Abstract
(1) Background: Peritoneal metastasis in gastric cancer is associated with a poor prognosis. Complete cytoreductive surgery including gastrectomy and complete removal of all peritoneal lesions followed by hyperthermic intraperitoneal chemotherapy (HIPEC) achieves promising results. There exists an immersive variety of approaches for HIPEC that makes it difficult to weigh different results obtained in the literature. In order to enable standardization and development of HIPEC, we here present a systematic review of different drug regimens and technical approaches. (2) Methods: PubMed, Embase, and the Cochrane Library were systematically searched on 26 May 2021 using the mesh terms “intraperitoneal chemotherapy AND gastric cancer”. Under consideration of systematic review guidelines, articles reporting on HIPEC in combination with CRS were selected. Data on duration, drugs, dosage, and other application parameters as well as morbidity and long term survival data were extracted for subsequent statistical analysis, tabulation, and descriptive synthesis. We assessed the risk of bias due to inhomogeneity of the patient cohort and incompleteness of report of HIPEC parameters. (3) Results: Out of 1421 screened publications, 42 publications presenting data from 1325 patients met the criteria. Most of the publications were single institutional retrospective cohort studies. The most common HIPEC regimen is performed after gastrointestinal anastomosis and consists of 50–200 mg/m2 cisplatinum and 30–40 mg/m2 mytomycin C at 42–43 °C for 60–90 min in a closed abdomen HIPEC system with three tubes. Almost every study reported incompletely on HIPEC parameters. Lower rates of anastomotic leakage were reported in studies that performed HIPEC after gastrointestinal anastomosis. Studies that performed open HIPEC and integrated a two-drug regimen indicated better overall survival rates. (4) Discussion: This is an exhaustive overview of the use of drug regimens and techniques for HIPEC after CRS for gastric cancer peritoneal metastasis. Other indications and application modes of intraperitoneal chemotherapy such as prophylactic or palliative HIPEC apart from CRS were not addressed. (5) Conclusion: Complete report of HIPEC parameters should be included in every publication. A consensus for dose expression either per BSA or as flat dose is desirable for comparison of the drug regimens. Despite numerous variations, we identified the most common regimens and techniques and their advantages and disadvantages according to the data in the literature. More phase I/II studies are needed to identify the best approach for HIPEC. (6) Other: This review was not supported by third parties.
Keywords: PRISMA, peritoneal metastasis, gastric cancer, intraperitoneal chemotherapy, hyperthermic intraperitoneal chemotherapy (HIPEC), cytoreductive surgery
1. Introduction
Despite recent efforts in prevention and early detection, gastric cancer (GC) still remains one of the most common and lethal neoplastic diseases worldwide, accounting for more than one million new cases in 2020 and 7.7% of all cancer-related deaths [1].
The peritoneum represents the second most common site of gastric cancer metastasis and is the most common site of cancer recurrence [2,3]. Peritoneal metastasis of gastric cancer (pmGC) has very poor median survival rates of only 3–6 months [4,5,6]. Palliative systemic intravenous chemotherapy still represents the standard treatment strategy for pmGC.
Hyperthermic intraperitoneal chemotherapy (HIPEC) combines the concept of direct delivery of the chemotherapeutic agent to the peritoneum, enabling the application of higher local doses with low systemic toxicity and the enhancement of its cytotoxic effects using hyperthermia [7,8,9]. HIPEC even offers the possibility of cure for a highly selected cohort of patients in cases of complete surgical resection of all peritoneal metastases and simultaneous oncologic gastrectomy with tumor-free resection margins and D2-lymphadenectomy [10,11].
HIPEC has proven promising survival outcomes in many tumor entities such as peritoneal mesothelioma [12], pseudomyxoma peritonei [13], and ovarian cancer [14], with acceptable morbidity and mortality rates and costs [15,16]. Promising results were achieved for pmGC with different technical and drug regimens of HIPEC [17].
Although the standardized use of HIPEC in the treatment algorithm of pmGC has not yet been integrated in national and international guidelines, CRS and HIPEC are performed worldwide with an immersive variety of technical and pharmacological approaches. On this account, it is still difficult to compare the results of both the existing randomized controlled studies (RCTs) and retrospective cohorts [18]. Kusamura et al. have identified eight aspects that can be influenced when performing HIPEC: type, combination and concentration of drugs, carrier solution and its volume, temperature, duration, and technique (open or closed abdomen) [19], leaving countless possibilities for pharmacological studies on HIPEC. Recently, standardization of HIPEC has been identified as an important element in order to find the ideal regime and technical approach and to further test the survival benefit intraperitoneal chemotherapy might offer for pmGC [20,21,22].
In an effort to contribute to this standardization of HIPEC regimens, we present a systematic review of all existing RCTs and prospective and retrospective trials with respect to technical approaches and drugs used for HIPEC in the context of CRS for pmGC.
2. Materials and Methods
2.1. Literature Search and Selection of Records
This review was conducted taking into account the 2020 PRISMA guidelines for systematic reviews [23]. with respect to different nominations of HIPEC in the past [24] and without limiting the publication date, we systematically searched PubMed, Embase, and the Cochrane Library on 26 May 2021 using the search terms “intraperitoneal chemotherapy AND gastric cancer” as MeSH Terms, as these terms represent the widest definition of intraabdominal locoregional chemotherapeutic drug therapy for gastric cancer peritoneal metastases and include the following translations:
intraperitoneal: “intraperitonal” [All Fields] OR “intraperitonally” [All Fields] OR “intraperitoneal” [All Fields] OR “intraperitoneally” [All Fields]
chemotherapy: “chemotherapy’s” [All Fields] OR “drug therapy” [MeSH Terms] OR (“drug” [All Fields] AND “therapy” [All Fields]) OR “drug therapy” [All Fields] OR “chemotherapies” [All Fields] OR “drug therapy” [Subheading] OR “chemotherapy” [All Fields]
gastric cancer: “stomach neoplasms” [MeSH Terms] OR (“stomach” [All Fields] AND “neoplasms” [All Fields]) OR “stomach neoplasms” [All Fields] OR (“gastric” [All Fields] AND “cancer” [All Fields]) OR “gastric cancer” [All Fields]
After exclusion of other species than human and other languages than English, French, or German, 828 publications were eligible for title, abstract, and full-text screening, which was performed independently by F.G. and F.O. without the use of an automation tool. In case of a discordance between F.G. and F.O., B.R. and L.F. helped to solve the conflict by discussion.
We limited our analysis to studies that performed HIPEC directly after cytoreductive surgery. For this reason, we excluded studies performing HIPEC without CRS or for cytology-positive GC, as well as early postoperative intraperitoneal chemotherapy (EPIC) and neoadjuvant intraperitoneal and/or systemic chemotherapy (NIPS). Further exclusion criteria were introduction of intraperitoneal chemotherapy via direct single or multiple injections or an implanted port-system without an in- and outflow perfusion system, as these procedures are normally performed separately from CRS as well [25]. Articles presenting the results of patient cohorts including multiple tumor entities were excluded when the GC cohort comprised fewer than 6 patients, as we considered such small groups as not representative. We excluded publications when there was no information available on the drug regimen used for HIPEC for pmGC. We considered this aspect crucial for this systematic review. We did not include data from other reviews.
In cases of multiple publications from the same group, we selected the newest or the one with the most complete patient cohort.
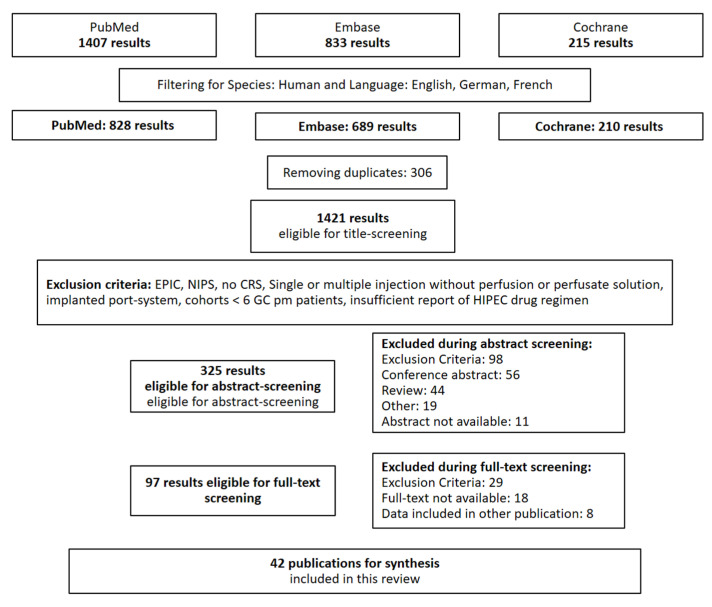
To identify study protocols for RCTs testing HIPEC for pmGC, we included the study protocols identified during the article screening and performed a PubMed search using the terms “study protocol AND hipec AND gastric cancer” as well as a search in clinicaltrials.gov using the terms “gastric cancer peritoneal metastasis”, “gastric cancer stage iv”, “intraperitoneal chemotherapy”, “HIPEC”, and “peritoneal metastasis”. We adopted the same exclusion criteria as for the already published studies and further only included phase III studies that have not published their results yet (see Figure 1).
Figure 1.
Methodology for article screening.
