Abstract
The processes involved in cell death are complex, and individual techniques measure specific fractions of the total population. The interaction of Candida albicans with amphotericin B was measured with fluorescent probes with different cellular affinities. These were used to provide qualitative and quantitative information of physiological parameters which contribute to fungal cell viability. SYBR Green I and 5,(6)-carboxyfluorescein were used to assess membrane integrity, and bis-(1,3-dibutylbarbituric acid)trimethine oxonol and 3,3-dihexyloxacarbocyanine iodide were used to evaluate alterations in membrane potential. The fluorescent indicators were compared with replication competency, the conventional indicator of viability. By using these tools, the evaluation of the response of C. albicans to amphotericin B time-kill curves delineated four categories which may represent a continuum between alive and dead. The data showed that replication competency (CFU per milliliter) as determined by conventional antifungal susceptibility techniques provided only an estimate of inhibition. Interpretation of fluorescent staining characteristics indicated that C. albicans cells which were replication incompetent after exposure to greater than 0.5 μg of amphotericin B per ml still maintained degrees of physiological function.
Candida albicans is both a commensal and opportunistic pathogen of humans. Morbidity and mortality associated with systemic infections caused by C. albicans remain unacceptably high because of difficulties in diagnosis and treatment (11). A mainstay of treatment for patients with invasive mycoses is the polyene macrolide antifungal amphotericin B (AmB). AmB binds to ergosterol, the principal sterol in the fungal cytoplasmic membrane. AmB molecules are believed to insert into the fungal cytoplasmic membrane and form pore-like structures, which culminate in osmotic instability, loss of membrane integrity, and metabolic disruption (4, 6).
Antifungal susceptibility testing remains dependent on the enumeration of replication-competent yeast cells with long incubation times and semiquantitative and subjective endpoints (11, 22, 28). Better, direct methods are required to evaluate yeast viability and the processes of fungal cell death and replicative deactivation to further our understanding of fungus-drug interactions. The process of cell replication deactivation as envisaged by Jones (16) involves a stepwise change in the physiochemical state of a cell which renders an intermediate form incapable of initiating replicative processes but still capable of metabolism. Measurements of qualitative and quantitative characteristics essential to fungal cell viability can be achieved with great precision by utilizing fluorescent probes which have specific cellular affinities (8, 13, 25). In combination, these vitality- and mortality-specific dyes monitor several physiological processes, such as membrane integrity, monitored with the fluorescent intercalating dye SYBR Green I, intracellular enzyme activity, monitored with the fluorogenic substrate 5,(6)-carboxyfluorescein diacetate (CFDA), and alterations in membrane potential, monitored with the fluorescent potentiometric probes bis-(1,3-dibutylbarbituric acid)trimethine oxonol [DiBAC4(3)] and 3,3-dihexyloxacarbocyanine iodide [DiOC6(3)]. AmB-treated C. albicans was investigated by comparing the levels of fluorescence from the four different probes to a standard time-kill curve. The results are consistent with the presence of four different phenotypic states that are dependent on the concentration of AmB and the exposure time.
MATERIALS AND METHODS
Yeast strains.
C. albicans ATCC 90028 (AmB MIC, 0.5 μg/ml) was obtained from the American Type Culture Collection (Rockville, Md.). C. albicans 97-150 (AmB MIC, 0.5 μg/ml) and 96-90 (AmB MIC, 0.5 μg/ml) were obtained from the National Center for Mycology, Division of Microbiology and Public Health, Edmonton, Alberta, Canada. The MIC of AmB for these strains was determined by broth microdilution (22).
Culture conditions and kill curve.
C. albicans from frozen stock cultures was subcultured twice on Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.) prior to use. The yeast strains were grown aerobically in yeast-peptone-dextrose (YPD) broth (1% mycological peptone, 1% yeast extract, 3% d-glucose) on a rotary shaker at 35°C for 10 to 12 h until the desired concentration of ∼4 × 106 cells/ml (confirmed by plate counts) was obtained. A total of 100 ml of culture was decanted into 500-ml Erlenmeyer flasks, and the appropriate concentration of AmB (Fungizone; Squibb Canada) was added from a −75°C stock of 10,000 μg/ml in dimethyl sulfoxide (DMSO). The culture flasks containing a range of AmB concentrations were then returned to incubation at 35°C in the dark. Each experiment included a control culture that was not exposed to AmB. The cells in these cultures were present as blastoconidia. Each incubating culture flask was sampled at 1.5, 4.5, and 10 h and then assayed directly (1-ml samples) to quantitate intracellular ATP, total number of cells per milliliter, CFU per milliliter, and vitality- and mortality-specific dye fluorescence.
Plate counts.
