Abstract
Lyme disease is the most prevalent vector-borne disease in the United States. Ixodes scapularis, commonly referred to as the blacklegged tick, is the primary vector of Lyme disease spirochetes, Borrelia burgdorferi sensu lato (s.l.), in the eastern United States. Connecticut has pervasive populations of I. scapularis and remains a hotspot for Lyme disease. A primary aim of this study was to determine if passively collected data on human-biting I. scapularis ticks in Connecticut could serve as a useful proxy for Lyme disease incidence based on the cases reported by the Connecticut Department of Public Health (CDPH). Data for human-biting I. scapularis ticks submitted to the Tick Testing Laboratory at the Connecticut Agricultural Experiment Station (CAES-TTL), and tested for infection with B. burgdorferi s.l., were used to estimate the rate of submitted nymphs, nymphal infection prevalence, and the rate of submitted infected nymphs. We assessed spatiotemporal patterns in tick-based measures and Lyme disease incidence with generalized linear and spatial models. In conjunction with land cover and household income data, we used generalized linear mixed effects models to examine the association between tick-based risk estimates and Lyme disease incidence. Between 2007 and 2017, the CAES-TTL received 26,116 I. scapularis tick submissions and the CDPH reported 23,423 Lyme disease cases. The rate of submitted nymphs, nymphal infection prevalence, the rate of submitted infected nymphs, and Lyme disease incidence all decreased over time during this eleven-year period. The rate of submitted nymphs, the rate of submitted infected nymphs, and Lyme disease incidence were spatially correlated, but nymphal infection prevalence was not. Using a mixed modeling approach to predict Lyme disease incidence and account for spatiotemporal structuring of the data, we found the best fitting tested model included a strong, positive association with the rate of submitted infected nymphs and a negative association with the percent of developed land for each county. We show that within counties, submissions of B. burgdorferi s.l. infected nymphs were strongly and positively associated with inter-annual variation in reported Lyme disease cases. Tick-based passive surveillance programs may be useful in providing independent measures of entomological risk, particularly in settings where Lyme disease case reporting practices change substantially over time.
Keywords: Ixodes scapularis, Borrelia burgdorferi sensu lato, Lyme disease, Passive surveillance, Connecticut
1. Introduction
First described in 1977 following the investigation of a cluster of children with arthritis-like symptoms in Lyme, Connecticut (Steere et al., 1977), Lyme disease is now the most prevalent vector-borne disease in the United States, with an estimated 330,000 human cases occurring annually (Hinckley et al., 2014; Nelson et al., 2015; Schwartz et al., 2017). Ixodes scapularis, commonly referred to as the blacklegged tick or deer tick, is the primary vector of Lyme disease spirochetes, Borrelia burgdorferi sensu lato (s.l.), and several other human disease-causing pathogens in the Eastern United States (Burgdorfer et al., 1982; Eisen and Eisen, 2018). Connecticut has pervasive populations of I. scapularis (Dennis et al., 1998; Eisen et al., 2016), and remains a high-incidence state for Lyme disease (Schwartz et al., 2017). In 2015, Connecticut was among the 14 states from which 95% of Lyme disease cases in the United States were reported, had the 5th highest number of reported cases (n = 1873), and concurrently has the 5th highest incidence (52.2 per 100,000 population) (Centers for Disease Control and Prevention, 2017).
Surveillance for Lyme disease cases can be complemented by conducting active or passive tick surveys to better understand spatial and temporal risk of human exposure to tick bites. Active tick surveillance is the collection of ticks in the environment, for example through drag or flag sampling or examination of captured rodents. Entomological risk measures generated through active tick surveillance include the density of host-seeking infected nymphal ticks (DIN), calculated as the product of the density of nymphs (DON) and nymphal infection prevalence (NIP) which is the proportion of nymphs that test positive for B. burgdorferi s.l. (or another pathogen of interest). DIN is generally considered the best predictor of human Lyme disease risk (Mather et al., 1996; Diuk-Wasser et al., 2012; Pepin et al., 2012).
