Abstract
The effect of contrast media (CM), delivered prior to- and during transcatheter aortic valve implantation (TAVI), on kidney function, following the procedure, is debatable. Consequently, the performance of CM-based, acute kidney injury (AKI) risk prediction models is also questionable. We retrospectively studied 210 patients that underwent TAVI. We recorded the dose of CM used prior and during TAVI, calculated the results of different AKI risk assessment models containing a CM module, and tested their association with AKI after the procedure. AKI was diagnosed in 38 patients (18.1%). The baseline estimated glomerular filtration rate (eGFR) was lower in the AKI+ group compared to AKI− group (51 ± 19.3 versus 64.5 ± 19 mL/min/1.73 mr2, respectively). While the dose of CM delivered prior to TAVI, during TAVI or the cumulative amount of both did not differ between the groups, the results of all tested risk models were higher in AKI+ patients. However, by multivariable analysis, only eGFR had a consistent independent association with AKI. We suggest that the dose of CM delivered prior or during TAVI is not associated with AKI and that the predictive power of CM based AKI risk models is, in all probability, limited to eGFR alone.
Keywords: transcatheter aortic valve implantation (TAVI), acute kidney injury (AKI), contrast media (CM), risk models
1. Introduction
Acute kidney injury (AKI) following transcatheter aortic valve implantation (TAVI) is frequent and strongly associated with adverse outcomes [1,2,3,4,5]. Therefore, it is imperative to recognize the parameters that inflict an increased risk for AKI following TAVI. Previous studies have linked, although inconsistently, the volume of radiographic contrast media (CM) administered during TAVI, with the development of AKI after the procedure [5,6,7,8,9,10]. It is possible, although not tested thus far, that preparatory exposure to CM, before TAVI, may impose additional deleterious effects on post-TAVI kidney function. Risk assessment tools, incorporating CM volume, have proved useful in predicting the development of AKI following percutaneous coronary procedures [11,12,13,14]. However, the utility of such risk models in predicting post-TAVI AKI is less established [6,15,16].
We investigated the association between the total volume of CM delivered during TAVI as well as during near-past CM utilizing procedures and the occurrence of post-TAVI AKI. We additionally tested the ability of CM based AKI risk assessment models, previously verified with percutaneous coronary interventions (PCI), to predict post-TAVI AKI. We also examined whether including previous exposures to CM, before TAVI, would improve the accuracy of those risk models in predicting AKI after TAVI.
2. Materials and Methods
We conducted a retrospective analysis using patient data from Poriya Medical Center’s (PMC) local TAVI database. The study complied with the ethical guidelines of the Declaration of Helsinki, was approved by the Ethical Review Board at PMC, and each patient provided written informed consent before the intervention. Data were recorded prospectively during the index hospitalization as well as during follow-up visits. Patients were excluded from the analysis if they had one or more of the following: (1) treatment with renal replacement therapy prior to TAVI, (2) missing data about the volume of CM prior or during TAVI, (3) a death within seven days of TAVI without being diagnosed with AKI, (4) missing follow-up AKI data, or (5) additional exposures to CM between one day and seven days after the completion of TAVI.
We grouped and compared the patients according to the diagnosis of AKI (AKI+), following TAVI, or freedom from AKI (AKI-). AKI was defined according to the Valve Academic Research Consortium-3 standardized endpoint definitions as follows: an increase in serum creatinine (SCr) ≥ 150–200% within seven days compared with baseline or increase of ≥0.3 mg/dL within 48 h of the index procedure was listed as AKI stage-1. An increase in SCr > 200–300% within seven days compared with baseline was recorded as AKI stage-2, an increase in SCr > 300% within seven days compared with baseline or SCr ≥ 4.0 mg/dL with an acute increase of ≥0.5 mg/dL was indexed as AKI stage-3, and AKI requiring new temporary or permanent renal replacement therapy was indexed as AKI stage-4 [17]. AKI+ depicts AKI stages 1 through 4, while AKI− represents the absence of AKI.
The following parameters were recorded: (1) baseline clinical and echocardiography data, (2) the volume of CM delivered during TAVI, and (3) the total volume of CM administered during the time intervals that started either seven days or 30 days before TAVI and ended at the completion of TAVI. We included all CM delivered during percutaneous diagnostic and interventional coronary and peripheral procedures as well as during computed tomography angiography (CTA) performed within the previously stated time intervals. In the cases where there were additional exposures to CM, within 24 h of TAVI, the volume of CM delivered during those exposures was added to the volume of CM administered during TAVI.
We used Omnipaque™ 350 (GE Healthcare, Cork, Ireland) in all procedures. Omnipaque™ 350 is a low-osmolar, non-ionic, water-soluble, radiographic CM containing 755 mg/mL of Iohexol equivalent to 350 mg/mL of organic iodine (Osmolality 844 mOsm/kg water, Osmolarity 541 mOsm/L, absolute viscosity at 37 °C 10.4 cp, specific gravity at 37 °C 1.406).
The estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [18]. The CKD category was determined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria [19]. The risk of a post-procedural AKI was calculated using various, previously published risk models utilizing CM volume delivered during TAVI. We also calculated those risk models using the cumulative volume of CM administered during a seven-day or 30-day interval, as described above. Additionally, when possible, we calculated a modified version of the risk model in which the CM volume was excluded. The following AKI risk prediction models were tested: (1) CM volume (in ml) divided by the creatinine clearance (CrCl) (in mL/min/1.73 m2) [11], (2) CM (in ml) × Serum Creatinine (SCr) (in mg/dL)/Body weight (BW) (in kg) [10], (3) CM (in ml) × SCr (in mg/dL)/body mass index (BMI) (in kg/m2) [16], (4) The Mehran risk score was calculated by adding the following: congestive heart failure, 5 points; hypotension, 5 points; intra-aortic balloon pump use, 5 points; age over 75 years, 4 points; anemia, 3 points; diabetes mellitus, 3 points; CM volume, 1 point per 100 mL; eGFR, 2 points for 40–59 mL/min/1.73 m2, 4 points for 20–39 mL/min/1.73 m2 and 6 points for <20 mL/min/1.73 m2) [13], and (5). The CR4EATME3AD3 model was calculated as follows: contrast volume > 200 mL, 2 points; eGFR < 60 mL/min/1.73 m2, 4 points; emergency procedure, 2 points; age > 70 years, 2 points; hypotension, 2 points; previous myocardial infarction, 2 points; left ventricular ejection fraction < 45%, 3 points; anemia, 2 points; diabetes mellitus, 3 points [14].