2.2. Quality Assessment
As we did not intend meta-analysis or other forms of comparative statistical analysis of the outcome data obtained with each drug regimen or technical approach, we did not perform a quality assessment of each study with a PICO (patient cohort, intervention, comparator, outcome)-based tool such as RoB-2 or ROBINS-I [26,27]. Instead, our goal was to provide an exhaustive overview of regimens and techniques used for HIPEC for pmGC and the respective morbidity and survival results. We therefore assessed each publication for the homogeneity of the patient cohort with regard to tumor entity (gastric cancer) and stage (peritoneal metastasis), as well as completeness of report of HIPEC parameters, as we deemed these parameters crucial for an exact overview of adapted techniques. A completed table with a quality and risk of bias assessment due to missing data is attached in the supplements (Table S1).
2.3. Data Extraction
F.G. and F.O. independently extracted the data. In cases of a discrepancy during data extraction, again L.F. and B.R. helped to solve the conflict by discussion. We systematically extracted the median PCI, sequence of HIPEC before or after gastrointestinal anastomosis, duration in minutes, open or closed technique, maximum heat, drug regimen including drug name, sequence of application and dose, perfusate solution and volume, flow rate, number of in- and outflow tubes, and the use of simultaneous bidirectional intravenous chemotherapy. The following outcome data were extracted, as well the rate of anastomotic leakage, median overall survival (median OS), median PCI, and major complication rate ≥ III° according to the Clavien–Dindo classification [28]. If only the abstract was available or information was missing, we did not contact the authors.
2.4. Data Synthesis
The data were synthesized in a table, and basic statistics were calculated using Microsoft Office (Microsoft, Redmond, WA, USA) and SPSS (Version 25.0. IBM Corp., Armonk, NY, USA). All information collected from the studies can be found in Table 1, Table 2 and Table 3 in this review. For each parameter, the range and distribution of frequency is displayed in the text, as well as the number of missing values. If there were different technical or drug regimens used in one study, all were included in the synthesis, and if available, the percentage of patients treated in each approach was indicated. Concerning the outcome data, the median and the range were compared between the different subgroups and regimens in order to describe the given differences in the literature.
Table 1.
Full list of published studies included.
| Reference | Year | Single- or Multi-Institutional | Type of Study | Number of Patients Included | Number of Patients with pmGC and CRS + HIPEC |
|---|---|---|---|---|---|
| Fujimoto [29] | 1988 | single | PCS | 15 | 9 |
| Fujimoto [30] | 1997 | single | RCS | 48 | 30 |
| Chen [31] | 1997 | single | RCS | 42 | 6 |
| Sayag-Beaujard [32] | 1999 | single | pCTII | 83 | 18 |
| Loggie [33] | 2000 | single | pCTII | 84 | 22 |
| Glehen [34] | 2004 | single | RCS | 49 | 21 |
| Yonemura [35] | 2005 | single | RCS | 107 | 42 |
| Kusamura [36] | 2006 | single | RCS | 209 | 12 |
| Roviello [37] | 2006 | single | RCS | 59 | 6 |
| Scaringi [38] | 2008 | single | RCS | 37 | 26 |
| Yang [39] | 2009 | single | RCS | 21 | 12 |
| Piso [40] | 2009 | single | RCS | 37 | 11 |
| Yang [41] | 2010 | single | pCTII | 28 | 28 |
| Li [42] | 2010 | single | RCS | 128 | 10 |
| Glehen [43] | 2010 | multiple | RCS | 159 | 147 |
| Yang [44] | 2011 | single | RCT | 68 | 34 |
| Cotte [45] | 2011 | single | pCTI | 12 | 12 |
| Mizumoto [46] | 2012 | single | RCS | 250 | 16 |
| Wu [47] | 2013 | single | RCS | 62 | 32 |
| Yarema [48] | 2014 | single | RCS | 98 | 20 |
| Tabrizian [49] | 2014 | single | RCS | 170 | 12 |
| Rudloff [50] | 2014 | single | RCT | 17 | 9 |
| Magge [51] | 2014 | single | RCS | 23 | 23 |
| Levine [52] | 2014 | single | RCS | 1000 | 46 |
| Kim [53] | 2014 | single | RCS | 112 | 9 |
| Königsrainer [54] | 2014 | single | RCS | 18 | 13 |
| Graziosi [55] | 2014 | single | RCS | 36 | 15 |
| Polanco [56] | 2015 | single | PCS | 370 | 24 |
| Desantis [57] | 2015 | single | RCS | 356 | 14 |
| Wu [58] | 2016 | single | RCS | 50 | 50 |
| Kopanakis [59] | 2017 | single | RCS | 14 | 14 |
| Rihuete Caro [60] | 2018 | single | RCS | 35 | 32 |
| Montori [61] | 2018 | single | RCS | 150 | 26 |
| Yarema [62] | 2019 | multiple | RCS | 117 | 70 |
| Solomon [63] | 2019 | single | RCS | 268 | 18 |
| Rau [4] | 2019 | single | RCS | 88 | 58 |
| Manzanedo [64] | 2019 | multiple | RCS | 88 | 84 |
| Kimbrough [65] | 2019 | multiple | RCS | 28 | 28 |
| Hotopp [66] | 2019 | single | RCS | 26 | 26 |
| Bonnot [17] | 2019 | multiple | RCS-PSm | 275 | 180 |
| Braeuer [67] | 2020 | single | RCS | 109 | 37 |
| Koemans [68] | 2021 | single | pCT I–II | 25 | 23 |
PCS: prospective cohort study; RCS: retrospective cohort study; RCT: randomized controlled trial; pCTI: prospective clinical trial phase I; pCTII: prospective clinical trial phase II; PSm: propensity score matched.
Table 2.
Study protocols for phase III studies evaluating CRS and HIPEC with unpublished results.
| NCT Number | Patients | End Until | Location | Arm Intervention | Arm Control | Open/Closed | HIPEC Drug | HIPEC Solution/Duration | Temperature | Primary Outcome Measures | Secondary Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03023436 | 220 | 22 June | China | CRS + HIPEC + sCTx | single arm | closed | DTX 120 mg | 5 L saline; 70 min | 43 ± 0.5 °C | MS 2-year (24 months) | 1. 2-year OS; 2. 2-year PFS; 3. M&M (30 d; 24 months) |
| NCT02158988 | 105 | 21 September | Germany | CRS + HIPEC + sCTx | CRS + sCTx | open/closed | MMC 15 mg/m2 CDDP 75 mg/m2 | 5 L saline; 60 min | 41–42 °C | OS (2.5 years) | 1. PFS; 2. M&M (30 d; 24 months) 3. MFS; 4. QoL (every 6 months) |
| NCT03348150 | 182 | 22 October | The Netherlands | CRS + HIPEC + sCTx | palliative sCTx | open | OX 460 mg/m2 DTX 50 mg/m2 | ns; 30 + 90 min | 41–42 °C + 37 °C | OS (5 years) | 1. PFS 2. toxicity 3.cost and health benefits |
MMC: mitomycin C; CDDP: cisplatin; MS: median survival; OS: overall survival; PFS: progression-free survival; QoL: quality of life; DTX: docetaxel; OX: oxaliplatin; sCTx: systemic chemotherapy; M&M: morbidity and mortality.
Table 3.
Parameters of HIPEC in the included studies.