Active tick surveillance is labor intensive, which limits the geographic coverage of sampling locations. Moreover, tick abundance and density estimated through active tick surveillance (i.e., tick dragging) is highly variable and unreliable if not based on repeated measures (Clow et al., 2018). Additionally, human behavior (such as how humans use the landscape, to what extent they take protective measures, and for how long ticks remain attached before detection and removal) mediates the relationship between DIN and Lyme disease acquisition (Rossi et al., 2015; Eisen and Eisen, 2016). Several studies have found a positive relationship between DIN and Lyme disease cases (Mather et al., 1996; Nicholson and Mather, 1996; Stafford et al., 1998; Pepin et al., 2012). However, in some cases the relationship was weak or equivocal (Nicholson and Mather, 1996; Pepin et al., 2012; Ripoche et al., 2018), and in other studies no association was reported (Connally et al., 2006; Prusinski et al., 2014). These discrepant findings likely reflect differences across studies in human behavior or the scale of the analysis, with the strength of the relationship between DIN and Lyme disease weakening with increased spatial resolution (Connally et al., 2006; Pepin et al., 2012).
Compared with active surveillance, there has been less focus on understanding how well tick measures obtained through passive surveillance estimate reported Lyme disease cases. Passive surveillance can include assessing tick abundance or infection rates in ticks submitted from the public, physicians or veterinarians. Testing for pathogens in ticks engorged or partially engorged with human blood is offered at no cost to residents of Connecticut by the Tick Testing Laboratory at the Connecticut Agricultural Experiment Station (CAES-TTL). This testing service promotes voluntary tick submissions from Connecticut residents. Secondarily, it provides passive surveillance data to estimate the frequency of human exposure to ticks, as well as tick infection prevalence, on a broader scale than more focal active tick surveillance (Xu et al., 2016). Compared to active surveillance of ticks in the environment, passive surveillance is economical, more epidemiologically relevant, covers a larger geographical area and may better detect tick populations at low densities. Drawbacks of passive surveillance include (1) limitations of a presence-only dataset, (2) potential for waning interest over time (participation fatigue) or variable knowledge across communities of the surveillance program, (3) spatial bias to more versus less populated areas, and (4) difficulty in detecting immature tick life stages on humans and pets (Koffi et al., 2012; Nelder et al., 2014; Soucy et al., 2018). Nevertheless, passive tick surveillance has been used to better understand the epidemiology of tick-borne diseases and assess the risk of human infection (Stromdahl et al., 2001; Ogden et al., 2006, 2010; Koffi et al., 2012; Nelder et al., 2014; Rossi et al., 2015; Gasmi et al., 2016; Xu et al., 2016; Ripoche et al., 2018). Previous studies have found associations between passive tick surveillance metrics and Lyme disease cases, and provided insights into spatiotemporal trends of actual human exposure to bites by infected ticks (Johnson et al., 2004; Rand et al., 2007; Waller et al., 2007; Rossi et al., 2015; Shelton, 2015; Ripoche et al., 2018; Gasmi et al., 2019; Jordan and Egizi, 2019).
Here we use passive surveillance data, based on I. scapularis tick submissions to the CAES-TTL and tick testing results for B. burgdorferi s.l., and reported Lyme disease cases to describe spatiotemporal patterns of disease risk at two spatial scales (town and county) in Connecticut between 2007 and 2017. Over this eleven-year period, we aim to describe tick-based risk measures and Lyme disease incidence and examine the relationship between passive tick surveillance-derived tick-based risk metrics and Lyme disease incidence.
2. Materials and methods
2.1. Study area
Connecticut is the southernmost state in New England, a small state of about 14,356 km2 and a population of 3.6 million people (United States Census Bureau, 2017). The state has eight counties and 169 towns. Overall, approximately 58% of the state is forested and even in the most urban counties forest cover is roughly 50% (Wharton et al., 2004; The Community Health Foundation, 2007; Butler, 2017).
2.2. Lyme disease data
Lyme disease case data for each town and year were provided by the Connecticut Department of Public Health (CDPH) Epidemiology and Emerging Infections Program. Notably, Lyme disease surveillance methods in Connecticut have changed over time. Mandatory laboratory reporting was instated in 1998 to monitor the efficacy of the Lyme disease vaccine, but this requirement ended when the vaccine was withdrawn in 2002 and was not reinstated until 2007 (Ertel et al., 2012).
