Abstract
Ischemic heart disease (IHD) is the leading cause of mortality in women. While traditional cardiovascular risk factors play an important role in the development of IHD in women, women may experience sex-specific IHD risk factors and pathophysiology, and thus female-specific risk stratification is needed for IHD prevention, diagnosis and treatment. Emerging data from the last two decades have significantly improved the understanding of IHD in women, including mechanisms of ischemia with no obstructive coronary arteries (INOCA) and myocardial infarction with no obstructive coronary arteries (MINOCA). Despite this progress, sex-differences in IHD outcomes persist, particularly in young women. This review highlights the contemporary understanding of coronary arterial function and disease in women with no obstructive coronary arteries, including coronary anatomy and physiology, mechanisms of INOCA and MINOCA, noninvasive and invasive diagnostic strategies, and management of IHD.
Keywords: Cardiovascular Disease, Coronary Artery Disease, Coronary Circulation, Women, Sex, Gender
INTRODUCTION
Ischemic heart disease (IHD) is estimated to affect 67 million women globally and is the leading cause of mortality in women.1 IHD has traditionally been considered a problem of postmenopausal women, but the annual incidence of acute MI hospitalizations has been increasing in young women <55 years.2 While traditional cardiovascular risk factors play an important role in the development of IHD in women, women may experience sex-specific IHD risk factors and pathophysiology. Thus female-specific risk stratification is needed for IHD prevention, diagnosis, and treatment. Recently, a clinical practice guideline for the evaluation and diagnosis of chest pain underscored the “uniqueness” of chest pain in women.3
Emerging data from the last two decades have significantly improved the understanding of IHD in women, including mechanisms of ischemia with no obstructive coronary arteries (INOCA) and myocardial infarction with no obstructive coronary arteries (MINOCA). This review highlights the contemporary understanding of coronary arterial function and disease in women with no obstructive coronary arteries, including risk perception and stratification, coronary anatomy and physiology, mechanisms of INOCA and MINOCA, noninvasive and invasive diagnostic strategies, and management of IHD.
RISK PERCEPTION AND STRATIFICATION
In the past decade, the conventional “typical” vs “atypical” distinction of angina has been a source of controversy in the evaluation of IHD in women vs men, given gendered sociocultural language differences in symptom expression4 as well as gender bias in chest pain diagnosis and treatment.5 Atypical angina may be more prevalent than typical angina in women with INOCA.6 Determination of “atypical angina” may thus contribute to misdiagnosis and delayed recognition and treatment of ischemia in women.3, 6 In recognition of this, the recently published chest pain guidelines recommend the term “atypical” should be avoided.3 Additional challenges to the diagnosis and prognosis of IHD based on symptom presentation include poor correlation of angina with evidence of myocardial ischemia in women and men.7, 8
Furthermore, commonly used primary prevention cardiovascular risk scores fail to predict cardiovascular event rates in women with INOCA,9 compared to secondary prevention risk scores which either over- or underestimate observed INOCA risk.10 Ongoing efforts to improve risk factor stratification has led to national guideline addition of women-specific cardiovascular risk enhancing conditions, such as premature menopause, autoimmune inflammatory diseases, and history of pregnancy-associated conditions such as hypertensive disorders of pregnancy (including preeclampsia), preterm labor, gestational diabetes, and small for gestational age delivery.11 Rigorous studies are needed to determine whether incorporation of these risk enhancers improve cardiovascular risk prediction and outcomes in women.
CORONARY ANATOMY AND PHYSIOLOGY
Women have smaller epicardial coronary arteries than men, even when adjusted for smaller body habitus and left ventricular mass.12–14 Women also have lower prevalence of obstructive coronary atherosclerosis,15, 16 even with comparable levels of ischemia17, 18 and are less likely to have adverse plaque characteristics and less atherosclerotic plaque of all subtypes compared to men.19 Interestingly, sex differences in CAD severity and plaque composition are most evident among young adults and attenuated in older adults,20–22 thought to be related to the protective role of endogenous estrogen in premenopausal women. These sex differences in coronary size and atherosclerosis development appear to contribute to higher prevalence of INOCA in women.23
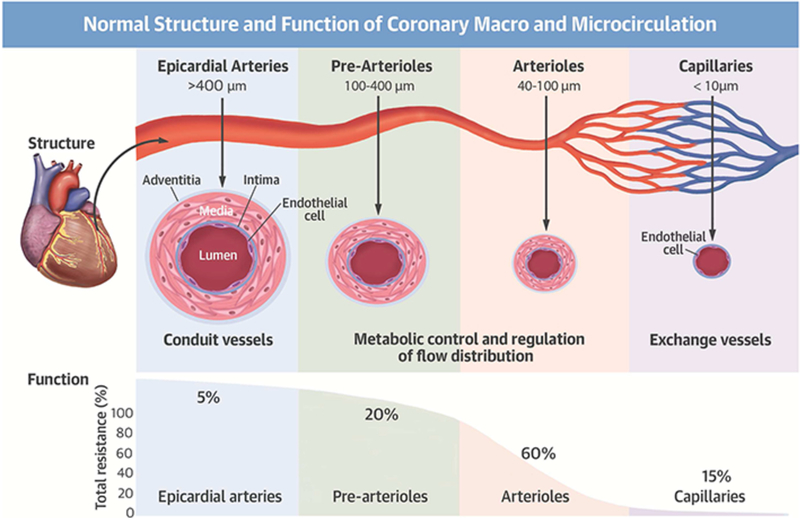
Increasing recognition of INOCA and MINOCA has shifted the focus from identifying epicardial coronary artery disease (CAD) to understanding coronary anatomy and physiology (Figure 1).3, 24, 25 The proximal epicardial coronary arteries (>400 μm) are the conduit vessels, providing low resistance to coronary flow under physiologic conditions and dilating in response to arterial wall shear stress, an endothelium-dependent mechanism. The epicardial arteries represent only 10% of the coronary circulation volume, while the microvasculature (pre-arterioles and arterioles) represents approximately 90%. The pre-arterioles (100 to 400 μm) and intramural arterioles (<100 μm) interface directly with the coronary capillary bed (<10 μm) and control the majority of coronary vascular resistance. These resistance vessels are responsible for the dynamic regulation and distribution of coronary blood flow; the pre-arterioles and proximal arterioles maintain a steady level of shear stress and pressure by endothelial-dependent and non-endothelial dependent dilatation, while the distal arterioles play a critical role in the metabolic regulation of coronary blood flow.26 Arterioles dilate in response to changes in intramyocardial concentration of metabolites as a result of increased oxygen consumption, decreasing coronary vascular resistance and triggering further dilation of upstream vessels.26, 27 These arterioles are also responsive to the effects of vasoactive drugs.
Figure 1. Normal Structure and Function of Coronary Macro- and Microcirculation.
Reprinted from Taqueti VR et al25 with permission.
In healthy vessels, coronary blood flow remains constant over a wide range of coronary perfusion pressures through dynamic changes in resistances within the different microvascular territories via distinct regulatory mechanisms.27 An increase in metabolic demand must be met by an increase in coronary blood flow. Based on studies in men, coronary blood flow can increase at least three to five-fold in normal conditions,28, 29 primarily regulated by the coronary endothelium by releasing vasodilator substances such as nitric oxide, which then produces a guanylyl-cyclase-mediated relaxation of the vascular smooth muscle cells.30 A normal functioning endothelium is needed not only for appropriate dilation of the coronary arteries but also for vascular growth, repair and thromboresistance.31 Nonendothelial-dependent coronary microvascular dilatation also occurs due to a myogenic response to reduced pressure.26 In addition, adrenergic and muscarinic receptors regulate vasoconstriction or vasodilation of the coronary arterioles in response to sympathetic and parasympathetic innervation.24
Invasive and noninvasive interrogation studies of coronary flow and myocardial perfusion have suggested sex differences in coronary physiology. Middle-aged women have higher resting coronary blood flow compared to men,32–35 possibly related to sex differences in autonomic function.7 Higher resting coronary blood flow may explain lower coronary flow reserve (CFR) values, defined as the ratio of maximal hyperemic coronary blood flow to resting blood flow, in women compared to men in invasive studies,34, 36 although noninvasive studies suggest similar CFR distribution in women and men.17, 35 On the other hand, premenopausal women have nearly two-fold better coronary blood flow response than postmenopausal women and age-matched men,37, 38 consistent with evidence linking estradiol with the production of nitric oxide and estradiol receptor mediated vasodilation.39 Another coronary physiologic measure is fractional flow reserve (FFR), the ratio of mean distal coronary pressure to mean aortic pressure and a measure of the physiologic significance of an epicardial coronary stenosis; FFR is dependent not only on the severity of the stenosis and subtended myocardium but also on hyperemic microvascular resistance.40, 41 For a given epicardial stenosis severity, FFR tends to be higher in women than men.42 Higher FFR in women could possibly related to sex differences in microvascular resistance or vasomotion although the finding of similar index of microvascular resistance (IMR) by sex in INOCA may refute this explanation.34
ISCHEMIA WITH NO OBSTRUCTIVE CORONARY ARTERIES (INOCA)
The definition of INOCA is broad and refers to ischemia with stable or unstable symptoms in the setting of normal or nonobstructive coronary arteries (<50% stenosis). INOCA is a common but heterogenous condition associated with elevated risk for cardiovascular events in both women and men.24 In a large retrospective Danish cohort study, compared to an asymptomatic reference population without IHD, INOCA was associated with an increased risk of all-cause mortality even after adjusting for traditional cardiac risk factors and similar in women and men (normal coronary arteries, adjusted HR 1.29; non-obstructive CAD, adjusted HR 1.52).16 Among symptomatic women in the Women’s Ischemia Syndrome Evaluation (WISE), cardiac mortality rates were 6% (for no CAD) and 11% (for minimal CAD) within 10 years of their angiographic evaluation, again demonstrating that INOCA is not benign.
Standardized diagnostic criteria have been proposed by the Coronary Vasomotor Disorders International Study Group (COVADIS) for the two primary mechanisms of INOCA: coronary microvascular dysfunction (CMD) and coronary vasospasm.43, 44 In the absence of obstructive CAD, CMD has an estimated prevalence of up to 53% in individuals undergoing non-invasive stress tests.35, 45 In patients with suspected INOCA undergoing invasive evaluation, CMD and/or coronary vasospasm are diagnosed in up to 4 in 5 patients;46–50 these patients are often female (50–80%).47, 48, 50–52 Prevalence estimates may change over time as more centers adopt invasive evaluation of INOCA. Long-term prognosis is impaired in women with CMD and/or vasospasm,35, 53–57 with a 5% annualized major adverse cardiac event (MACE) rate (including death, nonfatal MI, heart failure hospitalization, late revascularization or nonfatal stroke) in INOCA women with CMD.35, 53–58 When microvascular angina and epicardial vasospastic angina co-exist, this combination is associated with worse prognosis.59 An international cohort study recently found no sex or ethnic difference in prognosis of patients with microvascular angina, but women had lower quality of life scores.60
Coronary Microvascular Dysfunction (CMD): Definition and Pathophysiology
CMD should be suspected when exertional or rest angina is present in the absence of obstructive CAD (<50% diameter reduction or FFR>0.80), particularly in the presence of objective evidence of myocardial ischemia, such as 1) dynamic ischemic electrocardiographic changes during an episode of angina or 2) stress-induced angina and/or ischemic electrocardiographic changes in the presence of absence of transient/reversible abnormal myocardial perfusion and/or wall motion abnormality.43 CMD is diagnosed in the setting of impaired CFR<2.0 or 2.5, coronary microvascular spasm, abnormal coronary microvascular resistance indices (IMR<25 or hyperemic vascular resistance [hMR]<2.0), or coronary slow flow phenomenon.43 Although traditional cardiovascular risk factors are associated with CMD,47, 58, 61 they account for less than 20% of the observed variability in CFR in women with suspected CMD.61, 62 Postmenopausal status does not appear to significantly contribute to CFR, while systemic inflammation, prior adverse pregnancy outcomes, and prior breast cancer chemotherapy may play a role.62–65
CMD pathophysiology may be characterized by structural and functional abnormalities. Structural abnormalities may include luminal obstruction (e.g., microembolization during acute coronary syndrome or revascularization), vascular-wall infiltration (e.g., infiltrative heart disease such as Anderson-Fabry disease), vascular remodeling (e.g., arterial hypertension, hypertrophic cardiomyopathy), perivascular fibrosis (e.g., arterial hypertension, aortic stenosis), and capillary rarefaction (e.g., arterial hypertension, aortic stenosis, heart failure), while functional abnormalities include endothelial dysfunction, smooth-muscle dysfunction, and autonomic dysfunction.26 Sex differences in structural CMD pathophysiology have not been well studied in humans, although estrogen effects on preventing inflammation-induced apoptosis of vascular endothelial cells and perivascular fibrosis have been hypothesized to cause postmenopausal women to be more vulnerable to capillary rarefaction and impaired hypoxia-induced angiogenesis than premenopausal women.66 In the presence of atherosclerosis, the endothelium becomes dysfunctional and there may be blunted coronary vasodilation or increased vasoconstriction, resulting in blunted coronary blood flow augmentation or frank reduction in blood flow.67, 68 In response to increased oxidative stress, the endothelium may release vasoconstrictive substances (endothelin, thromboxane A2, prostaglandin H2, superoxide) that counter the endothelium’s vasodilating, anti-proliferative, and antithrombotic mediators (nitric oxide, prostacyclin, endothelium-derived hyperpolarizing factor).69 Diffuse nonobstructive epicardial CAD may result in a longitudinal pressure gradient, compensatory microvascular vasodilation to maintain myocardial perfusion, and a diminished coronary vasodilatory reserve.70 Furthermore, presence of positive remodeling, serial stenoses, and high-risk plaque such as large necrotic core can also contribute to ischemia in the absence of obstructive coronary stenosis.71, 72
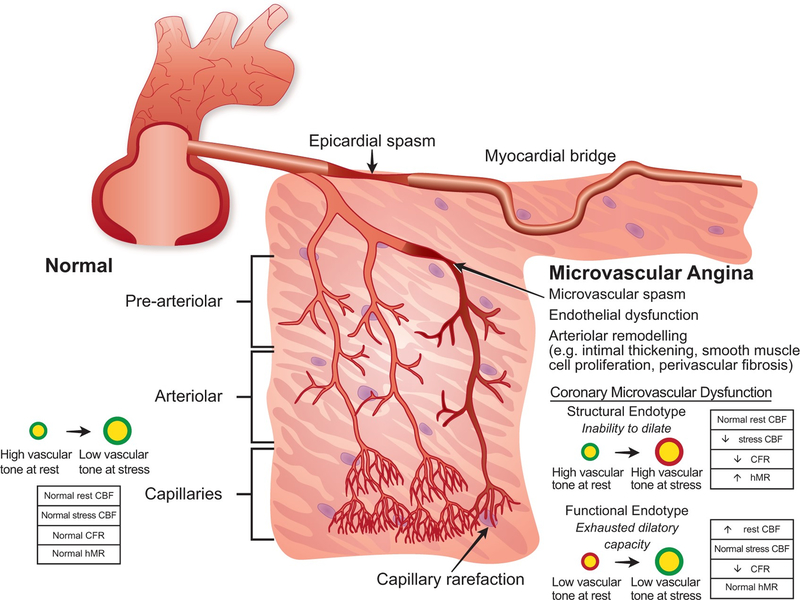
Recently, CMD has been further characterized by structural vs functional endotypes based on vascular tone at rest and stress (Figure 2).73 Patients with functional CMD endotype tend to have low vascular tone as reflected by elevated resting flow, normal microvascular resistance and higher nitric oxide synthase activity, while patients with structural CMD endotype tend to have high vascular tone as reflected by low resting flow, elevated microvascular resistance and endothelial dysfunction.74 The prognostic and therapeutic implications of each endotype, including any potential sex-specific implications, warrant further study.
