Abstract
BACKGROUND AND PURPOSE:
Prognostic factors of stroke-like migraine attacks after radiation therapy (SMART) syndrome have not been fully explored. This study aimed to assess clinical and imaging features to predict the clinical outcome of SMART syndrome.
MATERIALS AND METHODS:
We retrospectively reviewed the clinical manifestations and imaging findings of 20 patients with SMART syndrome (median age, 48 years; 5 women) from January 2016 to January 2020 at 4 medical centers. Patient demographics and MR imaging features at the time of diagnosis were reviewed. This cohort was divided into 2 groups based on the degree of clinical improvement (completely versus incompletely recovered). The numeric and categoric variables were compared as appropriate.
RESULTS:
There were statistically significant differences between the completely recovered group (n = 11; median age, 44 years; 2 women) and the incompletely recovered group (n = 9; median age, 55 years; 3 women) in age, months of follow-up, and the presence of steroid treatment at diagnosis (P = .028, .002, and .01, respectively). Regarding MR imaging features, there were statistically significant differences in the presence of linear subcortical WM susceptibility abnormality, restricted diffusion, and subcortical WM edematous changes in the acute SMART region (3/11 versus 8/9, P = .01; 0/11 versus 4/9, P = .026; and 2/11 versus 7/9, P = .022, respectively). Follow-up MRIs showed persistent susceptibility abnormality (11/11) and subcortical WM edematous changes (9/9), with resolution of restricted diffusion (4/4).
CONCLUSIONS:
Age, use of steroid treatment at the diagnosis of SMART syndrome, and MR imaging findings of abnormal susceptibility signal, restricted diffusion, and subcortical WM change in the acute SMART region can be prognostic factors in SMART syndrome.
Stroke-like migraine attacks after radiation therapy (SMART) syndrome is a rare delayed complication of radiation therapy. It can consist of subacute onset of stroke-like symptoms, including headache, seizure, visuospatial deficits, unilateral hemianopsia, facial droop, confusion, hemiparesis, or aphasia, often in conjunction with migraine attacks with or without an aura.1-3 The diagnostic criteria proposed by Black et al,1 in 2006, include the following: 1) remote history of cranial radiation; 2) prolonged, reversible unilateral cortical signs and symptoms beginning years after radiation with manifestations as listed above; 3) transient, diffuse, unilateral cortical gray matter enhancement sparing the WM; and 4) not attributed to any other disorder.1,3 The onset of symptoms varies from 1 to 37 years after completion of radiation therapy, and doses of ≥50 Gy have been used in many cases, though SMART syndrome can also occur with lower doses.3,4 Other reported delayed reversible neurologic complications of brain irradiation include peri-ictal pseudoprogression5 and acute late-onset encephalopathy after radiation therapy (ALERT) syndrome.6 Recently, it has been proposed that SMART syndrome, peri-ictal pseudoprogression, and ALERT syndrome are within the same spectrum of late-onset complications of brain irradiation.6
The characteristic MR imaging features of SMART syndrome include reversible unilateral gyral T2 and FLAIR hyperintensity with cortical enhancement in a distribution not consistent with vascular territories.1-6 However, there are additional conventional MR imaging features reported, such as superficial siderosis, diffusion restriction, and brain stem lesions.2,7,8 Other reported imaging findings include hypermetabolism of the lesion on [18F] FDG-PET/CT, increased CBV on perfusion imaging, and decreased NAA with increased Cr and Cho peaks on MRS.7,9-11 However, these imaging features are mainly reported by case reports or small case series because of the rarity of the disease.
Clinically, CSF testing and electroencephalogram (EEG) are often performed. Findings of CSF testing are usually normal or may demonstrate a mild CSF pleocytosis with elevated protein, and EEG may demonstrate slowing or epileptiform features.2,12 There is no specific treatment for SMART syndrome, but steroids and antiepileptic drugs have been used.5,12 The neurologic symptoms and characteristic MR imaging features typically resolve, but the symptoms can remain persistent despite long-time observation, with a high rate of recurrence.1,2,13,14 Still, the relationship between clinical outcomes and imaging features has not been fully investigated. Therefore, our study aimed to assess the factors associated with the outcome of clinical SMART syndrome by reviewing patient demographics, neurologic symptoms, and MR imaging features at diagnosis.