Statistical analysis: Categorical variables were reported as absolute numbers and percentages and were compared using the chi-square test. Continuous data with normal distribution were reported as the mean and standard deviation (SD) and were compared using an independent samples t-test. Continuous variables with a non-normal distribution were reported as medians and interquartile ranges (IQR), and were compared using the Mann–Whitney U test. Univariable and multivariable logistic regression analyses were performed to evaluate for predictors of AKI following TAVI with reported odds ratios (OR’s) and 95% confidence intervals (CI). We excluded any variable with more than 10% missing values. First, univariable associations were determined for all potential covariates. Covariates with a p value of less than 0.1 on univariate analyses were entered into the multivariable logistic regression models. Risk variables that were determined to be significant in the univariable analysis were subsequently tested with the multivariable modeling. A 2-sided p-value of ≤0.05 was considered statistically significant.
3. Results
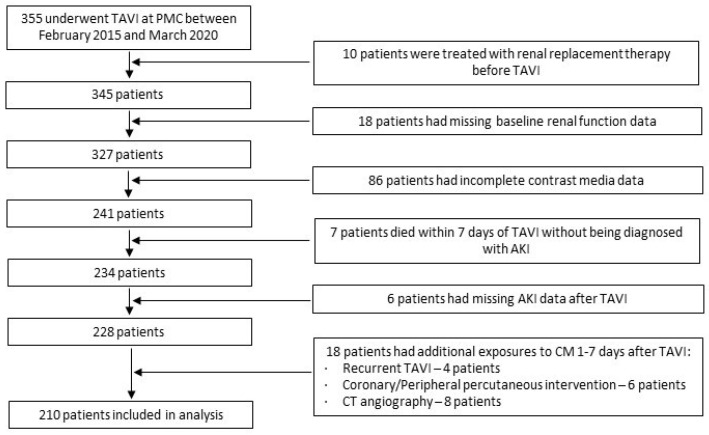
Between February 2015 and March 2020, a total of 355 patients underwent TAVI at PMC. One hundred and forty-five patients were excluded from the analysis. The reasons for excluding these patients are depicted in Figure 1.
Figure 1.
Patient selection flow chart.
The baseline data of the remaining 210 patients, which were included in the analysis, is summarized in Table 1. AKI was diagnosed in 38 patients (18.1%). Of them, 26 (68.4%) patients were classified as suffering from stage-1 AKI, eight (21.1%) patients were classified as stage-2 AKI, three (7.9%) patients had stage-3 AKI and one (2.6%) patient was diagnosed with stage-4 AKI. For the most part, the two groups had a similar baseline profile with the exception of a few significant differences. AKI+ patients, compared to AKI− patients, had a higher prevalence of prior neurological insult, higher surgical risk, as assessed by the STS score, lower hemoglobin levels and a higher rate of anemia (86.8% versus 69.2%, respectively, p = 0.028). They also had worse baseline kidney function with higher baseline creatinine and lower eGFR. Overall, 60.5% of AKI+ patients had eGFR ≤ 60 mL/min/1.73 m2 compared to 37.2% of AKI− patients (p = 0.008). Baseline echocardiographic data was not found to be different between the two groups.
Table 1.
Baseline characteristics.
| AKI (−) (n = 172) |
AKI (+) (n = 38) |
p Value | |||
|---|---|---|---|---|---|
| Clinical data | Age; Years | 79 ± 7.9 | 81.8 ± 8.2 | 0.1 | |
| Female; n (%) | 97 (56.4) | 21 (55.3) | 0.9 | ||
| Body mass index; (kg/m2) | 28.92 ± 5.17 | 28.2 ± 5.97 | 0.46 | ||
| Hypertension; n (%) | 151 (87.8) | 34 (89.5) | 0.77 | ||
| Dyslipidemia; n (%) | 133 (77.3) | 23 (60.5) | 0.032 | ||
| Diabetes mellitus; n (%) | 76 (44.2) | 20 (52.6) | 0.34 | ||
| Smoking history; n (%) | 26 (15.1) | 6 (15.8) | 0.92 | ||
| Cardio-vascular disease; n (%) | 96 (55.8) | 23 (60.5) | 0.596 | ||
| Coronary artery disease; n (%) | 91 (52.9) | 23 (60.5) | 0.728 | ||
| Peripheral arterial disease; n (%) | 19 (11) | 7 (18.4) | 0.211 | ||
| Past PCI; n (%) | 70 (40.4) | 15 (41.7) | 0.98 | ||
| Past CABG; n (%) | 20 (11.6) | 3 (7.9) | 0.51 | ||
| Past CVA/TIA; n (%) | 12 (7) | 7 (18.4) | 0.026 | ||
| Chronic obstructive pulmonary disease; n (%) | 22 (12.8) | 8 (21.1) | 0.19 | ||
| Atrial fibrillation/atrial flutter; n (%) | 43 (25) | 15 (39.5) | 0.071 | ||
| Pacemaker/CRT/ICD; n (%) | 23 (13.4) | 4 (10.5) | 0.64 | ||
| NYHA functional class; n (%) | 1 | 5 (2.9) | 1 (2.6) | 0.727 | |
| 2 | 133 (77.3) | 29 (76.3) | |||
| ≥3 | 8 (4.7) | 3 (7.9) | |||
| Urgent TAVI; n (%) | 40 (23.4) | 10 (26.3) | 0.7 | ||
| Surgical risk | STS, mortality; % | 3.79 ± 2.37 | 4.91 ± 2.27 | 0.009 | |
| EuroScore II; % | 3.83 ± 3.71 | 4.7 ± 3.48 | 0.22 | ||
| Laboratory data | Hemoglobin; g/dL | 11.55 ± 1.71 | 10.77 ± 1.4 | 0.01 | |
| Creatinine; mg/dL | 1.07 ± 0.48 | 1.35 ± 0.58 | 0.002 | ||
| eGFR; mL/min/1.73 m2 | 64.5 ± 19 | 51 ± 19.3 | <0.001 | ||
| CKD category; n (%) | 1–2 | 109 (63.4) | 15 (39.5) | <0.001 | |
| 3a–3b | 54 (31.4) | 17 (44.7) | |||
| ≥4 | 9 (5.2) | 6 (15.8) | |||
|
Echo-
cardiography data |
Left ventricle ejection fraction; % | 58 ± 10.5 | 58 ± 11.1 | 0.917 | |
| Aortic valve area; cm2, | 0.77 ± 0.16 | 0.82 ± 0.19 | 0.166 | ||
| Aortic valve area index; cm2/m2 | 0.43 ± 0.07 | 0.46 ± 0.06 | 0.081 | ||
| Aortic valve mean pressure gradient; mmHg | 43 ± 12 | 41 ± 14.8 | 0.58 | ||
| Severe mitral regurgitation; n (%) | 11 (6.9) | 3 (8.1) | 0.737 | ||
| Severe tricuspid regurgitation; n (%) | 6 (4) | 1 (2.7) | 0.79 | ||
| Severe pulmonary hypertension; n (%) | 14 (9.5) | 4 (11.4) | 0.634 | ||
| Procedural data | Trans-femoral access; n (%) | 170 (98.8) | 38 (100) | 0.99 | |
| THV type; n (%) | Evolut-R™ (Medtronic) | 124 (72.5) | 27 (71.1) | 0.676 | |
| SAPIEN-3™ (Edwards Lifesciences) | 28 (16.4) | 5 (13.2) | |||
| ACURATE-Neo™ (Boston Scientific) | 19 (11.1) | 6 (15.8) | |||
| Anesthesia Type; n (%) | General anesthesia | 72 (51.4) | 16 (48.5) | 0.12 | |
| Local anesthesia with sedation | 68 (48.6) | 17 (51.5) | |||
AKI—acute kidney injury; PCI—percutaneous coronary intervention; CABG—coronary artery bypass graft surgery; CVA—cerebrovascular accident; TIA—transient ischemic attack; CRT—cardiac resynchronization therapy; ICD—implantable cardioverter defibrillator; STS—society of thoracic surgeons; eGFR—estimated glomerular filtration rate; CKD—chronic kidney disease; THV—transcatheter heart valve.