| Reference | Before/After Anastomosis | Duration (min) | Open/Closed | Max. Heat (°C) | Drug First i.p. (mg/m2) | Drug Second i.p. (mg/m2) |
Perfusate | Flow Rate | Number of Tubes | Bidirectional Drugs i.v. |
|---|---|---|---|---|---|---|---|---|---|---|
| Fujimoto et al. [29] | ns | 120 | ns | 44.7–48.7 | MMC 10 µg/mL; 30 mg td | 3–5 L | ns | ns | ||
| Fujimoto et al. [30] | ns | 120 | closed | 43–45 | MMC 10 µg/mL; 100 mg/L | 3–4 L MWS | ns | 2 | ||
| Chen et al. [31] | after | 120 | closed | 40.5 | MMC 30–40 m td | 2–3 L RL | ns | 2|2 | ||
| Sayag-Beaujard et al. [32] | ns | 90 | ns | 46–49 | MMC 10 mg/L | 4–6 L | 400–500 mL/min | 2 | ||
| Loggie et al. [33] | ns | 120 | ns | 40.5 | MMC | ns | ns | ns | ||
| Glehen et al. [34] | after | 90 | closed | 46–48 | MMC 10 mg/mL | 4–6 L | 500 mL/min | 2|1 | ||
| Yonemura et al. [35] | after | 60 | open | 42–43 | MMC 30 mg td | td: CDDP 300 mg, Etoposid 150 mg | 8 L saline | 10 L/min | ns | |
| Kusamura et al. [36] | after | 60–90 | closed | 42–43 | CDDP 25 mg/m2/L | MMC 3.3 mg/m2/L | ns | ns | 4 | |
| Roviello et al. [37] | before | 60 | closed | 41–43 | MMC 25 | CDDP 100 | ns | 700–800 | 5 | |
| Scaringi et al. [38] | since 1998 before | 90–120 | o/c | 41–43 | MMC 120 | CDDP 200 | 12 L saline | ns | 2 | |
| Yang et al. [39] | after | 60–90 | open | 43 ± 0.5 | HCPT 20 mg td | MMC 30 mg td | 12 L saline | 200 mL/min | 1|1 | |
| Piso et al. [40] | after | 60 | closed | 42.5–43 | CDDP 75 | Doxorubicin 15 | ns | ns | ns | |
| Yang et al. [41] | after | 90–120 | open | 43 ± 0.5 | td: HCPT 20 mg CDDP 120 mg | MMC 30 mg td | 12 L saline | 200 mL/min | 1|1 | |
| Li et al. [42] | after | 60 | closed | 43 ± 1 | CDDP 50 µg/mL | MMC 5 µg/mL | 5–6 L | ns | 2|1 | |
| Glehen et al. [43] | ns | (1) 60–120 (2) 30 mean: 80.1 |
o/c | 40–43; mean: 42.6 | (1) MMC 30–50 (2) OX 360–460 |
(1) ±CDDP 100–200 (2) ±IRI 50–100 |
ns | ns | ns | 5-FU + FA |
| Yang et al. [44] | after | 60–90 | open | 43 ± 0.5 | CDDP 120 mg td | MMC 30 mg td | 6 L saline | 500 mL/min | 1|1 | ns |
| Cotte et al. [45] | after | 90 | closed | 44–46 | MMC 0.7 mg/kg | IRI 100 | 3–4 L GLC | 500 mL/min | 2|1 | |
| Mizumoto et al. [46] | after | 60 | ns | 41–42 | MMC 20 mg td | CDDP 100 mg td | saline | ns | 2|1 | |
| Wu et al. [47] | ns | 60 | ns | 43 ±0.5 | OX 460 | 3–4 L GLC | 500–800 mL/min | 3 | ||
| Yarema et al. [48] | after | 90 | open | 43 ± 1.3 | MMC 12.5 | CDDP 75 | ns | ns | ns | 5-FU |
| Tabrizian et al. [49] | ns | 60 + 30 | closed | 41–43 | MMC † | ns | ns | ns | ||
| Rudloff et al. [50] | before | 30 | closed | 41 | OX 460 | 3–4 L GLC | 2 l/min | ns | ||
| Magge et al. [51] | before | 100 | closed | 42 | MMC 30–40 mg td | 3 L saline | 800 mL/min | 2|1 | ||
| Levine et al. [52] | after | 60 + 60 | closed | 43 | MMC † | 3 L RL | 1 L /min | 2|2 | ||
| Kim et al. [53] | ns | 60 + 30 | ns | 41 | MMC † | ns | ns | ns | ||
| Königsrainer I. et al. [54] | after | 90 | open | 42 | CDDP 50 | ns | ns | ns | ||
| Graziosi et al. [55] | after | 60 | closed | 42 | CDDP 25 mg/L/m2 | MMC 3.3 mg/L/m2 | ns | ns | ns | |
| Polanco et al. [56] | ns | ns | closed | 42 | MMC 40 CDDP 50 |
ns | ns | ns | ||
| Desantis, M. [57] | before | 60 | open | 43 | CDDP 50 | ns | 800 mL/min | 5 | ||
| Wu, H. T. [58] | before | 60 | open | 43 ± 0.5 | Lobaplatin 50 | DTX 60 | 6 L saline | 400 mL/min | ns | |
| Kopanakis [59] | ns | 90 | ns | ns | CDDP 50 | Doxorubicin 50 | ns | ns | ns | |
| Rihuete Caro [60] | after | 90 | open | 42–43 | CDDP 100 | Doxorubicin 15 | ns | ns | ns | |
| Montori et al. [61] | before | 90 | open | 42–43 | CDDP 100 | Paclitaxel 175 | ns | ns | 1|4 | |
| Yarema, R. [62] | ns | 30–90 | closed | 42.7 ± 0.78 | (1) MMC 10–15 (2) OX 460 (3) CDDP 75 |
(1) CDDP 75 (2) Doxorubicin 15 |
ns | ns | ns | 5-FU |
| Solomon, D. [63] | before | 90 | closed | 41–43 | MMC 40 mg td | ns | ns | ns | ||
| Rau [4] | after | 60 | o/c | 41 | MMC 15 | CDDP 75 | ns | ns | ns | |
| Manzanedo [64] | ns | ns | open | CDDP (50%) | Doxorubicin (50%) | |||||
| Kimbrough, C. W. [65] | ns | ns | o/c | ns | MMC | ns | ns | ns | ||
| Hotopp [66] | ns | ns | open | OX 200 | DTX 80 | ns | 1500 mL/min | ns | ||
| Bonnot, P. E. [17] | ns | 30–120 | o/c | 41–43 | (1) MMC 30–50 (2) CDDP: 50–100 (3) OX: 300–460 |
(1) or (3) ±IRI 200 (1) +CDDP 100 (2) ±doxorubicin 15 |
ns | 500 mL/min | ns | 5-FU + FA |
| Braeuer, F. [67] | ns | 45–60 | closed | 42 | OX 400 | ns | ns | ns | ||
| Koemans, Willem J. [68] | before | 30 + 90 | open | 41–42 (OX), 37 (DTX) | OX 460 | DTX 0; 50, 75 | ns | ns |
HCPT: hydroxycampthothecin; MMC: mitomycin C; CDDP: cisplatin; OX: oxaliplatin; IRI: irinotecan; DTX: docetaxel; ns: not stated; 5-FU: 5-fluorouracil; FA: folinic acid/leucovorin; td: total dose; MWS: Maxwell solutions-1S; RL: Ringer’s lactate; GLC: glucose 5%; o/c: open/closed; † 30 mg for the first, 10 mg for the latter perfusion duration; n1|n2 n1 inflow tubes|n2 outflow tubes.
3. Results
3.1. Publications on CRS and HIPEC for pmGC
The oldest publication found in the initial search dates from 1957, the latest from 25 May 2021. In total, 42 publications published between 1988 and 2021 were included in this review, including 2 RCTs, 5 phase I/II clinical trials, two prospective cohort studies, and 33 retrospective studies reporting on 1325 patients treated with CRS and HIPEC for pmGC. Most of the studies were conducted as single-center studies, five publications presented the results of multi-center cohorts (see Table 1).
3.2. Study Protocols
Three ongoing phase III studies met our inclusion criteria. All of them were multi-centric and measured the long-term survival as a primary endpoint. The Chinese trial conducted by Li et al. was a one-armed study that performed closed HIPEC with 12 mg docetaxel in 5 L saline for 70 min at 43 °C. Rau et al. compared CRS only with CRS and HIPEC in the GASTRIPEC trial. They used mitomycin C 15–30 mg/m2 and cisplatin 75–150 mg/m2 in a maximum of 5 L perfusion. The PERISCOPE II trial by van Sandick et al. compared CRS and HIPEC with oxaliplatin 460 mg/m2 at 42 °C for 30 min followed by docetaxel 50 mg/m2 at 37 °C for 90 min in the intervention arm with standard palliative chemotherapy in the control arm. For more details of the study protocols please refer to Table 2.
3.3. Open or Closed HIPEC before or after Anastomosis
For an overview on the technical parameters of HIPEC that were utilized in each publication, please refer to Table 3. Most of the groups performed HIPEC after gastrointestinal anastomosis (17). However, this important information was not included in 38% of publications (16 studies). Nine groups reported to have started HIPEC before completion of anastomosis. Three of them reported anastomotic leakage rates ranging from 1% to 22% (median: 13%), which is higher than the 0% to 6.5% (median: 0%) rate indicated by 9 groups that performed HIPEC after gastrointestinal anastomosis. HIPEC after closure of the abdomen was more common than open HIPEC (17 vs. 13 publications), although five trials reported the use of both techniques and seven publications did not report on this parameter. Seven studies reported outcome results for their pmGC patients treated with CRS and closed HIPEC, ranging between 6.1 and 33.8 months (median: 11.75 months), which was lower than the median survival of 15 months stated by seven groups that performed open HIPEC (range: 8.9–21.2 months). However, this tendency should be carefully interpreted, as the patient cohorts might be completely different, and the median PCI was not available in most of the underlying studies.
3.4. Duration of HIPEC, Temperature, and Choice of Chemotherapeutic Regimen
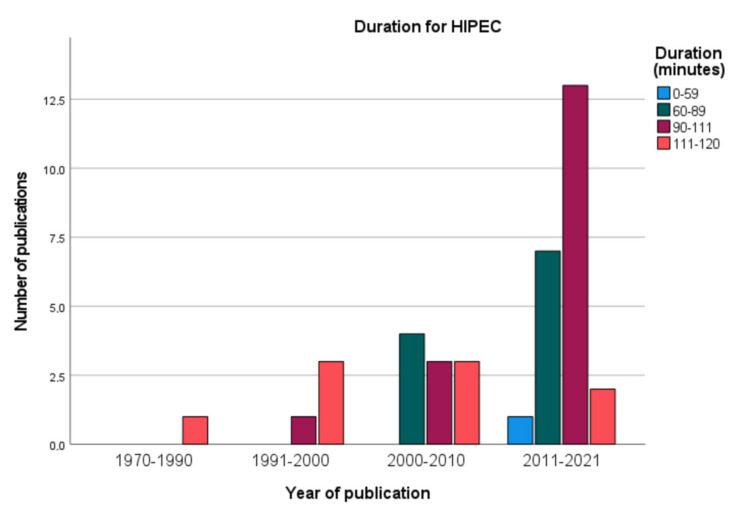
HIPEC perfusion lasted for 30 to 120 min within the peritoneal cavity. There has been a trend over the years towards longer durations of HIPEC, as Figure 2 shows. The overall median duration in the underlying publications was 90 min, which was reported by eleven studies. Ten studies used a duration of 60 min and nine studies reported various durations depending on the regimen that was used. Exposure time was shortened to 30 min for application of oxaliplatin in five studies, while MMC was applied for up to 120 min in seven studies. Only four publications did not mention the duration for HIPEC.