Figure 2. Coronary Vascular Dysfunction Mechanisms in Ischemia and No Obstructive Coronary Artery Disease.
CBF, coronary blood flow; CFR, coronary flow reserve; hMR, hyperemic index of microcirculatory resistance. Reprinted from de Silva R et al73 with permission.
Epicardial Coronary Vasospasm: Definition and Pathophysiology
Vasospastic angina should be suspected in the presence of nitrate-responsive angina, often occurring at rest between night and early morning, and suppressed by calcium channel blockers.44 Epicardial coronary vasospasm is defined as transient total or subtotal coronary artery occlusion (>90% constriction) with angina and ischemic electrocardiographic (ECG) changes either spontaneously or in response to a provocative stimulus.44 Compared to men, women were approximately two times more likely to be found to have inducible coronary vasospasm in an European study.48 However, coronary vasospasm has been found to be more common in Japanese men than women.75 Coronary vasospasm mechanisms include smooth muscle cell hyperreactivity (including through rho-kinase activation), inflammation, oxidative stress, deficiency of endogenous nitric oxide activity (including e-NOS polymorphisms), deficient variant aldehyde dehydrogenase genotype, and endothelial dysfunction.76–78 Vasospasm may be triggered in the setting of sex-specific factors, such as menstruation, and factors that affect both sexes, including sympathetic activity, non-selective beta-blockers, hyperventilation, allergic reactions (such as mast cell activation syndrome), alcohol, muscarinic agonists, ergot alkaloids, prostaglandins, exposure to cold or to smoke, and abnormal platelet activation.76, 77
Long-term prognosis of patients with coronary artery spasm on medical therapy are generally favorable (>95% survival over 5 years).56, 57 However, if comorbid obstructive CAD (FFR>0.80) is present, a high incidence of MACE has been reported in patients with coronary artery vasospasm despite calcium channel blockade treatment.79 Predictors of MACE include smoking, significant CAD, ST-elevation, multivessel spasm, reduction or withdrawal of anti-spasm medications, and history of cardiac arrest.57
NONINVASIVE RISK STRATIFICATION AND DIAGNOSIS
The evaluation of IHD in women can present unique challenges to clinicians.80–82 The accuracy of standard noninvasive ischemia testing can vary significantly when evaluated against a gold standard of finding anatomic obstructive CAD. This may be particularly problematic for female patients, whose symptoms are less likely to be explained by findings on invasive coronary angiography (ICA), and whose abnormal stress tests in the absence of obstructive CAD are more likely to be interpreted as ‘false positives’ when in fact they may represent INOCA.83 Over the last two decades, we have witnessed falling diagnostic yields for noninvasive stress testing84 and ICA,85 reflecting the referral of patients with lower risk and less prevalent obstructive CAD. The current American Heart Association (AHA)/American College of Cardiology (ACC) chest pain guidelines provide clinical pathways that may begin with anatomic or functional imaging, and either pathway may lead to a diagnosis of INOCA.3
Standard stress tests have low sensitivity for detecting CMD, although the presence of ischemic ECG changes, specifically ST depression that persists for at least 1 minute into recovery, in the absence of obstructive CAD should raise suspicion for CMD.86, 87 Exercise echocardiography may be preferred in women with myocardial bridging, to assess for end-systolic to early-diastolic septal wall buckling with apical sparing, a sign of dynamic focal ischemia of a compressed segment.88 The limited accuracy of conventional noninvasive testing for the diagnosis of CMD and its increased prevalence in women underscores the importance and complementary value of assessing both the macro- and microcirculation in symptomatic women with suspected coronary disease (Figure 3). Identification of women with INOCA should trigger evaluation of CMD with referral for specialized testing, such as with cardiac stress positron emission tomography (PET) and stress cardiac magnetic resonance (CMR) imaging, to confirm ischemia that, in the absence of epicardial CAD or artifact, represents coronary microvascular dysfunction.3, 25, 83 While transthoracic Doppler echocardiography has been used to diagnose CMD by assessing CFR in the left anterior descending artery in eligible patients with favorable anatomy, it is not commonly performed.3, 89 Additional assessments of atherosclerotic plaque burden using coronary computed tomography angiography (CCTA) may also further refine risk assessment and help focus preventive care strategies in women with INOCA.3
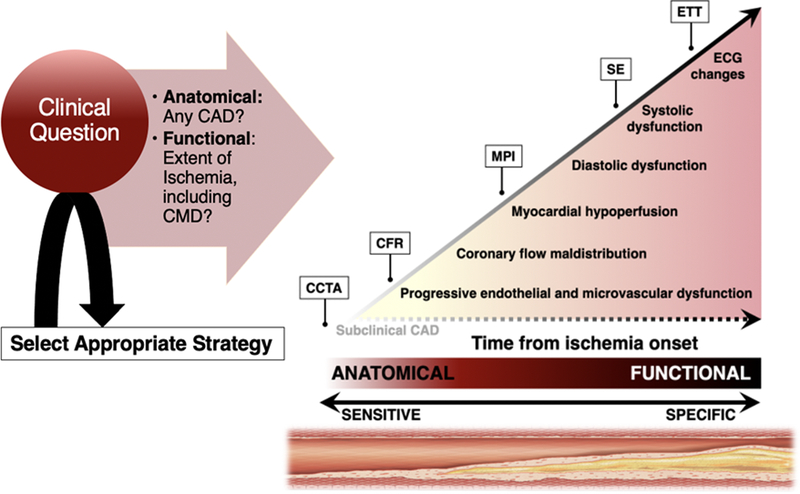
Figure 3. Noninvasive Diagnosis of Ischemic Heart Disease in Women.
Schematic of the ischemic cascade, a sequence of pathophysiologic events associated with CAD, and noninvasive multimodality imaging approaches for the evaluation of IHD in women. Anatomy-based (CCTA) and quantitative functional (CFR) imaging probes earlier events in the cascade than dose conventional testing, and as such may be especially useful in the evaluation of women. CCTA, coronary computed tomography angiography; CFR, coronary flow reserve; MPI, myocardial perfusion imaging; SE, stress echocardiography; ETT, exercise treadmill testing. Adapted from Taqueti VR et al81 with permission.
Coronary Computed Tomography Angiography (CCTA)
In asymptomatic patients, non-contrast computed tomography measurement of coronary artery calcium (CAC) score is highly predictive of MACE in women and men.90 However, women have significantly lower CAC scores than men, with 83–95% of women <50 years old demonstrating a zero CAC score.91 Measures beyond the CAC score have provided important insight to sex differences in atherosclerotic plaque and cardiovascular risk. The Coronary Artery Calcium Consortium found an increase in the proportion of women with detectable CAC occurring at approximately 46 years of age, which is nearly 10 years older than in men.92 Despite having lower CAC scores, women had greater calcified plaque volume and higher calcified plaque density than men within CAC subgroups. In addition, more extensive diffuse calcification across multiple vessels or larger calcified plaque volume was associated with higher cardiovascular mortality among women than men, despite women having less obstructive disease.92 Although noncalcified plaque is missed by CAC scores, a zero CAC score represents very low annual MACE rates in both women and men.90, 93 Since statin therapy might be withheld or deferred in patients with zero CAC scores, this impact on long-term cardiovascular prevention in women is currently poorly understood.
CCTA has dramatically increased test sensitivity for CAD diagnosis and enabled early characterization of plaque morphology. Mounting evidence supports that: (1) the presence of any atherosclerotic plaque, obstructive or not, portends increased risk of events, and (2) the higher the overall plaque burden present, the higher the risk.94, 95 From the international multicenter CONFIRM registry, nonobstructive CAD was associated with increased MACE in both women and men.96 Women and men with comparable risk and extent of CAD had comparable prognosis, as there was no interaction of sex on the association between per-vessel extent of obstructive CAD and MACE.96 Nonetheless, prevalent patterns of disease do differ between women and men, with more symptomatic women than men manifesting nonobstructive rather than obstructive CAD.15–17 Subsequent analysis of the CONFIRM data demonstrated that women with nonobstructive left main disease had a nearly 80% higher risk for MACE than men with nonobstructive left main disease, potentially due to smaller luminal area size and more positive remodeling associated with vulnerable plaque in women.97 In a secondary analysis of the International Study of Comparative Health Effectiveness of Medical and Invasive Approaches (ISCHEMIA) trial, among those who had obstructive CAD on CCTA, women demonstrated less extensive CAD as compared to men (36% vs. 47% with 3-vessel disease), despite experiencing more frequent angina.18
In nonobstructive CAD, CCTA also allows for characterization of high-risk plaque features, including positive remodeling, low-attenuation plaque, or napkin-ring sign. In an observational substudy98 of the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial, the presence of high-risk plaque in patients with nonobstructive CAD increased the adjusted risk of adverse events by 1.6-fold, and high-risk plaque was more strongly associated with events in women than in men. Nonetheless, the positive predictive value of high-risk plaque detection in these patients was low (only 4.8% for future events), limiting its current clinical applicability. Although the PROMISE trial found that a strategy of initial CCTA, as compared with functional testing, did not improve clinical outcomes over a median follow-up of 2 years, half of the cardiovascular events occurred in patients with no obstructive CAD or negative stress testing, highlighting the clinical impact of INOCA.99 PROMISE investigators found that women were more likely to have positive stress tests than positive CCTA for obstructive CAD compared to men, again suggesting the role of INOCA in these women.100 More recently, among patients with nonobstructive CAD followed with serial CCTA over >2 years in the Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging (PARADIGM) study, baseline percent atheroma volume independently predicted the development of obstructive CAD, whereas presence of high-risk plaque (positive remodeling, spotty calcification, low-attenuation plaque) did not.101 Analysis of sex differences in PARADIGM demonstrated that women had greater calcified plaque volume progression but slower noncalcified plaque progression and less development of high-risk plaques than men, suggesting that CCTA may provide additional sex-specific risk stratification in patients with nonobstructive CAD.102 In patients presenting with known or suspected angina, a higher proportion of women than men will have a normal angiogram or no obstructive atherosclerosis.103 Yet a substantial number of these patients may have underlying CMD microvascular angina.