After TAVI, AKI+ patients, compared to their counterparts, had higher SCr and lower eGFR during their post procedural hospitalization both at short-term (i.e., 30 days) and long-term (i.e., 12-month) follow-up (Table 2).
Table 2.
Post-TAVI renal function data.
| AKI (−) (n = 172) |
AKI (+) (n = 38) |
p Value | ||
|---|---|---|---|---|
| Creatinine; mg/dL | Highest (In-hospital) | 1.08 ± 0.31 | 2 ± 1 | <0.001 |
| 30-day | 1.12 ± 0.42 | 1.45 ± 0.55 | 0.011 | |
| 12-month | 1.21 ± 0.5 | 2 ± 1 | 0.014 | |
| eGFR; mL/min/1.73 m2 | Lowest (In-hospital) | 62 ± 20 | 27 ± 12 | <0.001 |
| 30-day | 60.9 ± 20.6 | 44.9 ± 19.2 | 0.005 | |
| 12-month | 58.4 ± 21.3 | 44 ± 20.9 | 0.035 | |
TAVI—transcatheter aortic valve implantation; AKI—acute kidney injury; eGFR—estimated glomerular filtration rate.
CM data and risk models data, at the different tested time intervals, indexed according to the presence or absence of AKI after TAVI, are presented in Table 3. AKI+ patients and AKI− patients did not significantly differ in the volume of CM administered during TAVI. Nor did they differ in the amount of CM delivered, prior to TAVI, within a seven-day or a 30-day periods or in the cumulative amount of CM administered during all procedures. The scores for all tested risk models were significantly higher for AKI+ patients compared to AKI− patients either when they included the volume of CM administered during TAVI or the cumulative volume of CM as described earlier. Additionally, the modifications of Mehran risk model and CR4EATME3AD3 model, which were calculated without the CM element, were also higher in AKI+ patients.
Table 3.
Contrast media and risk models data.
| Within 30 Days | Within 7 Days | During TAVI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AKI (−) (n = 38) |
AKI (+) (n = 172) |
p Value | AKI (−) (n = 38) |
AKI (+) (n = 172) |
p Value | AKI (−) (n = 38) |
AKI (+) (n = 172) |
p Value | |
| CM volume; mL | 319 ± 92 | 320 ± 105 | 0.97 | 246 ± 89 | 259 ± 111 | 0.54 | 187 ± 53 | 201 ± 83 | 0.316 |
| CM volume prior to TAVI; mL | 133 ± 74 | 119 ± 65 | 0.41 | 59 ± 73 | 58 ± 71 | 0.93 | NR | NR | NR |
| Mehran score | 13.52 ± 4.15 | 16.07 ± 3.63 | 0.004 | 12.76 ± 4.06 | 15.46 ± 3.73 | 0.025 | 12.2 ± 4.05 | 14.72 ± 3.75 | 0.004 |
| Modified Mehran score ^ | NR | NR | NR | NR | NR | NR | 10.33 ± 3.97 | 12.87 ± 3.87 | 0.004 |
| CR4EATME3AD3 | 10.09 ± 3.88 | 12.03 ± 3.75 | 0.007 | 9.56 ± 3.87 | 11.55 ± 3.63 | 0.004 | 9.09 ± 3.87 | 11.13 ± 3.78 | 0.004 |
| Modified CR4EATME3AD ^ | NR | NR | NR | NR | NR | NR | 8.45 ± 3.81 | 10.45 ± 3.85 | 0.006 |
| (CM × SCr)/BMI | 11.83 ± 6.27 | 15.52 ± 9.8 | 0.004 | 9.1 ± 5.25 | 12.36 ± 9.78 | 0.005 | 7.02 ± 4.29 | 9.33 ± 5.6 | 0.005 |
| (CM × SCr)/BW | 4.5 ± 2.41 | 5.85 ± 3.44 | 0.005 | 3.48 ± 2.04 | 4.69 ± 3.48 | 0.005 | 2.66 ± 1.61 | 3.58 ± 2.13 | 0.004 |
| CM/CrCl | 5.45 ± 2.66 | 7.04 ± 3.6 | 0.002 | 4.18 ± 2.25 | 5.71 ± 3.56 | <0.001 | 3.24 ± 1.95 | 4.41 ± 2.56 | 0.002 |
AKI—acute kidney injury; TAVI—transcatheter aortic valve implantation; NR—not relevant; CM—contrast media; SCr—serum creatinine; CrCl—creatinine clearance, BMI—body mass index, BW—body weight. ^ Calculated without CM module.
Table 4 shows the results of univariable analysis. Baseline eGFR < 45 mL/min/1.73 m2, hemoglobin level and the STS score were significantly associated with AKI following TAVI. Additionally, all risk models, whether computed without the CM volume module or with it, as administered during TAVI or during the prespecified seven-day and 30-day intervals, were also predictive of AKI. In contrast, neither the administered volume of CM during TAVI nor the seven-day or 30-day cumulative dose of CM were associated with AKI development.
Table 4.
