ADC histogram metrics are associated with progression-free survival and response to treatment with selumetinib in pediatric low-grade gliomas.
Abstract
BACKGROUND AND PURPOSE:
Selumetinib is a promising MAP (mitogen-activated protein) kinase (MEK) 1/2 inhibitor treatment for pediatric low-grade gliomas. We hypothesized that MR imaging–derived ADC histogram metrics would be associated with survival and response to treatment with selumetinib.
MATERIALS AND METHODS:
Children with recurrent, refractory, or progressive pediatric low-grade gliomas who had World Health Organization grade I pilocytic astrocytoma with KIAA1549–BRAF fusion or the BRAF V600E mutation (stratum 1), neurofibromatosis type 1–associated pediatric low-grade gliomas (stratum 3), or sporadic non-neurofibromatosis type 1 optic pathway and hypothalamic glioma (OPHG) (stratum 4) were treated with selumetinib for up to 2 years. Quantitative ADC histogram metrics were analyzed for total and enhancing tumor volumes at baseline and during treatment.
RESULTS:
Each stratum comprised 25 patients. Stratum 1 responders showed lower values of SD of baseline ADC_total as well as a larger decrease with time on treatment in ADC_total mean, mode, and median compared with nonresponders. Stratum 3 responders showed a greater longitudinal decrease in ADC_total. In stratum 4, higher baseline ADC_total skewness and kurtosis were associated with shorter progression-free survival. When all 3 strata were combined, responders showed a greater decrease with time in ADC_total mode and median. Compared with sporadic OPHG, neurofibromatosis type 1–associated OPHG had lower values of ADC_total mean, mode, and median as well as ADC_enhancement mean and median and higher values of ADC_total skewness and kurtosis at baseline. The longitudinal decrease in ADC_total median during treatment was significantly greater in sporadic OPHG compared with neurofibromatosis type 1–associated OPHG.
CONCLUSIONS:
ADC histogram metrics are associated with progression-free survival and response to treatment with selumetinib in pediatric low-grade gliomas.
Pediatric low-grade gliomas (pLGGs) are the most commonly occurring childhood brain tumor and comprise 40%–50% of all childhood CNS tumors.1,2 World Health Organization (WHO) grade I pilocytic astrocytoma is the most frequent primary brain tumor in individuals 0–19 years of age, accounting for 15% of children’s and adolescents’ (0–19 years) and 17.8% of primary childhood (0–14 years) brain tumors.1 pLGGs are biologically distinct from their adult counterparts.3 Unlike adult low-grade gliomas that occur mostly in the cerebral hemispheres and transform into higher-grade gliomas, pLGGs can occur throughout the central nervous system, are molecularly distinct, rarely undergo malignant transformation,3,4 and require different therapies.
Total surgical resection is the first-line of treatment for pLGGs and can be curative.2 However, this is not always feasible due to tumor location, particularly in the brain stem or optic pathway and hypothalamic gliomas (OPHGs), and other therapies are required. Radiation therapy can be effective but may cause considerable neurocognitive deficits in young children and may increase the risk of developing a secondary malignancy, particularly in children with neurofibromatosis type 1 (NF1).5 Several chemotherapy regimens have shown promise in pLGGs, with 5-year progression-free survival (PFS) and overall survival rates of 35%–45% and 85%–100%, respectively, in sporadic pLGG and 5-year 60%–70% PFS and 90%–100% overall survival rates in NF1-associated pLGG.6 Selumetinib is a potent orally available MAP (mitogen-activated protein) kinase (MEK) 1/2 inhibitor that has recently shown great efficacy in refractory, recurrent, and progressive pLGGs.7,8 We explored the associations of advanced diffusion MR imaging metrics with response and survival in pLGGs treated with selumetinib in a large prospective Phase II Pediatric Brain Tumor Consortium (PBTC) trial, PBTC029B.