Between 1996 and 2007, 16% more Lyme disease cases were reported by physicians in years when laboratory reporting was mandated (Ertel et al., 2012). Therefore it is pragmatic to restrict the epidemiological data to 2007 2017 when both laboratory and physician surveillance were conducted. Physician reported cases tend to include early onset manifestations (e.g., erythema migrans), whereas laboratory reported cases tend to comprise later manifestations such as those involving the musculoskeletal, neurological, or cardiovascular systems (Ertel et al., 2012). We therefore use the combined surveillance metric, which we call total cases (confirmed and probable physician and laboratory-based surveillance cases) for analysis as it provides a more comprehensive estimate of Lyme disease cases (Ertel et al., 2012). We used the US Census estimates from 2000 to calculate incidence per 100,000 population for each year from 2007 to 2009 and the 2010 US Census estimates to calculate incidence per 100,00 population for each year from 2000 to 2017 (United States Census Bureau, 2017).
2.3. Tick-based data
The CAES-TTL started testing ticks for evidence of infection with B. burgdorferi s.l. in 1996. Ticks are submitted by residents, health departments, and physicians offices. All submitted ticks are examined under a dissecting microscope and identified with standard morphological keys and taxonomic references (Keirans and Litwak, 1989; Durden and Keirans, 1996). Engorged or partially engorged female and nymphal I. scapularis ticks (showing evidence of at least some ingested blood) are screened for infection with B. burgdorferi s.l. as described below.
Two methodologies have been used for screening of I. scapularis ticks for evidence of infection with B. burgdorferi s.l. from 1996 to 2017. From 1996 to 2014, polymerase chain reaction (PCR) amplification combined with Southern blot hybridization was used. Briefly, ticks were homogenized, genomic DNA extracted, and a portion of the OspA gene was amplified (Persing et al., 1990). PCR-amplified products were then analyzed by gel electrophoresis, followed by Southern blot hybridization (Persing et al., 1990). In 2014, Southern blot hybridization was removed from the methodology due to the potential health and safety hazards associated with using 32 P-labled probes. Since 2014, screening of engorged or partially engorged ticks was conducted by extracting genomic DNA using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA), or DNA-zol BD (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturers recommendations with some modifications (Molaei et al., 2006), followed by PCR amplification of the Vagellin (Barbour et al., 1996), 16S rRNA (Gazumyan et al., 1994), and OspA (Persing et al., 1990) genes. A more detailed description of these methods is provided elsewhere (Williams et al., 2018). Comparison between the two methods, PCR-Southern blot hybridization and PCR using three diagnostic genes on a subset of DNA extracts from ticks with known and unknown infection status with B. burgdorferi s.l. produced comparable results (data not shown). Although this assay is not specific to B. burgdorferi sensu stricto (s.s.), a human-pathogenic member of the bacterial genospecies complex B. burgdorferi s.l., it is agreed upon that B. burgdorferi s.s. accounts for the vast majority of Lyme disease infections in Connecticut and throughout North America (Waddell et al., 2016). Moreover, a recent study capable of distinguishing B. burgdorferi s.s. from other B. burgdorferi s.l. spirochetes found all infected I. scapularis nymphs from Connecticut, and nearly all from neighboring New York, to represent B. burgdorferi s.s. (Feldman et al., 2015).
On the submission form to the CAES-TTL, the person submitting the tick must enter their, or their patient’s town of residence and provide information on the likely town the tick was acquired if it is known to be different from the town of residence. Ticks acquired outside of Connecticut or from a Connecticut county other than the county of the submitter’s residence were excluded from the analysis. These actions served to minimize error introduced by travel-related tick exposures, which can be problematic in a passive surveillance program based on human tick bites (Xu et al., 2018). We further narrowed the dataset to submissions of female and nymphal ticks, excluding males and larvae. Because nymphs are considered the primary vectors of Lyme disease spirochetes to humans in the Northeast (Falco et al., 1999), we estimated the rate of submitted nymphs per 100,000 population, NIP, and the rate of submitted infected nymphs per 100,000 population at two spatial scales (town and county) for each year from 2007 to 2017. To calculate the rate of submitted nymphs per 100,000 population, we used the 2000 and 2010 United States Census estimates (United States Census Bureau, 2017). NIP was calculated as the number of positive nymphs divided by the total number of tested nymphs. The rate of submitted infected nymphs recovered from humans was calculated as the rate of submitted nymphs multiplied by the NIP.