MATERIALS AND METHODS
Study Population
This international multicenter retrospective study was approved by each institutional review board, and the requirement for informed consent was waived. Data were acquired in compliance with all applicable Health Insurance Portability and Accountability Act regulations.
We retrospectively reviewed 20 cases of SMART syndrome (median age, 48 years; age range, 29–69 years; 5 women, 15 men) with available MR imaging at diagnosis from April 2014 to January 2020 in multiple centers. Patients were followed clinically for 1–70 months (median, 8.5 months). We applied the diagnostic criteria by Black et al.1 Clinical follow-up was performed and assessed by the neurology department at each institution. On the basis of this assessment, the cohort was divided into 2 groups: the completely recovered group and the incompletely recovered group. Biopsy was not performed in any of the cases.
The completely recovered group (n = 11) was composed of patients whose neurologic symptoms were completely resolved or back to baseline during the follow-up period, while the incompletely recovered group (n = 9) was composed of patients whose neurologic symptoms were not completely resolved or who were clinically determined to have a fixed or persistent residual deficit. The follow-up period was defined as the time from diagnosis of SMART syndrome to the last neurologic or MR imaging assessment. The neurologic symptoms included seizure, migraine-type headache, hemiparesis, speech impairment, visual disturbance, confusion, and lethargy.
The treatments included steroid (intravenous administration including pulse therapy; dose period, 3–5 days), antiepileptic drugs and/or antimigraine drugs (levetiracetam, verapamil, divalproex sodium, phenytoin, valproate, aspirin), and/or an antiangiogenic drug (bevacizumab) for the acute episode of SMART syndrome.
Patient Demographics
Patient demographics were reviewed from the electronic medical records and included the following information: age and sex, original oncologic diagnosis, history of surgical resection of a tumor, the presence of concurrent chemotherapy at the original oncologic diagnosis, radiation dose and its distribution, time since the completion of brain irradiation, neurologic symptoms at a SMART syndrome episode, the presence of a migraine during a SMART syndrome episode, drug treatment during the SMART syndrome episode, the follow-up period, the results of EEG and lumbar puncture (LP) examinations, and recurrence of SMART syndrome after the first episode.
MR Imaging Acquisition
MR imaging studies in all cases were performed during the acute symptomatic phase of SMART syndrome. MR imaging studies were acquired on multiple scanners including the following: 1.5T scanners (Achieva, n = 6, Philips Healthcare; Signa Excite, n = 1, and Signa HDxt, n = 3, GE Healthcare; Avanto, n = 1, Siemens); and 3T scanners (Magnetom Vida, n = 4, Siemens). MR imaging was performed with contrast for all patients. Sequences including axial T2WI, axial FLAIR, axial pre- and postcontrast T1WI, echo-planar DWI, SWI/gradient recalled-echo T2*WI, and DSC MR imaging (relative CBV [rCBV]) (6 cases) were interpreted. The last follow-up MR imaging for each case (range, 1–20 months) was evaluated.
MR Imaging Features
Two board-certified neuroradiologists with 7 and 9 years of experience interpreted all MR imaging sequences independently. They were aware of the diagnosis, but were blinded to the clinical information. Both radiologists evaluated the following imaging features:
Gyral enhancement evaluated on pre- and postcontrast T1-weighted images (yes/no)
Cortical edema evaluated on T1-weighted images, T2-weighted images, and FLAIR (yes/no)
Restricted diffusion evaluated on DWI and ADC (yes/no), relative to the surrounding parenchyma
Hypointensity on SWI/T2*WI in the subcortical WM along the cortex (yes/no); in 3 cases in which SWI was not available, gradient recalled-echo T2*WI was used
Increased rCBV (yes/no) relative to surrounding parenchyma
The presence of a cavernoma or microhemorrhage in the whole brain remote from the acute lesion (yes/no)
Subcortical WM edematous change evaluated on T2-weighted images and FLAIR (yes/no).