MATERIALS AND METHODS
Subjects
This was a multicenter, National Cancer Institute–sponsored Phase II study conducted by the PBTC using the MEK 1/2 inhibitor selumetinib in patients with pediatric low-grade gliomas treated at 11 PBTC member hospitals.8,9 Patients 3–21 years of age with a Lansky/Karnofsky Performance Status score of >60 and the presence of recurrent, refractory, or progressive pediatric low-grade glioma after at least 1 standard therapy, including chemotherapy and radiation therapy, were eligible for inclusion. Patients were assigned to 6 unique strata according to histology, tumor location, NF1 status, and BRAF aberration status.8,9 Clinical analyses of strata 1, 3, and 4 have been completed,8,9 and the imaging data were used in this study. Stratum 1 comprised patients with WHO grade I pilocytic astrocytoma with either KIAA1549-BRAF fusion or the BRAF V600E (Val600Glu) mutation. Stratum 3 comprised patients with imaging- or biopsy-proved pLGGs and a clinical or genetic diagnosis of NF1. Stratum 4 comprised OPHG pLGGs not associated with NF1.
Selumetinib was provided as capsules given orally at a dose of 25 mg/m2 twice daily in 28-day courses for up to 26 courses. The primary end point was the proportion of patients with a stratum-specific objective response (partial response or complete response), as assessed by the local site and sustained for at least 8 weeks. All responses were confirmed by central review.8,9
The protocol was approved by the Cancer Therapy Evaluation Program (CTEP) as well as each site’s institutional review board. All patients or legal guardians provided written, informed consent when applicable based on institutional guidelines.
Imaging and Image Analysis
Standard MR Imaging Evaluation.
Standard MR imaging, which included T2-FLAIR and axial pre- and postcontrast T1-weighted images, as well as DTI, was performed primarily on 3T scanners at the start of treatment followed by every 2 months during the first year of therapy and then every 3 months thereafter. Additionally, high-resolution MR imaging of the orbits and optic pathway was performed for optic pathway tumors.
Patients whose tumors achieved an MR imaging response (complete or partial response) assessed locally underwent central radiographic review at the PBTC Neuroimaging Center. A complete response was defined as complete tumor disappearance on T2-FLAIR images, no new lesions, and disappearance of all enhancement on T1 postcontrast imaging. A partial response was defined as at least 50% tumor reduction (in a 2D area calculated as a product of 2 perpendicular linear measurements) on T2-FLAIR. Stable disease was defined as neither a sufficient increase nor a reduction to qualify as a partial response or progressive disease. Progressive disease was a >25% increase or the development of new lesions. These response criteria, while similar to the latest Response Assessment in Pediatric Neuro-Oncology recommendations,10 were developed earlier by a PBTC consensus panel and used both for local and central imaging response assessments.
DTI Acquisition and ADC Histogram Analysis.
DTI data were acquired with the following acquisition parameters on a 3T scanner: section thickness = 2.2 mm, TR = 8800 ms, TE = 88 ms, FOV = 220 mm, b-value = 1000 s/mm2, 35 directions. By means of the mutual information algorithm in FSL (http://www.fmrib.ox.ac.uk/fsl),11 ADC and postcontrast T1 volumes were registered to FLAIR volume as described previously.12 3D ROIs comprising the total tumor volume from the FLAIR images and the enhancing tumor volume from postcontrast T1 were automatically generated using the thresholding feature in Fiji (https://fiji.sc),13 and the corresponding ADC was used to generate the ADC_total and ADC_enhancement volumes, respectively. These volumes were thresholded using a uniform range of 600–2600 × 10−6 mm2/s to automatically exclude cysts, necrosis, and hemorrhage; corresponding ADC_total and ADC_enhancement histograms were generated.12
Histogram metrics used for statistical analysis were the mean, SD, mode, median, skewness, and kurtosis of these histograms at baseline and 6, 12, 18, and 24 months into treatment and also at progression. In studies with multiple lesions, the primary target lesion was evaluated for all analyses.
For each of the imaging metrics, the baseline value and the time-dependent longitudinal change in the metrics during the course of treatment were examined for associations with tumor volume, response, and PFS.
Statistical Methods
The Wilcoxon rank-sum test was used to test the differences in ADC parameters at baseline between 2 groups. Subjects with at least 1 follow-up MR imaging after baseline were eligible for time-dependent longitudinal analyses. Tumor volumes were correlated within the patients because the measures were longitudinal. The mixed model was used to investigate the correlation between tumor volume and ADC metrics, taking intrapatient correlation into account. Mixed-effects models were used to estimate trends across time in the ADC parameters as well as differences in longitudinal changes between groups. Cox models were used to investigate the association between PFS and ADC parameters at baseline as well as with time. The latter incorporated time-dependent ADC parameters. Q values were calculated within each stratum for PFS and response-based analyses separately to adjust for multiplicity via the false discovery rate if >1 parameter was significant.14 A q-value of 10% (0.1) was considered significant. Similarly, q-values were also used for cross-strata comparisons.