2.4. Covariates
To assess the influence of selected underlying conditions on the variability in the (infected) rate of submitted nymphs and Lyme disease incidence in Connecticut, we measured median household income and extent of developed land cover. We speculated that these variables influence tick submission to the CAES-TTL and/or Lyme disease incidence. Median household income may underlie access to or knowledge of services for tick testing or Lyme disease diagnosis and the degree of developed land cover may explain some of the variability in human-tick encounters (Cortinas and Spomer, 2014). To estimate town and county level median household income, we used United States Census (2012 2016) American Community Survey 5-year estimates of median household income (United States Census Bureau, 2017). To determine the extent of developed land cover for each town and county, we used the 2011 National Land Cover Database (NLCD) (Homer et al., 2015). We used the land cover classes considered developed (developed open space, developed low intensity, developed medium intensity, and developed high intensity) to create a binary raster grid at 30 meter spatial resolution of developed and undeveloped land. Using this binary raster grid we then determined the percentage of developed land for each town and county using the zonal statistics as table tool from the spatial analysis toolbox in ArcGIS 10.1 (ESRI, 2011). We investigated the relationship of these two covariates to tick-based risk measures and Lyme disease incidence through correlation analyses.
2.5. Data analysis
Passive surveillance data from the CAES-TTL is available since 1996 and we used the full record (1996 2017) to describe submission patterns including seasonality of submissions. To compare tick-based risk measures to Lyme disease incidence, we restricted the analyses to the years 2007 2017. To ensure that this restricted dataset was reflective of the entire dataset, we performed a Spearman’s rank correlation test.
To assess temporal patterns in tick-based risk metrics and Lyme disease incidence, we summarized the data across the state for annual estimates. To test for temporal differences in the rate of submitted nymphs, NIP, the rate of submitted infected nymphs, and Lyme disease incidence, we used generalized linear models (family = Poisson; link = log) with year structured as an ordinal integer. To test for spatial patterns, we summarized the data across all years for each town (n = 169) and calculated the Global Moran’s I in ArcGIS 10.1. For robust estimation of Global Moran’s I at least thirty observations are needed; therefore, we were unable to calculate spatial clustering at the county (n = 8) level.
To assess the relationship between Lyme disease incidence and tick-based metrics, we used generalized linear mixed effects models (GLMER; family = Poisson; link = log) with year and county as grouping variables to explicitly account for spatiotemporal structure in the data. We compared GLMER model fits by Akaike Information Criterion (AIC). Lower scores indicate better model fits; a two-point difference is significant. To determine how accurately the GLMER models predicted Lyme disease incidence, we calculated Spearman’s rank correlation coefficient between predicted and observed Lyme disease cases. Further, we used leave-one-out (LOO) cross validations across years and counties. Each year (or county) of data was iteratively omitted from the analysis and the compiled sets of predictions from the LOO models were then compared with predictions based on the full record using root mean square error (RMSE). RMSE gives the standard deviation of the model prediction error; smaller values indicate better model performance. For data processing and analyses we used R (R Core Team, 2017) and for mixed effects modeling we employed the lme4 package (Bates et al., 2014).
3. Results and discussion
3.1. Lyme disease data, 2007 2017
A total of 31,471 Lyme disease cases (including confirmed and probable) has been reported from Connecticut during 2007 to 2017. Of these, 8048 were excluded due to unknown town of residence. Of the remaining 23,423 cases, 13,331 (57%) were initiated through laboratory-based surveillance and 10,092 (43%) through physician-based reporting.
3.2. Tick-based data, 1996 2017
A total of 91,671 I. scapularis ticks was submitted to the CAES-TTL between 1996 and 2017, most of which (91,409; 99.7%) by Connecticut residents. The majority of these ticks were females (48,747) or nymphs (39,236) but there were also submissions of males (1027) and larvae (2399).
Although we did not assess the precise location the tick was acquired, human tick encounters were traced to the town of residence or the likely town the tick was acquired, if known (see Methods). We found a high degree of agreement between the locations of a submitter’s residence and where the tick was thought to be acquired 73,312 (80%) ticks were acquired and submitted from the same town and 81,171 (89%) were acquired and submitted from the same county. The finding that the vast majority of ticks were acquired and submitted in the same town supports the importance of peridomestic risk for tick-borne disease transmission (Connally et al., 2006; Eisen et al., 2016; Jordan and Egizi, 2019). Nymphal submissions were markedly higher between 1996 and 2006 compared with between 2007 and 2017 (Table 1); however we have no explanation for this change.
Table 1.