Statistical Analysis
Numeric variables, such as age, follow-up period, and the time since the completion of brain irradiation were compared using the Mann-Whitney U test. The remaining binary variables (presence of surgical resection of the tumor, presence of concurrent chemotherapy at the original oncologic diagnosis, presence of migraine during a SMART syndrome episode, presence of each drug treatment during the SMART syndrome episode, and presence of recurrence of SMART syndrome after first episode), neurologic symptoms at the time of diagnosis, and MR imaging features were compared between the 2 groups using the Fisher exact test.
For MR imaging features, interreader agreement was assessed by κ analysis, which was interpreted as follows: <0.40, poor-to-fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and 0.81–1.00, almost perfect agreement.15
All statistical calculations were conducted with JMP Pro, Version 15.0.0 (SAS Institute). Variables with P < .05 were considered statistically significant.
RESULTS
Patient Demographics
The completely recovered group (n = 11; median age, 44 years; 2 women) received radiation therapy for metastatic melanoma (2 cases), anaplastic oligodendroglioma, teratoma of the third ventricle, pineoblastoma, diffuse large B-cell lymphoma, oligodendroglioma, astrocytoma, anaplastic astrocytoma, medulloblastoma, and ganglioglioma.
The incompletely recovered group (n = 9; median age, 55 years; 3 women) received radiation therapy for glioblastoma (2 cases), medulloblastoma, pineal teratoma, lymphoplasmacytic lymphoma, diffuse large B-cell lymphoma, nasal cavity squamous cell carcinoma, oligodendroglioma, and atypical teratoid/rhabdoid tumor.
Table 1 summarizes overall patient demographics. The Online Supplemental Data summarize individual information of age and sex, neurologic symptoms at the diagnosis, original pathology, dose and distribution of radiation, and the result of EEG and LP of each group.
Table 1:
Patient demographicsa
| Total | Completely Recovered | Incompletely Recovered | P Value | |
|---|---|---|---|---|
| No. of patients | 20 | 11 | 9 | NA |
| Sex (female/total) | 5/20 | 2/11 | 3/9 | .62 |
| Age (yr) | 48 (36–55) | 44 (35–48) | 55 (51–63) | .028 |
| Time since the completion of RT (yr) | 10.5 (5–20) | 12 (7–20) | 8 (5–14) | .62 |
| Period of follow-up (mo) | 8.5 (1–18) | 1 (1–2) | 18 (10–22) | .002 |
| Surgical resection | 15/20 | 8/11 | 7/9 | 1 |
| Concurrent chemotherapy at the first diagnosis | 16/20 | 9/11 | 7/9 | 1 |
| Episode of migraine at the diagnosis | 12/20 | 8/11 | 4/9 | .36 |
| Steroid treatment | 11/20 | 3/11 | 8/9 | .01 |
| Antiepileptic or antimigraine drugs | 12/20 | 7/11 | 5/9 | 1 |
| Antiangiogenic drug | 1/20 | 1/11 | 0/9 | 1 |
| Recurrent episode of SMART syndrome | 11/20 | 6/11 | 5/9 | 1 |
Note:—RT indicates radiation therapy; NA, not applicable.
Values were described as median (interquartile range).
There were significant differences in age (median, 44 versus 55 years; P = .028) and the follow-up period (median, 1 versus 18 months; P = .002) between the 2 groups. There was also a significant difference in the presence of steroid treatment during the SMART syndrome episode (completely recovered versus incompletely recovered, 3/11 versus 8/9; P = .01). Otherwise, there was no significant difference in any other patient demographics. EEG findings were abnormal in 75% (15/20). Spinal fluid showed nonspecific high protein in 25% (4/16) in the Online Supplemental Data.
Neurologic symptoms at the diagnosis of SMART syndrome included hemiparesis (13/20; completely recovered versus incompletely recovered 5/11 versus 8/9; P = .07), seizure (11/20; 7/11 versus 4/9; P = .65), migraine-type headache (12/20; 8/11 versus 4/9; P = .36), speech impairment (9/20; 6/11 versus 3/9; P = .41), visual disturbance (4/20; 2/11 versus 2/9; P = 1), confusion (2/20; 1/11 versus 1/9; P = 1), and lethargy (1/20; 0/11 versus 1/9; P = .45). Residual symptoms of the incompletely recovered group included hemiparesis (8/9), speech impairment (2/9), visual disturbance (1/9).
