Abstract
Isoxyl (ISO), a thiourea (thiocarlide; 4,4′-diisoamyloxythiocarbanilide), demonstrated potent activity against Mycobacterium tuberculosis H37Rv (MIC, 2.5 μg/ml), Mycobacterium bovis BCG (MIC, 0.5 μg/ml), Mycobacterium avium (MIC, 2.0 μg/ml), and Mycobacterium aurum A+ (MIC, 2.0 μg/ml), resulting in complete inhibition of mycobacteria grown on solid media. Importantly, a panel of clinical isolates of M. tuberculosis from different geographical areas with various drug resistance patterns were all sensitive to ISO in the range of 1 to 10 μg/ml. In a murine macrophage model, ISO exhibited bactericidal killing of viable intracellular M. tuberculosis in a dose-dependent manner (0.05 to 2.50 μg/ml). The selective action of ISO on mycolic acid synthesis was studied through the use of [1,2-14C]acetate labeling of M. tuberculosis H37Rv, M. bovis BCG, and M. aurum A+. At its MIC for M. tuberculosis, ISO inhibited the synthesis of both fatty acids and mycolic acids (α-mycolates by 91.6%, methoxymycolates by 94.3%, and ketomycolates by 91.1%); at its MIC in M. bovis BCG, ISO inhibited the synthesis of α-mycolates by 87.2% and that of ketomycolates by 88.5%; and the corresponding inhibitions for M. aurum A+ were 87.1% for α-mycolates, 87.2% for ketomycolates, and 86.5% for the wax-ester mycolates. A comparison with isoniazid (INH) and ethionamide (ETH) demonstrated marked similarity in action, i.e., inhibition of the synthesis of all kinds of mycolic acids. However, unlike INH and ETH, ISO also inhibited the synthesis of shorter-chain fatty acids. ISO showed no acute toxicity against primary macrophage cell cultures as demonstrated by diminution of redox activity. A homologous series of ISO derivatives were synthesized. Most derivatives were as effective or more effective than the parent compound in the agar proportion assay. Thus, these thioureas, like INH and ETH, specifically inhibit mycolic acid synthesis and show promise in counteracting a wide variety of drug-sensitive and -resistant strains of M. tuberculosis.
Despite the availability of effective chemotherapies, tuberculosis, among those infectious diseases caused by a single etiology, is still a leading cause of death (22, 36). The human immunodeficiency virus pandemic, which contributes substantially to the morbidity and mortality from tuberculosis (2, 6), and the emergence of multidrug-resistant strains of Mycobacterium tuberculosis (9, 37) have compounded the problem. Although infections with drug-sensitive strains of M. tuberculosis can be successfully cured with the currently used combination of iosoniazid (INH), rifampin, pyrazinamide, and ethambutol or streptomycin (8), the problem of drug resistance and the continuing rise in disease incidence have prompted research on new drug developments, particularly the search for new drug targets and the definition of mechanisms of drug resistance.
INH, which is one of the most efficient and the most widely used antituberculosis drug (51), has been the subject of intensive research on its modes of action and mechanisms of resistance. Both M. tuberculosis and Mycobacterium bovis BCG are extremely susceptible to INH, which is active in the range of 0.02 to 0.2 μg/ml (3). Early work demonstrated that INH specifically inhibits synthesis of mycolic acids in M. tuberculosis (39, 41, 45, 48). INH is a prodrug which requires activation by the endogenous mycobacterial enzyme catalase-peroxidase (KatG) (20, 52) to form an electrophilic species (13, 46, 47) before reacting with targets such as InhA (1). Other targets of the activated INH have been suggested to include two components of the type II fatty acid synthase system, a 12-kDa acyl carrier protein (ACP) designated AcpM and β-ketoacyl ACP synthase (KasA) (18, 19). Ethionamide (ETH), a structural analog of INH, is a useful second-line antituberculosis drug (47), and the two drugs have almost-identical effects in strongly inhibiting the synthesis of mycolic acids, slightly decreasing the synthesis of bound nonmycolic acids, and stimulating the synthesis of soluble lipids in susceptible species of mycobacteria (26, 49). ETH is inhibitory for M. tuberculosis in liquid medium at about 1 μg/ml and can be active against INH-resistant strains (47). The work of Banerjee and colleagues demonstrated that a single mutation in the inhA gene, which is now known to encode an NADH-dependent 2-trans-enoyl ACP reductase, conferred resistance to both INH and ETH, leading to the impression that the modes of action of the drugs were identical (1). However, there is not complete accord in the resistance patterns of ETH and INH; strains resistant to ETH can still be sensitive to INH, while, conversely, strains resistant to INH can show slightly increased sensitivity to ETH (30, 47).
Isoxyl (ISO) (4,4′-diisoamyloxydiphenylthiourea; 4,4′-diisoamyloxythiocarbanilide; thiocarlide) (47) is an old drug used for the clinical treatment of tuberculosis in the 1960s. Urbancik (43, 44) and Titscher (42) demonstrated modest therapeutic efficacy of ISO monotherapy in cases of untreated pulmonary tuberculosis of various degrees of difficulty. The drug was able to convert about 25% of bacteriologically chronically positive cases to negative after 6 to 8 weeks of 6 g of ISO daily. However, when the treatment was extended to 10 to 18 weeks, about 50% of the patient population was converted to sputum negative (14). Schmid (33) concluded that combined INH and ISO was more effective than monotherapy with either drug. It had been noted in the early 1950s that ISO exhibited strong antimycobacterial activity in vitro (47). A note from Winder et al. in 1971 (49) showed that, like INH and ETH, ISO strongly inhibited mycolic acid synthesis in M. bovis during 6 h of exposure to 10 μg/ml. ISO also partially inhibited the synthesis of the fatty acids of free lipids, which were stimulated by INH and ETH. This is the extent of published work conducted on the mechanisms of action of ISO. Consequently, we examined the efficacy of ISO in an attempt to decipher its mode of action.
MATERIALS AND METHODS
Growth and maintenance of mycobacterial strains.
M. tuberculosis H37Ra (TMCC 25711), M. bovis BCG 1173P2, and Mycobacterium avium 724 were grown in 250-ml tissue culture flasks containing 50 ml of liquid Sauton medium and were incubated without agitation. Cells were grown to mid-exponential phase (for M. tuberculosis, ∼21 days; for M. bovis BCG, ∼14 days; and for M. avium, ∼10 days) and harvested, and sterile glycerol was added to a final concentration of 10%. Cell suspensions were dispensed into tubes and stored at −70°C until required. Thawed suspensions were added to 50 ml of Sauton medium to yield identical cultures for further studies. The fast-growing Mycobacterium aurum A+ (from GlaxoWellcome, Stevenage, United Kingdom), which is sensitive to INH, was grown in nutrient broth (Difco Laboratories, Detroit, Mich.) containing 0.05% Tween 80. Cells were incubated to mid-log phase (∼5 days) at 37°C with shaking, as previously described (25). Mycobacterium smegmatis mc2 155 was grown in 250-ml Erlenmeyer flasks containing 100 ml of Sauton medium. Cells were incubated at 37°C with shaking for 4 days, and growth was monitored by measuring the A600. Virulent M. tuberculosis H37Rv (TMCC 102) and Erdman (TMCC 107) were grown in 250-ml Erlenmeyer flasks containing 100 ml of Sauton medium and incubated to mid-exponential phase at 37°C with shaking. A variety of human clinical isolates of M. tuberculosis had been stored in 2-ml aliquots and frozen at −70°C until used. The frozen stocks were counted by serial dilution in saline and plating onto 7H11 agar. The varied drug resistance patterns of these strains are shown in Table 1. Drug resistance profiles were identified at the time of collection, as described elsewhere (24, 29).
TABLE 1.
