Abstract
The pharmacokinetics of gatifloxacin (400 mg orally) and the influence of the antacid aluminum magnesium hydroxide (20 ml of Maalox 70) on the bioavailability of gatifloxacin in 24 healthy volunteers were assessed. In an open, randomized, six-period crossover study, the volunteers received either gatifloxacin alone (treatments A and D); aluminum magnesium hydroxide concomitant with gatifloxacin (treatment C); or aluminum magnesium hydroxide 2 h before (treatment B), 2 h after (treatment E), or 4 h after gatifloxacin administration (treatment F). Gatifloxacin concentrations were measured by a validated bioassay and high-performance liquid chromatography. Pharmacokinetics of a single 400-mg dose of gatifloxacin alone were characterized as follows (mean ± standard deviation): peak concentration (Cmax), 3.8 ± 0.5 (treatment A) and 3.4 ± 0.9 (treatment D) μg/ml; time to Cmax, 1.4 ± 0.8 (treatment A) and 1.7 ± 0.7 (treatment D) h; area under the curve from time zero to infinity (AUC0–∞), 33.5 ± 5.9 (treatment A) and 31.4 ± 3.4 (treatment D) μg · h/ml; urine recovery, (83 ± 6)% (treatment A) and (84 ± 8)% (treatment D). Comparison of the results obtained by bioassay showed a good correlation. Aluminum magnesium hydroxide administration 2 h before (treatment B) or concomitant with (treatment C) gatifloxacin decreased the Cmax by 45% (2.1 ± 1.2 μg/ml) or even 68% (1.2 ± 0.4 μg/ml) highly significantly (P < 0.01). AUC0–∞ was significantly reduced from 33.5 ± 5.9 to 19.4 ± 6.9 μg · h/ml (by 42%) or even to 11.9 ± 3.3 μg · h/ml (by 64%) (P < 0.01). If aluminum magnesium hydroxide was given 2 h after gatifloxacin (treatment E), there was no significant reduction of concentration in serum but AUC0–∞ was significantly reduced from 31.4 ± 3.4 to 25.9 ± 5.3 μg · h/ml (18%) (P < 0.01). Aluminum magnesium hydroxide given 4 h after gatifloxacin (treatment F) showed no influence on the gatifloxacin pharmacokinetics. Therefore, the optimal time between gatifloxacin application and the intake of an aluminum-containing antacid should be 4 h.
Gatifloxacin is a new fluoroquinolone with a 3-methylpiperazinyl side chain at position 7 and a methoxy group at position 8 of the quinolone ring. Gatifloxacin is available as a racemate for therapeutic use. Previous studies showed enhanced activity against gram-positive cocci comparable to those of sparfloxacin (25) and ciprofloxacin (26). Gatifloxacin was more active than ciprofloxacin against anaerobic gram-negative bacilli (1, 26) and Helicobacter pylori and Campylobacter jejuni (1). Preliminary studies suggested that gatifloxacin showed enhanced activity against members of the family Enterobacteriaceae in comparison with ciprofloxacin (25) and sparfloxacin and trovafloxacin (26). The good antibacterial activity against Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae will be of clinical interest for the treatment of community-acquired respiratory infections, especially of pneumonia.
Our group provided the first report on the interaction of antacids with quinolones (7); continuing our interest in this field, we established this study protocol. The main objective was to evaluate the absorption and disposition of the new fluoroquinolone gatifloxacin in healthy male volunteers and possible interactions with an antacid containing aluminum (Maalox) obtained at various relative times. Since the formation of complexes with metal cations through carboxyl and carbonyl groups of quinolones is an important factor affecting the absorption of quinolones, the disposition of gatifloxacin may be affected by these mechanisms. There was an additional purpose of evaluating whether both bioassay and high-performance liquid chromatography (HPLC) would provide the same exact measurements of gatifloxacin concentrations.
MATERIALS AND METHODS
Volunteers.