MR Imaging Features
There was a significant difference in the presence of linear hypointensity on SWI/T2*WI in the acute lesion between the completely recovered and the incompletely recovered groups (3/11 versus 8/9; P = .01). T2*WI was used in 3 cases of the incompletely recovered patients. There were also significant differences in restricted diffusion and subcortical WM edematous change in the SMART episode lesion between the 2 groups (0/11 versus 4/9, P = .026; 2/11 versus 7/9, P = .022, respectively). Otherwise, there was no significant difference in the remaining analyzed MR imaging features. In total, gyriform enhancement (20/20), cortical edematous change (19/20), restricted diffusion (4/20), local linear hypointensity on SWI/T2*WI (11/20), local increased rCBV (4/6), and distant cavernomas/microhemorrhages (13/20) were observed. Table 2 summarizes the results of MR imaging features. Representative cases of a completely recovered patient and 2 incompletely recovered patients are shown in Figs 1 and 2, respectively. In the follow-up MRIs, there was resolution of gyriform enhancement (20/20), cortical edematous changes (17/19), restricted diffusion (4/4), and increased rCBV (4/4), with residual local hypointensity on SWI/T2*WI (11/11) and subcortical WM edematous changes (9/9).
Table 2:
MR imaging features of the lesions of SMART syndrome
| Total | Completely Recovered | Incompletely Recovered | P Value | |
|---|---|---|---|---|
| Gyriform enhancement | 20/20 | 11/11 | 9/9 | 1 |
| Cortical edematous change | 19/20 | 11/11 | 8/9 | .45 |
| Restricted diffusion (high DWI and low ADC) | 4/20 | 0/11 | 4/9 | .026 |
| Linear subcortical WM SWI (17)/T2*WI (3) hypointensity | 11/20 | 3/11 | 8/9 | .01 |
| Increased rCBV (DSC MR imaging) | 4/6 | 2/3 | 2/3 | 1 |
| Complication of brain irradiation (cavernoma or microhemorrhage) | 13/20 | 8/11 | 5/9 | .64 |
| Subcortical WM edematous change | 9/20 | 2/11 | 7/9 | .022 |
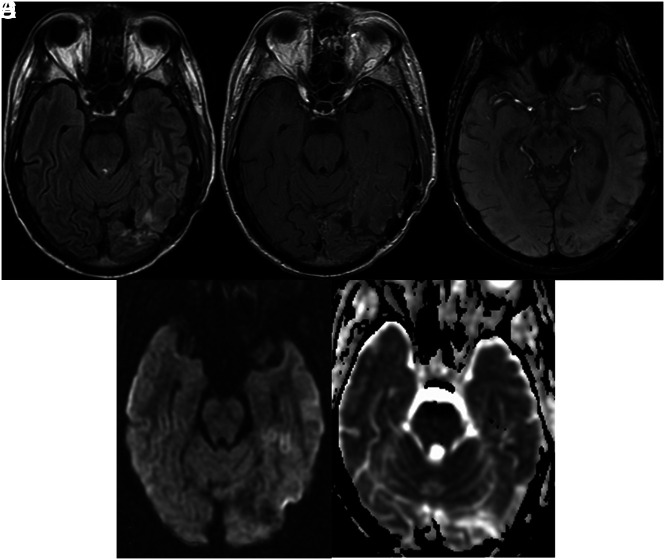
FIG 1.
A 36-year-old man with a history of metastatic melanoma treated with 40 Gy of whole-brain radiation therapy and a partial brain boost of 10 Gy presented with headache, seizure, visual disturbance, and speech impairment. A and B, Cortical edematous changes with gyriform enhancement is seen in the left temporal and occipital lobes. C, SWI shows no convincing focal WM hypointensity. D and E, DWI and ADC show no restricted diffusion. He completely recovered.
