3%, and 7.3%, respectively. Thirteen patients (29.5%) were already under antithrombotic medication before the stroke episode (8 with single antiplatelet therapy and 5 with a direct oral anticoagulant). The initial median NIHSS score and ASPECTS were 12 (interquartile range [IQR] = 9) and 8 (IQR = 2), respectively. Prior IVT was administered in 13 patients (29.5%), including 12 treated with alteplase and 1 with tenecteplase. Detailed occlusion locations were as follows: M1 segment in 12 (27.3%), M2 segment in 4 (9.1%), ICA terminus in 7 (15.9%), anterior circulation tandem occlusion in 7 (15.9%), isolated cervical ICA occlusion in 3 (6.8%), and vertebrobasilar circulation in 11 cases (25.0%). The median time from onset to puncture was 253 minutes (IQR = 166 minutes). A cardioembolic etiology was suspected in 5 (12.5%), while most etiologies were a suspected atherosclerosis cause (87.5%; n = 35/40). Favorable reperfusion was obtained in 90.9% (n = 40). The median number of passes was 2 (IQR = 2). The median time from puncture to reperfusion was 100 minutes (IQR = 78 minutes). Adjunctive aspirin and heparin were administered in, respectively, 75% (n = 33/44) and 40.9% (n = 18/44) of procedures. Intracranial stent placement was performed in 54.5% (n = 24/44), and cervical stent placement, in 27.3% (n = 12/44). The perioperative complication rate was 6.8% (n = 3/44; 2 emboli in a new territory and 1 groin hematoma with a secondary pseudoaneurysm requiring endovascular repair). Early neurologic improvement was recorded in 65.9% (n = 29/44). Favorable outcome (mRS 0–2) at 90 days was reached in 51.2% (n = 21/41). The mortality rate was 24.4% (n = 10/41). Symptomatic ICH and parenchymal hematoma rates were both 9.5% (n = 4/42). Details regarding sICH are provided in the Online Supplemental Data. The treated artery was patent on day 1 imaging in 82.5% (n = 33/40).
Meta-analysis
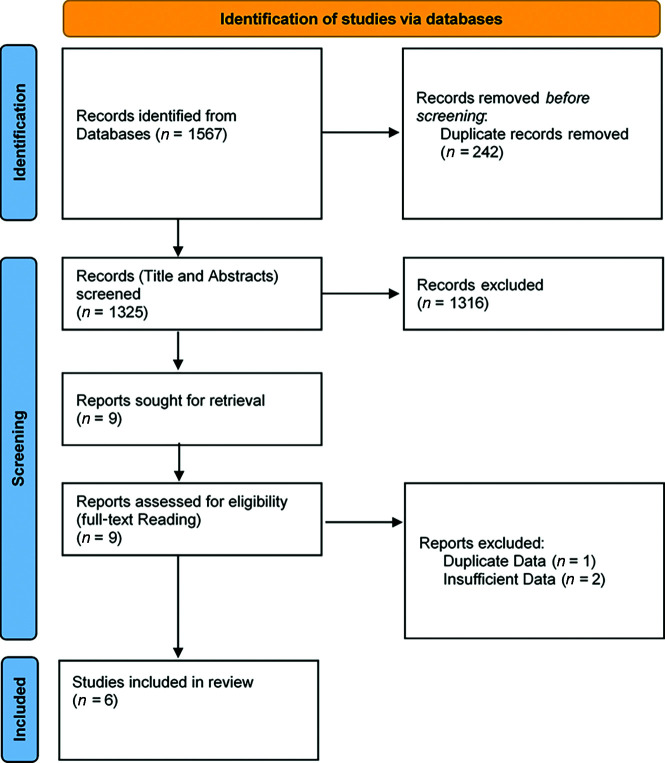
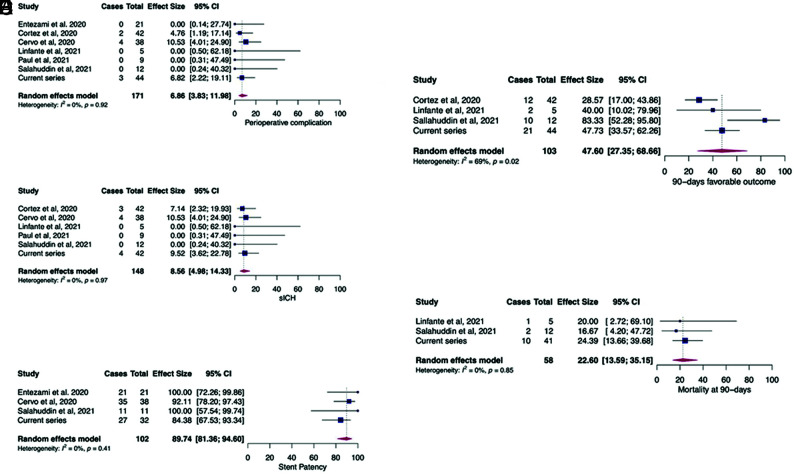
The global literature search identified 1567 citations. After we explored titles and abstracts, 9 articles were retained for complete reading. Duplicate removal and/or insufficient data allowed the final inclusion of 6 published studies in addition to our presented study (see the PRISMA flowchart, Fig 1). We analyzed a total of 171 patients: 127 from the systematic review and 44 patients from the ETIS Registry. The median age ranged from 56 to 68.5 years (Online Supplemental Data). The initial median NIHSS score and ASPECTS ranged, respectively, from 8 to 15.5 and 8 to 9. Prior IVT was administered in 29.5%–46.6% of patients (Online Supplemental Data). An additional perioperative aspirin bolus was given in 28.9%–100% of cases. From 25.0% to 54.7% and 27.7% to 68.4% of patients, respectively, underwent intracranial and cervical stent