Twenty-four volunteers completed the study as 12 healthy young male Caucasians in each of two parts. The age ranged from 20 to 43 years (mean, 28.8 ± 5.5 years), the average weight was 78.9 ± 9.1 kg, and the average body surface was 1.99 ± 0.1 m2. The volunteers were included after physical examination, electrocardiograms, and laboratory screening including testing for drugs of abuse, hepatitis and human immunodeficiency virus serology, and hematological and biochemical parameters. All volunteers had normal hepatic and renal functions (mean creatinine clearance of 112.5 ± 10.4 ml/min/1.73 m2). Further inclusion criteria were as follows: no history of gastrointestinal disease or surgery, no medication of any kind within 1 week and no alcohol ingestion within 48 h of study initiation, no allergy or intolerance to any drugs (especially to quinolones), no blood donation, and no participation in a clinical trial within 60 days of the study. After approval by the local ethics committee according to German law, informed written consent was obtained from all subjects.
Study design.
This was an open, randomized, single-oral-dose design. According to the six-period crossover parts with a 1-week washout period, each volunteer received the following drug combination of part 1 or part 2 in a random order: 400 mg orally of gatifloxacin alone (treatment A), two aluminum magnesium hydroxide (Maalox 70) flasks (each flask was 10 ml) 2 h before 400 mg of gatifloxacin (treatment B), two aluminum magnesium hydroxide flasks concomitant with 400 mg of gatifloxacin (treatment C), 400 mg of gatifloxacin alone (treatment D), two aluminum magnesium hydroxide flasks 2 h after 400 mg of gatifloxacin (treatment E), or two aluminum magnesium hydroxide flasks 4 h after 400 mg of gatifloxacin (treatment F).
Gatifloxacin (catalog no. 0037942, AM-1155; Grünenthal GmbH, Aachen, Germany) was given after 10-h overnight fasting with 240 ml of tap water. Each aluminum magnesium hydroxide single-use flask (catalog no. 75271; Rhône-Poulenc Rorer/Nattermann, Antony Cedex, France) containing 600 mg of magnesium hydroxide and 900 mg of aluminum oxide was rinsed with 10 ml of tap water. The fasting state (solid food) was maintained 4 h after the gatifloxacin dose, except for treatment F (6 h). Subjects abstained from caffeine and alcohol for 48 h after gatifloxacin administration.
Sampling.
Each blood sample consisted of 4 ml for treatments A to F for the HPLC and an additional 5 ml for treatments A and D for the bioassay method. The blood samples were taken through an indwelling cannula predose and 15, 30, and 45 min and 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 24, and 36 h after gatifloxacin administration on each profiling day. Immediately after collection of the blood samples for plasma in heparinized polypropylene tubes, they were placed in chipped ice, and the blood samples for serum in polypropylene tubes were stored for 15 min at room temperature. All samples were centrifuged for 15 min at 1,000 × g and 4°C to separate the plasma or serum and afterwards shock frozen below −20°C. All volunteers started with empty bladders after providing the last urine sample. The urine samples were collected at 0 to 4, 4 to 8, 8 to 12, 12 to 24, 24 to 36, and 36 to 48 h after dosing with gatifloxacin. Two 5-ml aliquots for treatments A to F and an additional 5 ml for treatments A and D were transferred into sterile tubes and immediately shock frozen (below −20°C). All specimens were protected against light during processing and storage.
Bioassay method.
The bioassay method was based on an agar plate diffusion technique previously described in detail by Reeves and Bywater (20). This method was used to determine gatifloxacin levels of treatments A and D (gatifloxacin, 400 mg alone) in serum and urine samples. Serum samples were assayed against standards prepared in activity-free pooled human serum. Phosphate buffer (pH 7.2) was used for the predilution of urine and urine standards. On each agar plate, four serum or urine samples, one control sample, and five standard samples were tested in triplicate. After prediffusion for 30 min at room temperature, the agar plates were incubated for 18 h at 30°C. The test strain was Escherichia coli (ATCC 25922), and Iso-Sensitest agar (Unipath CM471, pH 7.4; catalog no. 60043) was used. The lower limits of detection were determined to be 0.12 μg/ml in both urine and serum. The coefficient of variation, determined on three different days between concentrations of 0.16 and 5 μg/ml, was 4.2% for serum and 4.9% for urine.
HPLC.