The culture samples were grown on Sabouraud dextrose agar plates to assess reproductive competency (CFU per milliliter). After incubation at 35°C for 48 h, the colonies were counted. Samples were plated in triplicate after appropriate serial dilutions in 0.85% physiological saline.
Particle counts.
A 1-ml culture sample was centrifuged at 9,300 × g for 5 min at 25°C and resuspended in 1 ml of 0.1 M MOPS (3-[morpholino]propanosulfonic acid-sodium) (pH 7.0). The number of cells per milliliter in the sample was assayed with a Coulter M430 Counter (Coulter Electronics Inc., Hialeah, Fla.).
Vitality- and mortality-specific fluorescent dyes.
Fluorescent dyes were added after incubation of cultures in AmB for 1.5, 4.5, and 10 h, which eliminated uncertainties concerning growth-inhibiting effects of the staining process and also enhanced the ability to detect fungistatic activity (13). Pilot experiments established the most effective dye concentration, incubation time, temperature, pH, and number of wash steps. All samples were initially centrifuged at 9,300 × g for 5 min at the time of sampling. The stained culture samples were aliquoted (200 μl per well) in triplicate into a 96-well Nunc-Immuno PolySorp plate (Nunc, Nalge Nunc International, Rochester, N.Y.) and assayed for relative fluorescence intensity (RFU) with a FL500 microplate fluorescence reader (Bio-Tek Instruments Inc., Winooski, Vt.). All the fluorescent dyes could be optimally evaluated by using excitation and emission wavelengths of 485 and 530 nm, respectively. Stained cells were evaluated qualitatively by fluorescence microscopy.
(i) CFDA treatment.
C. albicans cells were resuspended in MOPS buffer (0.1 M MOPS, pH 7), washed two more times, and resuspended a final time in MOPS buffer (pH 3) plus 50 mM citric acid. CFDA (Sigma Chemical Co., St. Louis, Mo.) stock in DMSO was added, 10 μl of a 5-mg/ml concentration of stock, to each 1-ml sample for a final concentration of 50 μg/ml. Incubation with the stain was in the dark at 35°C, with shaking for 45 min. No additional wash step was then required.
(ii) DiOC6(3) treatment.
C. albicans cells were resuspended in MOPS buffer (pH 10). DiOC6(3) (Sigma Chemical Co.) stock in DMSO was added, 25.6 μl of a 20-μg/ml concentration, to each 1-ml sample for a final concentration of 0.5 μg/ml. Incubation with the stain was in the dark at room temperature for 30 min. The sample was then diluted 1:30 with Nonidet P-40 detergent (Sigma Chemical Co.) and incubated for 1 h with shaking in the dark at room temperature. Samples were then washed twice by centrifugation at 9,300 × g for 5 min and resuspension in MOPS buffer (pH 7.0). Samples were maintained on ice.
(iii) DiBAC4(3) treatment.
C. albicans cells were resuspended in MOPS buffer (pH 7.0). DiBAC4(3) (Molecular Probes Inc., Eugene, Oreg.) stock in 100% ethanol was added, 2 μl of a 1-mg/ml concentration, to each 1-ml sample for a final concentration of 2 μg/ml. Incubation with the stain was in the dark at room temperature with shaking for 1 h. The samples were then washed in MOPS buffer (pH 7.0) two times, as described above. Samples were maintained on ice.
(iv) SYBR Green I treatment.
C. albicans cells were resuspended in MOPS buffer (pH 7.0). SYBR Green I (14) in MOPS buffer (pH 7.0) was added, 15 μl of a 1:100 dilution of stock, to each 1-ml sample. Incubation with the stain was in the dark on ice at 4°C for 1 h. The samples were then washed two times in MOPS buffer (pH 7.0), as described above. Samples were maintained on ice.
Bioluminescence assay of ATP.
The luciferase ATP assay was used to assay the effect of AmB on viable C. albicans cell biomass (24). The viable-cell count of C. albicans exposed to an antifungal agent has been shown to be directly related to intracellular ATP levels (2). ATP was assayed by measuring luminescence produced by the oxidation of luciferin in the presence of luciferase and ATP.
(i) Analytical equipment and reagents.
Light emission from the bioluminescence assay was measured in a Bio-Orbit (Turku, Finland) 1258 microplate luminometer. The luminescence reaction temperature was set internally to 21°C. The ATP assay mix (Sigma Chemical Co.) containing luciferin and luciferase was prepared fresh according to the manufacturer. Apyrase (purified grade I; Sigma Chemical Co.) was used to eliminate extracellular ATP before the extraction of intracellular ATP.
(ii) Elimination of extracellular ATP.
A culture sample of 1 ml was centrifuged at 9,300 × g and resuspended in Tris-EDTA buffer (0.1 M Tris buffer [pH 7.8] containing 2 mM EDTA). The washed sample, 50 μl, was incubated for 15 min at 37°C with 50 μl of 0.04% apyrase ATPase.