Cardiac Positron Emission Tomography (PET)
Neither conventional ICA nor CCTA can detect CMD, which is defined as a reduced CFR in the absence of flow-limiting CAD.25 Global CFR, calculated as the ratio of hyperemic to rest absolute myocardial blood flow averaged over the left ventricle, is an integrated marker of coronary vasomotor dysfunction that measures the hemodynamic effects of focal, diffuse, and small-vessel CAD on myocardial tissue perfusion104, 105. Observational data have shown that CFR measurements using cardiac PET distinguish patients at low or high risk for MACE and cardiac mortality,106–109 beyond other clinical variables.110–112 Evidence now supports that: (1) the existence of impaired CFR, whether in the presence or absence of obstructive CAD, portends increased risk of cardiovascular events, and (2) the more severely impaired the overall global CFR, the higher the risk. Whereas a CFR≥2 effectively excluded high-risk angiographic CAD and was associated with low rates of annualized cardiac death,113 event rates for patients with CFR<2 increased steeply as CFR decreased, even in the absence flow-limiting CAD.35, 108 As such, noninvasive quantification of myocardial blood flow may be particularly useful for diagnosis and risk assessment of symptomatic women with suspected CMD.
PET evidence supports that women with ischemia and very low CFR<1.6 may be at an especially increased risk for MACE. In symptomatic patients referred for ICA after cardiac PET, women had a lower burden of obstructive CAD relative to men and still experienced worse outcomes, which were found to be mediated by impaired CFR, not obstructive CAD.17 Therefore, patients with impaired CFR and less obstructive CAD may be at significantly increased risk of MACE despite having access to revascularization, which is fundamentally targeted at management of obstructive CAD. Compounding this risk is the recognition that many of these patients may receive falsely reassuring (or perceived “false positive”) evaluations using conventional diagnostic testing approaches. This phenomenon may be especially relevant in women with obesity,114 cardiometabolic and inflammatory disease risk factors,115 heart failure and preserved ejection fraction,111 and INOCA.116
Cardiac Magnetic Resonance Imaging (CMR)
Stress-rest CMR myocardial perfusion reserve and coronary sinus flow reserve have been emerging tools for CMD diagnosis and prognosis over the last decade.117, 118 CMR semiquantitative myocardial perfusion reserve index (MPRI) <1.84 and quantitative myocardial perfusion reserve <2.19 have been shown to have high sensitivity and specificity for CMD in women and men.119, 120 In addition, MPRI has been found to be an independent predictor of MACE in patients with INOCA, with MPRI≤1.47 as the optimal cutoff value.121 CMR-derived coronary sinus flow reserve has also been found to be prognostic in patients with known or suspected CAD even in the absence of ischemic perfusion abnormalities, supporting its predictive role in INOCA patients.119 CMR’s strength lies in its superior spatial resolution allowing for the comprehensive assessment of cardiovascular structure and function, including the quantification of myocardial scar using late gadolinium enhancement (LGE). In women with INOCA, the prevalence of LGE has been reported to be 8%, with one-third of affected women lacking a prior diagnosis of MI despite the majority of patients demonstrating a scar pattern typical of CAD.122 As such, CMR is useful in the identification of MINOCA.123, 124
INVASIVE CORONARY ANGIOGRAPHY (ICA)
ICA remains a cornerstone for investigation of patients with suspected CAD. ICA allows for diagnosis of high-risk coronary anatomy needing revascularization but can also reveal non-obstructive causes of ischemia or infarction with additional specialized testing.89, 124 ICA can also identify lesions that may benefit from further interrogation using advanced imaging and physiological techniques. There are specific challenges that are associated with ICA in women, as they are more likely to experience access site complications and access site crossover from the radial to femoral approach.125 This is likely related to smaller caliber radial arteries and subsequent predisposition to radial spasm.126 Nevertheless, the use of radial access is associated with a reduction in vascular complications, with a greater absolute reduction seen in women when compared with men.127
ICA is limited by the two-dimensional depiction of the vascular lumen and does not provide information regarding plaque characteristics or the result of PCI.128 The use of advanced coronary imaging techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) have allowed for visualization of the coronary vasculature and coronary plaque, which is particularly useful when evaluating women with MINOCA.124 Furthermore, imaging guidance for stent implantation has been shown to reduce MACE and cardiovascular death.129
Intravascular Ultrasound (IVUS)
IVUS utilizes a miniature ultrasound transducer on a monorail catheter to visualize the coronary lumen and vascular wall. IVUS can assess for the presence of plaque disruption (encompassing plaque rupture, erosion and calcific nodules) of non-obstructive plaque.124 Interestingly, disruption rarely occurs in the largest, eccentric or remodeled plaques; highlighting the need for imaging assessment to identify the culprit lesion.
Optical Coherence Tomography (OCT)
OCT uses the backscattering of infrared light to produce an image of the coronary vascular wall, allowing a higher spatial resolution but lower penetration depth than IVUS.130 OCT requires the use of contrast media to clear blood from the vessel to ensure optimal image quality, which may preclude some patients who cannot tolerate large contrast volumes. OCT can be used in a similar fashion to IVUS to assess plaque morphology, optimal stent sizing and the post-PCI result, and has been shown to be non-inferior to IVUS in this context.131 The higher spatial resolution can help identify subtle plaque disruptions such as plaque erosion.124
Invasive Coronary Artery Function Testing
Invasive coronary function testing is recommended for selected patients with frequent or persistent stable chest pain and suspected INOCA for the comprehensive evaluation of CMD and coronary vasospasm.3, 89
Coronary Flow Reserve (CFR)
CFR describes the ratio between hyperemic coronary blood flow velocity and resting coronary flow velocity which can be measured invasively using a coronary Doppler wire.58 A thermodilution method using coronary pressure wires has also been validated.132 Although initially devised to analyze intermediate epicardial stenoses, CFR has been adapted to assess for CMD as it can interrogate the entire coronary circulation including epicardial arteries and the microvasculature.43
CFR measurements in healthy asymptomatic subjects are usually greater than 3.0, indicating that the coronary circulation can triple coronary blood flow when required.104 CFR is generally lower in women compared to men, possibly due to sex differences in resting coronary flow.34, 36 A CFR cutoff of <2.0 has been identified as significantly correlated with ischemia identified on SPECT imaging, with high sensitivity and specificity.133, 134 Therefore, this is often considered the threshold for abnormal microcirculatory function.89, 135 Among women with suspected INOCA, CFR<2.32 was found to be the best discriminating threshold for MACE.58
Index of Microcirculatory Resistance (IMR)
IMR is a measurement of coronary microvascular function by using a coronary pressure wire as a thermistor to calculate overall microcirculatory resistance at maximal hyperemia.136 This is calculated based on Ohm’s Law, as microvascular resistance is proportional to the pressure differential between the distal coronary bed and the venous pressure; and inversely proportional to coronary flow as measured by thermodilution.137 IMR is potentially more useful for assessing CMD as it is specific to the microvasculature and does not incorporate measurements from the epicardial vessels. IMR is also not affected by changes in patient hemodynamics. A normal IMR is considered <25.137 Doppler-derived hMR correlates modestly with IMR and CFR, and an hMR threshold <2 is considered normal.36, 138
An abnormal IMR or hMR has been reported in 21–35% of patients with suspected INOCA, suggestive of a microvascular cause of symptoms.36, 50, 139 Elevated IMR in concert with low CFR has been associated with increased MACE in patients with INOCA; however, an isolated elevated IMR did not portend worse outcomes.139
Coronary endothelial dysfunction
Coronary endothelial dysfunction is characterized by an attenuated increase or decrease in coronary blood flow and coronary artery diameter in response to low dose acetylcholine.68 Impaired endothelial-dependent CMD is often defined as a percentage increase in coronary blood flow ≤50%, while endothelial-dependent epicardial dysfunction is typically defined as coronary artery dilation <5% or any significant vasoconstriction.47, 49 Although endothelial dysfunction may affect both the epicardial and microvascular coronary circulation,49 there may be a dissociation between the epicardial and microvascular function such that coronary blood flow may be preserved or even increased in the setting of coronary epicardial vasoconstriction in response to acetylcholine.68 Invasively determined coronary endothelial dysfunction portends increased MACE and mortality risk in women with INOCA.55
Coronary vasospasm
Epicardial or microvascular vasospasm is assessed using intracoronary injection of sequentially higher doses of acetylcholine or ergonovine and can be found in up to 60% of patients with suspected INOCA.56 Epicardial vasospasm is typically defined as ≥90% angiographic vasoconstriction from baseline44 with reproduction of angina and transient ischemic ECG changes, while microvascular vasospasm is defined as angina and transient ischemic ECG changes in the absence of epicardial vasospasm.56 When performed by experienced operators, no fatal or irreversible nonfatal complications of intracoronary acetylcholine provocation testing have been reported, but testing may be associated with arrhythmias, including self-limiting pause, symptomatic bradycardia and atrial fibrillation.46, 140
Myocardial bridging
Myocardial bridging is an intramyocardial segment of an epicardial coronary artery with a prevalence of approximately 25% in women and men by autopsy studies, although reported prevalence differs based on method of evaluation.141 During ICA, myocardial bridge is often diagnosed when there is evidence of systolic compression, often appearing worse after nitroglycerin administration. IVUS can play an important role in confirming myocardial bridging by detecting a characteristic “half-moon” appearance in the bridged segment and identifying concomitant atherosclerosis..142. While myocardial bridging is generally considered benign, it can be associated with angina and adverse cardiovascular outcomes.141, 143, 144 Concomitant endothelial dysfunction or vasospasm may contribute to symptoms in those who present in adulthood.50, 145 Beta-blocker and calcium-channel blocker therapy are usually recommended medical therapy for symptomatic patients, with surgical unroofing the preferred surgical strategy in refractory cases.146
Doppler measurements in bridged coronary segments show a characteristic pattern that reflects abrupt early diastolic flow acceleration, mid-to-late diastolic plateau and retrograde flow in systole.147 CFR measurements have been shown to be abnormal distal to the bridged segment, despite being normal or slightly reduced in the proximal coronary segment, and vasospasm may often be provoked at bridge sites.147, 148 Invasive hemodynamic assessment is typically performed using inotropic stimulation with dobutamine. Diastolic FFR ≤0.76 during dobutamine provocation has the best sensitivity and specificity for identifying myocardial bridging associated with stress-induced myocardial ischemia.149 Another diastole-specific index, instantaneous wave-free ratio (iFR), has been shown to be superior to conventional-FFR in the assessment of myocardial bridging, although the ischemic cut-off values of iFR are unknown.150
STRATIFIED MEDICAL THERAPY OF INOCA
Currently, the therapeutic strategy for INOCA remains unclear due to a paucity of clinical trial evidence.151 Considering patients presenting with stable angina, clinical trials and practice guidelines have historically focused on obstructive CAD. However, this narrow focus promulgates under-recognition and undertreatment of INOCA, widening gender inequalities in cardiovascular care.89 Novel personalized therapeutic approaches are urgently needed. Stratified medicine is relevant to INOCA, since patients with initially undiagnosed disease may pass through clinical services that are providing care for relatively unselected (undifferentiated) populations.
Evidence from CorMicA
The British Heart Foundation Coronary Microvascular Angina (CorMicA) trial was a randomized, controlled, blinded clinical trial of stratified medical therapy versus usual care in patients with angina undergoing clinically indicated ICA (Figure 4). Patients with no obstructive CAD were randomized (n=151) to stratified medicine or standard, angiography-guided management. Baseline characteristics were notable for a median age 61 years, majority female (73.5%), and moderate angina frequency. Coronary function testing using thermodilution and acetylcholine vasoreactivity testing were undertaken in all patients and the results were disclosed in the intervention group but not in the blinded control group. The diagnostic options for all patients were 1) a non-cardiac diagnosis, 2) microvascular angina, 3) vasospastic angina, or 4) both microvascular and vasospastic angina. The treatment options aligned with the diagnosis and contemporary practice guidelines for the management of stable IHD and for coronary artery spasm.
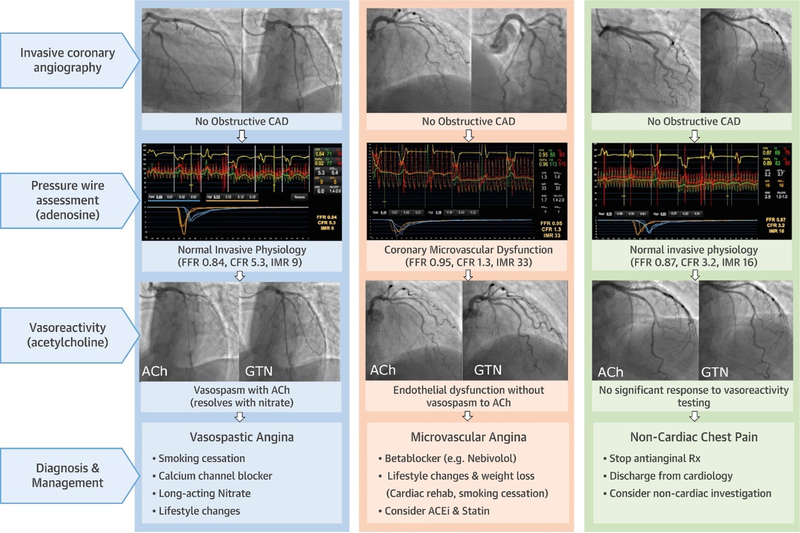
Figure 4. Stratified Medical Therapy Using Invasive Coronary Function Testing in INOCA.