The concentration of gatifloxacin in plasma and urine samples was determined by HPLC validated and described as a standard operating procedure at Cephac Bioanalytical Research Centre in Saint-Benoit, France (3). It involved a liquid-liquid extraction of gatifloxacin and of an internal standard at pH 6.8 followed by back-extraction in 1 N hydrochloric acid. The extract was then chromatographed and analyzed by fluorimetric detection. Concentrations versus peak area curves were linear in the following ranges: 0.01 to 10 μg/ml for plasma and 0.1 to 50 μg/ml for urine. The lower limit of quantification was 0.01 μg/ml in plasma and 0.1 μg/ml in urine. Precision within series was 7.9% in plasma (concentration, 0.01 μg/ml) and 11.7% in urine (concentration, 0.1 μg/ml). Precision between series (coefficient of variation) was 10.8 to 5.6% in plasma (concentration range, 0.04 to 8.0 μg/ml) and 13.4 to 6.1% in urine (concentration range, 0.15 to 45 μg/ml). The mean accuracy from plasma was 102.9%; that from urine was 102.0%.
Pharmacokinetic analysis.
The estimation of peak concentration (Cmax), time of peak concentration (Tmax), terminal half-life, the area under the curve from time zero to infinity (AUC0–∞), absorption rate constant, elimination rate constant, and the time between drug administration and the beginning of absorption were based on an open two-compartment model. The choice of the particular model was based on the Schwarz criterion (6, 9, 22). All other parameters were analyzed noncompartmentally (total area under the data [AUDtot], volume of distribution at steady state [VSS], and mean residence time). The AUDtot was calculated by the trapezoidal rule. Dose-dependent parameters (AUDtot, AUC0–∞, and Cmax) were based on a dose per 70 kg of body weight. The renal clearance was calculated by total clearance × total (extrapolated) urinary recovery. The computer programs used were Microsoft Excel and REVOL as described by Koeppe and Hamann (10).
Statistical analysis.
Differences in the pharmacokinetic parameters between the groups were identified by t test. The Wilcoxon test (matched-pairs signed rank test) for HPLC and the Whitney test (U test for two independent samples) for the comparison of bioassay and HPLC were also used. All groups were compared by analysis of variance by the Student-Newman-Keuls procedure for multiple comparisons of sample means. A P value of <0.05 was considered significant (17, 21).
RESULTS
Comparison of analysis methods.
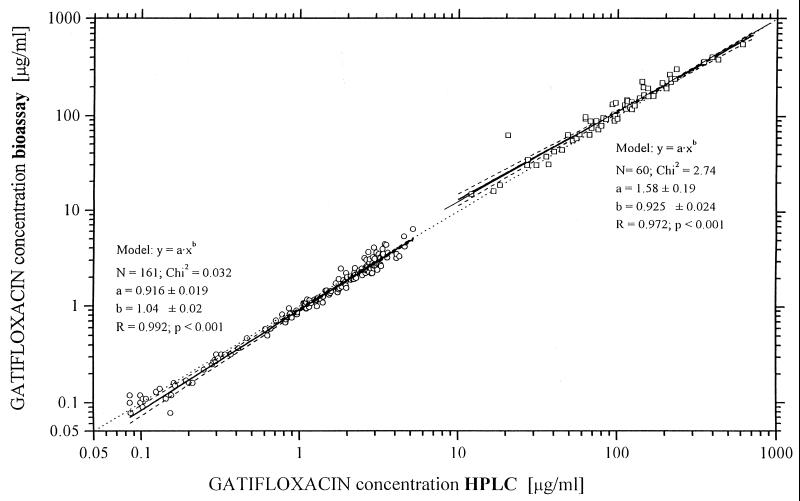
The correlation between the results of bioassay and those of HPLC was good. The regression lines of plasma or even serum concentration are very similar in both study groups, but urine values for the bioassay were slightly higher than those for HPLC. The bioassay values for serum were slightly lower than those with HPLC (Fig. 1). The bioassay values for urine were 16% (mean value) higher than those for HPLC, but the standard deviation was higher (±26%) and therefore the ratio = 1 (ideal) was between the 95% confidence intervals. The coefficient of correlation (r) was 0.99 for plasma and serum and 0.97 for urine.