placement. Perioperative complications occurred in 6.9% of patients (95% CI, 3.8%–12.0%; I2 = 0%, P = .92) (Fig 2). Reported sICH occurred in 8.6% of patients (95% CI, 5.0%–14.3%; I2 = 0%, P = .97). Day 1 artery patency was observed in 89.7% (95% CI, 81.4%–94.6%; I2 = 0%, P = .41). After 3 months, favorable outcome (mRS 0–2) was reached in 47.6% of patients (95% CI, 27.4%–68.7%; I 2= 69%, P = .02) with a mortality rate of 22.6% (95% CI, 13.6%–35.2%; I2 = 0%, P = .85). Heterogeneity was not significant (I2 < 50%) for all variables assessed except for favorable outcome at 3 months (I2 = 69%, P = .02). We found no evidence suggestive of publication bias by examining the funnel plots (Online Supplemental Data).
FIG 1.
Flow chart of patients included in the meta-analysis.
FIG 2.
Pooled estimates for periprocedural complications (A), symptomatic intracranial hemorrhage (B), stent patency at day 1 (C), mRS 0-2 at 90 days (D), and mortality at 90 days (E).
Quality Assessment
Most studies were found to be of low-to medium quality (Online Supplemental Data). Their main limitations were retrospective design, small sample size, mixed anterior and posterior circulation, and absence of blinded assessment of clinicoradiologic outcomes.
DISCUSSION
Our meta-analysis including our multicentric national clinical registry data found high successful recanalization rates and favorable outcome after cangrelor administration for EVT of LVOS. The pooled rates of sICH and favorable clinical outcome were 8.5% (95% CI, 5.0%–14.3%) and 47.6% (95% CI, 27.4%–68.7%), respectively.
These results are particularly interesting considering the especially complex endovascular presentations in which cangrelor would to be used; therefore, they cannot be compared with the results of EVT outcomes in standard LVOS. Indeed, cangrelor was administered in cases of refractory intracranial occlusion, recanalization failure after a standard EVT approach, and cervical or intracranial atherosclerosis with acute stent placement.18-20 With the broadening of EVT indications and the growing number of treated patients, such endovascular configurations are increasingly encountered. The literature regarding “intense” rescue management after failure or in addition to standard EVT is increasing.2-7 Among the adjunctive complementary tools that have recently emerged, acute antiplatelet therapies (cangrelor, GP IIb/IIIa inhibitors, and others) are becoming essential components of the acute reperfusion strategy in patients with stroke. Given its pharmacologic properties, cangrelor may now be a first-line intravenous antiplatelet therapy in comparison with GP IIb/IIIa inhibitors. Thus, we recently published a comparison between these 2 acute antiplatelet therapies.21 Yet, data for safety and efficacy profiles still have to be explored.