Figure 2.
Duration for HIPEC.
The median temperature of HIPEC in the underlying studies was 42.5 °C; 26 studies reported temperatures between 42 and 43°. Five earlier publications describe a maximum heat of 45–49 °C, while in eight publications, temperatures below 42° were used. Four studies did not report on the applied temperature. If docetaxel was part of the HIPEC regimen as in one study, the inflow temperature was decreased to 37 °C, as taxanes do not necessitate heat activation [61,63].
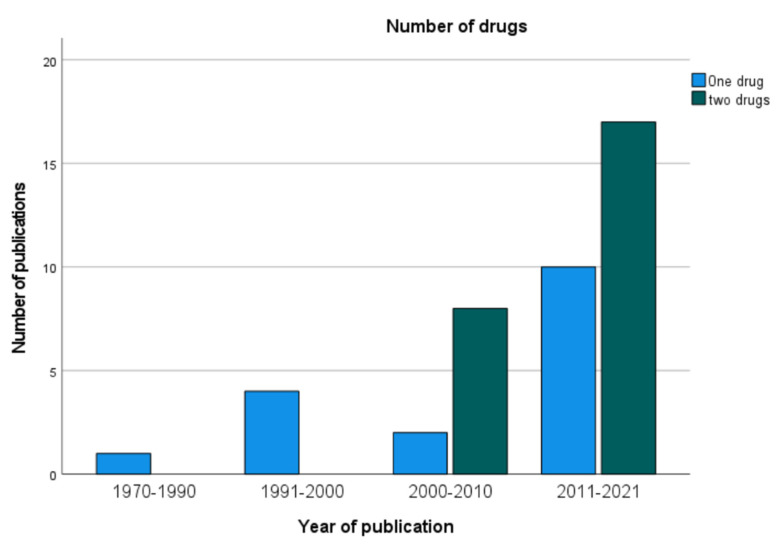
In 54.1% of the studies, two chemotherapeutic drugs were used for HIPEC, mostly a combination of MMC and cisplatin (CDDP), as in 11 studies. Other duplet combinations were CDDP and doxorubicin (6 studies) and oxaliplatin combined with either irinotecan or docetaxel (both in 2 studies). Monotherapy was less common (19 studies) and consisted in 14 studies of MMC, which was part of 30 regimens in total. Figure 3 depicts the development and growing importance of a duplet drug regime since the 2000s.
Figure 3.
Number of drugs used for HIPEC.
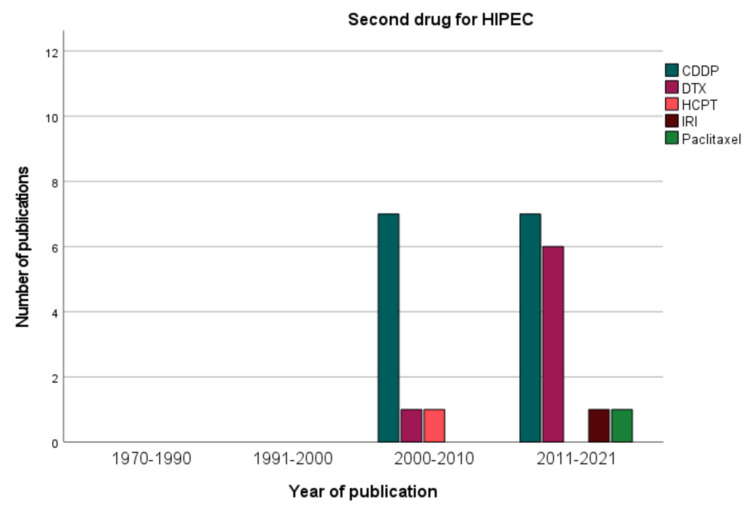
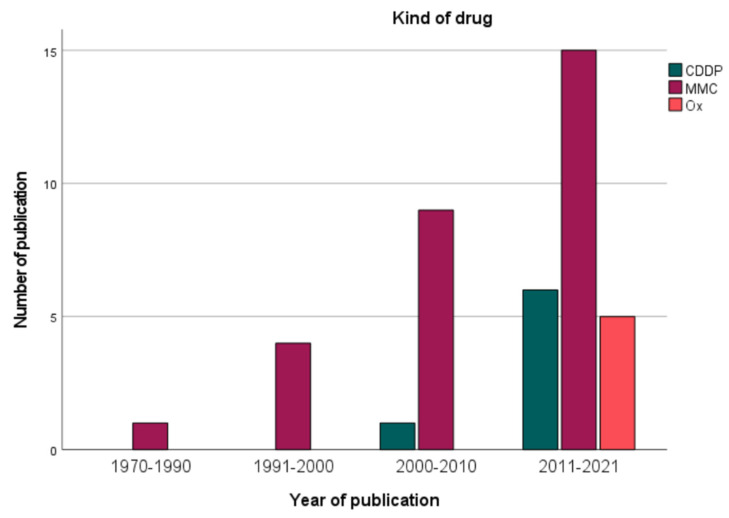
Among the groups that used two drugs for HIPEC, the median survival was reported by 10 groups and ranged between 9.2 and 21.2 months (median: 15 months). The median survival after mono-drug HIPEC seems to be lower and was indicated in 11 studies with a range of 6.1 to 33.8 months (median: 11 months), but median PCI was rarely stated for the pmGC patients. Oxaliplatin (OX) or CDDP monotherapy was used in 4 studies and in 3 studies, respectively. CDDP was part of the HIPEC regimen in 20 studies in doses between 50 and 200 mg/m2 body surface (median: 75 mg/m2), mostly in combination with other drugs, as Figure 4 shows. Dosage for MMC was ranging between 10 and 120 mg/m2, for OX between 200 and 460 mg/m2 (median: 460 mg/m2), and for doxorubicin between 15 and 50 mg/m2. Some studies assessed the maximum tolerated dose of irinotecan or doxorubicin, which is more of a trend, as Figure 4 illustrates. Four groups simultaneously applied intravenous 5-fuorouracil with or without leucovorin to enhance cytotoxicity of intraperitoneal oxaliplatin [22]. Figure 5 depicts the evolution of chemotherapy regimens for HIPEC. While the first studies used MMC, CDDP was introduced in the 2000s and oxaliplatin in the last decade. The dosage was difficult to compare between the studies, as some studies did not express the dose per BSA but as a flat dose in total or even per liter of perfusion solution.
Figure 4.
Second drug for HIPEC.
Figure 5.
Kind of drug.
3.5. Number of Tubes, Perfusate, and Flow Rate
Authors rarely described the placement as well as the numbers of tubes in the abdominal cavity for in- and outflow of the HIPEC perfusion. Only in 43.9% reported this aspect. Most commonly, as in six publications, respectively, two or three tubes in total were used. Three studies described the use of four or five tubes in total. Most groups (n = 23) did not report the type and volume perfusion solution that they used; however, saline (8 studies) was the most common one. Glucose-containing solution was used in three studies, as oxaliplatin necessitates chloride-free solutions [69]. The volume was ranging between 2 and 12 L, with a median of 4 L (eight studies). Only 38.1% (16) of the underlying publications reported the flow rate of HIPEC perfusion, with a median around 500 mL/min, as stated in six studies. A higher flow rate up to 10 L/min was applied in seven studies, a lower flow rate between 200 and 400 mL/min in four studies.
4. Discussion
Our aim was to collect the latest literature from both prospective and retrospective trials and to systematically analyze the given data on the technical approaches, therapy protocols, drug, and parameter selection.
With an immersive variation, HIPEC attracts a lot of attention worldwide. In the 42 publications that were analyzed in this systematic review, every group used a different approach and drug regimen. However, closed HIPEC was more common than open, with most groups performing HIPEC after suture of gastrointestinal anastomosis.
The delivered temperature ranged from 40 to 49 °C but mostly around 43 °C and measured mainly intra-abdominally. Temperatures differed due to the number and localization of measurement points. This made interpretation of results difficult.
HIPEC drug regimens preferred CDDP and MMC as a duplet regimen, which was most frequently used, although the dosages varied greatly. In this context, the different outcome results are difficult to weigh.
Open HIPEC and duplet therapy according to the data reported in this literature review might lead to an improved survival. Open HIPEC as described by Sugarbaker et al. offers the theoretical advantage of a uniform distribution of heat and liquid chemotherapy [70]. It also allows direct agitation of the chemotherapeutic solution by the surgeon and continuous visualization of the small bowel in order to identify possible penetrations [71]. However, more heat is necessary to maintain hyperthermia, and there is a potential higher risk for the operating staff than with the closed HIPEC [71]. The closed HIPEC in contrast allows an increased intra-abdominal pressure that may lead to an increased tissue penetration of the chemotherapy [71]. Addressing the number of in- and outflow catheters in vivo studies show that four inflow tubes might lead to a more stable and uniform hyperthermia within the abdominal cavity [72]. Nevertheless, several unknown confiders such as PCI, underlying localization of gastric cancer, completeness of cytoreduction, etc. make interpretation difficult and should be discussed with caution (Table 4).
Table 4.