RESULTS
Stratum 1
Twenty-five patients (mean age at study entry, 9.2 years; range, 3.9–20.8 years; 12 males and 13 females) with WHO grade I pilocytic astrocytoma with either 1 of the 2 most common BRAF aberrations (KIAA1549–BRAF fusion or the BRAF V600E [Val600Glu] mutation) were enrolled. Eighteen of these had a KIAA1549–BRAF fusion, and the remaining 7 had the BRAFV 600E mutation. Nine patients showed sustained partial response, 9 had stable disease, and 7 showed progressive disease. Two-year PFS in stratum 1 was 70%. These results were reported previously.8
None of the baseline ADC_total histogram parameters were associated with PFS. Only the SD of ADC_total at baseline was significantly associated with response (P = .009, q = 0.04), with responders showing lower values than nonresponders. The best response achieved was used in all analyses. There were significant associations of response with longitudinal change across time on treatment in the ADC_total mean (P = .02, q = 0.05), ADC_total mode (P = .02, q = 0.07), and ADC_total median (P = .03, q = 0.1), with responders showing a larger decrease in these parameters across time than nonresponders. Five of the 8 responders showed a transient increase in ADC followed by a decrease by 24 months as seen in Fig 1. Of the 21 enhancing tumors in stratum 1, sixteen were eligible for longitudinal analysis and 8 of these showed a response to selumetinib. None of the histogram metrics of ADC_enhancement were associated with a response in the longitudinal analyses.
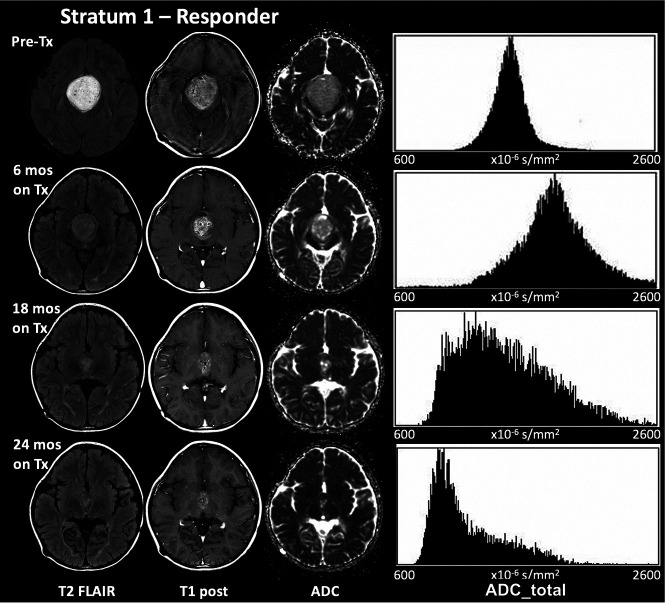
FIG 1.
The decrease at 24 months in the mean, median, and mode of ADC_total with treatment in a 6-year-old boy with a WHO grade I glioma with a BRAF aberration who responded to selumetinib. The initial transient increase in these metrics seen here was observed in 5 of the 8 responders in stratum 1 and is possibly linked to increased water mobility due to early cell death, followed by tissue consolidation later in treatment. Tx indicates treatment.
No group differences were found in ADC histogram metrics between the BRAF fusion and the BRAF mutation groups either at baseline or in the longitudinal analysis.
Stratum 3
Twenty-five patients (mean age at study entry, 10.2 years; range, 3.5–16.6 years; 15 males and 10 females) with any NF1-associated pediatric low-grade glioma (WHO grades I and II) were enrolled in stratum 3. Nine patients showed sustained partial response, 15 had stable disease, and 1 showed progressive disease. The 2-year PFS in stratum 3 was 96%. These results were reported previously.8
None of the baseline ADC histogram metrics were associated with response or PFS. Longitudinal change with time on treatment of ADC_total mode was significantly associated with response (P = .03, q = 0.07), with responders showing a greater decrease across time than nonresponders. Five of 10 responders showed a transient increase in the ADC followed by a decrease by 24 months.