The Coronary Microvascular Angina (CorMicA) Trial randomized patients with angina and no obstructive CAD to invasive coronary function testing linked to stratified medical therapy or standard care. Invasive coronary function testing included assessment of coronary flow reserve (CFR), index of microcirculatory resistance (IMR), fractional flow reserve (FFR), and vasoreactivity testing with acetylcholine (Ach) and nitroglycerin (GTN). Stratified medical therapy based on diagnosis of microvascular angina, vasospastic angina, vs noncardiac chest pain was associated with improved angina and quality of life. Reprinted from Ford TJ et al46 with permission.
Stratified medicine informed by coronary function testing significantly improved angina and quality of life at six months and one year as compared to a usual care approach without knowledge of coronary reactivity test findings.152 There was no difference in MACE at 6 months or after a median follow-up duration of 19 months.46, 152 Evidence from CorMicA supported Class IIA guideline recommendations for invasive coronary function testing in patients with suspected INOCA.3, 89
Pharmacologic Treatment of INOCA
Multiple factors have contributed to a paucity of clinical evidence for pharmacotherapy in INOCA. The reasons include limitations in the accuracy of traditional diagnostic tests (noninvasive stress tests, CCTA and ICA) for coronary vasomotor disorders. The tests with comparatively high accuracy for INOCA (PET, CMR and invasive coronary function tests) may have limited adoption because of costs, logistics, education, and training. Finally, the clinical evidence pertaining to INOCA has mainly been developed in observational studies (with the notable exception of a small WISE clinical trial of angiotensin-converting enzyme inhibition [ACE-I]).153 Therefore, lack of clinical evidence and, relatedly, limitations in practice guideline recommendations, has underpinned an empirical approach to the management of INOCA, and sometimes, therapeutic nihilism. Since most patients with microvascular and/or vasospastic angina are female, these evidence gaps underpin sex-related disparities in healthcare.154 Sex-specific therapeutic trials are lacking, thus the following treatment options are generalized for women and men.
Guideline-indicated, preventive pharmacological therapies to control cardiovascular risk factors is a key part of management of INOCA.3, 89 For symptomatic patients with nonobstructive CAD, intensification of preventive therapies is recommended.3 Statins and ACE-I/angiotensin receptor blocker therapy is suggested to improve microvascular function. Lifestyle counseling, including stress management, should be also supported as outlined in the stable IHD guidelines.155
Microvascular angina can be treated with a beta-blocker, calcium channel blockers, nicorandil, and lifestyle modification.89 If vasospasm is diagnosed or suspected, a calcium channel blocker should be prescribed in preference to a beta-blocker. A low dose should be prescribed initially to give the best chance of tolerance and adherence. The dose can then be titrated to enhance efficacy and symptom relief, as clinically appropriate.
Coronary artery spasm should be treated with calcium channel blockade. The choice of calcium channel blocker should be personalized according to heart rate, blood pressure, and drug interactions. In patients with persisting symptoms, dual calcium channel blocker therapy may be helpful. Long-acting oral nitrate therapy is also indicated, with short acting nitrates on demand for chest pain.89, 156
Emerging Pharmacological Therapies in INOCA
Ongoing randomized clinical trials may expand pharmacologic therapies or strategies in INOCA and will provide critically needed evidence regarding the potential impact of treatment on cardiovascular events. Novel therapies include a selective, potent endothelin receptor A antagonist zibotentan (NCT04097314) and intracoronary autologous CD34+ cell treatment (NCT04614467). The Women’s IschemiA Trial to Reduce Events in Non-ObstRuctIve CORonary Artery Disease (WARRIOR) is a multicenter, prospective, randomized, open blinded end-point trial evaluating the effect of an intensive statin/ACE-I (or ARB)/aspirin treatment strategy vs. usual care strategy on first occurrence of MACE in women with suspected INOCA (NCT03417388).
Non-pharmacologic Options and Emerging Clinical Strategies in INOCA
Lifestyle measures represent the cornerstone of management for patients with INOCA.89 Non-pharmacological approaches should be personalized according to the needs and preferences of the individual. A shared-care approach should consider different forms of exercise, stress management, yoga, meditation and dietary modification for weight loss, including referral to cardiac rehabilitation.
Device therapy is an emerging new paradigm for INOCA patients with refractory symptoms. Percutaneously implantable coronary sinus devices mechanically narrow the coronary sinus to achieve an increase in coronary sinus pressure and redistribution of coronary blood flow to reduce myocardial ischemia. An ongoing randomized trial will assess improvement in microvascular angina at six months after coronary sinus reducer implantation versus optimal medical therapy (NCT04606459).
Novel clinical strategies are also being investigated in randomized controlled trials, comparing standard CCTA- or ICA-guided therapy with invasive coronary function testing-guided stratified medicine in patients with suspected INOCA (CorCTCA, NCT03477890; iCorMicA, NCT04674449). Similarly, CorCMR is a randomized controlled trial of stratified medicine informed by stress perfusion CMR or standard, angiography-guided care in patients with suspected INOCA (NCT04805814).
MYOCARDIAL INFARCTION IN WOMEN
Risk stratification, diagnosis, and treatment of women with IHD (including INOCA) are targeted to prevent MACE, including MI. In the United States, approximately 430,000 women are hospitalized with acute coronary syndromes each year.157 Compared with men, women tend to be older at the time of first MI presentation (72.0 vs. 65.6 years).157 Atypical MI symptoms in women may not be promptly recognized and may contribute to pre-hospital delays in care.158, 159 Women age <55 years were more likely than men to present >6 hours after the onset of MI symptoms.160 Sex-specific decision limits of high sensitivity troponin improve the accuracy of diagnosis of MI in women.161 Among patients with non-ST segment elevation MI (NSTEMI), women are less likely to undergo ICA within the first 24 hours of admission compared to men.162 Similarly, in STEMI, door-to-device times are longer in women versus men.163, 164 Guideline-directed medical therapies for MI are underused in women compared with men, both in the acute setting and at hospital discharge.162, 165, 166 Clinical outcomes reflect these disparities in care. In-hospital mortality is higher in women than in men with MI.157, 167 The greatest sex differences in MI mortality are observed among younger individuals, and differences attenuate with older age.168 Sex disparities in MI outcomes may improve using standardized protocols.169
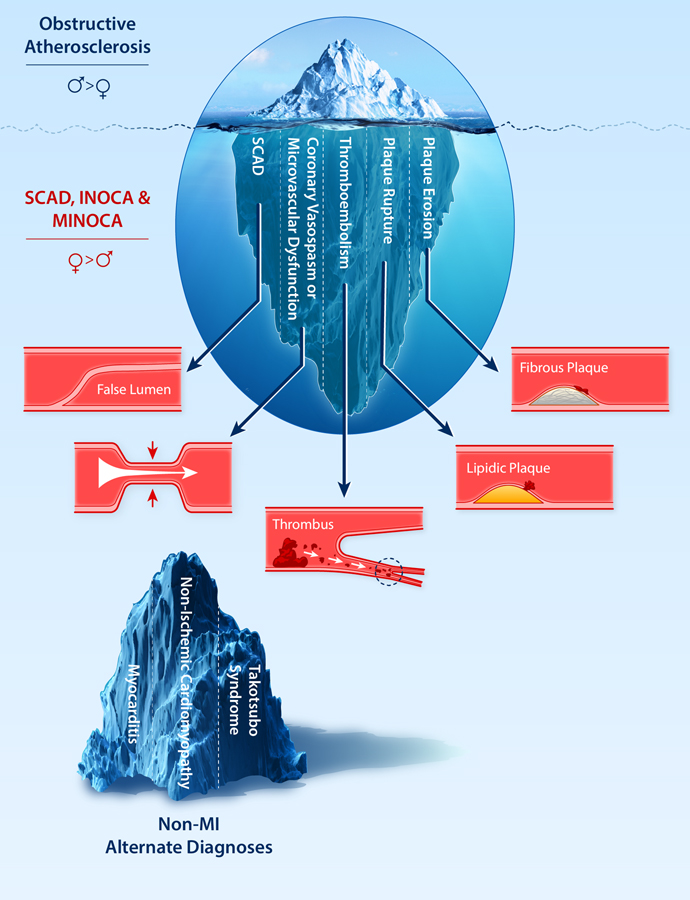
The pathophysiology, management, and outcomes of MI in women vary considerably by subtype, which are broadly stratified based on the presence or absence of obstructive CAD and coronary dissection at the time of coronary angiography (Figure 6).
Figure 6. The Diverse Pathogenesis of Myocardial Infarction and Ischemia in Women.
While obstructive atherosclerosis is the most common etiology of MI and ischemia in women, it is just the tip of the iceberg when considering the diverse pathological mechanisms of coronary arterial function and disease in women. Coronary microvascular dysfunction and coronary vasospasm are common causes of ischemia with no obstructive coronary arteries (INOCA). Spontaneous coronary artery dissection (SCAD) is a cause of MI that can have an obstructive or nonobstructive angiographic appearance. MI in the setting of no obstructive coronary arteries (MINOCA) can be attributed to coronary vasospasm (epicardial or microvascular), thromboembolism, plaque rupture, or plaque erosion. Elevated troponin in women may also be due to non-MI etiologies, including myocarditis, nonischemic cardiomyopathy, and Takotsubo Syndrome. INOCA, ischemia with no obstructive coronary arteries; MI, myocardial infarction; MINOCA, myocardial infarction with no obstructive coronary arteries; SCAD, spontaneous coronary artery dissection. (Illustration credit: Julia Huang and Ben Smith).
Myocardial Infarction with No Obstructive Coronary Arteries (MINOCA):
MINOCA is reported in ~6% of spontaneous MI, and is more common among women than men.168, 170 In a large national registry of acute MI in the United States, MINOCA was identified in approximately 1 of every 9 women without a history of established obstructive CAD.168 MINOCA is defined based on a <50% diameter stenosis in all major epicardial coronary arteries, in the absence of a specific alternative cause for the clinical presentation, such as pulmonary embolism or myocarditis.124 Among women with MINOCA, relatively few (14%) present with STEMI, but ST elevation is associated with higher risk of adverse outcomes.168 Although MINOCA confers a more favorable prognosis than MI with obstructive CAD (MI-CAD), it can be fatal, and pre-hospital deaths have been reported.171 Mortality associated with MINOCA is ~1% in-hospital, 2–5% at 1 year, and ~11% at 5 years.168, 170, 172–174 MACE occur in nearly 25% of individuals with MINOCA at 4-year follow-up.175 The risk of adverse outcomes is higher in patients aged 65 and older, with up to 12% 1-year mortality and nearly 19% 1-year rate of MACE.173
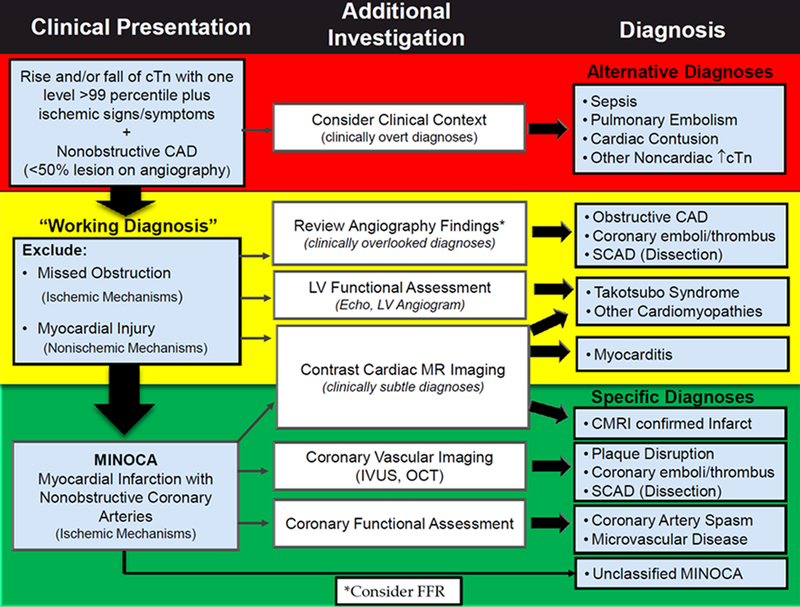
The pathogenesis of MINOCA is heterogeneous and can include atherosclerotic plaque rupture, plaque erosion, coronary spasm, coronary thromboembolism, and rarely, coronary dissection.124, 176 Patients with Takotsubo Syndrome, acute myocarditis, or non-ischemic cardiomyopathy are not considered to have MINOCA once the alternate diagnosis has been established.124 Since the optimal management of MINOCA may vary based on the underlying mechanism of infarction, a systematic approach to diagnosis is critical. Clinical algorithms for the diagnosis of MINOCA (Figure 5)124 highlight the importance of a careful review of coronary angiographic findings for clinically overlooked diagnoses, left ventricular functional assessment to evaluate for wall motion abnormalities characteristic for Takotsubo Syndrome, and contrast CMR to diagnose myocarditis or identify the territory of acute infarction or injury.124, 177 Intracoronary imaging with OCT or IVUS to identify atherosclerotic culprit lesions, and coronary functional assessments to diagnose epicardial or microvascular coronary spasm, should also be considered to enhance diagnostic sensitivity and potentially tailor therapy.124 These additional tests may be undertaken at the time of initial diagnostic angiography or during a subsequent cardiac catheterization. CCTA may be useful to identify the presence of coronary atherosclerosis but does not currently provide imaging resolution sufficient to identify plaque rupture or erosion.
Figure 5. “Traffic light” clinical algorithm for the diagnosis of MINOCA.