Annual Ixodes scapularis tick submissions to the CAES-TTL, 1996 2017.
| No. submitted | No. tested (% positive) | |||
|---|---|---|---|---|
|
| ||||
| Year | Nymph | Adult | Nymph | Adult |
| 1996 | 2563 | 1789 | 2403 (15%) | 1565 (29%) |
| 1997 | 1195 | 1133 | 1113 (12%) | 1041 (27%) |
| 1998 | 1877 | 1938 | 1764 (19%) | 1824 (33%) |
| 1999 | 3235 | 2870 | 3138 (16%) | 2737 (32%) |
| 2000 | 3178 | 2545 | 3085 (17%) | 2402 (32%) |
| 2001 | 2464 | 2550 | 2388 (17%) | 2448 (36%) |
| 2002 | 3401 | 2481 | 3386 (21%) | 2447 (39%) |
| 2003 | 1684 | 3768 | 1673 (23%) | 3694 (35%) |
| 2004 | 1599 | 2478 | 1596 (35%) | 2438 (42%) |
| 2005 | 3193 | 1983 | 3174 (23%) | 1936 (36%) |
| 2006 | 1557 | 2525 | 857 (16%) | 1149 (27%) |
| 2007 | 806 | 1358 | 540 (36%) | 684 (33%) |
| 2008 | 996 | 1606 | 566 (20%) | 731 (26%) |
| 2009 | 1094 | 1979 | 659 (41%) | 905 (34%) |
| 2010 | 663 | 1221 | 461 (34%) | 597 (29%) |
| 2011 | 622 | 1716 | 424 (16%) | 824 (27%) |
| 2012 | 366 | 1210 | 270 (15%) | 556 (20%) |
| 2013 | 1142 | 959 | 824 (29%) | 520 (33%) |
| 2014 | 520 | 1492 | 339 (28%) | 789 (27%) |
| 2015 | 847 | 1646 | 718 (27%) | 1297 (33%) |
| 2016 | 740 | 1543 | 561 (19%) | 1239 (33%) |
| 2017 | 758 | 2832 | 693 (16%) | 2610 (36%) |
| Total | 34500 | 43622 | 30632 (21%) | 34433 (33%) |
Of those ticks that were submitted and acquired from the same county between 1996 and 2017, 43,622 were adult females and 34,500 were nymphs (Table 1). A total of 65,056 partially or fully engorged ticks (34,433 females and 30,632 nymphs) recovered while biting humans were tested for the presence of B. burgdorferi s.l. The overall prevalence of B. burgdorferi s.l. infection in I. scapularis ticks was 21% for nymphs and 33% for adult females (see Table 1 for annual values). These results are similar to passive surveillance-derived I. scapularis infection prevalence (all stages combined) in Massachusetts (30% between 2006 and 2012) (Xu et al., 2016) and in New Jersey (38% of adult females and 22% of nymphs between 2006 and 2016) (Jordan and Egizi, 2019)
Submissions of nymphal and adult female I. scapularis ticks followed a distinct seasonal pattern (Fig. 1). Nymphal tick submissions peaked in June, while submissions of adult female ticks showed a bimodal pattern with a major peak in April-May and a minor peak in November. The June peak of nymphal submissions coincides with the June July peak in reported Lyme disease cases in Connecticut (Ertel et al., 2012). This finding further supports the understanding that nymphal bites are responsible for the majority of Lyme disease cases in the Northeast (Mather et al., 1996; Falco et al., 1999). Nymphal tick submissions in June alone represented 25% of the total I. scapularis submissions, underscoring the temporally focused nature of Lyme disease risk in Connecticut and throughout the Northeastern United States.
Fig. 1.
Submission phenology. Submission phenology of adult female and nymph Ixodes scapularis ticks to the CAES-TTL by month (1996 2017).
3.3. Tick-based data, 2007 2017
When comparing the tick-based risk measures to Lyme disease incidence, we restricted the analyses to the years 2007 2017. Over this eleven-year period there were 26,116 submissions of female and nymphal I. scapularis ticks that were submitted and acquired from the same county in Connecticut. Partially or fully engorged ticks tested for presence of B. burgdorferi s.l. (n = 16,807; 64% of all submitted ticks) included 10,752 females and 6055 nymphs. Tick-based risk measures calculated for this temporally restricted dataset were well correlated, assessed with Spearman’s rank correlation coefficient, with those calculated for the 1996 2017 period at both the town and county levels (town rate of submitted nymphs: = 0.79, p < 0.001; town NIP: = 0.59, p < 0.001; county rate of Submitted nymphs:= 0.98, p < 0.001; and county NIP: = 0.90, p = 0.002).