The use of antithrombotic therapy at the acute phase of ischemic stroke must target a perfect balance between promoting recanalization and a reasonable hemorrhagic risk, especially ICH. Our study provides somewhat reassuring data with an overall sICH rate of 8.6% (95% CI, 5.0%–14.3%). Considering the poor prognosis of patients with intracranial reperfusion failure, a reasonable ICH risk could probably be taken into account in the benefit risk-ratio. In our study, the ICH rate should also be observed, considering that cangrelor was mostly administered in association with other antithrombotic drugs (aspirin and/or heparin). Given the absence of validated guidelines, aspirin or heparin were administered either as a first-line antithrombotic strategy or as complementary antithrombotic therapy and could increase the bleeding risk. In our clinical registry, aspirin and heparin were given in 75% and 40% with cangrelor, respectively. In daily practice, the introduction of cangrelor was often considered when heparin and aspirin were not deemed sufficient or relevant in the setting of stent placement or rescue treatments. Nevertheless, despite the association of aspirin or heparin with cangrelor, we did not observe a particularly high ICH rate. To date, we are not able to assess whether the administration of cangrelor alone may reduce the risk of bleeding and mortality without decreasing efficiency. Still, the task of improving the knowledge about cangrelor for acute ischemic stroke should also aim to clarify the real need for other adjunctive antithrombotic therapies to limit, as much as possible, the hemorrhagic risk. In addition, sICH rates here were in line with the current literature regarding rescue strategies after EVT failure using acute intravenous antiplatelet therapy.4-6,22,23
To date, data regarding ICH following cangrelor use are too limited to statistically identify specific risk factors for parenchymal hematoma and sICH. In the ETIS Registry case series, 4 cases of sICH occurred. No singular clinical or radiologic pattern was observed (ages ranged from 44 to 90 years; initial ASPECTS, from 6 to 9; intracranial occlusions were intracranial ICA and tandem [n = 2] and vertebral artery; adjunctive aspirin was given in 3 of 4 patients, and no heparin was used). Still, cangrelor introduction should always be cautiously considered. For example, its administration may be reasonably considered in patients with identified risk factors for sICH and/or parenchymal hematoma or with pre-existing intracranial hemorrhagic lesions (for example, early ICH within the ischemic core or multiple microbleeds).24,25 ICH may also be hypothetically influenced by dose, treatment duration, and oral antiplatelet therapy bridging. Along with the operator’s experience and growing literature regarding cangrelor, there is an ongoing debate on optimizing the dose to reach biologic antiplatelet efficacy. It seems likely that low-dose administration, inferior to standard, may be sufficient to obtain an efficient antiplatelet effect.16,26 Among the available literature, distinct strategies and cangrelor dosages have been reported. The influence of cangrelor dosage on ICH risk remains to be properly evaluated.
Contrary to GP IIb/IIIa inhibitors, platelet aggregation will be restored within 30–60 minutes after stopping cangrelor infusion. In case of acute clinical or radiologic worsening related to ICH, potentially requiring surgical treatment (craniectomy, external shunt), cangrelor suspension may allow normalizing platelet function to limit ICH extension and perform a rapid and safe surgical treatment if necessary.27 In a comparable situation, the use of GP IIb/IIIa inhibitors might not offer a similar solution due to less favorable pharmacologic characteristics (restoration of platelet function taking from 4 to 8 hours with tirofiban and eptifibatide and 12 to 48 hours for abciximab) and a possibly poorer safety profile.23 In our registry, 2 patients finally underwent craniectomy, and 1 had a groin hematoma. In such situations, cangrelor use might be more advantageous and safer.
Efficacy markers were also promising, especially once again given the usual quite poor prognosis of LVOS when cangrelor was administered. Favorable outcome was reached in 51.2% in our registry and in 47.6% (95% CI, 27.4%–68.7%) in the literature. Then, the targeted artery was still patent on day 1 imaging in 89.7% (95% CI, 81.4%–94.6%), which is the main objective of acute cangrelor administration with or without mechanical treatment (angioplasty and/or stent placement). Indeed, in this particular setting, the risk of early reocclusion is high with a strong propensity to worsen clinical evolution.28,29 Cangrelor allows a continuous intravenous infusion, maintaining its antiplatelet efficacy until oral dual-antiplatelet therapy can be introduced. Efficacy regarding recanalization and especially patency durability is in line with the literature reporting alternative medications such as GP IIb/IIIa inhibitors.4,23
Our study has several limitations. First, despite derived from our prospective multicenter registry, we conducted a retrospective analysis. Then, the limited number of patients from our prospective registry and in the available literature might impact the statistical power of the analysis. Interpretation of results should remain cautious. In addition, angiographic and imaging data were not assessed by a core lab either in the registry or in the literature data. Cangrelor use in cases of EVT of LVOS remains quite recent, and there are no proved guidelines regarding rescue strategies in the setting of antithrombotic management during EVT of complex LVOS. Consequently, there may have been heterogeneities in antiplatelet therapy indications, administration protocol, postoperative dual antiplatelet relay, and patient selection, as well as the endovascular approach.