Antimycobacterial activity of ISO against clinical isolates of M. tuberculosis
| Strain designation | MDRa | Growth inhibition with the following concn of ISO (μg/ml):
|
|||
|---|---|---|---|---|---|
| 1.0 | 2.0 | 5.0 | 10.0 | ||
| CSU 15 | No | No | No | Yes | Yes |
| CSU 21 | Yes | No | No | Yes | Yes |
| CSU 22 | Yes | No | No | No | Yes |
| CSU 31 | Yes | No | No | No | Yes |
| CSU 32 | Yes | No | No | No | Yes |
| CSU 37 | No | No | No | Yes | Yes |
| CSU 39 | Yes | No | No | No | Yes |
| CSU 44 | Yes | No | No | No | Yes |
| W 670 | Yes | No | No | Yes | Yes |
| W 3432 | Yes | No | No | Yes | Yes |
| BB | No | Yes | Yes | Yes | Yes |
| LL | Yes | Yes | Yes | Yes | Yes |
Drug resistance profiles were as follows: CSU 15, INHr Rifs EMBs Strs Kans Cycs Caps ETHs PZAs; CSU 21, INHr Rifr EMBr Strr Rfbr Kans Cycs Caps; CSU 22, INHr Rifr EMBr Strr Kanr Cycs Capr PASs AMKr; CSU 31, INHr Rifr EMBr Strs Kans Cycs Caps PASs AMKs ETHs; CSU 32, INHr Rifr EMBr Strs Kans Cycr Caps ETHr PZAr; CSU 37, INHs Rifs EMBs Strs Kans Cycs Caps PASs AMKs ETHs; CSU 39, INHr Rifr EMBs Strr Kanr Cycs Caps PASs AMKr ETHr PZAs; CSU 44, INHr Rifr EMBr Strr Kanr Cycs Caps PASs AMKs ETHs PZAr; W 670, INHr Rifr EMBr Strr Kanr Cycs Caps ETHs PZAs; W 3432, INHr Rifr EMBr Strr Kanr Cycs Caps AMKr ETHr Cipr; BB, INHs Rifs EMBs Strs Kans Cycs Caps PASs AMKs ETHs PZAs Cips; LL, INHr Rifr EMBs Strr Kanr Cycs Caps PASs AMKr ETHs PZAr. Profiles were determined by a conventional proportion method (10, 11). Strains were classified as multidrug resistant (MDR) if they were resistant to the major antituberculosis drugs INH and rifampin (Rif) or were resistant to these major drugs and one or more of the following: ethambutol (EMB), streptomycin (Str), kanamycin (Kan), cycloserine (Cyc) para-aminosalicylic acid (PAS), rifabutin (Rfb), ETH, amikacin (AMK), capreomycin (Cap), and pyrazinamide (PZA). The following responses were not determined: Rfb for CSU 22, CSU 31, CSU 32, CSU 37, CSU 39, CSU 44, W 670, W 3432, BB, and LL; PAS for CSU 32, W 670, and W 3432; PZA for CSU 37 and W 3432; and AMK for W 670.
Determination of the MICs of ISO and its derivatives.
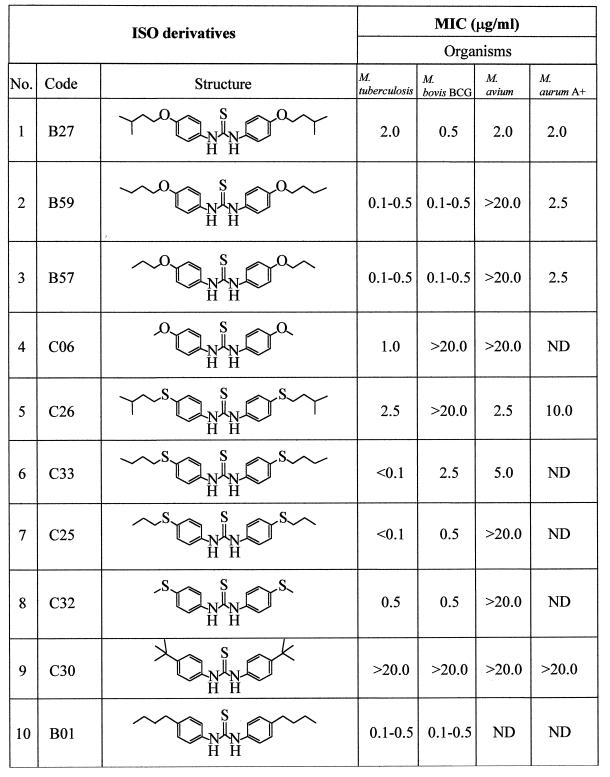
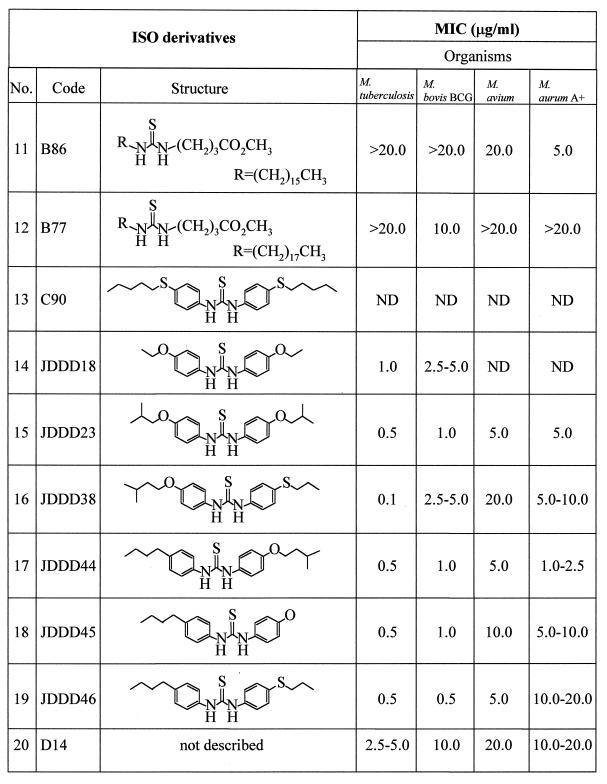
ISO was a gift from M. J. Colston and P. Draper, National Institute of Medical Research, London, United Kingdom. Derivatives of ISO included para-alkoxy, para-alkyl, and other substituted thioureas (Table 2); the synthesis of ISO and its derivatives will be documented separately. The MICs of ISO and its derivatives on solid medium were determined by the microdrop agar proportion test, which was modified from the method of McClatchy (17). Briefly, a series of 10-fold dilutions of cultures of M. tuberculosis H37Ra and H37Rv, M. tuberculosis Erdman, M. bovis BCG, and M. aurum A+ were prepared by using phosphate-buffered saline (8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4 in 1,000 ml of distilled H2O, adjusted to pH 7.4) as a diluent. An aliquot (5 μl) of each dilution was spotted on plates of 7H11 agar (Difco) containing oleic acid-albumin-dextrose-citric acid (OADC) as a supplement (7) and 0.1, 0.5, 1.0, 2.0, 2.5, 5.0, 10.0, and 20.0 μg of each tested drug per ml. The plates were incubated at 37°C (∼4 days for M. smegmatis mc2 155 and M. aurum A+, ∼12 days for M. avium, ∼14 days for M. bovis BCG, and ∼21 days for M. tuberculosis), and the number of viable bacteria was scored by counting colonies. The MIC was defined as the lowest concentration of ISO or its derivatives resulting in a 99% reduction in the number of colonies on that plate compared to those on a plate free of the drug at the same suspension of the culture dilution.
TABLE 2.