CAD, coronary artery disease; FFR, fractional flow reserve; LV, left ventricular; MINOCA, myocardial infarction with no obstructive coronary arteries; SCAD, spontaneous coronary artery dissection. Reprinted from Tamis-Holland JE et al124 with permission.
Invasive coronary imaging and pathology data demonstrate women to exhibit a higher prevalence of plaque erosion, lower prevalence of plaque rupture, less necrotic core and calcium, but similar plaque burden.178–180 Thin-cap fibroatheroma and plaque burden have been found to predict nonculprit MACE in women over time.180
A multi-modality imaging approach has a high likelihood of identifying abnormalities that provide insight into pathophysiology of MINOCA.123, 181–183 For example, in the largest multicenter observational study of women with MINOCA, 84.5% of patients undergoing multivessel OCT and CMR had an identifiable cause of the clinical presentation, of which 75.5% were ischemic and 24.5% of which were non-ischemic.123 The majority of non-ischemic presentations were due to myocarditis diagnosed solely on CMR. In general, myocarditis is more frequent among men.184 A coronary culprit lesion was identified in 46.2%, with atherosclerotic mechanisms, including intraplaque hemorrhage, layered plaque, and plaque rupture, most common.123 Thrombus without plaque rupture was also observed in a small number of cases.
Coronary artery spasm is common in patients with MINOCA. In studies of MINOCA in which patients underwent provocative testing with intracoronary acetylcholine or ergonovine, 24% to 65% of individuals had evidence of coronary spasm.181, 185–187 Despite concerns about provocative testing in the setting of acute MI, this approach was safe, with procedure-related arrhythmias reported in ~5% of patients, without any recurrent infarction or major adverse events reported.185
Mechanisms of MINOCA have been defined in small studies that predominantly enroll female participants; investigations to identify potential sex differences in MINOCA pathophysiology are currently ongoing (NCT02905357).
The preferred treatment of MINOCA is medical therapy, and there is no established role for coronary stents in the setting of non-obstructive coronary plaque disruption. However, impact of medical therapy for secondary prevention of MINOCA is unknown. Current clinical practice guidelines for the secondary prevention of MI are based largely on data from patients with obstructive CAD. Though it stands to reason that prognosis and best treatment may vary according to the underlying cause of MINOCA, this has yet to be proven. Consequently, although typical secondary prevention medications for MI are often administered at the time of discharge after a diagnosis of MINOCA, they are used less often than in MI-CAD, with substantial heterogeneity in clinical practice, reflecting provider equipoise.188, 189
In the absence of randomized controlled trials, observational studies provide some key insights into the effects of medical therapy in MINOCA, but few studies report sex specific findings.175 In a propensity-matched analysis of men and women with MINOCA, statins and angiotensin-converting enzyme inhibitors (ACE-I)/angiotensin receptor blockers (ARB) were associated with lower rate of MACE. Beta blockers were associated with a trend toward benefit, while no reduction in adverse events was observed with dual antiplatelet therapy.175 These findings were consistent in the subgroup of women with MINOCA. Still, observational data such as these are hypothesis generating and require confirmation in randomized trials. The ongoing Randomized evaluation of Beta blocker and ACE-I/ARB Treatment in patients with MINOCA trial (MINOCA-BAT) seeks to provide additional insights into effects of these two treatments on cardiovascular events (NCT03686696).190 Although calcium channel blocker use is reasonable in patients with presumed or documented coronary spasm as the mechanism of MI and is recommended in society statements, its role in unselected patients with MINOCA remains to be established. Additional efforts to define optimal medical therapy in patients with MINOCA are needed.
Spontaneous Coronary Artery Dissection (SCAD)
MI due to SCAD has a unique pathogenesis, in which spontaneous development of intramural hematoma, with or without an intimal tear, narrows the vessel lumen and limits myocardial perfusion.191 Dissection can occur between any of the three layers of arterial wall; specifically the intima, media or adventitia.192 SCAD is a non-atherosclerotic problem but causes narrowing that may be mistaken for MI due to atherosclerosis and thrombosis. Among patients with SCAD, the overwhelming majority (90%) are women, the average age is ~50 years, and few have traditional cardiovascular risk factors.193 Although often overlooked, SCAD may account nearly 35% of MI occurring in women age ≤50 years191 and is a leading cause of pregnancy-associated MI.194 Diagnosis of SCAD requires careful attention to the angiogram with a high index of suspicion, and intracoronary imaging may be considered in selected cases when a definitive diagnosis cannot be established although care must be taken to avoid propagation of further dissection.195
The preferred management of SCAD is conservative, with careful monitoring and medical therapy as the mainstay of treatment. This is both because angiographic healing of SCAD occurs in 70–97% cases in the weeks to months after the initial episode and because of the higher rate of PCI and bypass graft failure in the setting of SCAD as compared to MI due to CAD.191 Thus, coronary revascularization for SCAD should be reserved for patients with ongoing ischemia despite medical therapy or hemodynamic instability. When SCAD occurs in large-caliber, proximal vessels, CCTA may be useful for subsequent re-imaging to confirm angiographic resolution of the dissection.
Optimal medical management of SCAD has not been established. Some observational data indicate that beta-blockers reduce the risk of SCAD recurrence, but randomized trials have not been performed.191 The role of antiplatelet therapy also remains to be defined in clinical trials. Though dual antiplatelet therapy is prescribed commonly,196, 197 it increases bleeding risks and carries a potential for harm related to propagation of the intramural hematoma. Randomized trials evaluating medical therapy for SCAD are an area of unmet need in IHD with a disproportionate impact to women.
Myocardial Infarction with Obstructive Coronary Artery Disease (MI-CAD)
Despite the higher prevalence of MINOCA and SCAD in women versus men with MI, most women with MI have obstructive CAD (MI-CAD) due to atherosclerosis even if they did not have pre-existing obstructive lesions.168 Atherosclerotic MI-CAD are typically ascribed to plaque rupture or plaque erosion. Although a greater prevalence of plaque erosion was observed in young women than young men in autopsy studies, a prospective multicenter study of intracoronary OCT in age-matched men and women with STEMI found similar prevalence of plaque rupture (~50%) and plaque erosion (25%) in women and men.198,199
Women with atherosclerotic MI-CAD may be candidates for coronary revascularization, either by PCI with stent placement or coronary artery bypass grafting. Although few technical aspects of PCI are specific to women, women are at greater risk of major vascular access site complications after PCI for acute coronary syndromes.127 Women who undergo bypass surgery are less likely to receive guideline concordant revascularization with multiple arterial grafts or complete revascularization,200 and have a greater frequency of post-operative complications and early hospital readmission than men.201, 202
Takotsubo Syndrome
Takotsubo Syndrome is a reversible left ventricular dysfunction syndrome that presents as acute MI. Takotsubo Syndrome is characterized by wall motion abnormalities that are out of proportion to peak troponin, the absence of culprit coronary artery stenoses, frequent triggering by physical or emotional stress, and a predilection for postmenopausal women.203 Pathophysiology has not yet been identified as primarily vascular, though microvascular or epicardial spasm may contribute and may impact sex differences in treatment. Medical therapy with ACE-I/ARB is associated with improved 1-year survival in a large Takotsubo Syndrome registry (1750 patients; 90% women).204 Thus, Takotsubo Syndrome will not be discussed in depth in this compendium of coronary function and disease.
KNOWLEDGE GAPS
The Cardiovascular Disease in Women Committee of the ACC, in conjunction with interested parties (from the National Heart, Lung, and Blood Institute, AHA, and European Society of Cardiology), previously convened a working group to develop a consensus on the syndromes of INOCA.24 The report identified research gaps with recommendations addressing three overarching investigation goals: 1) formulate phenotypic classification of patients with INOCA based on clinical presentation, pathophysiological mechanisms, and prognosis; 2) develop diagnostic algorithms based on this classification system; 3) develop management approaches to reduce or prevent symptoms and to modify risk for adverse outcomes.
Expanded research recommendations from the prior report,24 to improve the understanding, diagnosis and management of coronary arterial dysfunction and disease in women include:
Discover markers for mechanisms of epicardial and/or microvascular coronary dysfunction to investigate environmental and biological determinants that may account for individual differences in vasomotor function, plaque micro-disruption, enhanced thrombus formation, sympathetic nervous system activation, and other potential triggering mechanisms for acute coronary syndromes.
Identify markers of risk that include clinical and advanced technology variables (e.g., proteomic, gene expression, cell-based, exosomes, miRNAs). Validate them in clinical settings and develop informatics platforms for prediction modeling that may require monitoring specific biological “signatures” periodically to discern which are perturbed before a clinical event (e.g., angina, HF hospitalization) and will be useful for predicting risk and directing new therapies. This should include, but not be limited to, new methods for predictive modeling using multidimensional data sets.
Using traditional and novel risk markers, new IHD risk scores can be developed in women and validated from ICA and CCTA and other sources to estimate near-term risk that also include clinical and behavioral variables, existing biomarkers, genetic, ‘omic, and imaging markers.
Test developed scores for use as targeted screening tools for women deemed to be at intermediate or higher cardiovascular risk by traditional risk scores to determine who would benefit from more intensive testing, monitoring, and therapeutic interventions.
Conduct adequately powered clinical trials in women, using standardized MINOCA/INOCA, epicardial and/or microvascular dysfunction definitions, on risk outcomes with existing strategies effective in obstructive CAD.
Conduct exploratory trials in the same populations using novel interventions based on new phenotypic and mechanistic understanding on risk outcomes.
Construct evidence-based therapeutic guidelines for INOCA, epicardial and/or microvascular coronary dysfunction. Develop physician education and fellowship training programs to enhance the understanding of these conditions and to encourage the use of novel risk assessment and management strategies. Develop programs to understand and overcome barriers to clinical implementation of these guidelines.
CONCLUSIONS
IHD remains the leading cause of death in women, and sex-differences in IHD outcomes persist. Evidence to date have established that coronary arterial function and disease are not limited to obstructive CAD in women. Increased understanding of female specific IHD risk factors and pathophysiology of INOCA and MINOCA has led to changes in guidelines for atherosclerotic cardiovascular disease prevention and for the diagnosis and management of acute and chronic coronary syndromes over the past five years. Next steps needed to address knowledge gaps in women include evidence-based approaches to IHD risk stratification and management, including conducting large randomized clinical trials for women with INOCA and MINOCA to inform therapeutic strategies.
Sources of Funding:
This work was supported by research funding from NIH R01HL146158 (C.N. Bairey Merz, J. Wei), R01HL124649 (C.N. Bairey Merz), R01HL153500 (J. Wei), U54AG065141 (C.N. Bairey Merz), K23HL150315 (N.R. Smilowitz), K23HL135438 (V.R. Taqueti), K23HL125941 (J. Wei), the American Heart Association Go Red for Women Strategically Focused Research Network grant 16SFRN27810006 (H. Reynolds), the British Heart Foundation (FS/17/26/32744; PG/18/52-33892; RE/18/6/34217) (C. Berry), the Medical Research Council (MR/SO18905/1) (C. Berry), the Lemann Foundation Cardiovascular Research Fellowship (A.C.A.H. Souza), the Barbra Streisand Women’s Cardiovascular Research and Education Program (C.N. Bairey Merz), the Linda Joy Pollin Women’s Heart Health Program (C.N. Bairey Merz), the Erika Glazer Women’s Heart Health Project (C.N. Bairey Merz), and the Adelson Family Foundation (C.N. Bairey Merz).