FIG. 1.
Comparison of bioassay and HPLC for serum-plasma and urine data for gatifloxacin (400-mg dose). ○, plasma; □, urine; ——, regression line according to model; –––––, 95% confidence limit; … …, Cbioassay = CHPLC.
Pharmacokinetics of gatifloxacin.
The pharmacokinetic data for gatifloxacin measured by HPLC are listed in Table 1. Gatifloxacin absorption reached a maximal concentration (Cmax) of 3.8 ± 0.5 μg/ml after 1.4 ± 0.8 h (Tmax) in group 1A. In the second group, the Cmax was slightly lower, at 3.4 ± 0.9 μg/ml after 1.7 ± 0.7 h (Tmax, 2D). The area under the curve (AUC0–∞) for gatifloxacin alone was 33.5 ± 5.9 μg · h/ml (group 1A) and 31.4 ± 3.4 μg · h/ml (group 2D). The VSS for group 1A was 121 ± 13 liters/70 kg. Group 2D showed a VSS of 115 ± 17 liters/70 kg. As measured by HPLC, (82.5 ± 6)% (group 1A) of the administered dose was recovered from urine as unchanged gatifloxacin. The total urinary recovery of the second group was very similar.
TABLE 1.
Pharmacokinetic parameters of gatifloxacin (400 mg orally) for different treatments determined by HPLCa
| Treatment | Cmax (μg/ml/70 kg) | Tmax (h) | Tlag (h) | Half-life (h) | MRT (h) | AUC0–∞ (μg · h/ml/70 kg) | Total urinary recovery (% of dose) | Renal clearance (ml/min/1.73 m2) | VSS (liters/70 kg) |
|---|---|---|---|---|---|---|---|---|---|
| 1A | 3.8 ± 0.5 | 1.4 ± 0.8 | 0.23 ± 0.02 | 8.6 ± 1.4 | 11.3 ± 1.9 | 33.5 ± 5.9 | 82.5 ± 6.0 | 164 ± 37 | 121 ± 13.4 |
| 1B | 2.1 ± 1.2b | 1.8 ± 1.0 | 0.22 ± 0.03 | 8.4 ± 1.9 | 12.0 ± 3.1 | 19.4 ± 6.9b | 53.2 ± 19b | 176 ± 30 | 260 ± 158b |
| 1C | 1.2 ± 0.4b | 1.6 ± 0.7 | 0.20 ± 0.06 | 9.5 ± 2.0 | 13.2 ± 3.0c | 11.9 ± 3.3b | 35.9 ± 10b | 195 ± 30c | 421 ± 156b |
| 2D | 3.4 ± 0.9 | 1.7 ± 0.7 | 0.23 ± 0.02 | 7.5 ± 1.2 | 10.3 ± 1.4 | 31.4 ± 3.4 | 84.2 ± 7.7 | 165 ± 20 | 115 ± 17 |
| 2E | 3.3 ± 1.4 | 1.5 ± 1.1 | 0.22 ± 0.03 | 7.0 ± 1.2 | 9.8 ± 1.3 | 25.9 ± 5.3b | 73.1 ± 11c | 175 ± 17 | 139 ± 47b |
| 2F | 3.5 ± 1.1 | 1.6 ± 1.0 | 0.21 ± 0.07 | 7.0 ± 0.9 | 10.0 ± 1.3 | 31.3 ± 4.0 | 79.8 ± 5.2 | 157 ± 14 | 111 ± 12 |
Treatment groups were as follows: 1A, gatifloxacin alone; 1B, aluminum magnesium hydroxide 2 h before gatifloxacin; 1C, aluminum magnesium hydroxide concomitant with gatifloxacin; 2D, gatifloxacin alone; 2E, aluminum magnesium hydroxide 2 h after gatifloxacin; 2E, aluminum magnesium hydroxide 4 h after gatifloxacin. Values are means ± standard deviations. MRT, mean residence time; Tlag, time between drug administration and beginning of absorption.
P < 0.01 versus gatifloxacin alone.
P < 0.05 versus gatifloxacin alone.