MICs of ISO and ISO derivatives against slow-growing (M. tuberculosis H37Ra, M. bovis BCG, and M. avium) and fast-growing (M. aurum A+) mycobacteriaa
The MICs of ISO derivatives against M. tuberculosis, M. bovis BCG, M. avium, and M. aurum A+ were determined on 7H11 agar medium. A series of 10-fold dilutions of cultures (M. tuberculosis, M. bovis BCG, M. avium, and M. aurum A+) in saline buffer were prepared, 5 μl of each culture dilution was spotted on plates of 7H11 containing OADC, and the compounds were tested at 0.1, 0.5, 1.0, 2.0, 2.5, 5.0, 10.0, and 20.0 μg/ml. The inoculated plates were incubated at 37°C as follows: M. tuberculosis for 21 days, M. bovis BCG for 14 days, M. avium for 12 days, and M. aurum A+ for 5 days. The number of colonies was then scored. The MIC was defined as the lowest concentration of compound that reduced 99% of the number of colonies on the plates compared to that on the control plate free of compound (38). ND, not done.
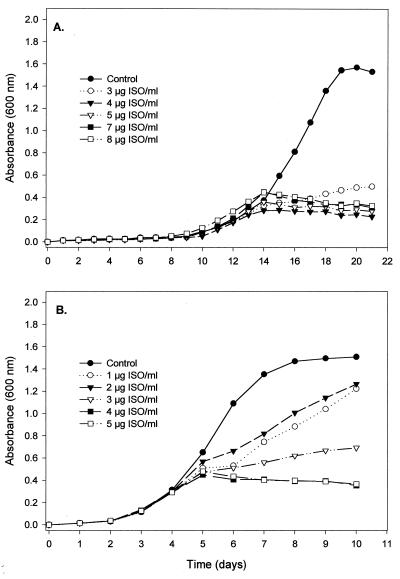
The analysis of growth curves and the estimation of broth MICs of ISO for both M. tuberculosis H37Ra and M. bovis BCG were performed in Sauton medium. Subcultures from master cell stocks were incubated to mid-exponential phase (A600, ∼0.600), and 500 μl of culture was inoculated in triplicate into 13- by 100-mm culture tubes containing 4.5 ml of fresh Sauton medium. The cultures were incubated at 37°C with gentle agitation until early exponential phase (A600, ∼0.250), at which time ISO was added to cultures to final concentrations of 1.0 to 8.0 μg/ml in 1% dimethyl sulfoxide (DMSO). The cultures were then subjected to gentle agitation with small magnetic stirrer bars and incubated at 37°C, and growth was monitored by measuring the A600 with a Bausch & Lomb Spectronic 1001 spectrophotometer once a day. The broth MIC was defined as the lowest concentration able to stop cell growth definitely after one doubling time (26). In all of the experiments, the stocks of ISO and its new derivatives were prepared in DMSO, and it was verified that at concentrations of up to 2% (vol/vol), the solvent had no effect on growth of these bacteria.
Drug susceptibility testing of drug-resistant clinical isolates of M. tuberculosis.
A panel of clinical isolates of drug-resistant strains of M. tuberculosis from different geographical areas with various drug resistance patterns were diluted in microplates with phosphate-buffered saline to a final concentration of 106 CFU/ml for each strain. A sample (5 μl) of the 106-CFU/ml dilution was then spotted on 7H11 supplemented with OADC and containing ISO at different concentrations (1.0, 2.0, 5.0, and 10.0 μg/ml and no ISO in a control plate). After inoculation of drug-resistant strains, the plates were incubated at 37°C for 21 days. Susceptibility of strains to ISO was defined as the absence of colonies on plates after exposure of organisms to ISO for 21 days.
Incorporation of [1,2-14C]acetate into fatty acids and mycolic acids.
M. bovis BCG and M. tuberculosis H37Rv were grown in 5 ml of Sauton medium in a set of culture tubes to early exponential phase, at which point ISO was added (other drugs tested included INH, ETH, and the butyl derivative of ISO). The cells were then incubated with gentle shaking for 10 h prior to addition of [1,2-14C]acetate (Na salt; 58 mCi/mmol; Dupont NEN, Boston, Mass.) at 1 μCi/ml to both control and drug-treated cultures, which were further incubated at 37°C with gentle agitation for an additional 24 h. The resulting 14C-labeled cells were harvested by centrifugation at 2,500 × g and washed twice with saline and once with sterile water (35). In parallel experiments, M. aurum A+ was grown in nutrient broth to early exponential phase, preincubated with ISO for 6 h, and exposed to [1,2-14C]acetate for 12 h.
Determination of the effects of ISO, INH, and ETH on mycolic acid biosynthesis.
The 14C-labeled control and drug-treated cells were resuspended in 2 ml of 15% tetrabutylammonium hydroxide (Sigma Chemical Co., St. Louis, Mo.) and saponified at 100°C overnight. After cooling, 2 ml of water, 3 ml of dichloromethane, and 300 μl of iodomethane (Aldrich Chemical Co., Milwaukee, Wis.) were added to the entire reaction mixture, which was then shaken on a rolling shaker for 1 h. After centrifugation, the upper layer was discarded and the lower organic phase was washed three times with 3 ml of water. The washed lower phase was dried by nitrogen flow, extracted with 4 ml of diethyl ether, sonicated for 5 min, and centrifuged at 2,500 × g (Beckman GPR desktop centrifuge). The ethereal extract was transferred into new 13- by 100-ml glass tubes, dried, and resuspended in 1.0 ml of dichloromethane for counting of radioactivity. Scintillation counting was conducted in vials containing 10 ml of EcoLume (ICN, Costa Mesa, Calif.) by using a Delta 300 scintillation system (Tracor Analytic, Elk Grove, Ill.). Equal volumes of this extract, which was composed of fatty acid methyl esters (FAMEs) and mycolic acid methyl esters (MAMEs), were applied to preparative thin-layer chromatography (TLC) plates of silica gel (5735 Silica Gel 60 F254; Merck, Darmstadt, Germany) and developed six times in petroleum-ether–acetone (95:5) (35). The radioactive bands on the plate were also located and scanned for radioactivity by the BioScan System 200 Imaging Scanner with the Autochanger 3000. Each band was read stepwise for 10 min. Autoradiograms were produced by overnight exposure at −70°C to Kodak X-Omat AR film to reveal the 14C-labeled FAME and MAME products. Separate bands of FAMEs and MAMEs were marked, cut from the TLC plates, and placed directly in 10 ml of EcoLume, and radioactivity was counted to estimate the degree of inhibition of the synthesis of FAMEs and individual population of MAMEs.
A more complete resolution of individual mycolate populations of M. bovis BCG, M. tuberculosis H37Rv, and M. aurum A+ was obtained by two-dimensional silver ion (Ag+) argentation TLC. To prepare TLC plates, 90% of a 10- by 10-cm silica gel plate was immersed in a 5% (wt/vol) aqueous silver nitrate solution, air dried, and activated at 100°C for 1 h prior to use. A known aliquot (ca. ∼80,000 cpm) of the 14C-labeled FAME-MAME mixture was then applied to the Ag+ TLC plate and developed in the first direction in hexane-ethyl acetate (95:5) two times. The plate was air dried and run in the second direction three times in petroleum ether-diethyl ether (85:15). The TLC plate was then exposed to Kodak X-Omat AR film at −70°C overnight, and individual FAMEs and MAMEs were marked.
In vitro murine macrophage model.