(iii) Extraction of intracellular ATP.
After elimination of the extracellular ATP, 50 μl of the apyrase-treated sample was pipetted into 500 μl of boiling Tris-EDTA buffer. After boiling for 90 s, the extracts were cooled and frozen at −75°C for later analysis.
(iv) Luciferase ATP assay.
The ATP assay mix (80 μl) was added to 200 μl of each thawed sample extract (in triplicate) in a nonstandard 96-well opaque plate (Corning Costar Corp., Cambridge, Mass.), and the intensity of the luminescence was determined for 10,000 ms after 1 min of incubation. The ATP concentration present in the sample extracts was determined with an ATP standard curve. ATP added to the extracts was used as an internal standard to correct for inhibition of the luciferase reaction. Correction for machine background luminescence was made by direct subtraction.
RESULTS
Replication competency and cell counts.
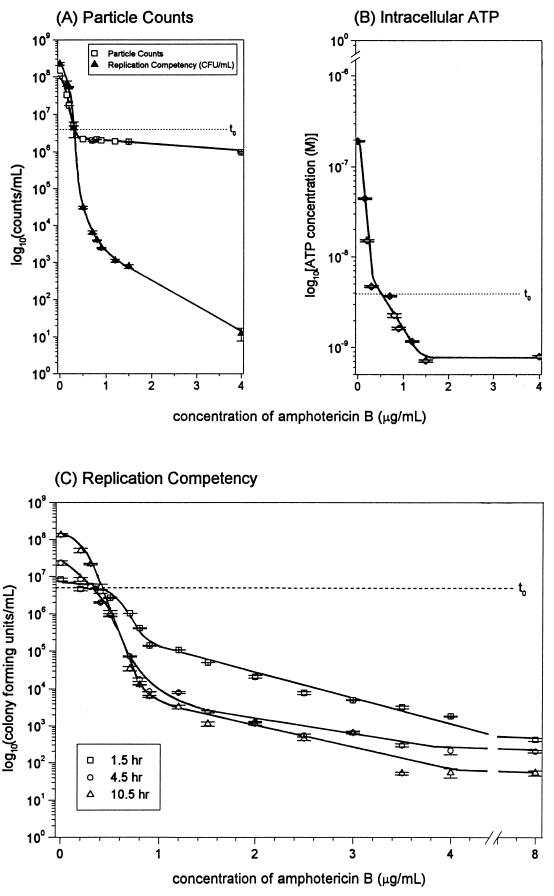
Increasing AmB concentration resulted in a dose-dependent reduction of replication competency for C. albicans to a maximum effect with 4 μg/ml at 10 h of incubation (Fig. 1A). AmB concentrations higher than 4 μg/ml did not increase the extent or rate of killing. The number of yeast cells present in cultures exposed to AmB as measured by particle counts (Fig. 1A) did not decrease below that present at the time of culture inoculation (t0), and an increase in particle counts after t0 coincided with growing cultures (0 to 0.3 μg of AmB per ml) (Fig. 1A). Replication competency and intracellular ATP content both decreased below t0 levels in cultures exposed to AmB concentrations greater than or equal to 0.5 μg/ml (Fig. 1A and B). Beyond 4.5 h, there was no significant additional decrease in replication competency in response to AmB (Fig. 1C). Growth (CFU per milliliter), albeit suboptimal in comparison to that of the control culture, was shown to occur at 4.5 and 10 h for cultures exposed to concentrations of AmB up to 0.3 μg/ml. C. albicans culture exposed to 0.4 μg of AmB per ml showed no significant increase or decrease in replication competency over the time course. A concentration of 0.4 μg of AmB per ml was thus fungistatic. A decrease in agar plate counts indicative of 99% inhibition of growth for C. albicans at 10 h occurred at 0.5 μg/ml (Fig. 1A), which corresponded to (i) the predetermined MIC and (ii) the lowest AmB concentration shown to be capable of causing a decrease in CFU per milliliter at 10 h (Fig. 1C).
FIG. 1.
Evaluation of incubation of C. albicans 96-90 with increasing concentrations of AmB, including replication competency and particle counts at 10 h (A), intracellular ATP at 10 h (B), and replication competency at 1.5, 4.5, and 10 h (C) t0 represents a measurement of the C. albicans culture prior to incubation with AmB. Error bars indicate standard error.
Intracellular ATP.
The decrease in intracellular ATP at 10 h reached a plateau at an AmB concentration of 1.2 μg/ml and was paralleled by a decrease in replication competency (Fig. 1A and B). The lowest AmB concentration tested that resulted in a 99% reduction of intracellular ATP concentration after 10 h of incubation was defined as the AmB minimum effective concentration and was determined to be 0.2 μg/ml. The detection limit for detecting intracellular ATP with this assay was 10−10 M.