Interactions of aluminum magnesium hydroxide with gatifloxacin.
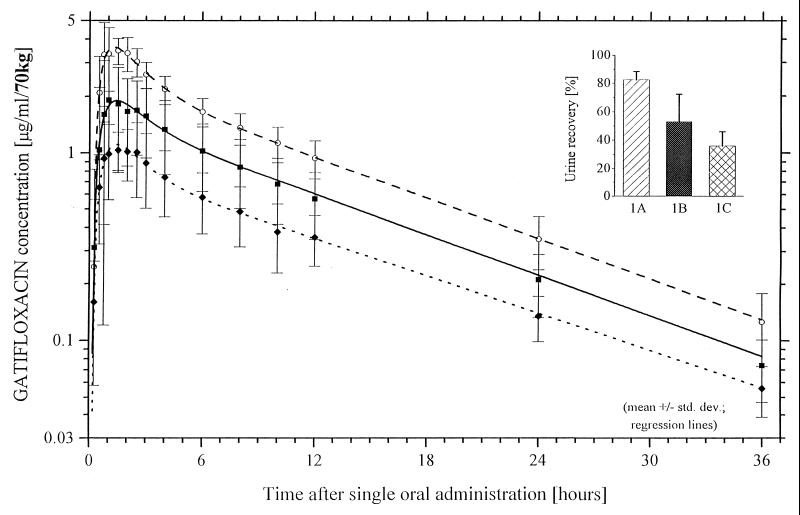
Drug concentrations and pharmacokinetic results in all figures and the table are given on the basis of HPLC (Fig. 2 and Table 1). Aluminum magnesium hydroxide administration 2 h before gatifloxacin significantly decreased Cmax from 3.8 ± 0.5 to 2.1 ± 1.2 μg/ml (45%) and AUC0–∞ from 33.5 ± 5.9 to 19.4 ± 6.9 μg · h/ml (42%) (P < 0.01). Concomitant administration of gatifloxacin and aluminum magnesium hydroxide reduced the Cmax highly significantly from 3.8 ± 0.5 to 1.2 ± 0.4 μg/ml (68%) and the AUC0–∞ from 33.5 ± 5.9 to 11.9 ± 3.3 μg · h/ml (64%) (P < 0.01). The terminal half-life and Tmax were not altered.
FIG. 2.
Gatifloxacin (400 mg) plasma concentration-time curves and urinary recovery levels for treatments A to C (group 1) determined by HPLC. ○, group 1, treatment A, gatifloxacin alone; ■, group 1, treatment B, gatifloxacin and two aluminum magnesium hydroxide single-use flasks 2 h before gatifloxacin; ⧫, group 1, treatment C, gatifloxacin concomitantly with two aluminum magnesium hydroxide single-use flasks.
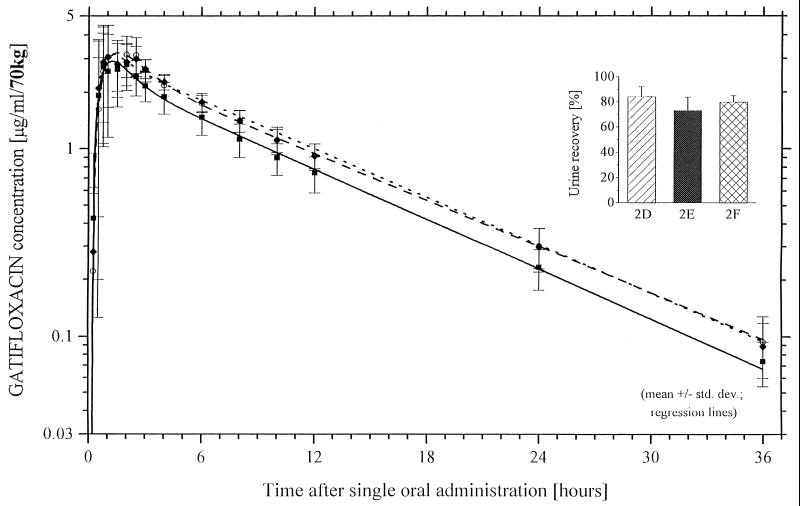
If aluminum magnesium hydroxide was given 2 h after gatifloxacin, there was no significant reduction of concentration in serum, but AUC0–∞ was significantly reduced from 31.4 ± 3.4 to 25.9 ± 5.3 μg · h/ml (18%) (P < 0.01). Aluminum magnesium hydroxide given 4 h after gatifloxacin showed no change in the bioavailability of the fluoroquinolone (Fig. 3).