Overview of the extracted outcome parameters.
| Reference | Year | Number of Patients with pmGC and CRS + HIPEC | Median PCI | Median OS | Clavien–Dindo ≥III° |
Anastomotic Leakage Rate |
|---|---|---|---|---|---|---|
| Fujimoto et al. [29] | 1988 | 9 | ns | ns | ns | ns |
| Fujimoto et al. [30] | 1997 | 30 | ns | ns | ns | ns |
| Chen et al. [31] | 1997 | 6 | ns | ns | ns | ns |
| Sayag-Beaujard et al. [32] | 1999 | 18 | ns | ns | ns | ns |
| Loggie et al. [33] | 2000 | 22 | ns | ns | ns | ns |
| Glehen et al. [34] | 2004 | 21 | ns | 10.3 months 1-year: 48.1% 2-year: 19.0% 5-year: 16.0% |
13 (27%) | 0/49 (0%) |
| Yonemura et al. [35] | 2005 | 42 | ns | 11.5 months 5-year: 6.7% |
ns | 7/107 (6.5%) |
| Kusamura et al. [36] | 2006 | 12 | ns | ns | ns | ns |
| Roviello et al. [37] | 2006 | 6 | ns | ns | ns | ns |
| Scaringi et al. [38] | 2008 | 26 | ns | 15 months | ns | ns |
| Yang et al. [39] | 2009 | 12 | ns | ns | ns | ns |
| Piso et al. [40] | 2009 | 11 | ns | ns | ns | 0/15 (0%) |
| Yang et al. [41] | 2010 | 28 | 12 | 1-year: 50.0% 2-year: 42.8% |
ns | ns |
| Li et al. [42] | 2010 | 10 | ns | 11.8 months 1-year: 52.5% 3-year: 13.2% 5-year: 5.5% |
ns | 0/10 (0%) |
| Glehen et al. [43] | 2010 | 147 | 9.4 (±7.7) | 9.2 months 1-year: 43% 3-year: 18% 5-year: 13% |
34.30% | ns |
| Yang et al. [44] | 2011 | 34 | 15 | 11 months 1-year: 41.2% 2-year: 14.7% 3-year: 5.9% |
ns | 0/35 (0%) |
| Cotte et al. [45] | 2011 | 12 | ns | ns | ns | 0/12 (0%) |
| Mizumoto et al. [46] | 2012 | 16 | 10 (±10) * | ns | 38% | ns |
| Wu et al. [47] | 2013 | 32 | ns | 15.5 months | ns | ns |
| Yarema et al. [48] | 2014 | 20 | 3.40 | 12 ± 1.6 months | ns | 1 (2%) |
| Tabrizian et al. [49] | 2014 | 12 | ns | 3-year: 16.6% | ns | ns |
| Rudloff et al. [50] | 2014 | 9 | ns | 11.3 months | 8 (89%) | 2 (22%) |
| Magge et al. [51] | 2014 | 23 | 10.5 | 9.5 months 1-year: 49.6% 3-year: 17.9% |
52% | 3 (13%) |
| Levine et al. [52] | 2014 | 46 | ns | 6.1 months | see Ref [53] | ns |
| Kim et al. [53] | 2014 | 9 | ns | 16 months | ns | ns |
| Königsrainer et al. [54] | 2014 | 13 | ns | 8.9 months | 11 (Grade 1–5) | 0 (0%) |
| Graziosi et al. [55] | 2014 | 15 | ns | ns | 4 (11.1%) | 0 (0%) |
| Polanco et al. [56] | 2015 | 24 | 13 | ns | see Ref [57] | ns |
| Desantis [57] | 2015 | 14 | ns | ns | ns | ns |
| Wu [58] | 2016 | 50 | 15 | 24.8 months | 12 (23.1%) | 1 (1%) |
| Kopanakis [59] | 2017 | 14 | 15 | ns | ns | ns |
| Rihuete Caro [60] | 2018 | 32 | ns | ns | 38% | ns |
| Montori et al. [61] | 2018 | 26 | 8 | 16 months 1-year: 70.8% 3-year: 21.3% |
9 (25.7%) | ns |
| Yarema [62] | 2019 | 70 | ns | PCI 0–6: 15 months PCI > 6: 8.2 months |
ns | 7 (6.5%) |
| Solomon [63] | 2019 | 18 | ns | 12 months | see Ref [63] | ns |
| Rau [4] | 2019 | 58 | 8.3 (±5.7) * | 9.8 months | 14 (62%) | ns |
| Manzanedo [64] | 2019 | 84 | 6 | 21.2 months 1-year: 79.9% 3-year: 30.9% |
30 (34.4%) | ns |
| Kimbrough [65] | 2019 | 28 | 12 | 10 months | 5 (18%) | 2 (7%) |
| Hotopp [66] | 2019 | 26 | 10 | 17 months | ns | 2 (7.7%) |
| Bonnot [17] | 2019 | 180 | 6 | 18.6 months | 53.70% | ns |
| Braeuer [67] | 2020 | 37 | 3.75 ±1.9 * | 33.8 months | 3 (37.5%) | ns |
| Koemans [68] | 2021 | 23 | 2 | 15 months | ns | ns |
* Mean (± standard deviation); OS: overall survival; ns: not stated.
Anastomotic leakage rate was reported higher in publications where gastrointestinal anastomosis was performed after HIPEC. This aspect was discussed in only a few publications. Leiting et al. retrospectively analyzed outcome parameters in a cohort of 1812 patients with mostly colorectal and appendiceal peritoneal metastases undergoing CRS and HIPEC. He found neither the open nor the closed technique to be an independent risk factor for post-operative complications or inferior long-term outcome [73], although others have detected decreased cardiac index, hepatic blood flow, and liver function due to the high abdominal pressure in closed abdomen HIPEC [74]. Somashekar et al. recently compared patients undergoing bowel anastomosis before and after HIPEC and found no significant difference in leakage or perforation rate between the two groups, but in this cohort only 7% pmGC patients were included [75].
Concerning the choice of drug for HIPEC, Woeste et al. have compared MMC and OHP for colorectal cancer peritoneal metastases. The choice of drug was not predictive of overall survival [76]. At the same time, three RCTs comparing intravenous OHP and CDDP for gastric cancer were compared in a meta-analysis by Montagnani et al., with a survival benefit and less adverse events in favor of OHP [77], possibly indicating a benefit in intraperitoneal delivery. The ideal drug for intraperitoneal chemotherapy shows a high concentration in the peritoneum, with a high penetration depth into the cancer nodule on one hand, and on the other hand, slow diffusion beyond the peritoneum blood membrane resulting in reduced systemic uptake in order to prevent systemic toxicity [22]. For DTX and OX, it was recently shown in pmGC patients undergoing CRS and HIPEC that systemic uptake is low after intraperitoneal application [78], while this has longer been known for CDDP, doxorubicin, and MMC [71,79].
CRS and HIPEC with intraperitoneal oxaliplatin at a dosage of 460 mg/m2 for 30 min at a temperature of 43 °C, as performed in the PRODIGE 7 trial, did not show significant benefit compared with CRS alone in peritoneal metastatic colorectal cancer [80]. However, pretreatment with oxaliplatin-containing systemic regimens reduces the sensitivity of colorectal cancer cells [81]. Additionally, low systemic uptake oxaliplatin is associated with postoperative hemorrhage [82], and cisplatin is associated with nephrotoxicity [83], which are both dose-limiting adverse effects. Concerning the nephrotoxicity, it will be interesting to see, whether sodium-thiosulfate, which has recently been shown to significantly reduce acute kidney injury [84], might allow increased intraperitoneal doses of cisplatin.
A handful of systematic reviews and meta-analyses addresses the indications and results of HIPEC in the context of cytoreductive surgery for gastric cancer peritoneal metastasis [85,86,87,88]. Granieri et al. performed a systematic review and meta-analysis of 12 randomized controlled trials in 2021 [89]. Eveno and Pocard reviewed the literature on this issue in 2016 and, as have other reviews, they only included data from randomized controlled trials [87,90,91] or did not focus on the different protocols for HIPEC [92,93].
The variations in different drug regimens and protocols have also been studied for colorectal cancer by Yurttas et al. [94]. Braam et al. reviewed the literature in June 2013 for different chemotherapeutical regimens in intraperitoneal chemotherapy delivery for pmGC [95]. Heldermann systematically reviewed different techniques used for intraperitoneal chemotherapy in general, but included only 29 publications until 2019 [96].
Brandl et al. recently presented a review concentrating on different therapeutic regimens of intraperitoneal chemotherapy, including NIPS, EPIC, and abdominal access port-based intraperitoneal chemotherapy. However, they did not include any study protocols of unpublished studies or retrospective cohorts with fewer than 50 patients, which represent 82.5% of the published studies we included in our analysis [25].
We think that the depicted differences in the regimens and outcomes should encourage further clinical trials on different HIPEC parameters, such as open vs. closed HIPEC before or after gastrointestinal anastomosis and more dose-finding phase I and II studies in order to identify the best approach for HIPEC in the treatment of pmGC.
5. Conclusions
This is the most current and comprehensive systematic overview of drug regimens and technical approaches for HIPEC that were used in the literature from 1988 to 2021. We here presented not only the most common HIPEC parameters but also the results achieved with the respective approaches. However, due to the great number of retrospective analyses, small number of patients, and lack of reported patient characteristics (for instance: no documentation of PCI in 25 of the 42 discussed papers (59.5%)) we only focused on HIPEC delivery.