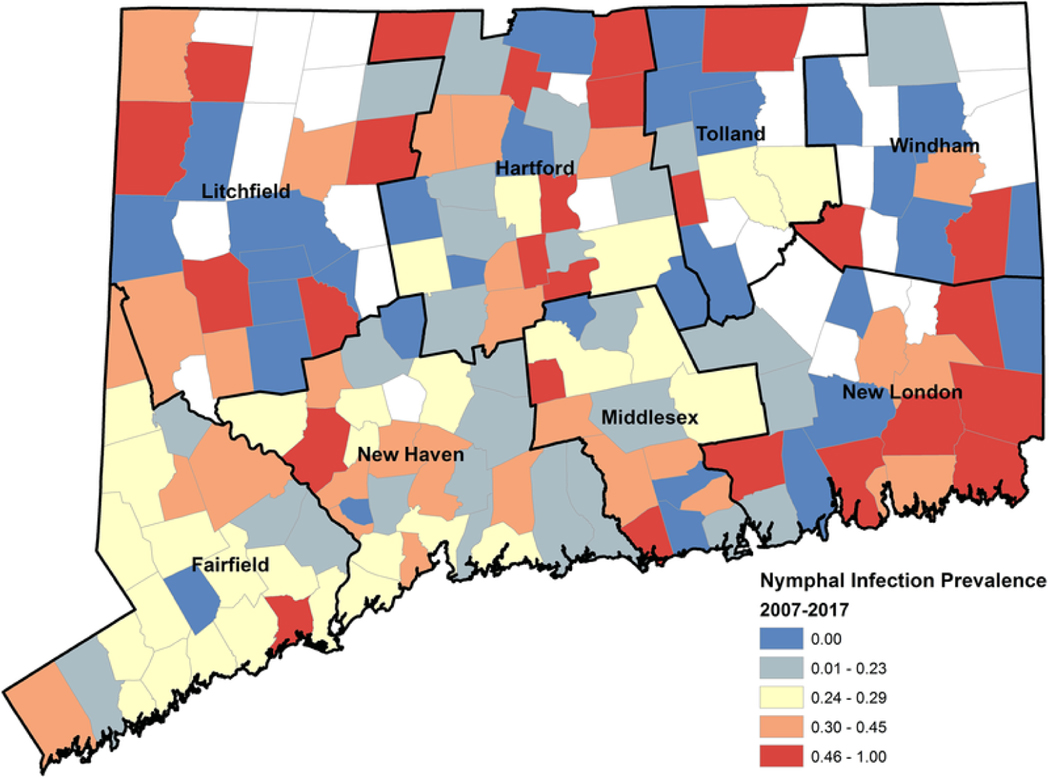
The rate of submitted nymphs, calculated as nymphal tick submissions per 100,000 population, ranged from 10.24 in 2012 to 32.12 in 2009 across the eleven-year period (mean = 22.12, SD = 6.99). Generally we note a slight decline in the annual rate of submitted nymphs, albeit with fluctuations (Fig. 2). We note that the rate of submitted nymphs per 100,000 population was much higher in Fairfield County compared to all other counties (Fig. 2). The rate of submitted infected nymphs, follows a similar trajectory decreasing over time and showing substantial spatial variability across counties (Fig. 2). NIP also generally decreased over time but remained markedly steady across counties (Fig. 2).
Fig. 2.
Descriptive spatial and temporal Lyme disease and tick-based risk measures. Cumulative Lyme disease incidence per 100,000 population, cumulative rate of submitted nymphs per 100,000 population, cumulative nymphal infection prevalence (%), and the cumulative rate of submitted infected nymphs by year and county for the years 2007 2017.
We assessed the association between NIP and the rate of submitted nymphs to determine if the downward trend in NIP over time is a result of decreasing submission rates. However, by testing for associations using Pearson’s product moment correlations, we did not Und an association at either the town (r = 0.003; p = 0.930) or the county (r = 0.028, p = 0.799) spatial scale.
3.4. Association of Lyme disease incidence and tick-based measures with household income and land cover
We found positive correlations between median household income and the rate of submitted nymphs (r = 0.50, p < 0.001) and the rate of submitted infected nymphs (r = 0.48, p < 0.001) at the town spatial scale but not at the county level. We did not find a relationship between NIP and median household income at either spatial scale, nor did we find a relationship between any tick-based risk measure and the degree of developed land at either spatial scale. We did not find a significant association between median household income and reported number of Lyme disease cases at either spatial scale. However, we did find a strong negative correlation between Lyme disease incidence and the degree of developed land at both the scale of town (r = 0.61, p < 0.001) and county (r = 0.91, p = 0.002).