Univariable logistic regression analysis for the probability of developing AKI following TAVI.
| n = 210 | ||||
|---|---|---|---|---|
| OR | 95% CI | p Value | ||
| eGFR reduction by 1 mL/min/1.73 m2 | 1.036 | 1.017–1.056 | <0.001 | |
| eGFR <45 mL/min/1.73 m2 | 4.14 | 1.93–8.9 | 0.003 | |
| Hemoglobin reduction by 1 g/dL | 1.33 | 1.06–1.66 | 0.013 | |
| STS score for mortality | 1.0387 | 1.009–1.069 | 0.009 | |
| Diabetes Mellitus | 1.4 | 0.694–2.835 | 0.345 | |
| Hypertension | 1.18 | 0.381–3.66 | 0.772 | |
| Body mass index | 0.974 | 0.91–1.04 | 0.458 | |
| Gender | 0.955 | 0.471–1.936 | 0.9 | |
| Age | 1.041 | 0.992–1.094 | 0.1 | |
| Left ventricle ejection fraction | 0.998 | 0.965–1.032 | 0.917 | |
| NYHA functional class | 1.252 | 0.474–3.303 | 0.65 | |
| Coronary artery disease | 1.836 | 0.881–3.824 | 0.105 | |
| Peripheral arterial disease | 1.818 | 0.704–4.695 | 0.217 | |
| Within 30 Days | CM volume | 1.001 | 0.997–1.003 | 0.971 |
| CM/CrCl | 1.182 | 1.058–1.321 | 0.004 | |
| (CM × Scr)/BMI | 1.062 | 1.014–1.113 | 0.011 | |
| (CM × Scr)/BW | 1.169 | 1.036–1.139 | 0.012 | |
| Mehran Score | 1.109 | 1.03–1.195 | 0.006 | |
| CR4EATME3AD3 score | 1.137 | 1.035–1.249 | 0.007 | |
| Within 7 Days | Volume of CM | 1.009 | 0.998–1.004 | 0.538 |
| CM/CrCl | 1.216 | 1.073–1.379 | 0.002 | |
| (CM × Scr)/BMI | 1.069 | 1.012–1.128 | 0.017 | |
| (CM × Scr)/BW | 1.19 | 1.036–1.367 | 0.014 | |
| Mehran Score | 1.118 | 1.037–1.205 | 0.004 | |
| CR4EATME3AD3 score | 1.146 | 1.041–1.262 | 0.005 | |
| TAVI | Volume of CM | 1.002 | 0.998–1.007 | 0.295 |
| CM/CrCl | 1.237 | 1.069–1.43 | 0.004 | |
| (CM × SCr)/BMI | 1.092 | 1.021–1.167 | <0.001 | |
| (CM × SCr)/BW | 1.275 | 1.069–1.521 | 0.007 | |
| Mehran Score | 1.102 | 1.013–1.2 | 0.025 | |
| CR4EATME3AD3 score | 1.147 | 1.043–1.261 | 0.005 | |
| Modified Mehran score ^ | 1.11 | 1.028–1.199 | 0.008 | |
| Modified CR4EATME3AD3 score ^ | 1.146 | 1.041–1.261 | 0.005 | |
AKI—acute kidney injury; TAVI—transcatheter aortic valve implantation; eGFR—estimated glomerular filtration rate; NYHA—New-York Heart Association; PAD—peripheral arterial disease, CVD—cardiovascular disease; CM—contrast media; SCr—serum creatinine; CrCl—creatinine clearance; BMI—body mass index, BW—body weight. ^ Calculated without contrast media module.
Each of the risk models was discretely entered into a multivariable logistic regression model together with the hemoglobin level, eGFR < 45 mL/min/1.73 m2 and STS score. By multivariable analysis none of the risk models predicted post-TAVI AKI. Neither did the hemoglobin level nor the STS score. Only pre-TAVI eGFR, in most of the analyses, was independently associated with AKI after it (Table 5).
Table 5.
Multivariable logistic regression analyses for the prediction of post-TAVI AKI.
| STS Score | Hemoglobin | eGFR ≤ 45 mL/min/1.73 m2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Within 30 Days | Mehran risk score | 1.02 (0.93–1.12) | 0.63 | 1.1 (0.94–1.29) | 0.24 | 0.83 (0.63–1.08) | 0.165 | 2.67 (1.1–6.5) | 0.03 |
| CR4EATME3AD3 | 1.04 (0.92–1.17) | 0.514 | 1.08 (0.92–1.27) | 0.355 | 0.84 (0.65–1.08) | 0.168 | 2.5 (1.01–6.28) | 0.049 | |
| CM/CrCl | 1.05 (0.9–1.26) | 0.539 | 1.08 (0.91–1.27) | 0.345 | 0.83 (0.64–1.06) | 0.137 | 2.35 (0.85–6.49) | 0.099 | |
| (CM× SCr)/BMI | 1.02 (0.97–1.08) | 0.397 | 1.09 (0.92–1.29) | 0.302 | 0.84 (0.65–1.08) | 0.163 | 2.48 (0.99–6.19) | 0.053 | |
| (CM × SCr)/BW | 1.04 (0.91–1.2) | 0.548 | 1.09 (0.92–1.28) | 0.328 | 0.84 (0.65–1.08) | 0.163 | 2.61 (1.04–6.56) | 0.041 | |
| Within 7 Days | Mehran risk score | 1.03 (0.94–1.13) | 0.518 | 1.07 (0.91–1.26) | 0.41 | 0.84 (0.65–1.09) | 0.194 | 2.62 (1.07–6.39) | 0.034 |
| CR4EATME3AD3 | 1.05 (0.93–1.18) | 0.417 | 1.08 (0.92–1.27) | 0.364 | 0.84 (0.65–1.08) | 0.17 | 2.46 (0.98–6.15) | 0.054 | |
| CM/CrCl | 1.01 (0.94–1.28) | 0.216 | 1.08 (0.91–1.27) | 0.364 | 0.83 (0.64–1.06) | 0.123 | 2.15 (0.8–5.7) | 0.125 | |
| (CM × SCr)/BMI | 1.02 (0.97–1.09) | 0.313 | 1.09 (0.92–1.28) | 0.323 | 0.83 (0.64–1.07) | 0.151 | 2.44 (1.0–6.1) | 0.049 | |
| (CM × SCr)/BW | 1.07 (0.92–1.24) | 0.377 | 1.08 (0.92–1.28) | 0.361 | 0.83 (0.64–1.07) | 0.15 | 2.54 (1.03–6.28) | 0.044 | |
| TAVI | Mehran risk score | 1.02 (0.92–1.12) | 0.761 | 1.1 (0.94–1.29) | 0.228 | 0.83 (0.64–1.08) | 0.173 | 2.76 (1.11–6.82) | 0.028 |
| CR4EATME3AD3 | 1.05 (0.93–1.18) | 0.429 | 1.08 (0.92–1.26) | 0.357 | 0.84 (0.65–1.08) | 0.175 | 2.46 (0.98–6.19) | 0.054 | |
| CM/CrCl | 1.08 (0.89–1.31) | 0.43 | 1.09 (0.92–1.28) | 0.309 | 0.83 (0.64–1.06) | 0.134 | 2.39 (0.85–6.7) | 0.1 | |
| (CM × SCr)/BMI | 1.03 (0.97–1.12) | 0.406 | 1.1 (0.93–1.29) | 0.262 | 0.83 (0.65–1.07) | 0.156 | 2.48 (0.93–6.45) | 0.07 | |
| (CM × SCr)/BW | 1.09 (0.88–1.35) | 0.414 | 1.09 (0.93–1.29) | 0.287 | 0.83 (0.64–1.07) | 0.159 | 2.47 (0.94–6.55) | 0.067 | |
| Modif-ied ^ | Mehran risk score | 1.00 (0.91–1.11) | 0.888 | 1.08 (0.92–1.27) | 0.373 | 0.83 (0.64–1.08) | 0.161 | 2.8 (1.13–6.93) | 0.026 |
| CR4EATME3AD3 | 1.03 (0.91–1.17) | 0.62 | 1.08 (0.92–1.26) | 0.362 | 0.83 (0.65–1.08) | 0.163 | 2.55 (1.0–6.11) | 0.051 | |
TAVI—transcatheter aortic valve implantation; AKI—acute kidney injury; CM—contrast media; CrCl—creatinine clearance; SCr—serum creatinine; BMI—body mass index; BW—body weight. ^ Calculated without contrast media module.