Disclosures: C.N. Bairey Merz has served as consultant for Sanofi, Abbott Diagnostics, and iRhythm. C. Berry has research funding and/or served as consultant for Abbott Vascular, Astra Zeneca, Boehringer Ingelheim, Dalcor, GSK, HeartFlow, Menarini, Neovasc, Siemens Healthcare and Valo Health. H. Reynolds has received nonfinancial support from Abbott Vascular, Siemens, and BioTelemetry. Dr Saw has received unrestricted research grant support (Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, National Institutes of Health, Michael Smith Foundation of Health Research, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, Boston Scientific, and Servier), speaker honoraria (AstraZeneca, Boston Scientific, and Abbott Vascular), consultancy and advisory board memberships (AstraZeneca, Abbott Vascular, Boston Scientific, Gore, Baylis, and FEops), and proctorship honoraria (Abbott Vascular and Boston Scientific). Dr. Smilowitz, Dr. Taqueti, and Dr. Wei have served as consultants and/or advisory board members for Abbott Vascular. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- ACE-I
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- CAD
coronary artery disease
- CCTA
coronary computed tomography angiography
- CMD
coronary microvascular dysfunction
- CFR
coronary flow reserve
- CMR
cardiac magnetic resonance
- ECG
electrocardiographic
- FFR
fractional flow reserve
- HMR
hyperemic microvascular resistance
- ICA
invasive coronary angiography
- IFR
instantaneous wave-free ratio
- IHD
ischemic heart disease
- IMR
index of microcirculatory resistance
- INOCA
ischemia with no obstructive coronary arteries
- IVUS
intravascular ultrasound
- LGE
late gadolinium enhancement
- MACE
major adverse cardiac events
- MI
myocardial infarction
- MINOCA
myocardial infarction with no obstructive coronary arteries
- MPRI
myocardial perfusion reserve index
- NSTEMI
non-ST elevation myocardial infarction
- OCT
optical coherence tomography
- PCI
percutaneous coronary intervention
- PET
positron emission tomography
- SCAD
spontaneous coronary artery dissection
- STEMI
ST elevation myocardial infarction
References:
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R, Rosamond WD, Bhatt DL, et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation. 2019;139:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, Blankstein R, Boyd J, Bullock-Palmer RP, Conejo T, et al. 2021. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021:CIR0000000000001030. [DOI] [PubMed] [Google Scholar]
- 4.Kreatsoulas C, Shannon HS, Giacomini M, Velianou JL and Anand SS. Reconstructing angina: cardiac symptoms are the same in women and men. JAMA Intern Med. 2013;173:829–31. [DOI] [PubMed] [Google Scholar]
- 5.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 6.Jones E, Delia Johnson B, Shaw LJ, Bakir M, Wei J, Mehta PK, Minissian M, Pepine CJ, Reis SE, Kelsey SF, et al. Not typical angina and mortality in women with obstructive coronary artery disease: Results from the Women’s Ischemic Syndrome Evaluation study (WISE). Int J Cardiol Heart Vasc. 2020;27:100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta PK, Bess C, Elias-Smale S, Vaccarino V, Quyyumi A, Pepine CJ and Bairey Merz CN. Gender in cardiovascular medicine: chest pain and coronary artery disease. Eur Heart J. 2019;40:3819–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds HR, Picard MH, Spertus JA, Peteiro J, Lopez Sendon JL, Senior R, El-Hajjar MC, Celutkiene J, Shapiro MD, Pellikka PA, et al. Natural History of Patients With Ischemia and No Obstructive Coronary Artery Disease: The CIAO-ISCHEMIA Study. Circulation. 2021;144:1008–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedlak T, Herscovici R, Cook-Wiens G, Handberg E, Wei J, Shufelt C, Bittner V, Reis SE, Reichek N, Pepine C, et al. Predicted Versus Observed Major Adverse Cardiac Event Risk in Women With Evidence of Ischemia and No Obstructive Coronary Artery Disease: A Report From WISE (Women’s Ischemia Syndrome Evaluation). J Am Heart Assoc. 2020;9:e013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E and Bairey Merz CN. Ischemia and No Obstructive Coronary Artery Disease ( INOCA ): What Is the Risk? J Am Heart Assoc. 2018;7:e008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiteshi AK, Li D, Gao Y, Chen A, Flores F, Mao SS and Budoff MJ. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol. 2014;37:605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SG, Apple S, Mintz GS, McMillan T, Canos DA, Maehara A and Weissman NJ. The importance of gender on coronary artery size: in-vivo assessment by intravascular ultrasound. Clin Cardiol. 2004;27:291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taqueti VR. Sex Differences in the Coronary System. Adv Exp Med Biol. 2018;1065:257–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. [DOI] [PubMed] [Google Scholar]
- 16.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H and Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. [DOI] [PubMed] [Google Scholar]
- 17.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S and Di Carli MF. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 2017;135:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, Picard MH, Kwong RY, Bairey-Merz CN, Cyr DD, et al. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, Adamson PD, Moss AJ, Alam S, Hunter A, et al. Sex-Specific Computed Tomography Coronary Plaque Characterization and Risk of Myocardial Infarction. JACC Cardiovasc Imaging. 2021;14:1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otaki Y, Gransar H, Cheng VY, Dey D, Labounty T, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, et al. Gender differences in the prevalence, severity, and composition of coronary artery disease in the young: a study of 1635 individuals undergoing coronary CT angiography from the prospective, multinational confirm registry. Eur Heart J Cardiovasc Imaging. 2015;16:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz-Garcia J, Lerman A, Weisz G, Maehara A, Mintz GS, Fahy M, Xu K, Lansky AJ, Cristea E, Farah TG, et al. Age- and gender-related changes in plaque composition in patients with acute coronary syndrome: the PROSPECT study. EuroIntervention. 2012;8:929–38. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Mintz GS, Witzenbichler B, Metzger DC, Rinaldi MJ, Duffy PL, Weisz G, Stuckey TD, Brodie BR, Inaba S, et al. Differences in Underlying Culprit Lesion Morphology Between Men and Women: An IVUS Analysis From the ADAPT-DES Study. JACC Cardiovasc Imaging. 2016;9:498–9. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekhar J and Mehran R. Sex-Based Differences in Acute Coronary Syndromes: Insights From Invasive and Noninvasive Coronary Technologies. JACC Cardiovasc Imaging. 2016;9:451–64. [DOI] [PubMed] [Google Scholar]
- 24.Bairey Merz CN, Pepine CJ, Walsh MN and Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taqueti VR and Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camici PG and Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 27.Chilian WM. Coronary microcirculation in health and disease. Summary of an NHLBI workshop. Circulation. 1997;95:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson RF, Marcus ML and White CW. Prediction of the physiologic significance of coronary arterial lesions by quantitative lesion geometry in patients with limited coronary artery disease. Circulation. 1987;75:723–32. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RF and White CW. Intracoronary papaverine: an ideal coronary vasodilator for studies of the coronary circulation in conscious humans. Circulation. 1986;73:444–51. [DOI] [PubMed] [Google Scholar]
- 30.Lerman A and Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–8. [DOI] [PubMed] [Google Scholar]
- 31.Boulanger CM. Endothelium. Arterioscler Thromb Vasc Biol. 2016;36:e26–31. [DOI] [PubMed] [Google Scholar]
- 32.Corban MT, Prasad A, Gulati R, Lerman LO and Lerman A. Sex-specific differences in coronary blood flow and flow velocity reserve in symptomatic patients with non-obstructive disease. EuroIntervention. 2021;16:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel MB, Bui LP, Kirkeeide RL and Gould KL. Imaging Microvascular Dysfunction and Mechanisms for Female-Male Differences in CAD. JACC Cardiovasc Imaging. 2016;9:465–82. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi Y, Fearon WF, Honda Y, Tanaka S, Pargaonkar V, Fitzgerald PJ, Lee DP, Stefanick M, Yeung AC and Tremmel JA. Effect of Sex Differences on Invasive Measures of Coronary Microvascular Dysfunction in Patients With Angina in the Absence of Obstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S, Mehta PK, Eshtehardi P, Hung OY, Koh JS, Kumar A, Al-Badri A, Rabah R, D’Souza M, Gupta S, et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheter Cardiovasc Interv. 2020. [DOI] [PubMed] [Google Scholar]
- 37.Mathews L, Iantorno M, Schar M, Bonanno G, Gerstenblith G, Weiss RG and Hays AG. Coronary endothelial function is better in healthy premenopausal women than in healthy older postmenopausal women and men. PLoS One. 2017;12:e0186448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halligan SC, Murtagh B, Lennon RJ, Pumper GM, Mathew V, Higano ST and Lerman A. Effect of long-term hormone replacement therapy on coronary endothelial function in postmenopausal women. Mayo Clin Proc. 2004;79:1514–20. [DOI] [PubMed] [Google Scholar]
- 39.Stanhewicz AE, Wenner MM and Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol. 2018;315:H1569–H1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia D, Harbaoui B, van de Hoef TP, Meuwissen M, Nijjer SS, Echavarria-Pinto M, Davies JE, Piek JJ and Lantelme P. Relationship between FFR, CFR and coronary microvascular resistance - Practical implications for FFR-guided percutaneous coronary intervention. PLoS One. 2019;14:e0208612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson NP, Kirkeeide RL and Gould KL. Same Lesion, Different Artery, Different FFR!? JACC Cardiovasc Imaging. 2019;12:718–721. [DOI] [PubMed] [Google Scholar]
- 42.Hoshino M, Hamaya R, Kanaji Y, Kanno Y, Hada M, Yamaguchi M, Sumino Y, Hirano H, Horie T, Usui E, et al. Sex Differences in Long-Term Outcomes in Patients With Deferred Revascularization Following Fractional Flow Reserve Assessment: International Collaboration Registry of Comprehensive Physiologic Evaluation. J Am Heart Assoc. 2020;9:e014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN and Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 44.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN and Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 45.Aribas E, van Lennep JER, Elias-Smale SE, Piek JJ, Roos M, Ahmadizar F, Arshi B, Duncker DJ, Appelman Y and Kavousi M. Prevalence of microvascular angina among patients with stable symptoms in the absence of obstructive coronary artery disease: a systematic review. Cardiovasc Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–2855. [DOI] [PubMed] [Google Scholar]
- 47.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO and Lerman A. Prevalence of Coronary Microvascular Dysfunction Among Patients With Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc Interv. 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 48.Aziz A, Hansen HS, Sechtem U, Prescott E and Ong P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J Am Coll Cardiol. 2017;70:2349–2358. [DOI] [PubMed] [Google Scholar]
- 49.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC and Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, Osborne MT, Seidelmann SB, Vita T, Bibbo CF, et al. Integrated Noninvasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients With Stable Coronary Artery Disease. Circulation. 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, et al. Ischemia and No Obstructive Coronary Artery Disease: Prevalence and Correlates of Coronary Vasomotion Disorders. Circ Cardiovasc Interv. 2019;12:e008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavella R, Cutri N, Tucker G, Adams R, Spertus J and Beltrame JF. Natural history of patients with insignificant coronary artery disease. Eur Heart J Qual Care Clin Outcomes. 2016;2:117–124. [DOI] [PubMed] [Google Scholar]
- 54.Kenkre TS, Malhotra P, Johnson BD, Handberg EM, Thompson DV, Marroquin OC, Rogers WJ, Pepine CJ, Bairey Merz CN and Kelsey SF. Ten-Year Mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seitz A, Gardezy J, Pirozzolo G, Probst S, Athanasiadis A, Hill S, Mahrholdt H, Bekeredjian R, Sechtem U and Ong P. Long-Term Follow-Up in Patients With Stable Angina and Unobstructed Coronary Arteries Undergoing Intracoronary Acetylcholine Testing. JACC Cardiovasc Interv. 2020;13:1865–1876. [DOI] [PubMed] [Google Scholar]
- 57.Takagi Y, Yasuda S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, et al. Clinical characteristics and long-term prognosis of vasospastic angina patients who survived out-of-hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ Arrhythm Electrophysiol. 2011;4:295–302. [DOI] [PubMed] [Google Scholar]
- 58.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G and Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suda A, Takahashi J, Hao K, Kikuchi Y, Shindo T, Ikeda S, Sato K, Sugisawa J, Matsumoto Y, Miyata S, et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J Am Coll Cardiol. 2019;74:2350–2360. [DOI] [PubMed] [Google Scholar]
- 60.Shimokawa H, Suda A, Takahashi J, Berry C, Camici PG, Crea F, Escaned J, Ford T, Yii E, Kaski JC, et al. Clinical characteristics and prognosis of patients with microvascular angina: an international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur Heart J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, Faber R, Host N, Gustafsson I, Hansen PR, et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women With Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. J Am Heart Assoc. 2016;5:e003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wessel TR, Arant CB, McGorray SP, Sharaf BL, Reis SE, Kerensky RA, von Mering GO, Smith KM, Pauly DF, Handberg EM, et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE). Clin Cardiol. 2007;30:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park K, Quesada O, Cook-Wiens G, Wei J, Minissian M, Handberg EM, Merz NB and Pepine CJ. Adverse Pregnancy Outcomes Are Associated with Reduced Coronary Flow Reserve in Women With Signs and Symptoms of Ischemia Without Obstructive Coronary Artery Disease: A Report from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. J Womens Health (Larchmt). 2020;29:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LE, Schapira J, Yang Y, et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging. 2011;4:27–33. [DOI] [PubMed] [Google Scholar]