Vitality-specific fluorescent staining.
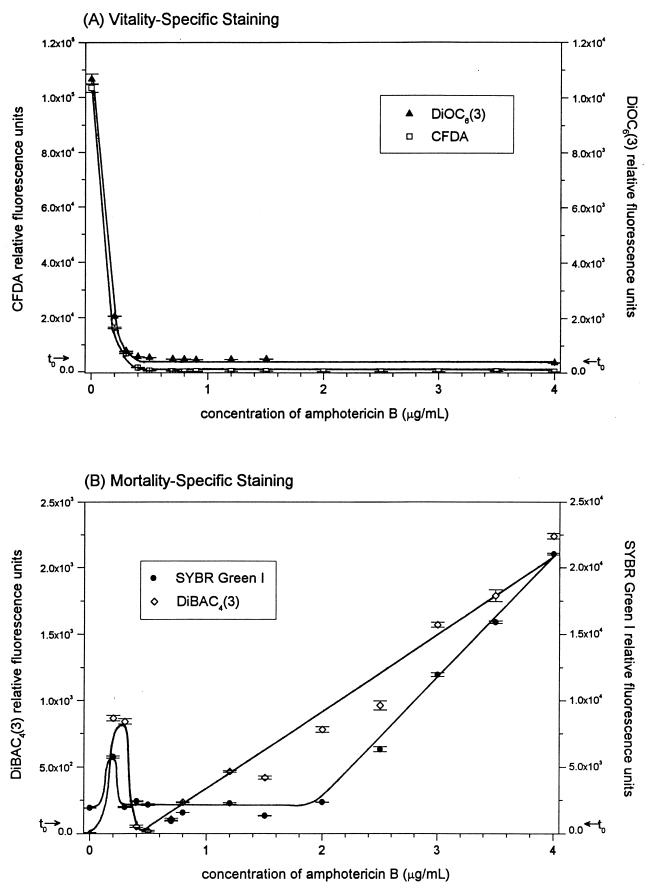
Fluorescent staining of AmB-treated C. albicans 96-90 with CFDA and DiOC6(3) (Fig. 2A) showed an exponential decrease in RFU in a dose-dependent manner, plateauing at a concentration of 0.5 μg of AmB per ml. The other two strains behaved similarly (data not shown). The decreased fluorescence intensity presumably corresponded to decreased intracellular sequestration of dye within the cell (CFDA) or a decreased membrane binding [DiOC6(3)].
FIG. 2.
Evaluation of incubation of C. albicans 96-90 with AmB at 10 h. Evaluation using vitality-specific fluorescent staining [DiOC6(3) and CFDA] (A) and mortality-specific fluorescent staining [DiBAC4(3) and SYBR Green I] (B) are shown. t0 represents a measurement of the C. albicans culture prior to incubation with AmB. Error bars indicate standard error.
Mortality-specific fluorescent staining. (i) SYBR Green I.
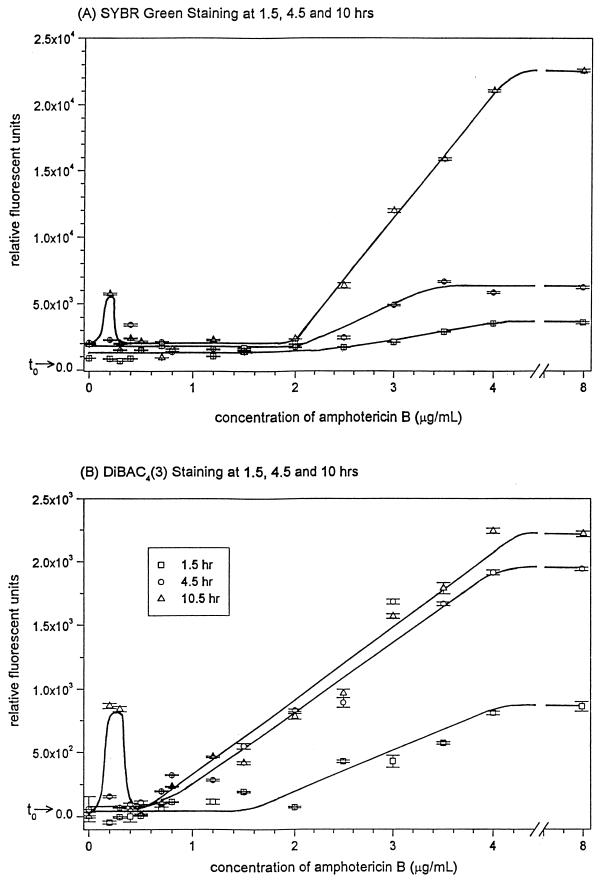
C. albicans 96-90 cells stained with SYBR Green I showed a linear increase in RFU with increased exposure to AmB at concentrations between 2.0 and 4.0 μg/ml, with a plateau in RFU between 0.4 and 2.0 μg/ml and a peak in RFU at 0.2 μg/ml (Fig. 2B). SYBR Green I mortality-specific staining showed a gradual increase in staining from 1.5 to 10 h for cultures exposed to greater than 2.0 μg of AmB per ml, with RFU values at an AmB incubation time of 4.5 h intermediate to those at 1.5 and 10 h (Fig. 3A). The peak in RFU present in the culture incubated with 0.2 μg of AmB per ml was shown to occur at 10 h of incubation and not earlier. SYBR Green I staining was similar for the other two C. albicans strains tested (data not shown).