4. Discussion
The main findings of the study were: (1) The volume of CM administered during TAVI and the cumulative volume of CM administered within seven days or within 30 days from TAVI were not associated with AKI post-TAVI; and (2) None of the tested risk assessment models that included a CM module, were independently associated with post-TAVI AKI. (3) eGFR was the only independent predictor for AKI.
AKI is a serious complication, commonly encountered following TAVI with an incidence of any level of AKI, reported from large registries and metanalyses, at around 20% [1,3]. The development of AKI, following TAVI, significantly increases the risk of both short-term and long-term deleterious complications, such as myocardial infarction, life threatening bleeding, permanent renal dysfunction necessitating renal replacement therapy, and mortality [1,3,4,5,20,21,22,23]. Various baseline characteristics, co-morbidities and peri-procedural factors have been linked with TAVI related AKI. Some of the most reported are the baseline decreasing level of hemoglobin, chronic kidney dysfunction, acute bleeding necessitating blood transfusions and hemodynamic instability during the procedure [2,5,22,24,25]. Still, there is much uncertainty with a multitude of proposed risk factors previously associated with AKI following other procedures in which CM is used, and the role they play in the development of AKI following TAVI with conflicting reported results. For instance, while some studies demonstrated a relationship between increasing age and AKI development following TAVI, other studies did not [3,6,7,10,25]. While in some studies LVEF was dis-concordantly associated with post-TAVI AKI, in other publications it was not [6,7,10,23,25,26]. Even decreasing baseline eGFR and chronic kidney dysfunction have an uncertain magnitude of effects on post-TAVI AKI [3,6,7,8,10,25,26,27]. The relationship between CM volume and TAVI-related AKI is also controversial. In several previous studies there was no significant difference in the volume of CM delivered during TAVI, between AKI+ patients and AKI− patients, nor between CM dose and post-procedural AKI [2,4,5,8,16]. Conversely, other studies described higher dosages of CM in AKI+ patients compared to AKI, and an association between the volume of CM delivered during TAVI and the subsequent development of AKI [6,9,10,25,26].
The uncertain association between administered CM volume and AKI has been described with other medical procedures utilizing radiographic CM. In patients with eGFR above 30 mL/min/1.73 m2 who underwent CT testing, there was no significant difference in the incidence of AKI between those who received intravenous CM and those who did not, and the results for patients with eGFR less than 30 mL/min/1.73 m2 were conflicting [28,29]. An analysis of observational studies concluded that radiocontrast use in CT scanning was not causally related to changes in kidney function [30]. These results were echoed in the most recent update of the American College of Radiology consensus statement in which it was declared that the risk of AKI developing following exposure to intravenous iodinated CM has been exaggerated and that the true risk of AKI, related to CM administration, remains uncertain even for patients with severe kidney disease [31]. It has been suggested that intravenous administration of CM, like with CT angiography, may impose a different risk of AKI than the one associated with arterial administration of CM, like with PCI and TAVI; however, the data is inconclusive [32,33,34,35,36,37].
CM related AKI has been extensively studied in the context of PCI. However, assessing the true impact of CM on the development of AKI, following PCI, has proven to be a difficult task. Studies that evaluated this coupling between CM and AKI widely differed in the type, chemical characteristics, pharmacokinetics and volume of CM, the route it was administered, the medical procedure it was given for and in the definition used to diagnose AKI [38]. Notably, a broad range of contrast volume (from below 100 mL to above 800 mL) has been associated with post-PCI nephropathy [12,13,39,40,41]. There are several fundamental differences between patients treated with TAVI and patients treated with PCI which, potentially, position the former at higher risk to develop AKI than the later. Patients undergoing TAVI are usually elderly, fragile, frequently suffering from multiple co-morbidities and have reduced GFR [7,42]. Additionally, PCI-treated patients are usually exposed to CM once during their index procedure. TAVI treated patients, however, are frequently exposed to a large cumulative volume of CM during several diagnostic and interventional procedures and over a relatively short period of time. Interestingly, despite these differences, Venturi et al. found that AKI actually occurred less frequently in patients undergoing TAVI than in patients undergoing PCI, even after propensity score matching [7]. Following TAVI, unlike other procedures that utilize CM, there is an immediate and sustained improvement in hemodynamics resulting in an increase in cardiac output and systemic perfusion [43,44,45]. Still, even though this can possibly translate into the reduced incidence of AKI following TAVI, the rate of immediate kidney function improvement after TAVI was found to be small in a recent publication (5%) [46].
The diagnosis of AKI tends to lag after the index exposure to CM, with gradual worsening of kidney function over the ensuing days. Accordingly, the assessment for CM associated nephrotoxicity was traditionally made within 48 to 72 h of exposure and according to VARC-3 consensus statements this time frame has been further extended to seven days [13,17,39]. Thus far, the effect of CM on kidney function was tested for CM delivered during TAVI alone, while the possible effect inflicted by near past exposures to CM was overlooked. This led us to test for an additive effect of preceding exposures to CM on the incidence of AKI following TAVI. In this study, the volume of CM administered during TAVI alone, during a 7-day period or during a 30-day period, did not significantly differ between AKI+ patients and AKI− patients, nor did it independently predict the development of AKI. Similarly, in a recent study, assessing the effect of multiple exposures to CM during recurrent diagnostic and interventional coronary procedures, although over a period of several years, worsening of renal function was associated with known risk factors for the progression of kidney disease but not with cumulative CM volume [47].