- 65.Hahn VS, Lenihan DJ and Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sickinghe AA, Korporaal SJA, den Ruijter HM and Kessler EL. Estrogen Contributions to Microvascular Dysfunction Evolving to Heart Failure With Preserved Ejection Fraction. Front Endocrinol (Lausanne). 2019;10:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeiher AM, Drexler H, Wollschlager H and Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. [DOI] [PubMed] [Google Scholar]
- 68.Hasdai D, Gibbons RJ, Holmes DR Jr., Higano ST and Lerman A Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–5. [DOI] [PubMed] [Google Scholar]
- 69.Pries AR and Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J. 2017;38:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, Gould KL and Wijns W. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation. 2001;104:2401–6. [DOI] [PubMed] [Google Scholar]
- 71.Imai S, Kondo T, Stone GW, Kawase Y, Ahmadi AA, Narula J and Matsuo H. Abnormal Fractional Flow Reserve in Nonobstructive Coronary Artery Disease. Circ Cardiovasc Interv. 2019;12:e006961. [DOI] [PubMed] [Google Scholar]
- 72.Ahmadi A, Leipsic J, Ovrehus KA, Gaur S, Bagiella E, Ko B, Dey D, LaRocca G, Jensen JM, Botker HE, et al. Lesion-Specific and Vessel-Related Determinants of Fractional Flow Reserve Beyond Coronary Artery Stenosis. JACC Cardiovasc Imaging. 2018;11:521–530. [DOI] [PubMed] [Google Scholar]
- 73.de Silva R and Cheng K. Microvascular angina: quo tendimus? Eur Heart J. 2021. [DOI] [PubMed] [Google Scholar]
- 74.Rahman H, Demir OM, Khan F, Ryan M, Ellis H, Mills MT, Chiribiri A, Webb A and Perera D. Physiological Stratification of Patients With Angina Due to Coronary Microvascular Dysfunction. J Am Coll Cardiol. 2020;75:2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato K, Takahashi J, Odaka Y, Suda A, Sueda S, Teragawa H, Ishii K, Kiyooka T, Hirayama A, Sumiyoshi T, et al. Clinical characteristics and long-term prognosis of contemporary patients with vasospastic angina: Ethnic differences detected in an international comparative study. Int J Cardiol. 2019;291:13–18. [DOI] [PubMed] [Google Scholar]
- 76.Lanza GA, Careri G and Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–82. [DOI] [PubMed] [Google Scholar]
- 77.Yasue H, Mizuno Y and Harada E. Coronary artery spasm - Clinical features, pathogenesis and treatment. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimokawa H and Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–75. [DOI] [PubMed] [Google Scholar]
- 79.Hao K, Takahashi J, Kikuchi Y, Suda A, Sato K, Sugisawa J, Tsuchiya S, Shindo T, Nishimiya K, Ikeda S, et al. Prognostic Impacts of Comorbid Significant Coronary Stenosis and Coronary Artery Spasm in Patients With Stable Coronary Artery Disease. J Am Heart Assoc. 2021:e017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, Kramer CM, Min JK, Newby LK, Nixon JV, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130:350–79. [DOI] [PubMed] [Google Scholar]
- 81.Taqueti VR, Dorbala S, Wolinsky D, Abbott B, Heller GV, Bateman TM, Mieres JH, Phillips LM, Wenger NK and Shaw LJ. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state-of-the-evidence and clinical recommendations. J Nucl Cardiol. 2017;24:1402–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, Mendelson MA, Wood MJ, Volgman AS and Mieres JH. Sex Differences in Ischemic Heart Disease: Advances, Obstacles, and Next Steps. Circ Cardiovasc Qual Outcomes. 2018;11:e004437. [DOI] [PubMed] [Google Scholar]
- 83.Wei J, Cheng S and Bairey Merz CN. Coronary Microvascular Dysfunction Causing Cardiac Ischemia in Women. JAMA. 2019;322:2334–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE and Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–65. [DOI] [PubMed] [Google Scholar]
- 85.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG and Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO and Lerman A. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lopez DM, Divakaran S, Gupta A, Bajaj NS, Osborne MT, Zhou W, Hainer J, Bibbo CF, Skali H, Dorbala S, et al. Role of Exercise Treadmill Testing in the Assessment of Coronary Microvascular Disease. JACC Cardiovasc Imaging. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin S, Tremmel JA, Yamada R, Rogers IS, Yong CM, Turcott R, McConnell MV, Dash R and Schnittger I. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:e000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41:3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hussain A, Ballantyne CM and Nambi V. Zero Coronary Artery Calcium Score: Desirable, but Enough? Circulation. 2020;142:917–919. [DOI] [PubMed] [Google Scholar]
- 92.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong ND, Cordola Hsu AR, Rozanski A, Shaw LJ, Whelton SP, Budoff MJ, Nasir K, Miedema MD, Rumberger J, Blaha MJ, et al. Sex Differences in Coronary Artery Calcium and Mortality From Coronary Heart Disease, Cardiovascular Disease, and All Causes in Adults With Diabetes: The Coronary Calcium Consortium. Diabetes Care. 2020;43:2597–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–91. [DOI] [PubMed] [Google Scholar]
- 95.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 96.Schulman-Marcus J, o Hartaigh B, Gransar H, Lin F, Valenti V, Cho I, Berman D, Callister T, DeLago A, Hadamitzky M, et al. Sex-Specific Associations Between Coronary Artery Plaque Extent and Risk of Major Adverse Cardiovascular Events: The CONFIRM Long-Term Registry. JACC Cardiovasc Imaging. 2016;9:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie JX, Eshtehardi P, Varghese T, Goyal A, Mehta PK, Kang W, Leipsic J, B OH, Bairey Merz CN, Berman DS, et al. Prognostic Significance of Nonobstructive Left Main Coronary Artery Disease in Women Versus Men: Long-Term Outcomes From the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter) Registry. Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, Meyersohn NM, Ivanov AV, Adami EC, Patel MR, et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018;3:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pagidipati NJ, Hemal K, Coles A, Mark DB, Dolor RJ, Pellikka PA, Hoffmann U, Litwin SE, Udelson J, Daubert MA, et al. Sex Differences in Functional and CT Angiography Testing in Patients With Suspected Coronary Artery Disease. J Am Coll Cardiol. 2016;67:2607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee SE, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F, Chinnaiyan K, Choi JH, Chun EJ, Conte E, et al. Differences in Progression to Obstructive Lesions per High-Risk Plaque Features and Plaque Volumes With CCTA. JACC Cardiovasc Imaging. 2020;13:1409–1417. [DOI] [PubMed] [Google Scholar]
- 102.Lee SE, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F, Chinnaiyan K, Choi JH, Chun EJ, Conte E, et al. Sex Differences in Compositional Plaque Volume Progression in Patients With Coronary Artery Disease. JACC Cardiovasc Imaging. 2020;13:2386–2396. [DOI] [PubMed] [Google Scholar]
- 103.Mangion K, Adamson PD, Williams MC, Hunter A, Pawade T, Shah ASV, Lewis S, Boon NA, Flather M, Forbes J, et al. Sex associations and computed tomography coronary angiography-guided management in patients with stable chest pain. Eur Heart J. 2020;41:1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2:1009–23. [DOI] [PubMed] [Google Scholar]
- 105.Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–53. [DOI] [PubMed] [Google Scholar]
- 106.Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG and Bengel FM. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52:726–32. [DOI] [PubMed] [Google Scholar]
- 107.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA and Kaufmann PA. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–6. [DOI] [PubMed] [Google Scholar]
- 108.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE and Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–8. [DOI] [PubMed] [Google Scholar]
- 110.Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S and Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R and Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med. 2014;55:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, et al. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J Am Coll Cardiol. 2018;72:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Motivala AA, Rose PA, Kim HM, Smith YR, Bartnik C, Brook RD, Muzik O and Duvernoy CS. Cardiovascular risk, obesity, and myocardial blood flow in postmenopausal women. J Nucl Cardiol. 2008;15:510–7. [DOI] [PubMed] [Google Scholar]
- 116.Schmaier AA and Taqueti VR. A Lack of Reserve: Recognizing the Large Impact of Small Vessels in the Heart. Circulation. 2018;138:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel AR, Salerno M, Kwong RY, Singh A, Heydari B and Kramer CM. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J Am Coll Cardiol. 2021;78:1655–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Indorkar R, Kwong RY, Romano S, White BE, Chia RC, Trybula M, Evans K, Shenoy C and Farzaneh-Far A. Global Coronary Flow Reserve Measured During Stress Cardiac Magnetic Resonance Imaging Is an Independent Predictor of Adverse Cardiovascular Events. JACC Cardiovasc Imaging. 2019;12:1686–1695. [DOI] [PubMed] [Google Scholar]
- 119.Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rahman H, Scannell CM, Demir OM, Ryan M, McConkey H, Ellis H, Masci PG, Perera D and Chiribiri A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:978–986. [DOI] [PubMed] [Google Scholar]
- 121.Zhou W, Lee JCY, Leung ST, Lai A, Lee TF, Chiang JB, Cheng YW, Chan HL, Yiu KH, Goh VK, et al. Long-Term Prognosis of Patients With Coronary Microvascular Disease Using Stress Perfusion Cardiac Magnetic Resonance. JACC Cardiovasc Imaging. 2021;14:602–611. [DOI] [PubMed] [Google Scholar]
- 122.Wei J, Bakir M, Darounian N, Li Q, Landes S, Mehta PK, Shufelt CL, Handberg EM, Kelsey SF, Sopko G, et al. Myocardial Scar Is Prevalent and Associated With Subclinical Myocardial Dysfunction in Women With Suspected Ischemia But No Obstructive Coronary Artery Disease: From the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. Circulation. 2018;137:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reynolds HR, Maehara A, Kwong RY, Sedlak T, Saw J, Smilowitz NR, Mahmud E, Wei J, Marzo K, Matsumura M, et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction With Nonobstructive Coronary Arteries in Women. Circulation. 2021;143:624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e891–e908. [DOI] [PubMed] [Google Scholar]
- 125.Keeley EC and Cornielle-Caamano VM. Radial artery access in women undergoing percutaneous coronary procedures. JACC Cardiovasc Interv. 2015;8:513–4. [DOI] [PubMed] [Google Scholar]
- 126.Rao SV, Hess CN, Barham B, Aberle LH, Anstrom KJ, Patel TB, Jorgensen JP, Mazzaferri EL Jr., Jolly SS, Jacobs A, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv. 2014;7:857–67. [DOI] [PubMed] [Google Scholar]
- 127.Pandie S, Mehta SR, Cantor WJ, Cheema AN, Gao P, Madan M, Niemela K, Rao SV, Schwalm JD, Valentin V, et al. Radial Versus Femoral Access for Coronary Angiography/Intervention in Women With Acute Coronary Syndromes: Insights From the RIVAL Trial (Radial Vs femorAL access for coronary intervention). JACC Cardiovasc Interv. 2015;8:505–12. [DOI] [PubMed] [Google Scholar]
- 128.Raber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–3300. [DOI] [PubMed] [Google Scholar]
- 129.Buccheri S, Franchina G, Romano S, Puglisi S, Venuti G, D’Arrigo P, Francaviglia B, Scalia M, Condorelli A, Barbanti M, et al. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention With Stent Implantation: A Systematic Review and Bayesian Network Meta-Analysis of 31 Studies and 17,882 Patients. JACC Cardiovasc Interv. 2017;10:2488–2498. [DOI] [PubMed] [Google Scholar]
- 130.McCabe JM and Croce KJ. Optical coherence tomography. Circulation. 2012;126:2140–3. [DOI] [PubMed] [Google Scholar]
- 131.Ali ZA, Maehara A, Genereux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, Guagliumi G, Meraj PM, Alfonso F, Samady H, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–2628. [DOI] [PubMed] [Google Scholar]
- 132.De Bruyne B, Pijls NH, Smith L, Wievegg M and Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–6. [DOI] [PubMed] [Google Scholar]
- 133.Miller DD, Donohue TJ, Younis LT, Bach RG, Aguirre FV, Wittry MD, Goodgold HM, Chaitman BR and Kern MJ. Correlation of pharmacological 99mTc-sestamibi myocardial perfusion imaging with poststenotic coronary flow reserve in patients with angiographically intermediate coronary artery stenoses. Circulation. 1994;89:2150–60. [DOI] [PubMed] [Google Scholar]
- 134.Meuwissen M, Siebes M, Chamuleau SA, van Eck-Smit BL, Koch KT, de Winter RJ, Tijssen JG, Spaan JA and Piek JJ. Hyperemic stenosis resistance index for evaluation of functional coronary lesion severity. Circulation. 2002;106:441–6. [DOI] [PubMed] [Google Scholar]
- 135.Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–41. [DOI] [PubMed] [Google Scholar]
- 136.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG and Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–32. [DOI] [PubMed] [Google Scholar]
- 137.Fearon WF and Kobayashi Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ Cardiovasc Interv. 2017;10. [DOI] [PubMed] [Google Scholar]
- 138.Williams RP, de Waard GA, De Silva K, Lumley M, Asrress K, Arri S, Ellis H, Mir A, Clapp B, Chiribiri A, et al. Doppler Versus Thermodilution-Derived Coronary Microvascular Resistance to Predict Coronary Microvascular Dysfunction in Patients With Acute Myocardial Infarction or Stable Angina Pectoris. Am J Cardiol. 2018;121:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES and Koo BK. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol. 2016;67:1158–1169. [DOI] [PubMed] [Google Scholar]
- 140.Ong P, Athanasiadis A, Borgulya G, Vokshi I, Bastiaenen R, Kubik S, Hill S, Schaufele T, Mahrholdt H, Kaski JC, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation. 2014;129:1723–30. [DOI] [PubMed] [Google Scholar]