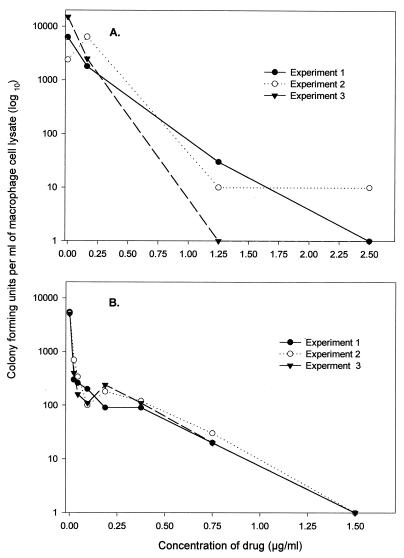
Six- to 8-week-old female specific-pathogen-free C57BL/6 mice were purchased from Charles River Laboratories (Walmington, Mass.) and sacrificed by cervical dislocation, and femurs were aseptically removed. The marrow was flushed out of the femurs with ice-cold Dulbecco’s minimal essential medium (DMEM) supplemented with 10 mM HEPES, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 250 ng of amphotericin B per ml, and 10% L-929 fibroblast conditioned medium (supplemented DMEM [sDMEM]) and with 10% heated-inactivated, low-endotoxin fetal calf serum (Summit Biotechnologies, Inc., Fort Collins, Colo.). All other tissue culture reagents were purchased from Sigma Chemical Co. The marrow plugs were disrupted by gentle pipetting, washed twice, and plated at 106 cells per well in 24-well tissue culture microplates (Falcon 3047; Becton Dickinson, Lincoln Park, N.J.). After 48 h of incubation at 37°C in a 5% CO2 and 96% humidity environment, the nonadherent cells were removed, and new sDMEM was added every 2 days. The sDMEM was changed every 2 days, and 2 days prior to infection, medium free of antibiotic (incomplete sDMEM) was added. Eight days after plating, macrophage cells were infected with 106 CFU of M. tuberculosis Erdman in 200 μl of medium for 2 h. Macrophages were then extensively washed to remove extracellular bacteria and incubated in incomplete sDMEM containing ISO at the concentrations given in Fig. 6A. The series of concentrations of the butyl derivatives of ISO used are given in Fig. 6B. Macrophage cells were lysed in 1 ml of distilled water with 0.05% Tween 80 after 6 days of incubation. Three 10-fold dilutions were made, and 0.1 ml from each dilution was plated on 7H11 medium (Difco) and incubated in a 37°C dry-air incubator. The number of viable bacteria in each well was scored by counting the number of colonies resulting from each dilution on 7H11 plates. As a control, cells in several wells were lysed immediately after the initial infection to determine the number of bacteria phagocytosed and to assess the extent of growth over time.
FIG. 6.
Bactericidal activity of ISO and its butyl derivative against M. tuberculosis Erdman in infected macrophage cell cultures. Murine bone marrow-derived macrophages were infected with 106 bacteria for 2 h, extracellular bacteria were removed, and infected macrophages were incubated with various concentrations of ISO or the butyl derivative for 6 days. Macrophage lysates were then individually plated on 7H11 agar to score the number of viable bacteria. (A) Bactericidal activity of ISO inside murine macrophage cells. ISO at a concentration of 2.5 μg/ml resulted in a 4-log-unit reduction in viable bacteria. (B) Bactericidal activity of the butyl derivative of ISO. At a concentration of 1.5 μg/ml, the butyl derivative completely killed intracellular mycobacteria, resulting in a 3.72-log-unit reduction in viable bacteria.
Determination of possible in vitro toxicities of ISO and the butyl derivative by microplate alamar blue assay.
Murine macrophages were prepared from C57BL/6 mice as described above. On day 6 after plating, the sDMEM was changed, and a fresh 2-ml aliquot of incomplete DMEM was added to each well. On day 8, the old medium was removed, and 1.8 ml of incomplete medium containing ISO or the butyl derivative of ISO at concentrations of 2.0, 1.0, 0.5, 0.25, 0.125, 0.0625, 0.032, and 0.017 μg/ml was added with 200 μl of alamar blue reagent (AccuMed International, Cleveland, Ohio), and plates were incubated at 37°C in the controlled-humidity and -CO2 environment. The color of the alamar blue dye mixed with incomplete DMEM and the cell morphology were observed periodically within 3 days.
RESULTS
Determination of MICs of ISO and ISO derivatives.
It is impossible to find accord in the literature on a standardized means of measuring MICs applicable to the different physical properties of drugs and different mycobacteria. Accordingly, MICs were evaluated under the defined conditions described in Materials and Methods, with species selected to reflect most of the known mycolic acid molecular types. ISO and a series of its derivatives with various substituted side chains were subjected to a preliminary evaluation of their in vitro activities against various species of mycobacteria by applying our defined quantitative agar plate proportion test involving use of 7H11 solid medium containing the OADC supplement. The MIC of ISO for M. tuberculosis was in the range of <1.0 to 2.5 μg/ml, compared to published values of 0.02 to 0.2 μg/ml for INH (3) and 5 to 10 μg/ml for ETH (Table 3). The fast-growing mycobacterium M. aurum A+ was also susceptible to ISO, at a MIC of 2.0 μg/ml. In contrast, M. smegmatis mc2 155 was resistant to ISO as demonstrated by the presence of a number of colonies on 7H11 plates containing concentrations of ISO of as high as 200 μg/ml (Table 3). Thus, M. smegmatis mc2 155 was excluded from further studies. The growth kinetics and the broth MIC of ISO for M. tuberculosis H37Ra were determined in Sauton medium. ISO at 3.0 μg/ml substantially reduced the growth rates of M. tuberculosis and M. bovis BCG, and it inhibited growth entirely at a concentration of 4.0 μg/ml, leading to the conclusion that the broth MIC of ISO for this strain of M. tuberculosis and M. bovis BCG is 4 μg/ml (Fig. 1). The MICs of most of the newly synthesized derivatives of ISO (Table 2) against M. tuberculosis were in the range of <0.1 to 2.5 μg/ml (Table 2). Most of the new derivatives were as effective or more so than ISO. We also examined the efficiency of ISO against a collection of drug-resistant clinical isolates of M. tuberculosis (Table 1). Under the test conditions, ISO exhibited potent antimycobacterial activity against all evaluated strains. In particular, the strains resistant to the major first-line drugs (INH and rifampin) were susceptible to ISO in the range of 1 to 10 μg/ml. No colonies of any of the drug-resistant strains were observed on plates containing 10 μg of ISO per ml (Table 1).
TABLE 3.
MICs of ISO against slow- and fast-growing species of mycobacteria on 7H11 mediuma
| Mycobacterium | MIC (μg/ml) |
|---|---|
| Slow growing | |
| M. tuberculosis Erdman | <1.0 |
| M. tuberculosis H37Rv | 2.5 |
| M. tuberculosis H37Ra | 2.0 |
| M. bovis BCG | 0.5 |
| M. avium | 2.0 |
| Fast growing | |
| M. aurum A+ | 2.0 |
| M. smegmatis mc2 155 | >200.0 |
MICs were determined on solid medium under defined conditions as described in Materials and Methods; i.e., the MIC of ISO was defined as the lowest concentration of ISO that completely inhibited 99% of the growth of the organism (38). Different mycobacterial species reflecting the various types of mycolic acids were included in the assay. The strains most susceptible to ISO then were chosen for further studies on its mode of action.
FIG. 1.
Growth characteristics of M. tuberculosis H37Ra (A) and M. bovis BCG (B) and effects of ISO on growth rates. Cells were grown in Sauton medium to an approximate A600 of 0.250 at 600 nm in a set of culture tubes. ISO in DMSO was added at the indicated concentrations; an equivalent amount of 1% DMSO was added to control cultures. Cells were further incubated and monitored for growth rates once a day. In the presence of 3 μg of ISO per ml, the growth rate of M. tuberculosis H37Ra was reduced, and at 4 μg/ml, ISO completely inhibited growth. Thus, the broth MIC of ISO against M. tuberculosis H37Ra was estimated to be 4 μg/ml. For M. bovis BCG in broth culture, the MIC of ISO was 4 μg/ml. Each data point represents the mean of triplicate readings of A600.
Selective effects of ISO and derivatives on inhibition of fatty acid and mycolic acid synthesis.