Stratum 4
Twenty-five patients (mean age at study entry, 9.4 years; range, 3.7–17.6 years; 12 males and 13 females) with sporadic non-NF1 pediatric optic pathway and hypothalamic low-grade gliomas (WHO grades I and II) were enrolled in stratum 4. Six patients showed sustained partial response, 14 had stable disease, and 5 showed progressive disease. The 2-year PFS in stratum 4 was 73.8% (SD 9.3%) as reported previously.9
At baseline, there were statistically significant associations of ADC_total skewness (P = .02, q = 0.06) and ADC_total kurtosis (P = .02, q = 0.06) with PFS in stratum 4. Patients in stratum 4 with higher baseline skewness or kurtosis of ADC_total had shorter PFS (Online Supplemental Data). None of the longitudinal ADC_total histogram metrics were associated with response or PFS. Of the 23 enhancing tumors in stratum 4, twenty were eligible for longitudinal analysis with only 4 responders in this group. While the entire cohort showed a decrease with time in ADC_enhancement mean (P = .02, q = 0.05), mode (P = .01, q = 0.04), and median (P = .008, q = 0.02), there was no difference between responders and nonresponders.
Combined Strata
None of the baseline ADC histogram metrics were associated with response or PFS when all strata were combined (n = 75). Response was still significantly associated with longitudinal change with time in treatment for ADC_total mode (P = .02, q = 0.06) and ADC_total median (P = .03, q = 0.09). A greater decrease in these metrics was found in patients showing a response to the study drug. Of the 64 enhancing tumors in the combined strata, 48 were eligible for longitudinal analysis, with 19 responders. The entire cohort showed a decrease with time for the mean (P = .01, q = 0.04), mode (P = .009, q = 0.03), and median (P = .009, q = 0.03) of ADC_enhancement in the longitudinal analysis, but there was no difference between responders and nonresponders. There were no group differences in ADC histogram metrics of tumors that progressed and those that showed no progression in the 24-month study period. Total tumor volume was found to be negatively associated with the SD of ADC_total (P = .008).
Optic Pathway and Hypothalamic Glioma: NF1 versus Sporadic
Twenty-five subjects with sporadic OPHGs in stratum 4 were compared with the cohort with 15 NF1-associated low-grade OPHGs from stratum 3. No difference was found in PFS between the 2 groups.9 Enhancing tumor volume and cyst volume at baseline were both significantly lower in the NF1-associated OPHG in stratum 3 compared with stratum 4 (P < .001), while there was no difference in FLAIR tumor volume between the groups.
Significant differences between the groups were found in baseline values of ADC_total mean (P = .005, q = 0.01), mode (P = .005, q = 0.01), median (P = .002, q = 0.003), skewness (P < .001, q < 0.001), and kurtosis (P = .01, q = 0.02). The sporadic stratum 4 cohort had higher values of ADC_total mean, mode, and median and lower values of skewness and kurtosis. Similarly, ADC_enhancement showed higher values in sporadic OPHG for mean (P = .045, q = 0.09) and median (P = .04, q = 0.09) compared with NF1-associated OPHG. In the longitudinal analysis of change with time on treatment, the ADC_total median decreased significantly more in the sporadic cohort in stratum 4 compared with NF1-associated stratum 3 OPHG (P = .02, q = 0.06) as shown in Fig 2, with the ADC_total mean similarly approaching significance (P = .04, q = 0.11).
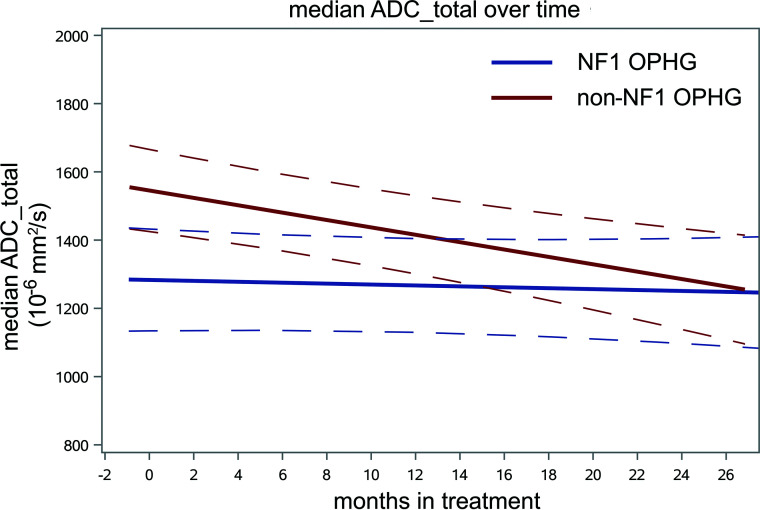
FIG 2.