FIG. 3.
Time-kill curves for C. albicans 96-90 incubated with AmB at 1.5, 4.5, and 10 h, as evaluated by mortality-specific staining. t0 represents a measurement of the C. albicans culture prior to incubation with AmB. Error bars indicate standard error.
(ii) DiBAC4(3).
Fluorescent staining of C. albicans 96-90 with DiBAC4(3) showed a linear increase in RFU in cell cultures incubated with increasing AmB concentrations between 0.8 and 4.0 μg/ml (Fig. 2B). This increase occurred at incubation times greater or equal to 4.5 h (Fig. 3B). At 1.5 h of exposure to AmB, mortality-specific staining did not increase below a concentration of 2.0 μg/ml. A peak in DiBAC4(3)-specific staining occurred in cultures exposed to 0.2 and 0.3 μg of AmB per ml, which coincided with the cultures which grew during AmB exposure. This peak in fluorescence was present at 10 h but not at the early times of 1.5 or 4.5 h (Fig. 3B). DiBAC4(3) staining was equally effective for the other two C. albicans strains tested (data not shown).
(iii) Replication competency and mortality-specific staining comparison.
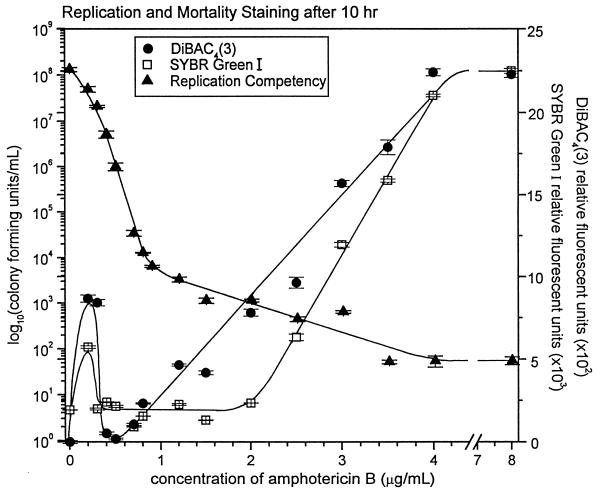
Over the time course of C. albicans culture incubation with AmB, the greatest mortality-specific fluorescent staining occurred after 10 h and only with cultures which had a significant reduction in replication competency. A direct graphical comparison of these results accentuates the observation that these cells, after 10 h of exposure to AmB at concentrations between 0.5 and 1.0 μg/ml, are not able to replicate on agar plates but do not take up mortality-specific dyes (Fig. 4).
FIG. 4.
Time-kill curves for C. albicans 96-90 incubated with AmB at 10 h, as evaluated by replication competency and mortality-specific staining. Error bars indicate standard error.
DISCUSSION
Yeast viability is a measure of the number of living cells, whereas vitality can be seen as a function of the total cell viability and the physiological state of that population (18). Difficulties in quantifying microbial killing are due in large part to which properties are attributed to the state of being alive, since the presence of dead microbes must be inferred retrospectively from estimates of these properties. For instance, reproductive competency as established through plate counts has long been the property considered to be the “gold standard” for cellular viability, even though it is recognized that only a fraction of viable cells replicate when stressed (21). Direct measurements of yeast cell vitality and mortality would provide a better understanding of antifungal activity and perhaps lead to advances in design of antifungals. Vitality- and mortality-specific fluorescent dyes, interpreted jointly, distinguish not only between live and dead microorganisms but also between the “vigorous, frail and injured” (19), potentially providing a summary evaluation of the true viability of each subpopulation affected by the drug.
The number of C. albicans cells, as detected by particle count, present in the AmB-exposed cultures did not decrease below that initially present at the time of culture inoculation, suggesting that the cells while incapable of replication on agar plates were still intact (Fig. 1A). Preliminary observations with scanning electron microscopy confirmed the presence of intact cells. Depending on their physiologic state, intact cells are able to take up and bind fluorescent dyes.