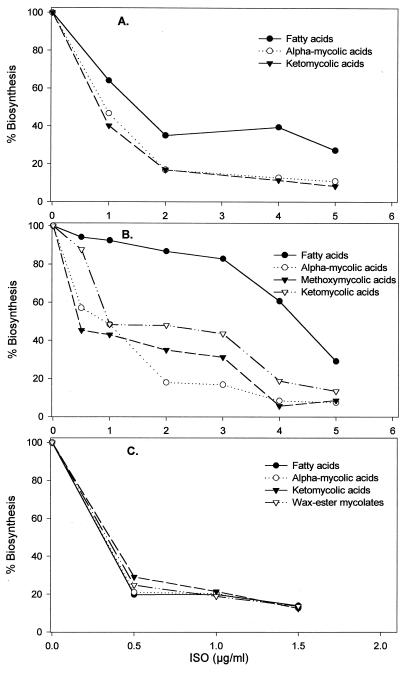
M. bovis BCG was grown in the presence or absence of ISO at various concentrations, following which cultures were labeled with [1,2-14C]acetate. Combined MAMEs and FAMEs were extracted, resolved, and fractionated on TLC plates. The results demonstrated a decrease in the incorporation of radioactivity into FAMEs and MAMEs in the presence of ISO (Fig. 2). Thus, the general effects of ISO were in accordance with those reported by Winder et al. (49), i.e., a generalized inhibition of fatty acid and mycolic acid synthesis. The approach was extended to an examination of the effects of ISO on the individual classes of mycolates. Initial two-dimension TLC demonstrated that the mycolic acid composition of M. bovis BCG consisted primarily of α-mycolates and ketomycolates (Fig. 3), and one-dimensional TLC revealed that, at the appropriate broth MIC of ISO for M. bovis BCG (4.0 μg/ml), the syntheses of α-mycolates and ketomycolates were inhibited by 87.20 and 88.49%, respectively (Table 4). A similar in vivo [1,2-14C]acetate labeling approach was extended to M. aurum A+, which has the advantage of containing α-mycolates, ketomycolates, and wax-ester mycolates, and to M. tuberculosis H37Rv, which contains α-mycolates, methoxymycolates, and ketomycolates (Fig. 4). ISO inhibited the syntheses of all of these classes of mycolic acids and also fatty acids of both M. tuberculosis H37Rv and M. aurum A+ (Fig. 4 and 5). According to the results of one-dimensional TLC, ISO at its respective MICs inhibited the synthesis of α-mycolate by 87.10%, of ketomycolate by 87.20%, and of wax-ester mycolate by 86.48% in the case of M. aurum A+ and inhibited the synthesis of α-mycolate by 91.61%, of methoxymycolate by 94.29%, and of ketomycolate by 91.12% in the case of M. tuberculosis H37Rv (Fig. 5).
FIG. 2.
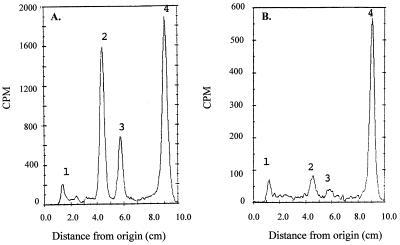
Radioactive scan of a TLC of the FAMEs and MAMEs synthesized by M. bovis BCG under conditions of ISO exposure. The samples applied were a mixture of esters of 14C-labeled fatty acids and mycolic acids from equal control cultures (A) and cultures of M. bovis BCG treated with ISO (5 μg/ml) (B). The time of preexposure of cells to ISO was 10 h prior to the labeling of cells, which lasted for 24 h. Families of FAMEs and MAMEs are indicated. Peak 1, sample origin; peak 2, ketomycolate; peak 3, α-mycolate; peak 4, fatty acid.
FIG. 3.
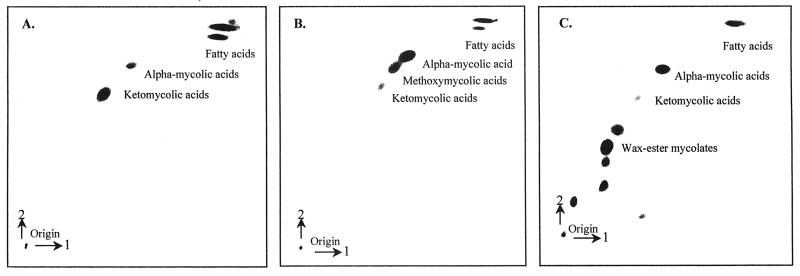
Two-dimensional silver ion argentation autoradiographic TLC of [1,2-14C]acetate-labeled cells of mycobacteria to resolve and identify FAMEs and the different types of MAMEs. (A) M. bovis BCG; (B) M. tuberculosis H37Ra; (C) M. aurum A+. Cells were grown in a set of culture tubes; [1,2-14C]sodium acetate was added to 5 ml of each culture for a final concentration of [14C]acetate of 1 μCi/ml, and cultures were further incubated with gentle agitation at 37°C. Labeled FAMEs and MAMEs were extracted as described in Materials and Methods. The labeling times were 12 h for M. aurum A+ and 24 h for M. tuberculosis H37Ra and M. bovis BCG. About 80,000 cpm of each extract was applied to two-dimensional silver ion argentation TLC plates, which were developed twice in one direction in hexane-ethyl acetate (95:5) and three times in a second direction in petroleum ether-diethyl ether (85:15). Autoradiograms were obtained after exposure to Kodak X-Omat AR film at −70°C for 24 h.
TABLE 4.
Effects of INH, ETH, ISO, and the butyl derivative of ISO on the incorporation of [1,2-14C]acetate into FAMEs and MAMEs of M. bovis BCG
| Agent and concn (μg/ml) | % Inhibitiona
|
||
|---|---|---|---|
| Fatty acids | Mycolic acids
|
||
| Alpha | Keto | ||
| INH | |||
| 0.01 | −12.23 | 14.72 | 16.75 |
| 0.02 | −25.60 | 34.43 | 39.91 |
| 0.10 | −70.00 | 84.36 | 90.54 |
| 1.00 | −46.88 | 97.83 | 97.42 |
| ETH | |||
| 1.00 | −34.62 | 38.15 | 58.95 |
| 2.00 | −47.82 | 63.84 | 99.08 |
| 5.00 | −48.82 | 75.70 | 85.82 |
| 10.00 | −66.88 | 87.76 | 92.51 |
| ISO | |||
| 1.00 | 35.94 | 53.27 | 59.81 |
| 2.00 | 64.90 | 83.07 | 83.09 |
| 4.00 | 60.43 | 87.20 | 88.49 |
| 5.00 | 72.75 | 89.07 | 91.65 |
| Butyl derivative of ISO | |||
| 0.50 | 23.50 | 45.57 | 60.94 |
| 1.00 | 25.57 | 62.44 | 75.92 |
| 5.00 | 40.94 | 72.78 | 91.12 |
Percent inhibition was determined based on the percent difference in incorporation of [1,2-14C]acetate into the free fatty acids and mycolic acids in the presence and absence of the drug. A negative value indicates stimulation of activity. All four drugs inhibited the synthesis of alpha- and ketomycolates. The difference in the effect on fatty acid synthesis is that INH and ETH stimulate the synthesis of short-chain fatty acids, whereas ISO and its derivative inhibit this activity.
FIG. 4.