The longitudinal trend of median ADC of the total tumor volumes for the cohort of 25 sporadic non-NF1 OPHGs from stratum 4 shown in red, compared with the 15 NF1-associated OPHGs shown in blue. Mixed-effects models were used to estimate trends with time in ADC parameters as well as differences in longitudinal changes between groups. Sporadic OPHG values are higher at baseline (P = .002, q = 0.003) and show a greater decrease with time (P = .02, q = 0.06) compared with NF1-associated OPHG.
DISCUSSION
The past decade has seen an explosion of molecular data showing that most pLGGs upregulate the RAS–mitogen-activated protein kinase pathway,15,16 most commonly including BRAF fusion or mutation of the BRAF gene17,18 and NF1 mutation.16 More than 80% of pilocytic astrocytomas have gene alterations in some component of the MEK signaling pathway;3 therefore, several therapies have been developed to target this pathway. BRAF resistance and tumor progression have been reported in some therapies such as sorafenib targeting non-V600e aberrations,19,20 leading to therapies targeting downstream pathway components like MEK. Selumetinib is one such potent orally available MEK 1/2 inhibitor that has shown promise in pLGG.7-9
Pilocytic astrocytoma is the most frequent primary brain tumor in children and can occur anywhere in the central nervous system, with the most common locations being the cerebellum (40%), followed by supratentorial locations (35%), the optic pathway and hypothalamus (11%), and the brain stem (9%).21 Histopathologically, pilocytic astrocytoma is a tumor of low-to-moderate cellularity with compact, densely fibrillated areas rich in Rosenthal fibers, as well as spongy loosely textured areas composed of multipolar cells that have varying degrees of mucoid background material, often with microcysts.22 Most OPHGs, including those associated with NF1, are pilocytic astrocytomas.23 The pathology of these tumors makes it highly likely that the extracellular matrix is an important contributor to the ADC of these tumors.
ADC maps derived from diffusion-weighted images measure the diffusivity of water molecules in tissue. In the context of brain tumors, ADC is influenced by tumor cellularity and the extracellular matrix, as well as the presence of edema, cystic components, and necrosis. Several studies have reported an inverse relationship between ADC and cellularity in a variety of pediatric brain tumors.24,25 As cellularity increases, there is less extracellular space for water diffusion, leading to a lower ADC. Jost et al26 examined the ADC in a cohort of 14 NF1-associated OPHGs and 13 sporadic OPHGs and found that ADC was not associated with either NF1 status or clinical aggressiveness. Yeom et al,27 however, found that in a retrospective study of a cohort of 5 patients with NF1 and 7 with sporadic pediatric OPHG, a higher baseline ADC was predictive of tumor progression and that ADC then declined following subsequent chemotherapy with a standard combination of carboplatin and vincristine. Similarly, Hsu et al28 reported that the ADC declined during the response to bevacizumab in a cohort of 8 patients with progressive pLGG. Whereas all the above studies used the mean ADC of the tumor volume, we used ADC histogram analyses to better characterize the ADC distribution in the tumors.
ADC histograms derived from the ADC of all voxels in a tumor volume are particularly well-suited to characterize the diffusion properties of the entire tumor volume in brain tumors and have been used to differentiate pediatric brain tumors by histologic type29 and tumor grade.30 ADC histogram parameters have also been associated with molecular subtype31 and response to therapy12,32 in diffuse intrinsic pontine gliomas in children. Herein, we report the use of ADC histogram metrics to identify prognosis and response criteria in refractory, recurrent, or progressive pLGGs treated with selumetinib.