FIG. 3.
Gatifloxacin (400 mg) plasma concentration-time curves and urinary recovery levels for treatments D to F (group 2) determined by HPLC. ○, group 2, treatment D, gatifloxacin alone; ■, group 2, treatment E, gatifloxacin and two aluminum magnesium hydroxide single-use flasks 2 h after gatifloxacin; ⧫, group 2, treatment F, gatifloxacin and two aluminum magnesium hydroxide single-use flasks 4 h after gatifloxacin.
Safety and tolerance.
The overall tolerance of gatifloxacin was good. None of the volunteers had to be withdrawn from the study. Most of the adverse effects were of a mild intensity; none of them were severe. The relation of the listed adverse events to the study drugs was mostly probable. One volunteer suffered from nausea with sweating and pale skin 30 min after aluminum magnesium hydroxide administration, which could be related to this drug. With one exception, all symptoms improved spontaneously. One case of balanitis caused by Candida spp. was successfully treated topically with 100,000 IU of nystatin for 7 days. One volunteer had a slightly increased alanine aminotransferase (ALT) level of 36 U/liter and an amylase level of 138 U/liter with no clinical relevance.
DISCUSSION
The pharmacokinetic profile of gatifloxacin found in the present study is similar to that described previously in a single-dose study (13). Gatifloxacin was rapidly absorbed in 1.3 to 1.8 h (Tmax), and the Tmax comparable to those of ciprofloxacin (1.1 h) and ofloxacin (1.4 h), the most commonly used quinolones (2). The Cmax in serum were achieved later by other newer fluoroquinolones, like trovafloxacin at 2.4 h (23) and sparfloxacin at 4.1 h (28). The Cmax of gatifloxacin (3.2 to 3.8 μg/ml) is similar to those of other quinolones, such as ofloxacin (4.0 μg/ml for 400 mg) and trovafloxacin (2.8 μg/ml for 300 mg), whereas the Cmax of ciprofloxacin (1.5 μg/ml for 400 mg) and sparfloxacin (1.3 μg/ml for 400 mg) are lower (2, 23, 28).
With regard to the relatively long terminal half-life of gatifloxacin (7.5 to 8.6 h), it is anticipated that a once-daily application will show sufficient clinical efficacy and patient compliance. The extensive urinary recovery of unchanged drug suggested that this drug was well absorbed from the gastrointestinal tract.
HPLC and bioassay methods were well comparable. The differences between the HPLC and bioassay measurements of urine could well reflect a bioactive metabolite. This is not known yet and would suggest further investigations.
Various studies have shown that aluminum-magnesium-containing antacids reduce the absorption of fluoroquinolone antibiotics (5, 7, 8, 15, 22, 27). The results of the present study are in line with these findings and demonstrate a significant reduction in the gastrointestinal absorption of gatifloxacin as well. The relative bioavailability was decreased by 64, 42, and even 18% when aluminum magnesium hydroxide was given concomitantly, 2 h before, or 2 h after gatifloxacin intake. Yamanaka-Yuen and Gantu (27) showed a reduction of bioavailability of norfloxacin by 2 and 77%, if aluminum magnesium hydroxide was given 5 min before or 2 h after norfloxacin. Lazzaroni et al. (11) described the interaction between rufloxacin and aluminum magnesium hydroxide. They found a substantial decrease of the relative bioavailability by 36% after administration of the antacid within 5 min before the intake of rufloxacin (11). In a previous study involving trovafloxacin (23), the AUC values were significantly decreased, by 66 and 28%, when administration of aluminum magnesium hydroxide was 30 min before or 2 h after trovafloxacin. Other authors observed interactions between ciprofloxacin and ofloxacin and the antacid (12, 15). Nix (15) showed a reduction of the ciprofloxacin absorption by 85% if aluminum magnesium hydroxide was given 5 or 10 min before ciprofloxacin. If the antacid was administered 2 or 4 h before the antibiotic agent, there was a reduction by 77 or 30%, respectively, of the bioavailability. The principal mechanism of the interaction between antacids containing polyvalent cations and fluoroquinolones is thought to be chelation of the antibiotic by the ions (8, 12, 15, 19). Aluminum, in particular, forms a very stable complex with quinolones, which are not easily soluble (24). Clinical significance was documented by Noyes and Polk (16), who reported one failure of norfloxacin treatment resulting from concomitant treatment with an aluminum- and magnesium-containing antacid suspension. Preheim et al. (18) suggested that antacids may interfere with the efficacy of ciprofloxacin, particularly in patients infected with Pseudomonas aeruginosa. The dose of magnesium-aluminum hydroxide in our study was a standard therapeutic dose, but the single-dose design used in the present study does not apply to real clinical situations with multiple doses of antacid. However, our results showed interaction problems of clinical relevance.