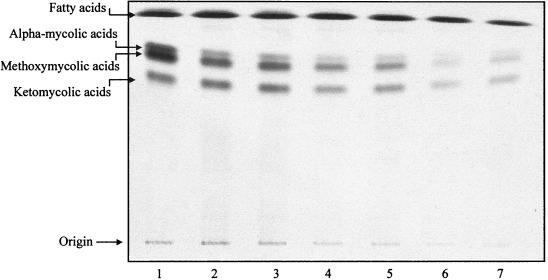
One-dimensional autoradiographic TLC of FAMEs and MAMEs from [1,2-14C]acetate-labeled M. tuberculosis H37Rv in the presence and absence of ISO. A mixture of FAMEs and MAMEs was isolated, and equal volumes of the extract (20 μl of 1 ml) were spotted on aluminum-backed TLC plates, which were developed six times in petroleum-ether–acetone (95:5) in one direction. The resulting radiograms were obtained after exposure to Kodak X-Omat film at −70°C for 24 h. Separated bands of FAMEs and MAMEs were cut out and placed directly into scintillation fluid for radioactivity counting. Degrees of inhibition of FAMEs and each type of MAMEs were determined. Lane 1, control; lane 2, 0.5 μg/ml; lane 3, 1.0 μg/ml; lane 4, 2.0 μg/ml; lane 5, 3.0 μg/ml; lane 6, 4.0 μg/ml; lane 7, 5.0 μg/ml.
FIG. 5.
Dose-response effects of ISO on fatty acid and mycolic acid synthesis in M. bovis BCG (A), M. tuberculosis H37Rv (B), and M. aurum A+ (C). Labeling of cultures was performed in triplicate and terminated by treatment with 15% tetrabutylammonium hydroxide at 100°C overnight. The FAME and MAME bands arising from each organism were isolated, and radioactivity was counted.
Comparisons of the effects of ISO with those of INH and ETH.
Comparison of the effects of ISO and its derivatives to those of INH and ETH on mycolic acid biosynthesis was studied through cell labeling with [1,2-14C]acetate. The effects of all of these drugs were similar in that the synthesis of mycolic acids was inhibited. However, as reported previously (49), both INH and ETH slightly stimulated fatty acid synthesis upon treatment of mycobacteria with INH at concentrations of 0.01, 0.02, 0.1, and 1.0 μg/ml and with ETH at 1.0, 2.0, 5.0, and 10 μg/ml, whereas ISO and its derivatives inhibited these types of lipids (Table 4).
Effect of ISO and the butyl derivative on viable M. tuberculosis in an in vitro bone marrow macrophage assay: absence of cytotoxicity.
The addition of ISO to macrophage cultures containing M. tuberculosis Erdman resulted in bacterial killing in a dose-dependent manner (Fig. 6). In the absence of ISO, viable bacteria grew to log 3.79 within 6 days, while in its presence, not only was growth inhibited but there was a reduction in the initial inoculum, indicating some bactericidal activity. The butyl derivative of ISO showed greater bactericidal activity in that it completely reduced the number of viable intracellular bacteria in macrophage cells at a concentration of 1.5 μg/ml (Fig. 6).
The alamar blue oxidation-reduction dye was applied as an indicator of the effects of ISO and the butyl derivative on macrophage cell viability. In this assay, the blue oxidized form becomes red due to the normal redox reactions within macrophage cells, and thus the red color represents cell viability. Mouse macrophage cells were grown in tissue microplates and treated with ISO or the butyl derivative. When the alamar blue dye was mixed with incomplete DMEM, the color was purple. All of the dilutions that contained ISO up to the highest tested concentration (2 μg/ml) exhibited the red color. Likewise, cells treated with the butyl derivative of ISO maintained their viability. Accordingly, this thiourea is apparently not acutely toxic for mouse macrophages.
DISCUSSION
The slow-growing mycobacteria used in this study, i.e., M. tuberculosis, M. bovis BCG, and M. avium, doubled about every 18 to 26 h and thus yielded colonies from single cells in 14 to 21 days. In contrast, the fast-growing mycobacteria, M. aurum A+ and M. smegmatis mc2 155, have doubling times of 2 to 3 h and yield colonies from a single cell in 3 to 4 days. The act of growing cells in tissue culture flasks without agitation in the case of M. tuberculosis, M. bovis BCG, and M. avium yielded high levels of reasonably unclumped viable cells. These species of Mycobacterium were included in preliminary experiments in order to identify ISO-susceptible and -resistant strains and to identify a set of suitable organisms for subsequent biochemical and genetic studies. The results of the MIC studies show that ISO is capable of inhibiting the growth of various mycobacteria within a narrow range of low concentrations. Importantly, a panel of virulent clinical isolates of M. tuberculosis also exhibited susceptibility to ISO when exposed to ISO in the range of 1.0 to 10.0 μg/ml. Some virulent strains appeared to be less susceptible, while others showed evidence of high susceptibility to ISO even at the very low concentration of 1.0 μg/ml. It is clear, however, that all of the clinical isolates of M. tuberculosis, which varied in patterns of resistance to other drugs (Table 1), were consistently susceptible to ISO at a concentration of 10 μg/ml. The suggestion is that ISO may be suitable for the treatment of tuberculosis, particularly the multidrug-resistant kind.
ISO (compound B27 in Table 2) is a substituted diacyl thiourea, and previous studies had demonstrated that the thiourea nucleus is required for antimycobacterial activity. In the hope of generating more-effective variants, random substitutions were made in the side chains attached to this key structure. This strategy resulted in an array of new ISO derivatives with variations in the symmetry and asymmetry of the side chains (Table 2) attached to the key structure. Some thioureas substituted in the para and para′ positions by alkyl, alkoxy, or sulfur functional groups were transformed from inactive thiocarbanilides into substances with considerable antimycobacterial activity. For instance, the butyl derivative of ISO (compound B01 in Table 2), the first synthesized ISO derivative, possessed low MICs (0.1 to 0.5 μg/ml) and was chosen for further evaluation of its effects on mycobacteria. Replacement of the oxygen with sulfur in the side chain(s) provided extremely high antibacterial activity against M. tuberculosis, as demonstrated by low MICs of <0.1 for B25 and B33 and 1.0 for JDD38. Thus, several of the ISO derivatives with various side chains of allyl, alkoxy, and alkylthio units were superior to the parent compound in their activities against M. tuberculosis. Other slow growers, including M. bovis BCG and M. avium, were also susceptible to ISO and its derivatives within a narrow range of low MICs. The present results suggest that a concerted approach to chemical modification of the basic thiourea nucleus of ISO would lead to even more-powerful inhibitors of M. tuberculosis and M. avium.
The range of mycobacteria selected for the present study was based on their susceptibility to ISO and range of constituent mycolic acids (21). The most-susceptible species, M. bovis BCG, M. tuberculosis H37Rv, and M. aurum A+, which also presented a representative spectrum of mycolic acids, were chosen to analyze the effects of ISO on mycolic acid synthesis through whole-cell labeling with [1,2-14C]acetate. Previous reports had indicated that prolonged exposure of mycobacteria to a low concentration of drugs, rather than short exposure to higher concentrations, provides a better gauge of its effects on bacterial metabolism (30, 47). Thus, the effects of ISO on mycolic acid synthesis could be clearly seen when the drug exposure times were 34 h for M. bovis BCG and M. tuberculosis H37Rv and 18 h for M. aurum A+. This approach allowed us to confirm that the mode of action of ISO is through the specific inhibition of mycolic acid synthesis and that the inhibitory effect of ISO on mycolic acid synthesis is dose dependent (Fig. 4 and 5). Based on the effects of ISO on the different species of mycobacteria, it can be concluded that ISO inhibited the synthesis of all types of mycolic acids, consistent with earlier observations (47). The naturally high resistance of M. smegmatis to ISO is striking and cannot be explained at this time.
The use of an in vitro macrophage model allowed an assessment of the ability of ISO and its butyl derivative to cross membranes and target viable bacteria within the confines of the macrophage and phagosome. The drugs also demonstrated strong intracellular bactericidal activity by reducing the initial inoculum of virulent M. tuberculosis, suggesting cidal rather than static action.