Pilocytic astrocytomas in stratum 1 that responded to selumetinib had a smaller SD of baseline ADC_total, suggesting that more homogeneous tumors responded better. In addition, the mean, median, and mode of ADC_total all decreased more during the course of treatment for responders compared with nonresponders. This decrease is similar to that found by Hsu et al28 in a small sample of recurrent or progressive pLGGs treated with bevacizumab. The transient increase in these ADC metrics seen in the first few months of treatment is possibly due to increased water mobility due to cell death, followed by tissue consolidation leading to a stable decrease in these values. Patients in stratum 1 with tumors that had the KIAA1549-BRAF fusion had a longer PFS than those with the BRAF V600E mutation, but there was no difference in the response rate between the groups.8 However, our analyses of ADC histogram metrics showed no difference between the BRAF fusion and BRAF mutation either at baseline or across time, possibly due to small sample size.
NF-1 associated pLGG in stratum 3 showed no association between baseline ADC histogram metrics and either PFS or overall survival. ADC_total mode decreased more with time during therapy in responders in stratum 3, similar to our findings in stratum 1.
Among the patients with sporadic OPHGs in stratum 4, higher baseline skewness and kurtosis of ADC_total were associated with shorter PFS, a finding similar to those in previous reports in pediatric diffuse intrinsic pontine glioma (DIPG).12 Higher skewness and kurtosis signify more homogeneous tumors with lower ADC and are usually associated with higher cellularity in high-grade tumors, but in these low-grade OPHGs, they may be indicative of a lower fraction of extracellular matrix. Longitudinal ADC_total histogram metrics were not associated with response or PFS.
When data from all 3 strata were combined, there were no associations between baseline ADC histogram metrics, and PFS or response, suggesting that strata-specific analyses may be more useful. In a study of newly diagnosed OPHGs treated with carboplatin and vincristine, Yeom et al27 found that tumors with a higher baseline mean had a shorter PFS. Our study comprised previously treated pLGGs not limited to OPHGs, which may explain why we found no baseline associations. However, a greater longitudinal decrease in mode and median ADC_total with time in treatment was seen in responders, similar to our findings in the individual strata. Tumors with smaller total tumor volume were found to have a higher SD of ADC_total, probably due to the higher proportion of cells on the tumor periphery in smaller tumors.
When the longitudinal trends in the individual and combined strata are considered as a whole, a consistent overall picture emerges, suggesting that a steeper drop in ADC_total values during treatment with selumetinib occurs in patients who respond to selumetinib. This finding confirms earlier reports in the literature from Yeom et al27 and Hsu et al,28 who saw similar trends in smaller samples of patients with pLGGs undergoing chemotherapy. This suggests that monitoring ADC during the course of treatment may provide some clinical value in assessing the response in pLGGs. A lower ADC has long been shown to be indicative of higher cellularity,33 and an increase in ADC has been shown to be a biomarker of cell death in response to treatment in high-grade gliomas in adults.34 Our longitudinal results linking a decrease in ADC with response is not consistent with the opposite behavior in high-grade gliomas because these are low-grade gliomas with a more extensive extracellular matrix. Tumor response with selumetinib in pLGGs may be the result of a decrease in extracellular space,27,35 leading to the decrease in ADC seen in responders in our study. The impressive response to selumetinib reported recently in plexiform neurofibromas,36 which are known to have high extracellular matrix content,37 may also be due to changes in the extracellular matrix.
Patients with OPHGs associated with NF1 are known to have longer PFS compared with those with sporadic OPHGs.38 When we compared the 25 sporadic OPHGs in stratum 4 with 15 NF1-associated OPHGs in stratum 3, no significant difference in PFS was seen, possibly due to the small sample size or the relatively short period of time of 24 months used in this analysis. Enhancing tumor volume as well as the volume of cysts were significantly higher in sporadic OPHGs compared with NF1-associated OPHGs, whereas there was no difference in overall tumor volume. The baseline mean, median, and mode of ADC_total as well as baseline mean and median of ADC_enhancement were higher in sporadic OPHGs compared with NF1-associated OPHGs. This result may be explained by the higher incidence of cystic components in sporadic OPHGs that we report, consistent with previous reports.39 The greater decrease with time in median ADC_total we report in sporadic OPHGs in stratum 4 compared with NF1-associated OPHG in stratum 3 (Fig 2) may also be associated with a decrease in cystic components of the tumor.
Although ours was larger than prior studies in the literature, the relatively small sample size and diverse prior treatment histories are limitations of this study.