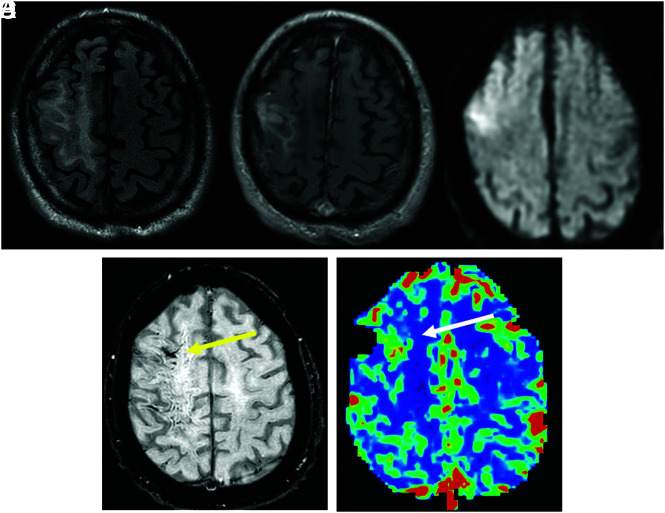
FIG 2.
A 62-year-old man with a history of lymphoplasmacytic lymphoma treated with resection and 40 Gy of whole-brain radiation therapy presented with seizure, left hemiparesis, and speech impairment. A and B, A FLAIR hyperintense region associated with gyriform enhancement and subcortical WM edematous change in the right frontal and parietal lobes. C and D, DWI and SWI show restricted diffusion (ADC not shown) and SWI hypointensity in the same area (yellow arrow). E, DSC MR imaging shows an increase in rCBV in the corresponding area (white arrow). The patient recovered incompletely, with remaining left hemiparesis.
Interreader agreement for tumor characteristics was substantial to almost perfect (κ = 0.7–1).
DISCUSSION
Our retrospective study was aimed at assessing which clinical and imaging features were prognostically relevant in predicting the clinical outcome of SMART syndrome. We compared clinical and imaging characteristics of completely recovered and incompletely recovered groups with SMART syndrome. Our clinical data showed that younger patients with SMART syndrome were more likely to completely recover from neurologic symptoms. Steroid treatment was related to worse clinical outcome. In addition, our imaging data showed that patients who did not fully recover were more likely to show local SWI/T2*WI hypointensity and restricted diffusion in the acute lesion than those in the completely recovered group.
Prior studies suggested that aging is related to a reduced capacity to repair radiation-induced damage,16 and this impaired repair response to radiation therapy with aging could help explain how age could be a prognostic factor for recovery from SMART syndrome.
Steroids are widely used in the clinical setting of increased intracranial pressure and edematous changes. The antiedema effect of steroids stems from their influence on endothelial cells and pericytes, which comprise the blood-brain barrier, and their anti-inflammatory effects from cytokine regulation.17 However, neither of these mechanisms nor the location of action is fully understood. There are some reported acute and delayed complications, including neuropsychiatric symptoms and cognitive impairment.17 Moreover, it has been reported that steroids could negatively influence the endogenous repair processes of the damaged myelin sheath by oligodendrocyte progenitor cells, which are responsible for myelination.18 Prior research in the setting of SMART syndrome has shown that the use of steroids was not significantly associated with worse clinical outcomes.13,19 One study has proposed that to control seizure activity, a short course of high-dose steroids can be considered for treatment.2 However, whether steroid introduction can result in better clinical outcomes has still not been established. Given our data of worse clinical outcomes in the SMART group with steroid use, the pros and cons of steroid use should be carefully considered, though our data may be confounded because steroids were possibly prescribed in more severe cases. Other treatments used in both groups were not related to the clinical outcome. There was a significant difference in the follow-up period between the 2 groups. This is thought to be because residual symptomatology in the incompletely recovered group required prolonged clinical and imaging follow-up from a clinical perspective, though clinical judgment for discontinuing follow-up could be affected by follow-up imaging findings. Otherwise, there were no other significant clinical differences. In our study, the presence of migraine during the SMART syndrome episode was 60% (12/20), which was a smaller percentage than in a previous study that reported an incidence of 73% in patients with SMART syndrome.2 Because migraine is not necessarily present in SMART syndrome, we believe that the presence of migraine should not be a necessary feature for diagnosis but should be regarded as one of the possible associated neurologic symptoms.