INH and ETH are specific antituberculosis drugs which clearly affect mycolic acid synthesis (19, 26, 47). INH, and apparently ETH, first requires conversion to an activated form, either an isonicotinic acyl anion (34) or an isonicotinic acyl radical (13), by the mycobacterial catalase-peroxidase enzyme (KatG) (13, 19, 52) before it exerts its lethal effect on mycolic acid biosynthesis. The activated form of INH is capable of attaching to NAD(H) as it is bound to the active site of InhA to generate a covalent INH-NAD adduct (31). InhA is a long-chain (C12 to C24) enoyl ACP-dependent reductase (27, 32) which catalyze the NADH-dependent reduction of a double bond at position 2 of a growing fatty acid chain linked to ACP. However, it had previously been observed (39–41) and was recently reconfirmed (19) that M. tuberculosis, in response to INH treatment, caused an accumulation of saturated hexacosanoic acid (C26:0). It is now known that this acyl group is attached to a 12-kDa ACP (AcpM) (19), and, according to this latest work, the β-ketoacyl ACP synthase in association with AcpM is the target of INH (19). Thus, at this point, the exact sites and mechanisms of action of INH for mycolic acid synthesis are varied and may be species dependent.
Unlike INH and ETH, ISO is not a nicotinamide derivative but has a higher molecular weight and is a thiourea modified by long hydrophobic side chains. It is difficult to envisage ISO as a prodrug capable of being converted into an electrophile. Hence, it is unlikely to form a complex with NAD+ at the InhA or any other active site. Moreover, the utilization of [1-2,14C]acetate as a precursor of fatty acid and mycolic acid synthesis demonstrated that ISO is distinct from INH and ETH in that it inhibits the synthesis of short-chain fatty acids (Table 4), a result that is consistent with the previous report on the mode of action of ISO (47) and suggests that the targets of ISO may lie at the points shared in the synthesis by both short-chain fatty acids and mycolic acids. The mycobacterial multifunctional FAS-I, the monofunctional FAS-II, and the largely undefined mycolic acid synthetase are responsible for fatty acid and mycolic acid synthesis. FAS-I is a single polypeptide with multiple catalytic activities that generate several shorter coenzyme A (CoA) esters from the acetyl-CoA primer (12, 15, 16, 23), and the primary products of the de novo FAS-I system are C16 to C18 and C24 to C26 fatty acyl-CoA derivatives (4, 5). Therefore, FAS-I creates the precursor for further elongation. FAS-II consists of dissociable enzyme components which act on a substrate bound to ACP. FAS-II is incapable of de novo fatty acid synthesis but instead elongates a C16 fatty acid primer (palmitoyl-ACP) to fatty acids ranging from C24 to C56 in length (28). Several different components of FAS-II were reported to be targets of INH, including the enoyl-ACP reductase (InhA) (1) and the ketoacyl-ACP synthase (KasA) in association with the ACP AcpM (19). It seems likely that ISO acts on other components of FAS-II, resulting in the inhibition of short-chain fatty acid synthesis, an effect distinct from that of INH. The mode of action of ISO remains elusive. An understanding of the specific mode of action of ISO is important in the search for new antimycobacterial drug targets and for the development of more effective chemotherapy. Furthermore, effects on fatty acid synthesis which differed from those of INH and ETH provide the prospects of identifying new fatty acid biosynthesis genes in addition to mycolic acid biosynthesis genes.
ACKNOWLEDGMENTS
We thank Ian M. Orme, Dean C. Crick, Christian Rittner, Brian Kelly, Jennifer DiTerro, and Jason Brooks for technical assistance and Marilyn K. Hein for preparing the manuscript.
This research was funded through Research Project Cooperative Agreement AI-38087 from the National Cooperative Drug Discovery Groups for the treatment of Opportunistic Infections, NIAID, NIH, and also by additional support from SmithKline Beecham Pharmaceuticals, Inc. (Collegeville, Pa.). B.P. was the recipient of a scholarship from the Royal Thai government.
G.S.B. and P.J.B. share responsibility for this research.
Footnotes
Dedicated to Frank G. A. Winder, Fellow, Trinity College, Dublin, Ireland, for his training of many graduate students, including one of us (P.J.B.), and seminal studies on the mechanisms of action of isoniazid and isoxyl.
REFERENCES
- 1.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um K S, Wilson T, Collins D, DeLisle G, Jacobs W R., Jr InhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P, Bloch A B, Davidson P T, Snider D E., Jr Tuberculosis in patients with immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein J, Lott W A, Steinberg B A, Yale H L. Chemotherapy of experimental tuberculosis: isonicotinic acid hydrazide (Nydrazid) and related compounds. Annu Rev Tuberc. 1952;65:357–364. doi: 10.1164/art.1952.65.4.357. [DOI] [PubMed] [Google Scholar]
- 4.Bloch K. Fatty acid synthetase from Mycobacterium phlei. Methods Enzymol. 1975;35:84–90. doi: 10.1016/0076-6879(75)35141-0. [DOI] [PubMed] [Google Scholar]
- 5.Bloch K. Control mechanisms for fatty acid synthesis in Mycobacterium smegmatis. Adv Enzymol. 1977;45:1–84. doi: 10.1002/9780470122907.ch1. [DOI] [PubMed] [Google Scholar]
- 6.Bloom B R, Murray C J L. Tuberculosis; commentary on a reemergent killer. Science. 1992;257:1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- 7.Cohn M L, Waggoner R F, McClatchy J K. The 7H11 media for the cultivation of mycobacteria. Am Rev Respir Dis. 1968;98:295–296. doi: 10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- 8.Combs D L, O’Brien R J, Geiter L J. USPHS tuberculosis short-course chemotherapy trial 21: effectiveness, toxicity, and acceptability: the report of final results. Ann Intern Med. 1990;112:397–406. doi: 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 9.Dooley S W, Jarvis W R, Martone W J, Snider D E., Jr Multidrug-resistant tuberculosis. Ann Intern Med. 1992;117:257–259. doi: 10.7326/0003-4819-117-3-257. [DOI] [PubMed] [Google Scholar]
- 10.Heifets L B. Drug susceptibility tests in management of chemotherapy. In: Heifets L B, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 99–101. [Google Scholar]
- 11.Heifets L B. General concepts in the testing drug susceptibility. In: Heifets L B, editor. Drug susceptibility in the chemotherapy of mycobacterial infections. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 2–11. [Google Scholar]
- 12.Jackowski S, Croman J E, Jr, Rock C O. Lipid metabolism in prokaryote. In: Vance D E, Vance J, editors. Biochemistry of lipids, lipoproteins and membranes. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1991. pp. 43–85. [Google Scholar]
- 13.Johnson K, Schultz P G. Mechanistic studies of the oxidation of isoniazid by the catalase peroxidase from Mycobacterium tuberculosis. J Am Chem Soc. 1994;116:7425–7426. [Google Scholar]
- 14.Kampelmann F. Results of monotherapy with isoxyl in untreated pulmonary tuberculosis cases of different difficulties and isoxyl in routine therapy. Antibiot Chemother. 1970;26:96–104. doi: 10.1159/000386808. [DOI] [PubMed] [Google Scholar]
- 15.Kolattukudy P E, Fernandes N D, Azad A K, Fitzmaurice A M, Sirakova T D. Biochemistry and molecular genetics of cell-wall lipid biogenesis in mycobacteria. Mol Microbiol. 1997;24:263–270. doi: 10.1046/j.1365-2958.1997.3361705.x. [DOI] [PubMed] [Google Scholar]