Metabolic pathways can be stoichiometrically related through the adenine nucleotide system (9), and thus intracellular ATP was used as a measure of C. albicans cell metabolic potential and was shown to decrease in a dose-dependent manner with increasing AmB concentration (Fig. 1B). The lowest concentration of AmB that was inhibitory to replication and caused a decrease in intracellular ATP content was 0.5 μg/ml, which corresponded to both the predetermined MIC and the minimum concentration of AmB which showed a dose-dependent decrease in fluorescent staining with the vitality-specific dyes CFDA and DiOC6(3) (Fig. 2A).
CFDA is a lipophilic, nonpolar substrate which traverses the cell membrane and is hydrolyzed by nonspecific intracellular esterases to the fluorescent anion carboxyfluorescein (27). Cells with compromised membranes rapidly leak carboxyfluorescein, even when residual esterase activity is retained intracellularly (15). DiOC6(3) is a lipophilic, cationic dye molecule that has an affinity for the negatively polarized membranes of living cells (20, 26). AmB concentrations between 0 and 0.5 μg/ml reduced the vitality of C. albicans cultures as defined by esterase activity and membrane integrity (CFDA), electrochemical potential [DiOC6(3)], and metabolic potential (ATP).
The AmB time-kill curves showed inhibition of replication competency (CFU per milliliter) with increasing concentrations of AmB between 0.5 and 4.0 μg/ml (Fig. 1A). Both of the fluorescent mortality-specific dyes, SYBR Green I and DiBAC4(3), showed increases in fluorescence for cultures exposed to increasing concentrations of AmB, reaching a maximum fluorescence at 4.0 μg of AmB per ml.
We observed several significant differences between the mortality-specific staining of SYBR Green I and that of DiBAC4(3). SYBR Green I is a nucleic acid-binding dye which increases in fluorescence after intercalation into double-stranded DNA. Intact membranes thus exclude the dye and prevent binding to the DNA, and we assume that significant damage to the cell membrane must take place to allow access. SYBR Green I did not bind to DNA significantly until cultures were exposed to concentrations of AmB above 2.0 μg/ml for a full 10 h (Fig. 3A). Above this concentration, cells also plateaued to a minimum intracellular ATP concentration, which may also indicate extensive damage to the cell membrane. A concentration of 2 μg of AmB per ml may represent the threshold required to induce sufficient membrane damage to saturate the cellular repair mechanism. At incubation times of 1.5 and 4.5 h, even concentrations of AmB above 2.0 μg/ml did not result in significant uptake of SYBR Green I.
DiBAC4(3) (Fig. 3B) is an anionic lipophilic dye sensitive to membrane potential. Normal cells have a negative internal charge and thus exclude the dye. Damaged cells depolarize, allowing the dye to penetrate, bind to lipid-rich intracellular components, and fluoresce (3). The loss of membrane potential is thus inferred from increased DiBAC4(3) cellular staining (8, 10). Similar to the results obtained with SYBR Green I, the cultures exposed to AmB for 1.5 h showed increasing dose-responsive staining only with AmB concentrations above 2.0 μg/ml. However, incubations of 4.5 and 10 h with AmB concentrations between 0.5 and 4.0 μg/ml resulted in dose-dependent increases in fluorescence. Thus, replication-incompetent cells that do not take up mortality-specific dyes can be induced to take them up with increased concentrations of AmB, increased incubation time, or a combination of the two. DiBAC4(3) detection of this transition requires at least 1 μg of AmB per ml for 4.5 h, and SYBR Green I detection requires at least 2 μg/ml for 10 h. It is reasonable to assume that while both of these populations are incapable of replication, they represent physiological states which are part of a viability continuum between alive and dead.
We have delineated four physiologic states as expressed by six different markers of viability after exposure of C. albicans to increasing concentrations of AmB (Table 1 and Fig. 4). These represent a progression along the continuum from viability to death. Cells exposed to AmB at concentrations between 0.5 and 1.0 μg/ml do not show uptake of vitality- or mortality-specific dyes and are replication incompetent but may represent cells capable of resuscitation. The possibility of resuscitation and outgrowth of these replication-deficient C. albicans cells in a systemic infection could represent an important therapeutic problem, especially in an immunocompromised host. Postantifungal growth suppression with subsequent recovery of C. albicans has been shown previously with AmB at these concentrations and incubation times and may in part explain this phenomenon (29). Gale et al. (12) have also shown that C. albicans cultures entering the stationary phase of growth develop phenotypic resistance to AmB due to ultrastructural changes in the cell wall. Possible resuscitation or phenotypic resistance of these replication-incompetent cells, which do not show uptake with either vitality- or mortality-specific dyes, has not yet been determined.