CONCLUSIONS
Cangrelor administration in the setting of EVT for complex LVOS was associated with encouraging safety and efficacy patterns. Its favorable pharmacodynamics may indicate cangrelor as a promising adjuvant agent for complex occlusions such as large-artery atherosclerosis (either intra- or extracranial) and/or rescue stent placement for refractory occlusions. Large prospective studies are needed to broaden the knowledge of cangrelor in this setting, to clarify its place within the therapeutic strategy, and to elaborate guidelines.
ABBREVIATIONS:
- ETIS
Endovascular Treatment in Ischemic Stroke
- EVT
endovascular therapy
- GP IIb/IIIa
glycoprotein IIb/IIIa
- ICH
intracranial hemorrhage
- IQR
interquartile range
- IVT
intravenous thrombolysis
- LVOS
large-vessel-occlusion stroke
- sICH
symptomatic intracranial hemorrhage
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Goyal M, Menon BK, Van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Baek JH, Kim BM, Kim DJ, et al. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke 2016;47:2360–63 10.1161/STROKEAHA.116.014073 [DOI] [PubMed] [Google Scholar]
- 3.Jia B, Feng L, Liebeskind DS, et al. ; EAST Study Group. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg 2018;10:746–50 10.1136/neurintsurg-2017-013489 [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Wu Y, Gao X, et al. Intraarterial versus intravenous tirofiban as an adjunct to endovascular thrombectomy for acute ischemic stroke. Stroke 2020;51:2925–33 10.1161/STROKEAHA.120.029994 [DOI] [PubMed] [Google Scholar]
- 5.Premat K, Dechartres A, Lenck S, et al. Rescue stenting versus medical care alone in refractory large vessel occlusions: a systematic review and meta-analysis. Neuroradiology 2020;62:629–37 10.1007/s00234-020-02360-9 [DOI] [PubMed] [Google Scholar]
- 6.Chang Y, Kim BM, Bang OY, et al. Rescue stenting for failed mechanical thrombectomy in acute ischemic stroke: a multicenter experience. Stroke 2018;49:958–64 10.1161/STROKEAHA.117.020072 [DOI] [PubMed] [Google Scholar]
- 7.Peng F, Wan J, Liu W, et al. Efficacy and safety of rescue stenting following failed mechanical thrombectomy for anterior circulation large vessel occlusion: propensity score analysis. J Neurointerv Surg 2020;12:271–73 10.1136/neurintsurg-2019-015154 [DOI] [PubMed] [Google Scholar]
- 8.Linfante I, Ravipati K, Starosciak AK, et al. Intravenous cangrelor and oral ticagrelor as an alternative to clopidogrel in acute intervention. J Neurointerv Surg 2021;13:30–32 10.1136/neurintsurg-2020-015841 [DOI] [PubMed] [Google Scholar]
- 9.Cervo A, Ferrari F, Barchetti G, et al. Use of cangrelor in cervical and intracranial stenting for the treatment of acute ischemic stroke: a “real life” single-center experience. AJNR Am J Neuroradiol 2020;41:2094–99 10.3174/ajnr.A6785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhorany M, Lenck S, Degos V, et al. Cangrelor and stenting in acute ischemic stroke: monocentric case series. Clin Neuroradiol 2021;31:439–48 10.1007/s00062-020-00907-0 [DOI] [PubMed] [Google Scholar]
- 11.Entezami P, Holden DN, Boulos AS, et al. Cangrelor dose titration using platelet function testing during cerebrovascular stent placement. Interv Neuroradiol 2021;27:88–98 10.1177/1591019920936923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguilar-Salinas P, Agnoletto GJ, Brasiliense LB, et al. Safety and efficacy of cangrelor in acute stenting for the treatment of cerebrovascular pathology: preliminary experience in a single-center pilot study. J Neuronterv Surg 2019;11:347–51 10.1136/neurintsurg-2018-014396 [DOI] [PubMed] [Google Scholar]
- 13.Abdennour L, Sourour N, Drir M, et al. Preliminary experience with cangrelor for endovascular treatment of challenging intracranial aneurysms. Clin Neuroradiol 2020;30:453–61 10.1007/s00062-019-00811-2 [DOI] [PubMed] [Google Scholar]