Concordant with previous studies that demonstrated a high rate of abnormal findings on EEG,2,12 our results also showed that 75% (15/20) of the patients with SMART syndrome had an abnormal EEG finding. This suggests that EEG can be a practical tool to gain clinical insight into this diagnosis. In contradiction, LP showed nonspecific high proteins in 25% (4/16), suggesting minimal utility of LP for the diagnosis of SMART syndrome. As for neurologic symptoms, there was no statistically significant difference in the completely recovered and the incompletely recovered patients. The most commonly associated neurologic symptoms include hemiparesis (13/20), migraine-type headache (12/20), seizure (11/20), and speech impairment (9/20), findings consistent with those in prior studies.2,13,14 Hemiparesis was the most prevalent remaining symptom at follow-up.
Regarding acute-phase MR imaging features, linear subcortical SWI/T2*WI hypointensity occurred at a significant rate in the incompletely recovered group. Although delayed radiation complications, microbleed, cavernoma, and superficial siderosis have previously been reported,7 the subcortical and linear distribution of this finding are thought to be distinct from the delayed radiation complication mentioned earlier and imply hemorrhagic transformation of lesions in the area acutely affected by SMART syndrome. This linear hypointensity could be a characteristic of a more severe form of SMART syndrome and, hence, could be associated with a worse prognosis.
Restricted diffusion also showed a significant difference between the 2 groups. Restricted diffusion is mainly caused by cytotoxic edema and may reflect more severe tissue damage in patients who incompletely recover, as has been suggested by a previous study.13 In our study, subcortical WM edematous change in the acute lesion was more frequently present in the incompletely recovered group than in the completely recovered group. These WM signal alterations are a known finding of SMART syndrome4,14 but have been not fully investigated. WM signal alteration can imply more severe local WM damage, though these WM changes can also be seen as a delayed effect of brain irradiation,20,21 and the imaging appearance of these lesions can overlap in the setting of SMART syndrome. Other MR imaging features were not significantly different between the 2 groups.
The increase of rCBV was observed in 66% (4/6) of individuals with SMART syndrome. This finding has been reported,10 but the incidence has not been revealed. Our relatively small sample size of DSC MR imaging perfusion suggests that increased rCBV is a feature of the acute phase of SMART syndrome, which can be used to help differentiate this entity from other diagnoses such as radiation necrosis, which is also a known delayed radiation complication. Most interesting, although the findings of gyriform enhancement, cortical edematous changes, restricted diffusion, and elevation of rCBV almost always resolve, associated linear susceptibility abnormality and subcortical WM edematous changes often remained. This result may suggest that the linear susceptibility abnormality and subcortical WM edematous changes do not resolve or demonstrate delayed recovery on imaging, acknowledging the difference in timing of follow-up MRIs.
Our study has several limitations. First, this was a retrospective study with few cases, mainly because of the rarity of the disease. Therefore, we were not able to perform a matched-paired analysis. Second, in 4 cases, details of the radiation therapy were not available due to the delayed onset of the disease and the long clinical course. Third, assessment of restricted diffusion and rCBV was performed on the basis of visual assessment because there were technical differences, including multiple MR imaging vendors and various field strengths, parameters, and sequences, due to the retrospective nature of the study and the different participating treatment centers, limiting quantitative assessment. Finally, there was a difference in when the follow-up MRIs were performed due to the nature of retrospective studies from multiple institutions.
CONCLUSIONS
The clinical outcome of SMART syndrome could be associated with increasing patient age, steroid administration, and MR imaging findings of linear subcortical WM susceptibility signal, restricted diffusion, and subcortical WM edematous changes. Caution should be used regarding the administration of steroids in the setting of SMART syndrome, though further research in a larger patient group is needed.