- 141.Lee MS and Chen CH. Myocardial Bridging: An Up-to-Date Review. J Invasive Cardiol. 2015;27:521–8. [PMC free article] [PubMed] [Google Scholar]
- 142.Tsujita K, Maehara A, Mintz GS, Doi H, Kubo T, Castellanos C, Liu J, Yang J, Oviedo C, Franklin-Bond T, et al. Comparison of angiographic and intravascular ultrasonic detection of myocardial bridging of the left anterior descending coronary artery. Am J Cardiol. 2008;102:1608–13. [DOI] [PubMed] [Google Scholar]
- 143.Rubinshtein R, Gaspar T, Lewis BS, Prasad A, Peled N and Halon DA. Long-term prognosis and outcome in patients with a chest pain syndrome and myocardial bridging: a 64-slice coronary computed tomography angiography study. Eur Heart J Cardiovasc Imaging. 2013;14:579–85. [DOI] [PubMed] [Google Scholar]
- 144.Ishikawa Y, Akasaka Y, Suzuki K, Fujiwara M, Ogawa T, Yamazaki K, Niino H, Tanaka M, Ogata K, Morinaga S, et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation. 2009;120:376–83. [DOI] [PubMed] [Google Scholar]
- 145.Arai R, Kano H, Suzuki S, Semba H, Arita T, Yagi N, Otsuka T, Matsuno S, Matsuhama M, Kato Y, et al. Myocardial bridging is an independent predictor of positive spasm provocation testing by intracoronary ergonovine injections: a retrospective observational study. Heart Vessels. 2020;35:474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boyd JH, Pargaonkar VS, Scoville DH, Rogers IS, Kimura T, Tanaka S, Yamada R, Fischbein MP, Tremmel JA, Mitchell RS, et al. Surgical Unroofing of Hemodynamically Significant Left Anterior Descending Myocardial Bridges. Ann Thorac Surg. 2017;103:1443–1450. [DOI] [PubMed] [Google Scholar]
- 147.Klues HG, Schwarz ER, vom Dahl J, Reffelmann T, Reul H, Potthast K, Schmitz C, Minartz J, Krebs W and Hanrath P. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997;96:2905–13. [DOI] [PubMed] [Google Scholar]
- 148.Nam P, Choi BG, Choi SY, Byun JK, Mashaly A, Park Y, Jang WY, Kim W, Choi JY, Park EJ, et al. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis. 2018;270:8–12. [DOI] [PubMed] [Google Scholar]
- 149.Aleksandric SB, Djordjevic-Dikic AD, Dobric MR, Giga VL, Soldatovic IA, Vukcevic V, Tomasevic MV, Stojkovic SM, Orlic DN, Saponjski JD, et al. Functional Assessment of Myocardial Bridging With Conventional and Diastolic Fractional Flow Reserve: Vasodilator Versus Inotropic Provocation. J Am Heart Assoc. 2021;10:e020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tarantini G, Barioli A, Nai Fovino L, Fraccaro C, Masiero G, Iliceto S and Napodano M. Unmasking Myocardial Bridge-Related Ischemia by Intracoronary Functional Evaluation. Circ Cardiovasc Interv. 2018;11:e006247. [DOI] [PubMed] [Google Scholar]
- 151.Marinescu MA, Loffler AI, Ouellette M, Smith L, Kramer CM and Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging. 2015;8:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ford TJ, Stanley B, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc Interv. 2020;13:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011;162:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berry C Stable Coronary Syndromes: The Case for Consolidating the Nomenclature of Stable Ischemic Heart Disease. Circulation. 2017;136:437–439. [DOI] [PubMed] [Google Scholar]
- 155.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:3097–137. [DOI] [PubMed] [Google Scholar]
- 156.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 157.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 158.Ting HH, Bradley EH, Wang Y, Lichtman JH, Nallamothu BK, Sullivan MD, Gersh BJ, Roger VL, Curtis JP and Krumholz HM. Factors associated with longer time from symptom onset to hospital presentation for patients with ST-elevation myocardial infarction. Arch Intern Med. 2008;168:959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nguyen HL, Saczynski JS, Gore JM and Goldberg RJ. Age and sex differences in duration of prehospital delay in patients with acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes. 2010;3:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.D’Onofrio G, Safdar B, Lichtman JH, Strait KM, Dreyer RP, Geda M, Spertus JA and Krumholz HM. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation. 2015;131:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, Sandeman D, Stables CL, Adamson PD, Andrews JPM, et al. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet. 2018;392:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.DeFilippis EM, Collins BL, Singh A, Biery DW, Fatima A, Qamar A, Berman AN, Gupta A, Cawley M, Wood MJ, et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the Mass General Brigham YOUNG-MI registry. Eur Heart J. 2020;41:4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huded CP, Johnson M, Kravitz K, Menon V, Abdallah M, Gullett TC, Hantz S, Ellis SG, Podolsky SR, Meldon SW, et al. 4-Step Protocol for Disparities in STEMI Care and Outcomes in Women. J Am Coll Cardiol. 2018;71:2122–2132. [DOI] [PubMed] [Google Scholar]
- 164.Stehli J, Martin C, Brennan A, Dinh DT, Lefkovits J and Zaman S. Sex Differences Persist in Time to Presentation, Revascularization, and Mortality in Myocardial Infarction Treated With Percutaneous Coronary Intervention. J Am Heart Assoc. 2019;8:e012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Poon S, Goodman SG, Yan RT, Bugiardini R, Bierman AS, Eagle KA, Johnston N, Huynh T, Grondin FR, Schenck-Gustafsson K, et al. Bridging the gender gap: Insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J. 2012;163:66–73. [DOI] [PubMed] [Google Scholar]
- 166.Koopman C, Vaartjes I, Heintjes EM, Spiering W, van Dis I, Herings RM and Bots ML. Persisting gender differences and attenuating age differences in cardiovascular drug use for prevention and treatment of coronary heart disease, 1998–2010. Eur Heart J. 2013;34:3198–205. [DOI] [PubMed] [Google Scholar]
- 167.Cenko E, Yoon J, Kedev S, Stankovic G, Vasiljevic Z, Krljanac G, Kalpak O, Ricci B, Milicic D, Manfrini O, et al. Sex Differences in Outcomes After STEMI: Effect Modification by Treatment Strategy and Age. JAMA Intern Med. 2018;178:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smilowitz NR, Mahajan AM, Roe MT, Hellkamp AS, Chiswell K, Gulati M and Reynolds HR. Mortality of Myocardial Infarction by Sex, Age, and Obstructive Coronary Artery Disease Status in the ACTION Registry-GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get With the Guidelines). Circ Cardiovasc Qual Outcomes. 2017;10:e003443. [DOI] [PubMed] [Google Scholar]
- 169.Wei J, Mehta PK, Grey E, Garberich RF, Hauser R, Bairey Merz CN and Henry TD. Sex-based differences in quality of care and outcomes in a health system using a standardized STEMI protocol. Am Heart J. 2017;191:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pasupathy S, Air T, Dreyer RP, Tavella R and Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–70. [DOI] [PubMed] [Google Scholar]
- 171.Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS and Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J. 2011;161:681–8. [DOI] [PubMed] [Google Scholar]
- 172.Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, Brass N, Armstrong PW and Kaul P. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): Insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. International Journal of Cardiology. 2018;264:12–17. [DOI] [PubMed] [Google Scholar]
- 173.Dreyer RP, Tavella R, Curtis JP, Wang Y, Pauspathy S, Messenger J, Rumsfeld JS, Maddox TM, Krumholz HM, Spertus JA, et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: outcomes in a Medicare population. Eur Heart J. 2020;41:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pelliccia F, Pasceri V, Niccoli G, Tanzilli G, Speciale G, Gaudio C, Crea F and Camici PG. Predictors of Mortality in Myocardial Infarction and Nonobstructed Coronary Arteries: A Systematic Review and Meta-Regression. Am J Med. 2020;133:73–83 e4. [DOI] [PubMed] [Google Scholar]
- 175.Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold A, Gard A and Jernberg T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation. 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 176.Iqbal SN, Feit F, Mancini GB, Wood D, Patel R, Pena-Sing I, Attubato M, Yatskar L, Slater JN, Hochman JS, et al. Characteristics of plaque disruption by intravascular ultrasound in women presenting with myocardial infarction without obstructive coronary artery disease. Am Heart J. 2014;167:715–22. [DOI] [PubMed] [Google Scholar]
- 177.Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 178.Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, Tuzcu EM, Nissen SE, King P and Nicholls SJ. Sex Differences in Nonculprit Coronary Plaque Microstructures on Frequency-Domain Optical Coherence Tomography in Acute Coronary Syndromes and Stable Coronary Artery Disease. Circ Cardiovasc Imaging. 2016;9. [DOI] [PubMed] [Google Scholar]
- 179.Yahagi K, Davis HR, Arbustini E and Virmani R. Sex differences in coronary artery disease: pathological observations. Atherosclerosis. 2015;239:260–7. [DOI] [PubMed] [Google Scholar]
- 180.Lansky AJ, Ng VG, Maehara A, Weisz G, Lerman A, Mintz GS, De Bruyne B, Farhat N, Niess G, Jankovic I, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S62–72. [DOI] [PubMed] [Google Scholar]
- 181.Gerbaud E, Arabucki F, Nivet H, Barbey C, Cetran L, Chassaing S, Seguy B, Lesimple A, Cochet H, Montaudon M, et al. OCT and CMR for the Diagnosis of Patients Presenting With MINOCA and Suspected Epicardial Causes. JACC Cardiovasc Imaging. 2020;13:2619–2631. [DOI] [PubMed] [Google Scholar]
- 182.Opolski MP, Spiewak M, Marczak M, Debski A, Knaapen P, Schumacher SP, Staruch AD, Grodecki K, Chmielak Z, Lazarczyk H, et al. Mechanisms of Myocardial Infarction in Patients With Nonobstructive Coronary Artery Disease: Results From the Optical Coherence Tomography Study. JACC Cardiovasc Imaging. 2018. [DOI] [PubMed] [Google Scholar]
- 183.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, Pena-Sing I, Axel L, Attubato MJ, Yatskar L, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124:1414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hausvater A, Smilowitz NR, Li B, Redel-Traub G, Quien M, Qian Y, Zhong J, Nicholson JM, Camastra G, Biere L, et al. Myocarditis in Relation to Angiographic Findings in Patients With Provisional Diagnoses of MINOCA. JACC Cardiovasc Imaging. 2020;13:1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Camma G, Lanza GA and Crea F. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–98. [DOI] [PubMed] [Google Scholar]
- 186.Choo EH, Chang K, Lee KY, Lee D, Kim JG, Ahn Y, Kim YJ, Chae SC, Cho MC, Kim CJ, et al. Prognosis and Predictors of Mortality in Patients Suffering Myocardial Infarction With Non-Obstructive Coronary Arteries. J Am Heart Assoc. 2019;8:e011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pirozzolo G, Seitz A, Athanasiadis A, Bekeredjian R, Sechtem U and Ong P. Microvascular spasm in non-ST-segment elevation myocardial infarction without culprit lesion (MINOCA). Clin Res Cardiol. 2020;109:246–254. [DOI] [PubMed] [Google Scholar]
- 188.Smilowitz NR, Dubner R, Hellkamp AS, Widmer RJ and Reynolds HR. Variability of discharge medical therapy for secondary prevention among patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) in the United States. PLoS One. 2021;16:e0255462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Maddox TM, Ho PM, Roe M, Dai D, Tsai TT and Rumsfeld JS. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3:632–41. [DOI] [PubMed] [Google Scholar]
- 190.Nordenskjold AM, Agewall S, Atar D, Baron T, Beltrame J, Bergstrom O, Erlinge D, Gale CP, Lopez-Pais J, Jernberg T, et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): Rationale and design. Am Heart J. 2021;231:96–104. [DOI] [PubMed] [Google Scholar]
- 191.Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Saw J, Mancini GBJ and Humphries KH. Contemporary Review on Spontaneous Coronary Artery Dissection. J Am Coll Cardiol. 2016;68:297–312. [DOI] [PubMed] [Google Scholar]
- 193.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI and Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). 2015;119:739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A and Roth A. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129:1695–702. [DOI] [PubMed] [Google Scholar]
- 195.Prakash R, Starovoytov A, Heydari M, Mancini GB and Saw J. Catheter-Induced Iatrogenic Coronary Artery Dissection in Patients With Spontaneous Coronary Artery Dissection. JACC Cardiovasc Interv. 2016;9:1851–3. [DOI] [PubMed] [Google Scholar]
- 196.Clare R, Duan L, Phan D, Moore N, Jorgensen M, Ichiuji A, Shen AY and Lee MS. Characteristics and Clinical Outcomes of Patients With Spontaneous Coronary Artery Dissection. J Am Heart Assoc. 2019;8:e012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saw J, Starovoytov A, Humphries K, Sheth T, So D, Minhas K, Brass N, Lavoie A, Bishop H, Lavi S, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40:1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J and Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–63. [DOI] [PubMed] [Google Scholar]
- 199.Guagliumi G, Capodanno D, Saia F, Musumeci G, Tarantini G, Garbo R, Tumminello G, Sirbu V, Coccato M, Fineschi M, et al. Mechanisms of atherothrombosis and vascular response to primary percutaneous coronary intervention in women versus men with acute myocardial infarction: results of the OCTAVIA study. JACC Cardiovasc Interv. 2014;7:958–68. [DOI] [PubMed] [Google Scholar]
- 200.Jawitz OK, Lawton JS, Thibault D, O’Brien S, Higgins RSD, Schena S, Vemulapalli S, Thomas KL and Zwischenberger BA. Sex Differences in Coronary Artery Bypass Grafting Techniques: A STS Database Analysis. Ann Thorac Surg. 2021. [DOI] [PubMed] [Google Scholar]
- 201.Hannan EL, Zhong Y, Lahey SJ, Culliford AT, Gold JP, Smith CR, Higgins RS, Jordan D and Wechsler A. 30-day readmissions after coronary artery bypass graft surgery in New York State. JACC Cardiovasc Interv. 2011;4:569–76. [DOI] [PubMed] [Google Scholar]
- 202.Kim C, Redberg RF, Pavlic T and Eagle KA. A systematic review of gender differences in mortality after coronary artery bypass graft surgery and percutaneous coronary interventions. Clin Cardiol. 2007;30:491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J. 2018;39:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929–38. [DOI] [PubMed] [Google Scholar]