- 14.Cortez GM, Monteiro A, Sourour N, et al. The use of cangrelor in neurovascular interventions: a multicenter experience. Neuroradiology 2021;63:925–34 10.1007/s00234-020-02599-2 [DOI] [PubMed] [Google Scholar]
- 15.Paul AR, Entezami P, Holden D, et al. Use of intravenous cangrelor and stenting in acute ischemic stroke interventions: a new single center analysis and pooled-analysis of current studies. Interv Neuroradiol 2021;27:837–42 10.1177/15910199211014417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salahuddin H, Dawod G, Zaidi SF, et al. Safety of low dose intravenous cangrelor in acute ischemic stroke: a case series. Front Neurol 2021;12:636682 10.3389/fneur.2021.636682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sible AM, Nawarskas JJ. Cangrelor: a new route for P2Y12 inhibition. Cardiol Rev 2017;25:133–39 10.1097/CRD.0000000000000142 [DOI] [PubMed] [Google Scholar]
- 18.Kaesmacher J, Gralla J, Mosimann PJ, et al. Reasons for reperfusion failures in stent-retriever-based thrombectomy: registry analysis and proposal of a classification system. AJNR Am J Neuroradiol 2018;39:1848–53 10.3174/ajnr.A5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim BM. Causes and solutions of endovascular treatment failure. J Stroke 2017;19:131–42 10.5853/jos.2017.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo LL, Bhogal P, Gopinathan A, et al. Why does mechanical thrombectomy in large vessel occlusion sometimes fail? A review of the literature. Clin Neuroradiol 2019;29:401–14 10.1007/s00062-019-00777-1 [DOI] [PubMed] [Google Scholar]
- 21.Marnat G, Delvoye F, Finitsis S, et al. ; ETIS Investigators. A multicenter preliminary study of cangrelor following thrombectomy failure for refractory proximal intracranial occlusions. AJNR Am J Neuroradiol 2021;42:1452–57 10.3174/ajnr.A7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maingard J, Phan K, Lamanna A, et al. Rescue intracranial stenting after failed mechanical thrombectomy for acute ischemic stroke: a systematic review and meta-analysis. World Neurosurg 2019;132:e235–45 10.1016/j.wneu.2019.08.192 [DOI] [PubMed] [Google Scholar]
- 23.Delvoye F, Maier B, Escalard S, et al. Antiplatelet therapy during emergent extracranial internal carotid artery stenting: comparison of three intravenous antiplatelet perioperative strategies. J Stroke Cerebrovasc Dis 2021;30:105521 10.1016/j.jstrokecerebrovasdis.2020.105521 [DOI] [PubMed] [Google Scholar]
- 24.Boisseau W, Fahed R, Lapergue B, et al. ; ETIS Investigators. Predictors of parenchymal hematoma after mechanical thrombectomy: a multicenter study. Stroke 2020;51:2364–70 10.1161/STROKEAHA.119.027977 [DOI] [PubMed] [Google Scholar]
- 25.Hao Y, Yang D, Wang H, et al. ; ACTUAL Investigators (Endovascular Treatment for Acute Anterior Circulation Ischemic Stroke Registry). Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke 2017;48:1203–09 10.1161/STROKEAHA.116.016368 [DOI] [PubMed] [Google Scholar]
- 26.Holden DN, Entezami P, Bush MC, et al. Characterization of antiplatelet response to low-dose cangrelor utilizing platelet function testing in neuroendovascular patients. Pharmacotherapy 2021;41:811–19 10.1002/phar.2619 [DOI] [PubMed] [Google Scholar]
- 27.Godier A, Mesnil M, De Mesmay M, et al. Bridging antiplatelet therapy with cangrelor in patients with recent intracranial stenting undergoing invasive procedures: a prospective case series. Br J Anaesth 2019;123:e2–e5 10.1016/j.bja.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 28.Abdalla RN, Cantrell DR, Shaibani A, et al. Refractory stroke thrombectomy: prevalence, etiology, and adjunctive treatment in a North American cohort . AJNR Am J Neuroradiol 2021;42:1258–63 10.3174/ajnr.A7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsang AC, Orru E, Klostranec JM, et al. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke 2019;50:1460–66 10.1161/STROKEAHA.119.024889 [DOI] [PubMed] [Google Scholar]