The difficulties in predicting AKI following TAVI led to the development of risk assessment models. Incorporating several parameters to form a risk model, can potentially improve the predictive power beyond that of the individual modules included in it. AKI risk prediction models have been developed, tested and validated for coronary procedures [11,12,13,14,48,49]. Sadly, only a few of these models have been specifically designed to assess for the risk of post-TAVI AKI. For instance, Zivcovic et al. introduced an AKI risk calculator that was meant to be utilized prior to TAVI and therefore did not include a CM component [27]. As in this study, several previous studies tested the effectiveness of CM-based, non-TAVI dedicated risk models in predicting AKI following TAVI [6,15,16,24,26,50]. In our study, none of the CM-based risk models we tested independently predicted post-TAVI AKI. Mach et al. reported that none of the six tested CM-based risk models (including the Mehran risk model) were significantly different between AKI+ and AKI− patients or, similarly to our results, independently predicted AKI [16]. Rosa et al. describes that all of their tested risk models, of which four included a CM module and two did not, had poor accuracy in terms of predicting the occurrence of any AKI. However, there was an improvement in AKI risk prediction for more advanced stages of AKI. By univariable logistic regression analysis, only risk models with a CM module were associated with AKI. The results of multivariable logistic regression coefficients of risk for any AKI or the different stages of AKI were not reported [15]. In contrast, a small study of 93 patients, of which AKI was diagnosed in 24 of them, found in a univariable analysis that the Mehran risk model, as well as CM volume, predicted post-TAVI AKI. The authors also reported that in multivariable analysis, CM volume, the Mehran score, SCr and eGFR were all independently associated with AKI [6]. However, the study was of limited sample size and, given the low number of endpoints achieved, was underpowered to accurately explore the aforementioned endpoints.
In our study, decreasing eGFR, as previously described by others, was significantly and independently associated with AKI across most analyses [5,8]. In fact, it was the only independent predictor for AKI. Therefore, with caution, we suggest that the AKI predictive power of risk score models containing a CM volume module, is almost entirely limited to pre-procedural eGFR.
Limitations: This is an individual center report and has the limitations associated with retrospective analysis. Given the relatively small number of AKI events, our study was underpowered to evaluate for the most severe stages of AKI. The assessment of baseline sCr was made prior to TAVI and not prior to the first exposure to CM within the assessed time interval. Additionally, most patients were discharged before seven days had elapsed from their procedure and therefore, even though it is our institutional practice to discharge the patient after kidney function has stabilized, it is possible that further deterioration in kidney function ensued after discharge without being recorded. The use of drugs with nephrotoxic properties close to contrast media exposure was also not recorded.
5. Conclusions
Neither the volume of CM delivered during TAVI nor the cumulative amount of CM delivered during a time period that starts either seven-days or 30 days before TAVI and ends with TAVI are associated with AKI. The power of non-TAVI dedicated CM based risk models, in predicting AKI, is limited to pre-procedural kidney dysfunction.
Author Contributions
Conceptualization, D.S. and I.M.; Methodology, D.S., L.G.-R., E.Y.B. and I.M.; Validation, D.S., L.G.-R., A.L. and I.M.; Formal Analysis, D.S., L.G.-R. and I.M.; Investigation, D.S., Y.D., F.K., A.L., M.G., W.K. and I.M.; Resources, D.S., W.K., E.H., M.G. and I.M.; Data Curation, D.S. and A.L.; Writing—Original Draft Preparation, D.S.; Writing—Review and Editing, D.S., L.G.-R., E.Y.B., A.L. and I.M.; Visualization, D.S., L.G.-R., A.L. and E.Y.B.; Supervision, E.Y.B. and I.M.; Project Administration, D.S.; All authors have contributed to preparing the manuscript in accordance with the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Padeh Medical Center (Protocol code POR-0099-14; Approval date: 30 December 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study was obtained from PMC’s local TAVI prospective registry and is available on request from the corresponding author.
Conflicts of Interest
D.S. received a speakers’ fee from Abbott Medical Laboratories, Sanofi, Novartis and Novo Nordisk; E.Y.B. received a speakers’ honoraria from CTS and Novo Nordisk received research support paid to the University of Pennsylvania by Medtronic Inc. and Impulse Dynamics Ltd.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gargiulo G., Sannino A., Capodanno D., Perrino C., Capranzano P., Barbanti M., Stabile E., Trimarco B., Tamburino C., Esposito G. Impact of postoperative acute kidney injury on clinical outcomes after transcatheter aortic valve implantation: A meta-analysis of 5,971 patients. Catheter. Cardiovasc. Interv. 2015;86:518–527. doi: 10.1002/ccd.25867. [DOI] [PubMed] [Google Scholar]
- 2.Gargiulo G., Capodanno D., Sannino A., Perrino C., Capranzano P., Stabile E., Trimarco B., Tamburino C., Esposito G. Moderate and severe preoperative chronic kidney disease worsen clinical outcomes after transcatheter aortic valve implantation: Me-ta-analysis of 4992 patients. Circ. Cardiovasc. Interv. 2015;8:e002220. doi: 10.1161/CIRCINTERVENTIONS.114.002220. [DOI] [PubMed] [Google Scholar]
- 3.Nunes Filho A.C., Katz M., Campos C.M., Carvalho L.A., Siqueira D.A., Tumelero R.T., Portella A.L.F., Esteves V., Perin M.A., Sarmento-Leite R., et al. Impact of Acute Kidney Injury on Short- and Long-term Outcomes After Transcatheter Aortic Valve Implantation. Rev. Esp. Cardiol. Engl. Ed. 2019;72:21–29. doi: 10.1016/j.recesp.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Barbash I.M., Ben-Dor I., Dvir D., Maluenda G., Xue Z., Torguson R., Satler L.F., Pichard A.D., Waksman R. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am. Hear. J. 2012;163:1031–1036. doi: 10.1016/j.ahj.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Elhmidi Y., Bleiziffer S., Deutsch M.-A., Krane M., Mazzitelli D., Lange R., Piazza N. Acute kidney injury after transcatheter aortic valve implantation: Incidence, predictors and impact on mortality. Arch. Cardiovasc. Dis. 2014;107:133–139. doi: 10.1016/j.acvd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Zungur M., Gul I., Tastan A., Damar E., Tavli T. Predictive Value of the Mehran Score for Contrast-Induced Nephropathy after Transcatheter Aortic Valve Implantation in Patients with Aortic Stenosis. Cardiorenal Med. 2016;6:279–288. doi: 10.1159/000443936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venturi G., Pighi M., Pesarini G., Ferrero V., Lunardi M., Castaldi G., Setti M., Benini A., Scarsini R., Ribichini F.L. Contrast-Induced Acute Kidney Injury in Patients Undergoing TAVI Compared with Coronary Interventions. J. Am. Heart Assoc. 