The applied dose of gatifloxacin was well tolerated in healthy volunteers. Vital signs, electrocardiograms, hematology, and urinalysis showed no change attributable to the trial medication. None of the subjects had to be withdrawn from the study. One volunteer had transient slightly increased ALT and amylase levels with no clinical relevance. Nakashima et al. also (13) described one volunteer with a transitory elevation of ALT.
In conclusion, the results of the present study indicate that the optimal time between gatifloxacin administration and the intake of an aluminum-containing antacid should be 4 h to avoid substantial interaction.
ACKNOWLEDGMENT
This study was supported by a grant from Grünenthal GmbH, Aachen, Germany.
REFERENCES
- 1.Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 2.Borner K, Lode H, Höffken G, Koeppe P, Olschewski P, Sievers B. Comparative pharmacokinetics of ofloxacin and ciprofloxacin. Rev Infect Dis. 1988;10(Suppl. 1):91–92. doi: 10.1093/jac/22.supplement_c.73. [DOI] [PubMed] [Google Scholar]
- 3.Cephac Standard Operating Procedure. No. 712. 1996. Unpublished. [Google Scholar]
- 4.Deppermann K M, Lode H. Fluoroquinolones: interaction profile during enteral absorption. Drugs. 1993;45(Suppl. 3):65–72. doi: 10.2165/00003495-199300453-00013. [DOI] [PubMed] [Google Scholar]
- 5.Flor S, Guay D R P, Opsahl J A, Tack K, Matzke G R. Effects of magnesium-aluminum hydroxide and calcium carbonate antacids on bioavailability of ofloxacin. Antimicrob Agents Chemother. 1990;34:2436–2438. doi: 10.1128/aac.34.12.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibaldi M. Biopharmaceutics and clinical pharmacokinetics. 4th ed. Philadelphia, Pa: Lea and Febiger; 1991. [Google Scholar]
- 7.Hoeffken G, Borner K, Glatzel P D, Koeppe P, Lode H. Reduced enteral absorption of ciprofloxacin in the presence of antacids. Eur J Clin Microbiol. 1985;3:345. doi: 10.1007/BF02013667. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 8.Hoeffken G, Lode H, Wiley R, Glatzel T D, Sievers D, Olschewski T, et al. Pharmacokinetics and bioavailability of ciprofloxacin and ofloxacin: effect of food and antacid intake. Rev Infect Dis. 1988;10(Suppl. 1):138–139. [Google Scholar]
- 9.Koeppe P. New regression function for absorption kinetics. Drug Res. 1988;38:1375–1377. [PubMed] [Google Scholar]
- 10.Koeppe P, Hamann C-M. REVOL—non-linear regression based on the strategy of evolution. EDV Med Biol. 1978;9:112–117. [Google Scholar]
- 11.Lazzaroni M, Imbimbo B P, Bargiggia S, Sangaletti O, Dal-Bo L, Broccali G, Porro G B. Effects of magnesium-aluminum hydroxide antacid on absorption of rufloxacin. Antimicrob Agents Chemother. 1993;10:2212–2216. doi: 10.1128/aac.37.10.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lode H. Drug interactions with quinolones. Rev Infect Dis. 1988;10(Suppl. 1):132–136. doi: 10.1093/clinids/10.supplement_1.s132. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima M, Uematsu T, Kosuge K, Kusajima H, Ooie T, Masuda Y, Ishida R, Uchida H. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob Agents Chemother. 