Machine learning methods have shown promise in differentiating BRAF fusion and mutation40 and could be useful in predicting response to therapy. This study was an observational and exploratory analysis and is the first study, to our knowledge, to look for any associations between treatment response and changes in ADC in pLGGs. Our population was heterogeneous with respect to molecular type as well as tumor location, and response to selumetinib varies by molecular type and tumor location. Future studies with the large homogeneous samples of each tumor type required for artificial intelligence and machine learning algorithms may definitively identify ADC histogram metrics as markers to identify response to selumetinib. Future work may also include exploring associations between ADC histogram metrics and standardized measures of visual outcomes in OPHGs as recommended by Response Assessment in Pediatric Neuro-Oncology.10
CONCLUSIONS
We hypothesized that ADC histogram metrics would be associated with response in pLGGs treated with selumetinib. ADC values in pLGGs decreased with time in treatment with selumetinib in responders compared with nonresponders. Compared with NF1-associated OPHG, ADC decreased with selumetinib treatment in sporadic OPHG. ADC histogram metrics were found to be associated with response of pLGG to selumetinib.
ABBREVIATIONS:
- MEK
MAP (mitogen-activated protein) kinase
- NF1
neurofibromatosis type 1
- OPHG
optic pathway and hypothalamic glioma
- PBTC
Pediatric Brain Tumor Consortium
- PFS
progression-free survival
- pLGG
pediatric low-grade glioma
- WHO
World Health Organization
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
This work was supported by the American Lebanese Syrian Associated Charities, AstraZeneca, US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, National Cancer Institute Cancer Therapy Evaluation Program, National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748, UM1CA081457.
References
- 1.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol 2019;21:v1–100 10.1093/neuonc/noz150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryall S, Tabori U, Hawkins C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol Commun 2020;8:30 10.1186/s40478-020-00902-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins KL, Pollack IF. Pediatric low-grade gliomas. Cancers 2020;12:1152 10.3390/cancers12051152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol 2007;25:682–89 10.1200/JCO.2006.06.8213 [DOI] [PubMed] [Google Scholar]
- 5.Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol 2006;24:2570–75 10.1200/JCO.2005.03.8349 [DOI] [PubMed] [Google Scholar]
- 6.Jones DTW, Kieran MW, Bouffet E, et al. Pediatric low-grade gliomas: next biologically driven steps. Neuro Oncol 2018;20:160–73 10.1093/neuonc/nox141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee A, Jakacki RI, Onar-Thomas A, et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol 2017;19:1135–44 10.1093/neuonc/now282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol 2019;20:1011–22 10.1016/S1470-2045(19)30277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fangusaro J, Onar-Thomas A, Poussaint TY, et al. A phase 2 trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: a Pediatric Brain Tumor Consortium Study. Neuro Oncol 2021;23:1777–88 10.1093/neuonc/noab047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fangusaro J, Witt O, Hernáiz Driever P, et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 2020;21:e305–16 10.1016/S1470-2045(20)30064-4 [DOI] [PubMed] [Google Scholar]
- 11.Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. Neuroimage 2012;62:782–90 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Poussaint TY, Vajapeyam S, Ricci KI, et al. Apparent diffusion coefficient histogram metrics correlate with survival in diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol 2016;18:725–34 10.1093/neuonc/nov256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–82 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics 2008;24:1461–62 10.1093/bioinformatics/btn209 [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, Pfister S, Bouffet E, et al. Pediatric low-grade gliomas: implications of the biologic era. Neuro Oncol 2017;19:750–61 10.1093/neuonc/now209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryall S, Zapotocky M, Fukuoka K, et al. Integrated molecular and clinical analysis of 1,000 pediatric low-grade gliomas. Cancer Cell 2020;37:569–83 10.1016/j.ccell.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones DT, Kocialkowski S, Liu L, et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene 2009;28:2119–23 10.1038/onc.2009.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lassaletta A, Zapotocky M, Mistry M, et al. Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol 2017;35:2934–41 10.1200/JCO.2016.71.8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karajannis MA, Legault G, Fisher MJ, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol 2014;16:1408–16 10.1093/neuonc/nou059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci U S A 2013;110:5957–62 10.1073/pnas.1219232110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkhard C, Di Patre PL, Schüler D, et al. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg 2003;98:1170–74 10.3171/jns.2003.98.6.1170 [DOI] [PubMed] [Google Scholar]