The most common HIPEC regimen for pmGC consists of CDDP 75 mg/m2 and MMC 30–40 mg/m2 dissolved in 4 L of saline solution applied after suture of gastrointestinal anastomosis at 42–43 °C for 60–90 min via three tubes with a flow rate of 500 mL/min in a closed abdomen HIPEC system. Open HIPEC and two-drug regimen showed better survival results according to the literature, but its use should be weighed with caution. HIPEC after gastrointestinal anastomosis reduces the leakage rate. Standardization of technical aspects of HIPEC such as open or closed abdomen, perfusate, number of tubes, temperature, and duration might help when comparing different drug regimens and might have an influence on survival and morbidity. Further comparison of technical approaches and different drug regimens in phase I/II trials are needed in order to identify the best approach for pmGC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11051456/s1, Table S1: Reasons for exclusions.
Author Contributions
Conceptualization, B.R., S.G.-M., O.G. and F.G.; methodology, B.R., L.V. and C.E.; software, F.G.; validation, L.F., F.O. and S.K.; formal analysis, S.K.; investigation, L.F., F.O. and S.K.; data curation, B.R.; writing—original draft preparation, F.G., O.G. and F.G.; writing—review and editing, L.F., S.G.-M., L.V., C.E., O.G., S.K. and B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be found in the cited literature, we did not include archived datasets.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Thomassen I., van Gestel Y.R., van Ramshorst B., Luyer M.D., Bosscha K., Nienhuijs S.W., Lemmens V.E., de Hingh I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer. 2014;134:622–628. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 3.Yoo C.H., Noh S.H., Shin D.W., Choi S.H., Min J.S. Recurrence following curative resection for gastric carcinoma. Br. J. Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 4.Rau B., Brandl A., Thuss-Patience P., Bergner F., Raue W., Arnold A., Horst D., Pratschke J., Biebl M. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer. 2019;22:1226–1237. doi: 10.1007/s10120-019-00969-1. [DOI] [PubMed] [Google Scholar]
- 5.Solon J.G., O’Neill M., Chang K.H., Deady S., Cahill R., Moran B., Shields C., Mulsow J. An 18 year population-based study on site of origin and outcome of patients with peritoneal malignancy in Ireland. Eur. J. Surg. Oncol. (EJSO) 2017;43:1924–1931. doi: 10.1016/j.ejso.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Glehen O., Mohamed F., Gilly F.N. Peritoneal carcinomatosis from digestive tract cancer: New management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–228. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 7.Kok H.P., Beck M., Löke D.R., Helderman R., van Tienhoven G., Ghadjar P., Wust P., Crezee H. Locoregional peritoneal hyperthermia to enhance the effectiveness of chemotherapy in patients with peritoneal carcinomatosis: A simulation study comparing different locoregional heating systems. Int. J. Hyperth. 2020;37:76–88. doi: 10.1080/02656736.2019.1710270. [DOI] [PubMed] [Google Scholar]
- 8.Helderman R.F.C.P.A., Löke D.R., Verhoeff J., Rodermond H.M., van Bochove G.G.W., Boon M., van Kesteren S., Garcia Vallejo J.J., Kok H.P., Tanis P.J., et al. The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines. Cells. 2020;9:1775. doi: 10.3390/cells9081775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Löke D.R., Helderman R.F.C.P.A., Franken N.A.P., Oei A.L., Tanis P.J., Crezee J., Kok H.P. Simulating drug penetration during hyperthermic intraperitoneal chemotherapy. Drug Deliv. 2021;28:145–161. doi: 10.1080/10717544.2020.1862364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandl A., Yonemura Y., Glehen O., Sugarbaker P., Rau B. Long term survival in patients with peritoneal metastasised gastric cancer treated with cytoreductive surgery and HIPEC: A multi-institutional cohort from PSOGI. Eur. J. Surg. Oncol. 2021;47:172–180. doi: 10.1016/j.ejso.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Chia C.S., You B., Decullier E., Vaudoyer D., Lorimier G., Abboud K., Bereder J.M., Arvieux C., Boschetti G., Glehen O. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016;23:1971–1979. doi: 10.1245/s10434-015-5081-3. [DOI] [PubMed] [Google Scholar]
- 12.Kusamura S., Kepenekian V., Villeneuve L., Lurvink R.J., Govaerts K., De Hingh I., Moran B.J., Van der Speeten K., Deraco M., Glehen O. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 2021;47:36–59. doi: 10.1016/j.ejso.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Govaerts K., Lurvink R.J., De Hingh I., Van der Speeten K., Villeneuve L., Kusamura S., Kepenekian V., Deraco M., Glehen O., Moran B.J. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021;47:11–35. doi: 10.1016/j.ejso.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 14.van Driel W.J., Koole S.N., Sikorska K., Schagen van Leeuwen J.H., Schreuder H.W.R., Hermans R.H.M., de Hingh I.H.J.T., van der Velden J., Arts H.J., Massuger L.F.A.G., et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 15.Hultman B., Lundkvist J., Glimelius B., Nygren P., Mahteme H. Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer. Acta Oncol. 2012;51:112–121. doi: 10.3109/0284186X.2011.594809. [DOI] [PubMed] [Google Scholar]
- 16.Coccolini F., Gheza F., Lotti M., Virzi S., Iusco D., Ghermandi C., Melotti R., Baiocchi G., Giulini S.M., Ansaloni L., et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013;19:6979–6994. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnot P.E., Piessen G., Kepenekian V., Decullier E., Pocard M., Meunier B., Bereder J.M., Abboud K., Marchal F., Quenet F., et al. Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019;37:2028–2040. doi: 10.1200/JCO.18.01688. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt A., de Hingh I., Van Der Speeten K., Hubner M., Deraco M., Bakrin N., Villeneuve L., Kusamura S., Glehen O. HIPEC Methodology and Regimens: The Need for an Expert Consensus. Ann. Surg. Oncol. 2021;28:9098–9113. doi: 10.1245/s10434-021-10193-w. [DOI] [PubMed] [Google Scholar]
- 19.Kusamura S., Dominique E., Baratti D., Younan R., Deraco M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2008;98:247–252. doi: 10.1002/jso.21051. [DOI] [PubMed] [Google Scholar]
- 20.Abdel Mageed H., Van Der Speeten K., Sugarbaker P. The many faces of intraperitoneal chemotherapy. Surg. Oncol. 2021;40:101676. doi: 10.1016/j.suronc.2021.101676. [DOI] [PubMed] [Google Scholar]
- 21.Ji Z.H., Peng K.W., Yu Y., Li X.B., Yonemura Y., Liu Y., Sugarbaker P.H., Li Y. Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. Int. J. Hyperth. 2017;33:562–570. doi: 10.1080/02656736.2017.1283065. [DOI] [PubMed] [Google Scholar]
- 22.Lemoine L., Sugarbaker P., Van der Speeten K. Drugs, doses, and durations of intraperitoneal chemotherapy: Standardising HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma. Int. J. Hyperth. 2017;33:582–592. doi: 10.1080/02656736.2017.1291999. [DOI] [PubMed] [Google Scholar]
- 23.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González-Moreno S. Peritoneal Surface Oncology: A progress report. Eur. J. Surg. Oncol. 2006;32:593–596. doi: 10.1016/j.ejso.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Brandl A., Prabhu A. Intraperitoneal chemotherapy in the treatment of gastric cancer peritoneal metastases: An overview of common therapeutic regimens. J. Gastrointest. Oncol. 2021;12:S32–S44. doi: 10.21037/jgo-2020-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 27.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimoto S., Shrestha R.D., Kokubun M., Ohta M., Takahashi M., Kobayashi K., Kiuchi S., Okui K., Miyoshi T., Arimizu N., et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann. Surg. 1988;208:36–41. doi: 10.1097/00000658-198807000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujimoto S., Takahashi M., Mutou T., Kobayashi K., Toyosawa T., Isawa E., Sumida M., Ohkubo H. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer. 1997;79:884–891. doi: 10.1002/(SICI)1097-0142(19970301)79:5<884::AID-CNCR3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Chen M.Y., Chiles C., Loggie B.W., Choplin R.H., Perini M.A., Fleming R.A. Thoracic complications in patients undergoing intraperitoneal heated chemotherapy with mitomycin following cytoreductive surgery. J. Surg. Oncol. 1997;66:19–23. doi: 10.1002/(SICI)1096-9098(199709)66:1<19::AID-JSO5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Sayag-Beaujard A.C., Francois Y., Glehen O., Sadeghi-Looyeh B., Bienvenu J., Panteix G., Garbit F., Grandclément E., Vignal J., Gilly F.N. Intraperitoneal chemo-hyperthermia with mitomycin C for gastric cancer patients with peritoneal carcinomatosis. Anticancer Res. 1999;19:1375–1382. [PubMed] [Google Scholar]
- 33.Loggie B.W., Fleming R.A., McQuellon R.P., Russell G.B., Geisinger K.R. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of gastrointestinal origin. Am. Surg. 2000;66:561–568. [PubMed] [Google Scholar]