1995;12:2635–2640. doi: 10.1128/aac.39.12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nix D E, Lebsack M E, Chapelsky M, Sedman A J, Busch J, Norman A. Effect of oral antacids on disposition of intravenous enoxacin. Antimicrob Agents Chemother. 1993;4:775–777. doi: 10.1128/aac.37.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nix D E, Watson W A, Lener M E, Frost R W, Kroll G, Goldstein H, Littieri J, Schentag J J. Effects of aluminum and magnesium antacids and ranitidine on the absorption of ciprofloxacin. Clin Pharmacol Ther. 1989;46:700–705. doi: 10.1038/clpt.1989.207. [DOI] [PubMed] [Google Scholar]
- 16.Noyes M, Polk R E. Norfloxacin and absorption of magnesium-aluminum. Ann Intern Med. 1988;109:168–169. doi: 10.7326/0003-4819-109-2-168_2. [DOI] [PubMed] [Google Scholar]
- 17.Ott L. An introduction to statistical methods and data analysis. 3rd ed. Boston, Mass: PWS-Kent Publishing Company; 1988. p. 199ff. [Google Scholar]
- 18.Preheim L C, Cuevas T A, Roccaforte J S, Mellencamp M A, Bittner M J. Ciprofloxacin and antacids. Lancet. 1985;i:48. doi: 10.1016/s0140-6736(86)92596-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 19.Radandt J M, Marchbanks C R, Dudley M N. Interactions of fluoroquinolones with other drugs: mechanisms, variability, clinical significance, and management. Clin Infect Dis. 1992;14:272–284. doi: 10.1093/clinids/14.1.272. [DOI] [PubMed] [Google Scholar]
- 20.Reeves D S, Bywater M J. Assay of antimicrobial agents. In: de Louvois J, editor. Selected topics in clinical bacteriology. London, United Kingdom: Bailliere Tindall; 1976. pp. 21–78. [Google Scholar]
- 21.Sachs L. Angewandte Statistik. 3rd ed. Berlin, Germany: Springer-Verlag; 1992. pp. 410–422. [Google Scholar]
- 22.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;2:461–464. [Google Scholar]
- 23.Teng R, Dogolo L C, Willavize S A, Friedman H L, Vincent J. Effect of Maalox and omeprazole on bioavailability of trovafloxacin. J Antimicrob Chemother. 1997;39(Suppl. B):93–97. doi: 10.1093/jac/39.suppl_2.93. [DOI] [PubMed] [Google Scholar]
- 24.Timmers K, Sternglanz R. Ionization and divalent cation dissociation constants of nalidixic and oxolinic acids. Bioinorg Chem. 1978;9:145–155. doi: 10.1016/s0006-3061(00)80286-0. [DOI] [PubMed] [Google Scholar]
- 25.Wakabayashi E, Mitsuhashi S. In vitro antibacterial activity of AM-1155, a novel 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1994;3:594–601. doi: 10.1128/aac.38.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise R, Brenwald N P, Andrews J M, Boswell F. The activity of the methylpiperazinyl fluoroquinolone CG 5501: a comparison with other fluoroquinolones. J Antimicrob Chemother. 1997;39:447–452. doi: 10.1093/jac/39.4.447. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka-Yuen N A, Gantu T G. Fluoroquinolone drug interactions. Am J Hosp Pharm. 1990;47:1270. [PubMed] [Google Scholar]
- 28.Zix J A, Geerdes-Fenge H F, Rau M, Vöckler J, Borner K, Koeppe P, Lode H. Pharmacokinetics of sparfloxacin and interaction with cisapride and sucralfate. Antimicrob Agents Chemother. 1997;8:1668–1672. doi: 10.1128/aac.41.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]