- 22.Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol 2015;129:775–78 10.1007/s00401-015-1410-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Listernick R, Louis DN, Packer RJ, et al. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol 1997;41:143–49 10.1002/ana.410410204 [DOI] [PubMed] [Google Scholar]
- 24.Gauvain KM, McKinstry RC, Mukherjee P, et al. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. AJR Am J Roentgenol 2001;177:449–54 10.2214/ajr.177.2.1770449 [DOI] [PubMed] [Google Scholar]
- 25.Koral K, Mathis D, Gimi B, et al. Common pediatric cerebellar tumors: correlation between cell densities and apparent diffusion coefficient metrics. Radiology 2013;268:532–37 10.1148/radiol.13121362 [DOI] [PubMed] [Google Scholar]
- 26.Jost SC, Ackerman JW, Garbow JR, et al. Diffusion-weighted and dynamic contrast-enhanced imaging as markers of clinical behavior in children with optic pathway glioma. Pediatr Radiol 2008;38:1293–99 10.1007/s00247-008-1003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeom KW, Lober RM, Andre JB, et al. Prognostic role for diffusion-weighted imaging of pediatric optic pathway glioma. J Neurooncol 2013;113:479–83 10.1007/s11060-013-1140-4 [DOI] [PubMed] [Google Scholar]
- 28.Hsu CH, Lober RM, Li MD, et al. Decreased tumor apparent diffusion coefficient correlates with objective response of pediatric low-grade glioma to bevacizumab. J Neurooncol 2015;122:491–96 10.1007/s11060-015-1754-9 [DOI] [PubMed] [Google Scholar]
- 29.Payabvash S, Aboian M, Tihan T, et al. Machine learning decision tree models for differentiation of posterior fossa tumors using diffusion histogram analysis and structural MRI findings. Front Oncol 2020;10:71 10.3389/fonc.2020.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vajapeyam S, Brown D, Johnston PR, et al. Multiparametric analysis of permeability and ADC histogram metrics for classification of pediatric brain tumors by tumor grade. AJNR Am J Neuroradiol 2018;39:552–57 10.3174/ajnr.A5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaimes C, Vajapeyam S, Brown D, et al. MR imaging correlates for molecular and mutational analyses in children with diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol 2020;41:874–81 10.3174/ajnr.A6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vajapeyam S, Brown D, Billups C, et al. Advanced ADC histogram, perfusion, and permeability metrics show an association with survival and pseudoprogression in newly diagnosed diffuse intrinsic pontine glioma: a report from the Pediatric Brain Tumor Consortium. AJNR Am J Neuroradiol 2020;41:718–24 10.3174/ajnr.A6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 1999;9:53–60 [DOI] [PubMed] [Google Scholar]
- 34.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 2000;92:2029–36 10.1093/jnci/92.24.2029 [DOI] [PubMed] [Google Scholar]
- 35.Hoyt WF, Baghdassarian SA. Optic glioma of childhood: natural history and rationale for conservative management. Br J Ophthalmol 1969;53:793–98 10.1136/bjo.53.12.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 2020;382:1430–42 10.1056/NEJMoa1912735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lévy P, Bièche I, Leroy K, et al. Molecular profiles of neurofibromatosis type 1-associated plexiform neurofibromas: identification of a gene expression signature of poor prognosis. Clin Cancer Res 2004;10:3763–71 10.1158/1078-0432.CCR-03-0712 [DOI] [PubMed] [Google Scholar]
- 38.Deliganis AV, Geyer JR, Berger MS. Prognostic significance of type 1 neurofibromatosis (von Recklinghausen Disease) in childhood optic glioma. Neurosurgery 1996;38:1114–18; discussion 1118–19 10.1097/00006123-199606000-00010 [DOI] [PubMed] [Google Scholar]
- 39.Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. AJNR Am J Neuroradiol 2001;22:1963–69 [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner MW, Hainc N, Khalvati F, et al. Radiomics of pediatric low-grade gliomas: toward a pretherapeutic differentiation of BRAF-mutated and BRAF-fused tumors. AJNR Am J Neuroradiol 2021;42:759–65 10.3174/ajnr.A6998 [DOI] [PMC free article] [PubMed] [Google Scholar]