ABBREVIATIONS:
- ALERT syndrome
acute late-onset encephalopathy after radiation therapy
- EEG
electroencephalogram
- LP
lumbar puncture
- rCBV
relative CBV
- SMART
stroke-like migraine attacks after radiation therapy
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Black DF, Bartleson JD, Bell ML, et al. SMART: stroke-like migraine attacks after radiation therapy. Cephalalgia 2006;26:1137–42 10.1111/j.1468-2982.2006.01184.x [DOI] [PubMed] [Google Scholar]
- 2.Black DF, Morris JM, Lindell EP, et al. Stroke-like migraine attacks after radiation therapy (SMART) syndrome is not always completely reversible: a case series. AJNR Am J Neuroradiol 2013;34:2298–2303 10.3174/ajnr.A3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartleson JD, Krecke KN, O’Neill BP, et al. Reversible, strokelike migraine attacks in patients with previous radiation therapy. Neuro Oncol 2003;5:121–27 10.1093/neuonc/5.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.April D, Lall N, Steven A. Stroke-like migraine attacks after radiation therapy syndrome. Ochsner J 2020;20:6–9 10.31486/toj.19.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rheims S, Ricard D, van den Bent M, et al. Peri-ictal pseudoprogression in patients with brain tumor. Neuro Oncol 2011;13:775–82 10.1093/neuonc/nor082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Stefano AL, Berzero G, Vitali P, et al. Acute late-onset encephalopathy after radiotherapy: an unusual life-threatening complication. Neurology 2013;81:1014–17 10.1212/WNL.0b013e3182a43b1f [DOI] [PubMed] [Google Scholar]
- 7.Pruitt A, Dalmau J, Detre J, et al. Episodic neurologic dysfunction with migraine and reversible imaging findings after radiation. Neurology 2006;67:676–78 10.1212/01.wnl.0000228862.76269.62 [DOI] [PubMed] [Google Scholar]
- 8.Ota Y, Leung D, Moritani T, et al. Atypical imaging findings of presumed stroke-like migraine attacks after radiation therapy syndrome in the brainstem. Neuroradiology 2021;63:1377–81 10.1007/s00234-021-02684-0 [DOI] [PubMed] [Google Scholar]
- 9.Nar Senol P, Gocmen R, Karli Oguz K, et al. Perfusion imaging insights into SMART syndrome: a case report. Acta Neurol Belg 2015;115:807–10 10.1007/s13760-015-0483-3 [DOI] [PubMed] [Google Scholar]
- 10.Biju RD, Dower A, Moon BG, et al. SMART (stroke-like migraine attacks after radiation therapy) syndrome: a case study with imaging supporting the theory of vascular dysfunction. Am J Case Rep 2020;21:e921795 10.12659/AJCR.921795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Cibeira E, Calleja-Castano P, Gonzalez de la Aleja J, et al. Brain magnetic resonance spectroscopy findings in the stroke-like migraine attacks after radiation therapy (SMART) syndrome. J Neuroimaging 2015;25:1056–58 10.1111/jon.12227 [DOI] [PubMed] [Google Scholar]
- 12.Fan EP, Heiber G, Gerard EE, et al. Stroke-like migraine attacks after radiation therapy: a misnomer? Epilepsia 2018;59:259–68 10.1111/epi.13963 [DOI] [PubMed] [Google Scholar]
- 13.Singh TD, Hajeb M, Rabinstein AA, et al. SMART syndrome: retrospective review of a rare delayed complication of radiation. Euro J of Neurol 2021;28:1316–23 10.1111/ene.14632 [DOI] [PubMed] [Google Scholar]
- 14.Di Stefano AL, Berzero G, Ducray F, et al. Stroke-like events after brain radiotherapy: a large series with long-term follow-up. Eur J Neurol 2019;26:639–50 10.1111/ene.13870 [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 16.Lee WH, Sonntag WE, Lee YW. Aging attenuates radiation-induced expression of pro-inflammatory mediators in rat brain. Neurosci Lett 2010;476:89–93 10.1016/j.neulet.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich J, Rao K, Pastorino S, et al. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 2011;4:233–42 10.1586/ecp.11.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarner T, Parabucki A, Beyer C, et al. Corticosteroids impair remyelination in the corpus callosum of cuprizone-treated mice. J Neuroendocrinol 2011;23:601–11 10.1111/j.1365-2826.2011.02140.x [DOI] [PubMed] [Google Scholar]
- 19.Jia W, Saito R, Kanamori M, et al. SMART (stroke-like migraine attacks after radiation therapy) syndrome responded to steroid pulse therapy: report of a case and review of the literature. eNeurologicalSci 2018;12:1–4 10.1016/j.ensci.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crossen JR, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol 1994;12:627–42 10.1200/JCO.1994.12.3.627 [DOI] [PubMed] [Google Scholar]
- 21.Walker AJ, Ruzevick J, Malayeri AA, et al. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 2014;10:1277–97 10.2217/fon.13.271 [DOI] [PMC free article] [PubMed] [Google Scholar]