2020;9:e017194. doi: 10.1161/JAHA.120.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J., Yu W., Zhou Y., Yang Y., Li C., Liu N., Hou X., Wang L. Independent Risk Factors Contributing to Acute Kidney Injury According to Updated Valve Academic Research Consortium-2 Criteria After Transcatheter Aortic Valve Implantation: A Meta-analysis and Meta-regression of 13 Studies. J. Cardiothorac. Vasc. Anesth. 2017;31:816–826. doi: 10.1053/j.jvca.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Madershahian N., Scherner M., Liakopoulos O., Rahmanian P., Kuhn E., Hellmich M., Mueller-Ehmsen J., Wahlers T. Renal impairment and transapical aortic valve implantation: Impact of contrast medium dose on kidney function and survival. Eur. J. Cardio-Thorac. Surg. 2012;41:1225–1232. doi: 10.1093/ejcts/ezr199. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M., Hayashida K., Mouillet G., Chevalier B., Meguro K., Watanabe Y., Dubois-Rande J.-L., Morice M.-C., Lefèvre T., Teiger E. Renal Function–Based Contrast Dosing Predicts Acute Kidney Injury Following Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2013;6:479–486. doi: 10.1016/j.jcin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Laskey W.K., Jenkins C., Selzer F., Marroquin O.C., Wilensky R.L., Glaser R., Holmes D.R., Jr., Cohen H.A. Volume-to-Creatinine Clearance Ratio: A Pharmacokinetically Based Risk Factor for Prediction of Early Creatinine Increase After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2007;50:584–590. doi: 10.1016/j.jacc.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 12.Freeman R.V., O’Donnell M., Share D., Meengs W.L., Kline-Rogers E., Clark V.L., DeFranco A.C., Eagle K.A., McGinnity J., Patel K., et al. Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am. J. Cardiol. 2002;90:1068–1073. doi: 10.1016/S0002-9149(02)02771-6. [DOI] [PubMed] [Google Scholar]
- 13.Mehran R., Aymong E.D., Nikolsky E., Lasic Z., Iakovou I., Fahy M., Mintz G.S., Lansky A.J., Moses J.W., Stone G.W. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 14.Fu N., Li X., Yang S., Chen Y., Li Q., Jin D., Cong H. Risk Score for the Prediction of Contrast-Induced Nephropathy in Elderly Patients Undergoing Percutaneous Coronary Intervention. Angiology. 2012;64:188–194. doi: 10.1177/0003319712467224. [DOI] [PubMed] [Google Scholar]
- 15.Rosa V.E., Campos C.M., Bacelar A., Abizaid A.A., Mangione J.A., Lemos P.A., Esteves V., Caramori P., Sampaio R.O., Tarasoutchi F., et al. Performance of Prediction Models for Contrast-Induced Acute Kidney Injury after Transcutaneous Aortic Valve Replacement. Cardiorenal Med. 2021;11:166–173. doi: 10.1159/000517058. [DOI] [PubMed] [Google Scholar]
- 16.Mach M., Hasan W., Andreas M., Winkler B., Weiss G., Adlbrecht C., Delle-Karth G., Grabenwöger M. Evaluating the Association between Contrast Medium Dosage and Acute Kidney Injury in Transcatheter Aortic Valve Replacement Using Different Pre-dictive Models. J. Clin. Med. 2020;9:3476. doi: 10.3390/jcm9113476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VARC-3 Writing Committee. Généreux P., Piazza N., Alu M.C., Nazif T., Hahn R.T., Pibarot P., Bax J.J., Leipsic J.A., Blanke P., et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021;42:1825–1857. doi: 10.1016/j.jacc.2021.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro A.F., III, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 20.Adamo M., Provini M., Fiorina C., Giannini C., Angelillis M., Testa L., Barbanti M., Merlanti B., Poli A., Ferrara E., et al. Interaction between severe chronic kidney disease and acute kidney injury in predicting mortality after transcatheter aortic valve implantation: Insights from the Italian Clinical Service Project. Catheter. Cardiovasc. Interv. 2020;96:1500–1508. doi: 10.1002/ccd.28927. [DOI] [PubMed] [Google Scholar]
- 21.Bagur R., Webb J.G., Nietlispach F., Dumont E., De Larochellière R., Doyle D., Masson J.-B., Gutiérrez M.J., Clavel M.-A., Bertrand O.F., et al. Acute kidney injury following transcatheter aortic valve implantation: Predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur. Hear. J. 2010;31:865–874. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuis R.-J.M., Van Mieghem N.M., Tzikas A., Piazza N., Otten A.M., Cheng J., van Domburg R.T., Betjes M., Serruys P.W., De Jaegere P.P. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2011;77:881–889. doi: 10.1002/ccd.22874. [DOI] [PubMed] [Google Scholar]
- 23.Pyxaras S.A., Zhang Y., Wolf A., Schmitz T., Naber C.K. Effect of Varying Definitions of Contrast-Induced Acute Kidney Injury and Left Ventricular Ejection Fraction on One-Year Mortality in Patients Having Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2015;116:426–430. doi: 10.1016/j.amjcard.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 24.Arai T., Morice M., O’Connor S.A., Yamamoto M., Eltchaninoff H., Leguerrier A., Leprince P., Laskar M., Iung B., Fajadet J., et al. Impact of pre- and post-procedural anemia on the incidence of acute kidney injury and 1-year mortality in patients undergoing transcatheter aortic valve implantation (from the French Aortic National CoreValve and Edwards 2 [FRANCE 2] Registry) Catheter. Cardiovasc. Interv. 2015;85:1231–1239. doi: 10.1002/ccd.25832. [DOI] [PubMed] [Google Scholar]
- 25.Ram P., Mezue K., Pressman G., Rangaswami J. Acute kidney injury post-transcatheter aortic valve replacement. Clin. Cardiol. 2017;40:1357–1362. doi: 10.1002/clc.22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uygur B., Celik O., Demir A.R., Sahin A.A., Guner A., Avci Y., Bulut U., Tasbulak O., Demirci G., Uzun F., et al. A simplified acute kidney injury predictor following transcatheter aortic valve implantation: The ACEF score. Kardiol. Pol. 2021;79:662–668. doi: 10.33963/KP.15933. [DOI] [PubMed] [Google Scholar]
- 27.Zivkovic N., Elbaz-Greener G., Qiu F., Arbel Y., Cheema A.N., Dvir D., Fefer P., Finkelstein A., Fremes S.E., Radhakrishnan S., et al. Bedside risk score for prediction of acute kidney injury after transcatheter aortic valve replacement. Open Heart. 2018;5:e000777. doi: 10.1136/openhrt-2018-000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davenport M.S., Khalatbari S., Cohan R.H., Dillman J.R., Myles J.D., Ellis J.H. Contrast material-induced nephrotoxicity and in-travenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268:719–728. doi: 10.1148/radiol.13122276. [DOI] [PubMed] [Google Scholar]
- 29.McDonald J.S., McDonald R.J., Carter R.E., Katzberg R.W., Kallmes D.F., Williamson E.E. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271:65–73. doi: 10.1148/radiol.13130775. [DOI] [PubMed] [Google Scholar]