TABLE 1.
Summary of the four physiological states of C. albicans as a consequence of incubation with increasing concentrations of amphotericin B (AmB) for 10 h
| Method of evaluation | Physiological parameter measured | Result at AmB concn (μg/ml) ofa:
|
|||
|---|---|---|---|---|---|
| 0–0.5 | 0.5–1.0 | 1.0–2.0 | 2.0–4.0 | ||
| Agar plate counts (<99% inhibition of control) | Replication competency | Yes | No | No | No |
| Intracellular ATP (<99% inhibition of control) | Metabolic potential | Yes | No | No | No |
| Vitality dyes | |||||
| DiOC6(3) | Negative membrane potential | Yes | No | No | No |
| CFDA | Intracellular enzyme activity | Yes | No | No | No |
| Mortality dyes | |||||
| DiBAC4(3) | Positive membrane potential | No | No | Yes | Yes |
| SYBR Green I | Loss of membrane integrity | No | No | No | Yes |
Yes, positive result for the physiological parameter being measured; no, negative result for the physiological parameter being measured.
With clinical use, peak serum concentrations of AmB with conventional intravenous doses are initially between 0.5 to 2.0 μg/ml, fall rapidly, and then slowly reach a plateau between 0.2 and 0.5 μg/ml (5). It is interesting that C. albicans exposed to this concentration range was shown to retain viability, and this may help explain the need for prolonged treatment and frequent clinical failure with this drug.
Cultures exposed to AmB concentrations between 0.2 and 0.3 μg/ml for 10 h permitted suboptimal growth to occur (Fig. 1C), and an increase in mortality-specific dye fluorescence was observed (Fig. 3). This peak in fluorescence may be the result of an increase in the number of nonviable cells present in stationary phase at 10 h, which accompanies the increase in total cell number from t0. Alternatively, the cytoplasmic membrane and/or cell wall of C. albicans synthesized during suboptimal growth in the presence of AmB concentrations between 0.2 and 0.3 μg/ml may have increased permeability and allowed access of the mortality-specific dye (17). Anomalous findings have been previously reported with C. albicans exposed to low, sublethal AmB concentrations, including increased CFU (7), reduced adherence and germ tube formation (23), and reduced synthesis of surface mannan (1).
It has been suggested that in cell populations under stress, an equilibrium exists whereby small disturbances are tolerated but extreme stress reduces vitality (19). This may result in a physiologic state where true viability is retained but normal cellular functions, such as replication or the maintenance of membrane potential, are reduced (19).
Increasing exposure of C. albicans cells for 10 h to AmB concentrations up to 0.5 μg/ml results in greatly decreased uptake of vitality-specific dyes, decreased concentrations of intracellular ATP, and greatly reduced replication. The remaining cells show gradually decreasing membrane potential with AmB concentrations above 1.0 μg/ml and decreased membrane integrity at concentrations above 2.0 μg/ml. Above 4.0 μg/ml, increasing AmB concentration or incubation time did not result in further decreases in membrane potential or membrane integrity.
Comparison of the time-kill curve to the fluorescent dye data of the AmB-exposed C. albicans cells allowed for a separate assessment of replication competency, vitality, and mortality. Using these indicators, we were able to describe four different response categories of C. albicans to AmB, which represented a progressive spectrum of AmB-induced cell damage (Table 1). This demonstrates that the processes taking place during the exposure of C. albicans to AmB occur gradually and that the failure of replication is only one measurement of the process, which may lead to cell death. The work described here provides evidence for the utility and potential clinical importance of evaluating mortality and vitality separately to develop an overall understanding of true viability. We believe that these findings indicate that further investigation of the physiological changes of C. albicans in response to AmB exposure is warranted.