- 34.Glehen O., Schreiber V., Cotte E., Sayag-Beaujard A.C., Osinsky D., Freyer G., Francois Y., Vignal J., Gilly F.N. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch. Surg. 2004;139:20–26. doi: 10.1001/archsurg.139.1.20. [DOI] [PubMed] [Google Scholar]
- 35.Yonemura Y., Kawamura T., Bandou E., Takahashi S., Sawa T., Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br. J. Surg. 2005;92:370–375. doi: 10.1002/bjs.4695. [DOI] [PubMed] [Google Scholar]
- 36.Kusamura S., Younan R., Baratti D., Costanzo P., Favaro M., Gavazzi C., Deraco M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: Analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106:1144–1153. doi: 10.1002/cncr.21708. [DOI] [PubMed] [Google Scholar]
- 37.Roviello F., Marrelli D., Neri A., Cerretani D., de Manzoni G., Pedrazzani C., Cioppa T., Nastri G., Giorgi G., Pinto E. Treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion (IHCP): Postoperative outcome and risk factors for morbidity. World J. Surg. 2006;30:2033–2040. doi: 10.1007/s00268-006-0038-0. [DOI] [PubMed] [Google Scholar]
- 38.Scaringi S., Kianmanesh R., Sabate J.M., Facchiano E., Jouet P., Coffin B., Parmentier G., Hay J.M., Flamant Y., Msika S. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: A single western center experience. Eur. J. Surg. Oncol. 2008;34:1246–1252. doi: 10.1016/j.ejso.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Yang X.J., Li Y., al-shammaa Hassan A.H., Yang G.L., Liu S.Y., Lu Y.L., Zhang J.W., Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: Results of 21 cases. Ann. Surg. Oncol. 2009;16:345–351. doi: 10.1245/s10434-008-0226-2. [DOI] [PubMed] [Google Scholar]
- 40.Piso P., Slowik P., Popp F., Dahlke M.H., Glockzin G., Schlitt H.J. Safety of gastric resections during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Ann. Surg. Oncol. 2009;16:2188–2194. doi: 10.1245/s10434-009-0478-5. [DOI] [PubMed] [Google Scholar]
- 41.Yang X.J., Li Y., Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J. Surg. Oncol. 2010;101:457–464. doi: 10.1002/jso.21519. [DOI] [PubMed] [Google Scholar]
- 42.Li C., Yan M., Chen J., Xiang M., Zhu Z.G., Yin H.R., Lin Y.Z. Surgical resection with hyperthermic intraperitoneal chemotherapy for gastric cancer patients with peritoneal dissemination. J. Surg. Oncol. 2010;102:361–365. doi: 10.1002/jso.21628. [DOI] [PubMed] [Google Scholar]
- 43.Glehen O., Gilly F.N., Arvieux C., Cotte E., Boutitie F., Mansvelt B., Bereder J.M., Lorimier G., Quenet F., Elias D., et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010;17:2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 44.Yang X.J., Huang C.Q., Suo T., Mei L.J., Yang G.L., Cheng F.L., Zhou Y.F., Xiong B., Yonemura Y., Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cotte E., Passot G., Tod M., Bakrin N., Gilly F.N., Steghens A., Mohamed F., Glehen O. Closed abdomen hyperthermic intraperitoneal chemotherapy with irinotecan and mitomycin C: A phase I study. Ann. Surg. Oncol. 2011;18:2599–2603. doi: 10.1245/s10434-011-1651-1. [DOI] [PubMed] [Google Scholar]
- 46.Mizumoto A., Canbay E., Hirano M., Takao N., Matsuda T., Ichinose M., Yonemura Y. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan. Gastroenterol. Res. Pract. 2012;2012:836425. doi: 10.1155/2012/836425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X.J., Yuan P., Li Z.Y., Bu Z.D., Zhang L.H., Wu A.W., Zong X.L., Li S.X., Shan F., Ji X., et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol. 2013;34:463–469. doi: 10.1007/s13277-012-0571-4. [DOI] [PubMed] [Google Scholar]
- 48.Yarema R.R., Ohorchak M.A., Zubarev G.P., Mylyan Y.P., Oliynyk Y.Y., Zubarev M.G., Gyrya P.I., Kovalchuk Y.J., Safiyan V.I., Fetsych T.G. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int. J. Hyperth. 2014;30:159–165. doi: 10.3109/02656736.2014.893451. [DOI] [PubMed] [Google Scholar]
- 49.Tabrizian P., Shrager B., Jibara G., Yang M.J., Romanoff A., Hiotis S., Sarpel U., Labow D.M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: Outcomes from a single tertiary institution. J. Gastrointest. Surg. 2014;18:1024–1031. doi: 10.1007/s11605-014-2477-5. [DOI] [PubMed] [Google Scholar]
- 50.Rudloff U., Langan R.C., Mullinax J.E., Beane J.D., Steinberg S.M., Beresnev T., Webb C.C., Walker M., Toomey M.A., Schrump D., et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014;110:275–284. doi: 10.1002/jso.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magge D., Zenati M., Mavanur A., Winer J., Ramalingam L., Jones H., Zureikat A., Holtzman M., Lee K., Ahrendt S., et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann. Surg. Oncol. 2014;21:1448–1455. doi: 10.1245/s10434-013-3327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levine E.A., Stewart J.H., Shen P., Russell G.B., Loggie B.L., Votanopoulos K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1000 patients. J. Am. Coll. Surg. 2014;218:573–585. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim K.W., Chow O., Parikh K., Blank S., Jibara G., Kadri H., Labow D.M., Hiotis S.P. Peritoneal carcinomatosis in patients with gastric cancer, and the role for surgical resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy. Am. J. Surg. 2014;207:78–83. doi: 10.1016/j.amjsurg.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 54.Königsrainer I., Horvath P., Struller F., Königsrainer A., Beckert S. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J. Gastric Cancer. 2014;14:117–122. doi: 10.5230/jgc.2014.14.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graziosi L., Mingrone E., Marino E., Cavazzoni E., Donini A. Analysis of operative morbidity in a single center initial experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Tumori. 2014;100:15–20. doi: 10.1700/1430.15809. [DOI] [PubMed] [Google Scholar]
- 56.Polanco P.M., Ding Y., Knox J.M., Ramalingam L., Jones H., Hogg M.E., Zureikat A.H., Holtzman M.P., Pingpank J., Ahrendt S., et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann. Surg. Oncol. 2015;22:1673–1679. doi: 10.1245/s10434-014-4111-x. [DOI] [PubMed] [Google Scholar]
- 57.Desantis M., Bernard J.L., Casanova V., Cegarra-Escolano M., Benizri E., Rahili A.M., Benchimol D., Bereder J.M. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) Langenbecks Arch. Surg. 2015;400:37–48. doi: 10.1007/s00423-014-1253-z. [DOI] [PubMed] [Google Scholar]
- 58.Wu H.T., Peng K.W., Ji Z.H., Sun J.H., Zhang Q., Yang X.J., Huang C.Q., Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center. Eur. J. Surg. Oncol. 2016;42:1024–1034. doi: 10.1016/j.ejso.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 59.Kopanakis N., Efstathiou E., Sarris D., Spiliotis J. Does upfront therapy with cytoreductive surgery and HIPEC confer a survival benefit in patients with synchronous gastric peritoneal carcinomatosis when compared with patients with metachronous gastric peritoneal carcinomatosis? J. Buon. 2017;22:1144–1147. [PubMed] [Google Scholar]
- 60.Rihuete Caro C., Manzanedo I., Pereira F., Carrion-Alvarez L., Serrano Á., Pérez-Viejo E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2018;44:1805–1810. doi: 10.1016/j.ejso.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 61.Montori G., Coccolini F., Fugazzola P., Ceresoli M., Tomasoni M., Rubicondo C., Raimondo S., Pinelli D., Colledan M., Frigerio L., et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in ovarian and gastrointestinal peritoneal carcinomatosis: Results from a 7-year experience. J. Gastrointest. Oncol. 2018;9:241–253. doi: 10.21037/jgo.2017.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yarema R., Mielko J., Fetsych T., Ohorchak M., Skorzewska M., Rawicz-Pruszyński K., Mashukov A., Maksimovsky V., Jastrzębski T., Polkowski W., et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: A retrospective cooperative Central-Eastern European study. Cancer Med. 2019;8:2877–2885. doi: 10.1002/cam4.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon D., DeNicola N., Feingold D., Liu P.H., Aycart S., Golas B.J., Sarpel U., Labow D.M., Magge D.R. Signet ring cell features with peritoneal carcinomatosis in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are associated with poor overall survival. J. Surg. Oncol. 2019;119:758–765. doi: 10.1002/jso.25379. [DOI] [PubMed] [Google Scholar]
- 64.Manzanedo I., Pereira F., Rihuete Caro C., Perez-Viejo E., Serrano A., Gutierrez Calvo A., Regueira F.M., Casado-Adam A., Cascales-Campos P.A., Arteaga X., et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP) Ann. Surg. Oncol. 2019;26:2615–2621. doi: 10.1245/s10434-019-07450-4. [DOI] [PubMed] [Google Scholar]
- 65.Kimbrough C.W., Beal E., Abdel-Misih S., Pawlik T.M., Cloyd J.M. Survival Outcomes Among Patients with Gastric Adenocarcinoma Who Received Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery. JAMA Surg. 2019;154:780–782. doi: 10.1001/jamasurg.2019.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hotopp T. HIPEC and CRS in peritoneal metastatic gastric cancer—Who really benefits? Surg. Oncol. 2019;28:159–166. doi: 10.1016/j.suronc.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Braeuer F., Fischer I., Brammen L., Pressl G., Fuegger R., Rohregger K., Wundsam H. Outcome in Patients Treated with Cytoreductive Surgery and HIPEC for Gastric Cancer with Peritoneal Carcinomatosis. Anticancer Res. 2020;40:2151–2156. doi: 10.21873/anticanres.14174. [DOI] [PubMed] [Google Scholar]