The positive associations between the rate of submitted nymphs and the rate of submitted infected nymphs with median household income imply that participation in the tick submission program increases with income. Perhaps wealthier communities have more knowledge of or access to the CAES-TTL. In contrast, the lack of an association between reported Lyme disease incidence and median household income suggests that Lyme disease case reporting is independent of the community’s wealth. Lot size has been shown to be associated with tick infestation and Lyme disease risk, with larger lots more likely to have a wooded area, higher numbers of ticks, and Lyme disease cases (Maupin et al., 1991; Cromley et al., 1998). The association between the rate of submitted infected nymphs and median household income may indicate that households with higher income tend to have larger lots with greater likelihood of including wooded areas. The degree of developed land use was associated with Lyme disease incidence but none of the tick-based metrics. The increase in reported Lyme disease incidence in less developed areas may therefore be due to human behavioral differences in urban versus rural areas. While we can only speculate on the differential mechanisms underlying these relationships, we are assured that, at least as they were measured, neither covariate confounds the relationship between these tick-based risk metrics and Lyme disease incidence.
3.5. Spatiotemporal patterns, 2007 2017
Overall, annual nymphal submissions were correlated (Spearman’s rank correlation) with annual reported Lyme disease incidence both at the town ( = 0.26, p < 0.001, n = 1859 observations) and the county ( = 0.66, p < 0.001, n = 88 observations) scales.
To explicitly assess temporal changes in the rate of submitted nymphs, NIP, the rate of submitted infected nymphs, and Lyme disease incidence, we used generalized linear models with year as an ordinal integer (Table 2). The models suggest that the rate of submitted nymphs, NIP, the rate of submitted infected nymphs, and Lyme disease incidence decreased over time between 2007 and 2017 (Table 2; s < 1).
Table 2.
Temporal trends.
| Year (95% CI) | |
|---|---|
| Rate of submitted nymphs | 0.974 (0.968, 0.981) |
| Nymphal infection prevalence | 0.950 (0.936, 0.964) |
| rate of submitted infected nymphs | 0.924 (0.855, 0.999) |
| Lyme disease incidence | 0.972 (0.968, 0.976) |
While Lyme disease cases have increased overall in the United States (Centers for Disease Control and Prevention, 2015), other researchers have noted a downward trend in Lyme disease incidence in states previously classified as high incidence (Schwartz et al., 2017). Such downward trends may be due to reporting fatigue, human behavioral changes (e.g., improved prevention and control), decreasing tick densities, among other factors.
The observation that NIP decreased over time between 2007 and 2017 differs from reports where infection prevalence in field-collected nymphs (Diuk-Wasser et al., 2012; Feldman et al., 2015) and passively collected I. scapularis ticks (Xu et al., 2016; Jordan and Egizi, 2019) remain relatively stable over time. In contrast to endemic areas, in areas of emergence infection prevalence has been shown to increase over time (Nelder et al., 2014; Gasmi et al., 2016). The fluctuations in rates of submitted (infected) nymphs are in agreement with changes in tick densities and the density of infected ticks over time, which in turn may be due to changes in host populations and climatic conditions (Stafford et al., 1998; Wilson, 1998; Killilea et al., 2008). However, in a hyperendemic Lyme disease state such as Connecticut we cannot rule out the possibility that tick submissions to the CAES-TTL have declined due to waning public interest.
We note differences in Lyme disease incidence across counties in Connecticut. Lyme disease incidence was highest in Windham, Tolland, and New London counties and lowest in New Haven, Fairfield, and Hartford counties (Fig. 2). At the town scale, we found evidence of spatial clustering for Lyme disease incidence (Moran’s I: 0.547, z = 10.307, p < 0.001); specifically, we note high incidence towns at the intersection of Tolland, Windham and New London Counties and low incidence towns in southwestern Hartford and northeastern New Haven Counties (Fig. 3).
Fig. 3.
Lyme disease incidence. Cumulative (2007 2017) total Lyme disease incidence (per 100,000) broken into quartiles and mapped by town.