REFERENCES
- 1.Al-Bassam T, Poulain D, Giummelly P, Lematre J, Bonaly R. Chemical and antigenic alterations of Candida albicans cell walls related to the action of amphotericin B sub-inhibitory doses. J Antimicrob Chemother. 1985;15:263–269. doi: 10.1093/jac/15.3.263. [DOI] [PubMed] [Google Scholar]
- 2.Ansehn S. In vitro synergistic action of antimycotics and antibiotics on Candida albicans. Curr Ther Res. 1977;22:92–99. [Google Scholar]
- 3.Bashford C L, Chance B, Smith J C, Yashida T. The behavior of oxonol dyes in phospholipid dispersion. Biophys J. 1979;25:63–85. doi: 10.1016/S0006-3495(79)85278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beggs W. Physiochemical cell damage in relation to lethal amphotericin B action. Antimicrob Agents Chemother. 1994;38:363–364. doi: 10.1128/aac.38.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett J. Antifungal agents. In: Mandell G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious diseases. 3rd ed. New York, N.Y: Churchill Livingstone; 1990. pp. 404–406. [Google Scholar]
- 6.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 7.Brajtburg J, Elberg S, Medoff G, Kobayashi G. Increase in colony-forming units of Candida albicans after treatment with polyene antibiotics. Antimicrob Agents Chemother. 1981;19:199–200. doi: 10.1128/aac.19.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter E, Paul F, Hunter P. Cytometric evaluation of antifungal agents. In: Lloyd D, editor. Flow cytometry in microbiology. London, United Kingdom: Springer-Verlag; 1993. pp. 111–120. [Google Scholar]
- 9.Chapman A, Atkinson D. Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol. 1977;15:253–306. doi: 10.1016/s0065-2911(08)60318-5. [DOI] [PubMed] [Google Scholar]
- 10.Dinsdale G, Lloyd D. Yeast vitality during cider fermentation: two approaches to the measurement of membrane potential. J Inst Brew. 1995;101:453–458. [Google Scholar]
- 11.Espinel-Ingroff A, Pfaller M A. Antifungal agents and susceptibility testing. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 1405–1414. [Google Scholar]
- 12.Gale E F, Ingram J, Kerridge D, Notario V, Wayman F. Reduction of amphotericin resistance in stationary phase cultures of Candida albicans by treatment with enzymes. J Gen Microbiol. 1980;117:383–391. doi: 10.1099/00221287-117-2-383. [DOI] [PubMed] [Google Scholar]
- 13.Green L, Petersen B, Steimel L, Haeber P, Current W. Rapid determination of antifungal activity by flow cytometry. J Clin Microbiol. 1994;32:1088–1091. doi: 10.1128/jcm.32.4.1088-1091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haugland R P. Handbook of fluorescent probes and research chemicals. 5th ed. Eugene, Oreg: Molecular Probes, Inc.; 1992. [Google Scholar]
- 15.Jepras R I, Carter J, Pearson S C, Paul F E, Wilkinson M J. Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl Environ Microbiol. 1995;61:2696–2701. doi: 10.1128/aem.61.7.2696-2701.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones R P. Measures of yeast death and deactivation and their meaning, parts I and II. Process Biochem. 1987;22:118–134. [Google Scholar]
- 17.Kwan B V, Medoff G, Kobayashi G, Schlessinger D. Potentiation of the antifungal effects of antibiotics by amphotericin B. Antimicrob Agents Chemother. 1974;2:61–65. doi: 10.1128/aac.2.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lentini A. A review of the various methods available for monitoring the physiological status of yeast: yeast viability and vitality. Fermentation. 1993;6:321–327. [Google Scholar]
- 19.Lloyd D, Hayes A. Vigour, vitality, and viability of microorganisms. FEMS Microbiol Lett. 1995;133:1–7. [Google Scholar]
- 20.Lloyd D, Moran C, Suller M, Dinsdale G, Hayes A. Flow cytometric monitoring of rhodamine 123 and cyanine dye uptake by yeast during cider fermentation. J Inst Brew. 1996;102:251–259. [Google Scholar]
- 21.Mason C A, Hamer G, Bryers J D. The death and lysis of microorganisms in environmental processes. FEMS Microbiol Rev. 1986;39:373–401. [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 23.Nugent K, Couchot K. Effects of sublethal concentrations of amphotericin B on Candida albicans. J Infect Dis. 1986;154:665–669. doi: 10.1093/infdis/154.4.665. [DOI] [PubMed] [Google Scholar]
- 24.Odds F C. Interactions among amphotericin B, 5-fluorocytosine, ketoconazole, and miconazole against pathogenic fungi in vitro. Antimicrob Agents Chemother. 1982;22:763–770. doi: 10.1128/aac.22.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Gorman M, Hopfer R. Amphotericin B susceptibility testing of Candida species by flow cytometry. Cytometry. 1991;12:743–747. doi: 10.1002/cyto.990120808. [DOI] [PubMed] [Google Scholar]
- 26.Ordonez J, Wehman N. Amphotericin B susceptibility of Candida species assessed by rapid flow cytometric membrane potential assay. Cytometry. 1995;22:154–157. doi: 10.1002/cyto.990220213. [DOI] [PubMed] [Google Scholar]
- 27.Pringle J, Preston R, Adams A, Stearns T, Drubin D, Haarer B, Jones E. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- 28.Rex J, Pfaller M, Rinaldi M, Polak A, Galgiani J. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnidge J D, Gudmundsson S, Vogelman B, Craig W A. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J Antimicrob Chemother. 1994;34:83–92. doi: 10.1093/jac/34.1.83. [DOI] [PubMed] [Google Scholar]