- 16.Magnuson K, Jackowski S, Rock C O, Croman J E., Jr Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993;57:522–524. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClatchy J K. Antimycobacterial drugs: mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biological fluids. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 181–222. [Google Scholar]
- 18.Mdluli K, Sherman D R, Hickey M J, Kreiswirth B N, Morris S, Stover C K, Barry C E. Biochemical and genetic data suggest that InhA is not the primary target for activated isoniazid in Mycobacterium tuberculosis. J Infect Dis. 1996;174:1085–1090. doi: 10.1093/infdis/174.5.1085. [DOI] [PubMed] [Google Scholar]
- 19.Mdluli K, Slayden R A, Zhu Y, Ramaswamy S, Pan X, Mead D, Crane D D, Musser J M, Barry C E., III Inhibition of a Mycobacterium tuberculosis β-ketoacyl ACP synthase by isoniazid. Science. 1998;280:1607–1610. doi: 10.1126/science.280.5369.1607. [DOI] [PubMed] [Google Scholar]
- 20.Middlebrook K. Sterilization of tubercle bacilli by isonicotinic acid hydrazide and the incidence of variants resistant to the drug in vitro. Am Rev Tuberc. 1952;64:765–767. [PubMed] [Google Scholar]
- 21.Minnikin D E, Minnikin S M, Parlett J H, Goodfellow M, Magnusson M. Mycolic acid patterns of some species of Mycobacterium. Arch Microbiol. 1984;139:225–231. doi: 10.1007/BF00402005. [DOI] [PubMed] [Google Scholar]
- 22.Murray C J L, Styblo K, Rouillon A. Disease control priorities in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:6–24. [PubMed] [Google Scholar]
- 23.Noto T, Miyakawa S, Oishi H, Endo H, Okazaki H. Thiolactomycin, a new antibiotic. III. In vitro antibiotic activity. J Antibiot (Tokyo) 1982;35:401–410. doi: 10.7164/antibiotics.35.401. [DOI] [PubMed] [Google Scholar]
- 24.Ordway D J, Sonnenberg M G, Donahue S A, Belisle J T, Orme I M. Drug-resistant strains of Mycobacterium tuberculosis exhibit a wide range of virulence for mice. Infect Immun. 1995;63:741–743. doi: 10.1128/iai.63.2.741-743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quemard A, Lacave C, Laneelle G. Isoniazid inhibition of mycolic acid synthesis by cell extracts of sensitive and resistant strains of Mycobacterium aurum. Antimicrob Agents Chemother. 1991;35:1035–1039. doi: 10.1128/aac.35.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quemard A, Laneelle G, Lacave C. Mycolic acid synthesis: a target for ethionamide in mycobacteria? Antimicrob Agents Chemother. 1992;36:1316–1321. doi: 10.1128/aac.36.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quemard A, Sacchettini J C, Dessen A, Vilcheze C, Bittman R, Jacobs W R, Jr, Blanchard J S. Enzymatic characterization of the target of isoniazid in Mycobacterium tuberculosis. Biochemistry. 1995;34:8235–8241. doi: 10.1021/bi00026a004. [DOI] [PubMed] [Google Scholar]
- 28.Ratledge C R. Lipids: cell composition, fatty acid biosyntheses. In: Ratledge C, Standford J, editors. The biology of mycobacteria. San Diego, Calif: Academic Press; 1982. pp. 53–94. [Google Scholar]
- 29.Rhoades E R, Orme I M. Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun. 1997;65:1189–1195. doi: 10.1128/iai.65.4.1189-1195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rist N. L’activitté antituberculeuse de l’ethionamide. Adv Tuberc Res. 1960;10:69–126. [Google Scholar]
- 31.Rozwarski D A, Grant G A, Barton D H R, Jacobs W R, Jr, Sacchettini J C. Modification of the NADH of the isoniazid target (inhA) from Mycobacterium tuberculosis. Science. 1998;279:98–102. doi: 10.1126/science.279.5347.98. [DOI] [PubMed] [Google Scholar]
- 32.Sacchettini J C, Blanchard J S. The structure and function of isoniazid target in M. tuberculosis. Res Microbiol. 1996;147:36–43. doi: 10.1016/0923-2508(96)80201-4. [DOI] [PubMed] [Google Scholar]
- 33.Schmid P C H. Clinical experiences in cases of primary tuberculosis with tuberculostaticum isoxyl. Antibiot Chemother. 1970;16:108–116. doi: 10.1159/000386810. [DOI] [PubMed] [Google Scholar]
- 34.Shoeb H A, Bowman B U, Jr, Ottolenghi A C, Merola A J. Peroxidase-mediated oxidation of isoniazid. Antimicrob Agents Chemother. 1985;27:399–403. doi: 10.1128/aac.27.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slayden R A, Lee R E, Armour J W, Cooper A M, Orme I M, Brennan P J, Besra G S. Antimycobacterial action of thiolactomycin: an inhibitor of fatty acid and mycolic acid synthesis in mycobacteria. Antimicrob Agents Chemother. 1996;40:2813–2819. doi: 10.1128/aac.40.12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snider D E, Jr, Raviglione M, Kochi A. Global burden of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 2–11. [Google Scholar]
- 37.Snider D E, Jr, Roper W L. The new tuberculosis. N Engl J Med. 1992;326:703–705. doi: 10.1056/NEJM199203053261011. [DOI] [PubMed] [Google Scholar]
- 38.Stone M S, Wallace R J, Jr, Swenson J M, Thornsberry C, Christensen L A. Agar disk elution method for susceptibility testing of Mycobacterium marinum and Mycobacterium fortuitum complex to sulfonamides and antibiotic. Antimicrob Agents Chemother. 1983;24:486–493. doi: 10.1128/aac.24.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takayama K. Selective action of isoniazid on the synthesis of cell wall mycolates in mycobacteria. Ann NY Acad Sci. 1974;235:426–438. doi: 10.1111/j.1749-6632.1974.tb43281.x. [DOI] [PubMed] [Google Scholar]
- 40.Takayama K, Schnoes H K, Armstrong E L, Boyle R W. Site of inhibitory action of isoniazid in the synthesis of mycolic aids in Mycobacterium tuberculosis. J Lipid Res. 1975;16:308–317. [PubMed] [Google Scholar]
- 41.Takayama K L, Wang L, David H L. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1972;2:29–35. doi: 10.1128/aac.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titscher R. Monotherapie mit isoxyl/DAT bei tuberculose-asylierungsfallen. Prax Pneumol. 1966;20:202. [PubMed] [Google Scholar]
- 43.Urbancik B. A clinical trial of thiocarlide (isoxyl) Tubercle. 1966;47:283–288. doi: 10.1016/s0041-3879(66)80007-7. [DOI] [PubMed] [Google Scholar]
- 44.Urbancik B. Clinical experiences with thiocarlide (isoxyl) Antibiot Chemother. 1970;16:117–123. doi: 10.1159/000386811. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Takayama K. Relation between the uptake of isoniazid and its action on in vivo mycolic acid synthesis in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1972;2:438–441. doi: 10.1128/aac.2.6.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winder F G. Catalase and peroxidase in mycobacteria. Am Rev Respir Dis. 1960;81:68–78. doi: 10.1164/arrd.1960.81.1P1.68. [DOI] [PubMed] [Google Scholar]
- 47.Winder F G. Mode of action of the antimycobacterial agents and associated aspects of the molecular biology of mycobacteria. In: Ratledge C, Standford J, editors. The biology of mycobacteria. Vol. 1. New York, N.Y: Academic Press, Inc.; 1982. pp. 353–438. [Google Scholar]
- 48.Winder F G, Collins P B. Inhibition by isoniazid of synthesis of mycolic acids in Mycobacterium tuberculosis. J Gen Microbiol. 1970;63:41–48. doi: 10.1099/00221287-63-1-41. [DOI] [PubMed] [Google Scholar]
- 49.Winder F G, Collins P B, Whelan D. Effects of ethionamide and isoxyl on mycolic acid synthesis in Mycobacterium tuberculosis BCG. J Gen Microbiol. 1971;66:379–380. doi: 10.1099/00221287-66-3-379. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Amitani R, Suzuki K, Tanaka T, Murayama T, Kuze F. In vitro bactericidal and in vivo therapeutic activities of a new rifamycin derivative, KRM-1648, against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:426–428. doi: 10.1128/aac.40.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Youatt J. A review of the action of isoniazid. Am Rev Respir Dis. 1969;99:729–749. doi: 10.1164/arrd.1969.99.5.729. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]