- 30.Aycock R.D., Westafer L.M., Boxen J.L., Majlesi N., Schoenfeld E.M., Bannuru R.R. Acute Kidney Injury After Computed Tomography: A Meta-analysis. Ann. Emerg. Med. 2018;71:44–53.e4. doi: 10.1016/j.annemergmed.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 31.Davenport M.S., Perazella M.A., Yee J., Dillman J.R., Fine D., McDonald R.J., Rodby R.A., Wang C.L., Weinreb J.C. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. 2020;2:85–93. doi: 10.1016/j.xkme.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schönenberger E., Martus P., Bosserdt M., Zimmermann E., Tauber R., Laule M., Dewey M. Kidney Injury after Intravenous versus Intra-arterial Contrast Agent in Patients Suspected of Having Coronary Artery Disease: A Randomized Trial. Radiology. 2019;292:664–672. doi: 10.1148/radiol.2019182220. [DOI] [PubMed] [Google Scholar]
- 33.Moideen A., Sajgure A., Dighe T., Bale C. Sun-001 A Comparative Study on The Incidence of Contrast Induced Nephropathy and Its Risk Factors Following Intra-Arterial Versus Intravenous Contrast Administration. Kidney Int. Rep. 2020;5:S205. doi: 10.1016/j.ekir.2020.02.523. [DOI] [Google Scholar]
- 34.McDonald J.S., Leake C.B., McDonald R.J., Gulati R., Katzberg R.W., Williamson E.E., Kallmes D.F. Acute kidney injury after intra-venous versus intra-Arterial contrast material administration in a paired cohort. Investig. Radiol. 2016;51:804–809. doi: 10.1097/RLI.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhury P., Armanyous S., Harb S.C., Provenzano L.F., Ashour T., Jolly S.E., Arrigain S., Konig V., Schold J.D., Navaneethan S.D., et al. Intra-Arterial versus Intravenous Contrast and Renal Injury in Chronic Kidney Disease: A Propensity-Matched Analysis. Nephron Karger Publ. 2019;141:31–40. doi: 10.1159/000494047. [DOI] [PubMed] [Google Scholar]
- 36.Nyman U., Almén T., Jacobsson B., Aspelin P. Are intravenous injections of contrast media really less nephrotoxic than in-tra-arterial injections? Eur. Radiol. 2012;22:1366–1371. doi: 10.1007/s00330-011-2371-4. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z., Ren K. Evaluation of iodine contrast-induced acute kidney injury via different injection routes using BOLD-MRI. Ren Fail Taylor Fr. 2019;41:341–353. doi: 10.1080/0886022X.2019.1604382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy S.W., Barrett B.J., Parfrey P.S. Contrast nephropathy. J. Am. Soc. Nephrol. JASN. 2000;11:177–182. doi: 10.1681/ASN.V111177. [DOI] [PubMed] [Google Scholar]
- 39.Dangas G., Iakovou I., Nikolsky E., Aymong E.D., Mintz G.S., Kipshidze N.N., Lansky A.J., Moussa I., Stone G.W., Moses J.W., et al. Contrast-Induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am. J. Cardiol. 2005;95:13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 40.McCullough P.A., Wolyn R., Rocher L.L., Levin R.N., O’Neill W.W. Acute Renal Failure After Coronary Intervention: Incidence, Risk Factors, and Relationship to Mortality. Am. J. Med. 1997;103:368–375. doi: 10.1016/S0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 41.Rihal C.S., Textor S.C., Grill D.E., Berger P.B., Ting H.H., Best P.J., Singh M., Bell M.R., Barsness G.W., Mathew V., et al. Incidence and Prognostic Importance of Acute Renal Failure After Percutaneous Coronary Intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.CIR.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Wahab M., Zahn R., Horack M., Gerckens U., Schuler G., Sievert H., Naber C., Voehringer M., Schäfer U., Senges J., et al. Transcatheter aortic valve implantation in patients with and without concomitant coronary artery disease: Comparison of characteristics and early outcome in the German multicenter TAVI registry. Clin. Res. Cardiol. 2012;101:973–981. doi: 10.1007/s00392-012-0486-5. [DOI] [PubMed] [Google Scholar]
- 43.Hahn R.T., Pibarot P., Stewart W.J., Weissman N.J., Gopalakrishnan D., Keane M.G., Anwaruddin S., Wang Z., Bilsker M., Lindman B.R., et al. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: A longitudinal study of echocardiog-raphy parameters in cohort A of the PARTNER trial (placement of aortic transcatheter valves) J. Am. Coll. Cardiol. 2013;61:2514–2521. doi: 10.1016/j.jacc.2013.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michail M., Hughes A., Comella A., Cameron J., Gooley R.P., McCormick L.M., Mathur A., Parker K.H., Brown A.J. Acute Effects of Transcatheter Aortic Valve Replacement on Central Aortic Hemodynamics in Patients with Severe Aortic Stenosis. Hypertension. 2020;75:1557–1564. doi: 10.1161/HYPERTENSIONAHA.119.14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anjan V.Y., Herrmann H.C., Pibarot P., Stewart W.J., Kapadia S., Tuzcu E.M., Babaliaros V., Thourani V.H., Szeto W.Y., Bavaria J.E., et al. Evaluation of Flow After Transcatheter Aortic Valve Re-placement in Patients with Low-Flow Aortic Stenosis: A Secondary Analysis of the PARTNER Randomized Clinical Trial. JAMA Cardiol. 2016;1:584–592. doi: 10.1001/jamacardio.2016.0759. [DOI] [PubMed] [Google Scholar]
- 46.Kliuk-Ben Bassat O., Sadon S., Sirota S., Steinvil A., Konigstein M., Halkin A., Bazan S., Grupper A., Banai S., Finkelstein A., et al. Assessment of Kidney Function After Transcatheter Aortic Valve Replacement. Can. J. Kidney Health Dis. 2021;8:1–9. doi: 10.1177/20543581211018029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sudarsky D., Naami R., Shehadeh F., Elias A., Kerner A., Aronson D. Risk of Worsening Renal Function Following Repeated Exposures to Contrast Media During Percutaneous Coronary Interventions. J. Am. Hear. Assoc. 2021;10:e021473. doi: 10.1161/JAHA.121.021473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranucci M., Castelvecchio S., Menicanti L., Frigiola A., Pelissero G. Reply to Letters Regarding Article, “Risk of Assessing Mortality Risk in Elective Cardiac Operations: Age, Creatinine, Ejection Fraction, and the Law of Parsimony”. Circulation. 2010;121:e227–e228. doi: 10.1161/CIR.0b013e3181d43879. [DOI] [PubMed] [Google Scholar]
- 49.Cigarroa R.G., Lange R.A., Williams R.H., Hillis D. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am. J. Med. 1989;86:649–652. doi: 10.1016/0002-9343(89)90437-3. [DOI] [PubMed] [Google Scholar]
- 50.Denegri A., Mehran R., Holy E., Taramasso M., Pasotti E., Pedrazzini G., Moccetti T., Maisano F., Nietlispach F., Obeid S. Post procedural risk assessment in patients undergoing trans aortic valve implantation according to the age, creatinine, and ejection fraction-7 score: Advantages of age, creatinine, and ejection fraction-7 in stratification of post-procedural outcome. Catheter. Cardiovasc. Interv. 2019;93:141–148. doi: 10.1002/ccd.27806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study was obtained from PMC’s local TAVI prospective registry and is available on request from the corresponding author.