- 68.Koemans W.J., van der Kaaij R.T., Wassenaar E.C.E., Boerma D., Boot H., Sikorska K., Los M., Grootscholten C., Hartemink K.J., Veenhof A., et al. Tumor characteristics and clinical outcome of peritoneal metastasis of gastric origin treated with a hyperthermic intraperitoneal chemotherapy procedure in the PERISCOPE I trial. J. Surg. Oncol. 2021;123:904–910. doi: 10.1002/jso.26366. [DOI] [PubMed] [Google Scholar]
- 69.Bouquet W., Ceelen W., Adriaens E., Almeida A., Quinten T., De Vos F., Pattyn P., Peeters M., Remon J.P., Vervaet C. In vivo toxicity and bioavailability of Taxol and a paclitaxel/beta-cyclodextrin formulation in a rat model during HIPEC. Ann. Surg. Oncol. 2010;17:2510–2517. doi: 10.1245/s10434-010-1028-x. [DOI] [PubMed] [Google Scholar]
- 70.Sugarbaker P.H., Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J. Gastrointest. Oncol. 2016;7:29–44. doi: 10.3978/j.issn.2078-6891.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rossi C.R., Mocellin S., Pilati P., Foletto M., Quintieri L., Palatini P., Lise M. Pharmacokinetics of intraperitoneal cisplatin and doxorubicin. Surg. Oncol. Clin. N. Am. 2003;12:781–794. doi: 10.1016/S1055-3207(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 72.Löke D.R., Helderman R., Sijbrands J., Rodermond H.M., Tanis P.J., Franken N.A.P., Oei A.L., Kok H.P., Crezee J. A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats. Cancers. 2020;12:3516. doi: 10.3390/cancers12123516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leiting J.L., Cloyd J.M., Ahmed A., Fournier K., Lee A.J., Dessureault S., Felder S., Veerapong J., Baumgartner J.M., Clarke C., et al. Comparison of open and closed hyperthermic intraperitoneal chemotherapy: Results from the United States hyperthermic intraperitoneal chemotherapy collaborative. World J. Gastrointest. Oncol. 2020;12:756–767. doi: 10.4251/wjgo.v12.i7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupont S., Schiffer E.R.C., White M.J., Diaper J.R.A., Licker M.J., Masouyé P.C. Changes in Hepatic Blood Flow and Liver Function during Closed Abdominal Hyperthermic Intraperitoneal Chemotherapy following Cytoreduction Surgery. Gastroenterol. Res. Pract. 2018;2018:8063097. doi: 10.1155/2018/8063097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Somashekhar S.P., Rohit K.C., Ramya Y., Zaveri S.S., Ahuja V., Namachivayam A.K., Ashwin K.R. Bowel Anastomosis After or Before HIPEC: A Comparative Study in Patients Undergoing CRS+HIPEC for Peritoneal Surface Malignancy. Ann. Surg. Oncol. 2022;29:214–223. doi: 10.1245/s10434-021-10661-3. [DOI] [PubMed] [Google Scholar]
- 76.Woeste M.R., Philips P., Egger M.E., Scoggins C.R., McMasters K.M., Martin R.C.G. Optimal perfusion chemotherapy: A prospective comparison of mitomycin C and oxaliplatin for hyperthermic intraperitoneal chemotherapy in metastatic colon cancer. J. Surg. Oncol. 2020;121:1298–1305. doi: 10.1002/jso.25920. [DOI] [PubMed] [Google Scholar]
- 77.Montagnani F., Turrisi G., Marinozzi C., Aliberti C., Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer. 2011;14:50–55. doi: 10.1007/s10120-011-0007-7. [DOI] [PubMed] [Google Scholar]
- 78.Koemans W.J., van der Kaaij R.T., Wassenaar E.C.E., Grootscholten C., Boot H., Boerma D., Los M., Imhof O., Schellens J.H.M., Rosing H., et al. Systemic exposure of oxaliplatin and docetaxel in gastric cancer patients with peritonitis carcinomatosis treated with intraperitoneal hyperthermic chemotherapy. Eur. J. Surg. Oncol. 2021;47:486–489. doi: 10.1016/j.ejso.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 79.Kuzuya T., Yamauchi M., Ito A., Hasegawa M., Hasegawa T., Nabeshima T. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J. Pharm. Pharmacol. 1994;46:685–689. doi: 10.1111/j.2042-7158.1994.tb03883.x. [DOI] [PubMed] [Google Scholar]
- 80.Quénet F., Elias D., Roca L., Goéré D., Ghouti L., Pocard M., Facy O., Arvieux C., Lorimier G., Pezet D., et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:256–266. doi: 10.1016/S1470-2045(20)30599-4. [DOI] [PubMed] [Google Scholar]
- 81.Nagourney R.A., Evans S., Tran P.H., Nagourney A.J., Sugarbaker P.H. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur. J. Surg. Oncol. 2021;47:738–742. doi: 10.1016/j.ejso.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 82.Charrier T., Passot G., Peron J., Maurice C., Gocevska S., Quénet F., Eveno C., Pocard M., Goere D., Elias D., et al. Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin Increases the Risk of Postoperative Hemorrhagic Complications: Analysis of Predictive Factors. Ann. Surg. Oncol. 2016;23:2315–2322. doi: 10.1245/s10434-016-5143-1. [DOI] [PubMed] [Google Scholar]
- 83.Ye J., Ren Y., Wei Z., Peng J., Chen C., Song W., Tan M., He Y., Yuan Y. Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: A retrospective cohort study. Surg. Oncol. 2018;27:456–461. doi: 10.1016/j.suronc.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 84.Kurreck A., Gronau F., Alberto Vilchez M.E., Abels W., Enghard P., Brandl A., Francis R., Föhre B., Lojewski C., Pratschke J., et al. Sodium Thiosulfate Reduces Acute Kidney Injury in Patients Undergoing Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy with Cisplatin: A Single-Center Observational Study. Ann. Surg. Oncol. 2021;29:152–162. doi: 10.1245/s10434-021-10508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta A.M., Van den Hoven J.M., Rosing H., Hillebrand M.J., Nuijen B., Huitema A.D., Beijnen J.H., Verwaal V.J. Stability of oxaliplatin in chloride-containing carrier solutions used in hyperthermic intraperitoneal chemotherapy. Int. J. Pharm. 2015;479:23–27. doi: 10.1016/j.ijpharm.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 86.Gill R.S., Al-Adra D.P., Nagendran J., Campbell S., Shi X., Haase E., Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011;104:692–698. doi: 10.1002/jso.22017. [DOI] [PubMed] [Google Scholar]
- 87.Eveno C., Pocard M. Randomized controlled trials evaluating cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention and therapy of peritoneal metastasis: A systematic review. Pleura Peritoneum. 2016;1:169–182. doi: 10.1515/pp-2016-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yap D.R.Y., Wong J.S.M., Tan Q.X., Tan J.W.-S., Chia C.S., Ong C.-A.J. Effect of HIPEC on Peritoneal Recurrence in Peritoneal Metastasis Treated with Cytoreductive Surgery: A Systematic Review. Front. Oncol. 2021;11:795390. doi: 10.3389/fonc.2021.795390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Granieri S., Bonomi A., Frassini S., Chierici A.P., Bruno F., Paleino S., Kusamura S., Germini A., Facciorusso A., Deraco M., et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021;47:2757–2767. doi: 10.1016/j.ejso.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 90.Chia C.S., Seshadri R.A., Kepenekian V., Vaudoyer D., Passot G., Glehen O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: A systematic review. Pleura Peritoneum. 2016;1:67–77. doi: 10.1515/pp-2016-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dominic J.L., Kannan A., Tara A., Hakim Mohammed A.R., Win M., Khorochkov A., Sultan W., Ahmed A., Kantamaneni K., Syzmanski M.W., et al. Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) for the prevention and control of peritoneal metastasis in patients with gastrointestinal malignancies: A systematic review of randomized controlled trials. EXCLI J. 2021;20:1328–1345. doi: 10.17179/excli2021-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Auer R.C., Sivajohanathan D., Biagi J., Conner J., Kennedy E., May T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A systematic review. Eur. J. Cancer. 2020;127:76–95. doi: 10.1016/j.ejca.2019.10.034. [DOI] [PubMed] [Google Scholar]
- 93.Kitai T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: A systematic review including evidence from Japan. Surg. Today. 2020;51:1085–1098. doi: 10.1007/s00595-020-02180-7. [DOI] [PubMed] [Google Scholar]
- 94.Yurttas C., Hoffmann G., Tolios A., Haen S.P., Schwab M., Königsrainer I., Königsrainer A., Beckert S., Löffler M.W. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. J. Clin. Med. 2018;7:567. doi: 10.3390/jcm7120567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Braam H.J., Schellens J.H., Boot H., van Sandick J.W., Knibbe C.A., Boerma D., van Ramshorst B. Selection of chemotherapy for hyperthermic intraperitoneal use in gastric cancer. Crit. Rev. Oncol. Hematol. 2015;95:282–296. doi: 10.1016/j.critrevonc.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Helderman R.F.C.P.A., Löke D.R., Kok H.P., Oei A.L., Tanis P.J., Franken N.A.P.K., Crezee J. Variation in Clinical Application of Hyperthermic Intraperitoneal Chemotherapy: A Review. Cancers. 2019;11:78. doi: 10.3390/cancers11010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting reported results can be found in the cited literature, we did not include archived datasets.