At the town scale, we found evidence of spatial clustering for the rate of submitted nymphs (Fig. 4; Moran’s I: 0.447, z = 8.776, p < 0.001), and the rate of submitted infected nymphs (Fig. 5; Moran’s I: 0.412, z = 7.997, p < 0.001). Indeed, the majority (81%) of submitted nymphs were from Fairfield and New Haven Counties (Fig. 2). There was little difference in NIP across towns (21.1%, 95%CI: 20.0%, 22.1%) or counties (21.0%, 95%CI: 19.4%, 22.5%) in Connecticut between 2007 and 2017 (Fig. 2) and NIP did not display spatial clustering (Fig. 6; Moran’s I: 0.07, z = 1.52, p = 0.13). NIP may be near uniform, at least at the spatial scale of counties or towns, in states or regions where I. scapularis is long established and ubiquitous (New York City Department of Health and Mental Hygiene, 2018). Of course, there is aggregation of estimates at the county and town levels. At smaller spatial scales, such as for individual households, there is likely a great deal of variability in tick-based risk measures (Ostfeld et al., 1996; Pardanani and Mather, 2004; Killilea et al., 2008). Interestingly the finding that NIP is relatively steady across Connecticut is different from previous study in Connecticut showing that before 1991 ticks infected with B. burgdorferi were concentrated to the coastline (Magnarelli et al., 1993), indicating a shift from emergent to endemic populations of I. scapularis. If it is true that NIP is fairly stable across the state within any year but changes over time, then repeated annual sampling in a few locations in an active tick surveillance program might provide sufficient information to quantify risk especially when resources are limited.
Fig. 4.
Rate of submitted nymphs. Cumulative (2007 2017) rate of submitted nymphs per 100,000 populations broken into quartiles and mapped by town.
Fig. 5.
Rate of submitted infected nymphs. Cumulative (2007 2017) rate of submitted infected nymphs per 100,000 population broken into quartiles and mapped by town.
Fig. 6.
Nymphal infection prevalence. Cumulative (2007 2017) nymphal infection prevalence broken into quartiles and mapped by town.
After accounting for population, we note higher Lyme disease incidence in more rural counties of Connecticut (as has been noted previously (Cromley et al., 1998)), such as Windham and Tolland, yet lower rates of submitted (infected) nymphs estimates that similarly account for population and similar NIP across counties (Fig. 2). Collectively, these findings suggest that human behavior is playing a large part in encounters with infected ticks and Lyme disease transmission risk (Nicholson and Mather, 1996). There may also be a need to better promote the CAES-TTL program in more rural parts of the state.
Future research should assess whether the rates of submitted nymphs are associated with the density of host-seeking nymphs. Furthermore, a comparison of infection prevalence in nymphal ticks collected from humans versus from the environment would be needed to determine if the trend for infection prevalence in nymphs removed from humans (in this case a decreasing trend) directly reflect that of nymphs in the environment, or if changes in human use of the landscape over time could have led to increased exposure to nymphs residing in microhabitats with lower tick density and less intense enzootic transmission of B. burgdorferi s.l., or if decreasing submission and case reports are simply explained by fatigue or reduced participation. Future studies should also explore whether passive (ticks on people) or active (drag sampling) surveillance provides better estimates of human disease risk. This comparison should also include a cost analysis to determine if any predictive improvement in active surveillance outweighs the added costs of these programs (Nelder et al., 2014). Finally, the findings that NIP decreases temporally between 2007 and 2017 but is geographically uniform, warrants further investigation.
3.6. Spatiotemporal modeling, 2007 2017
We found general declines in tick-based risk measures as well as Lyme disease incidence during the period 2007 2017. We also found divergent spatial patterns in the rates of submitted (infected) nymphs with those for Lyme disease incidence. We used a generalized linear mixed effects model to explicitly account for these spatiotemporal differences in tick-based risk measures and Lyme disease incidence to determine (1) if within each county (or town), there is a relationship between these tick-based risk measures and Lyme disease incidence and (2) if we can use these tick-based risk measures to predict Lyme disease for each county (or town).
At both the county and town spatial scales, we found that over the eleven years investigated an increase in the rate of submitted (infected) nymphs was predictive of increased Lyme disease incidence for each county (or town). Table 3 shows the coefficient estimates for each tick-based risk metric, the associated AIC score, and Spearman’s rank correlation coefficient for the model-predicted and observed Lyme disease incidence. Overall, we Und better model performance at the county compared to the town spatial scale. We note that the models with NIP are not significant, but that inclusion of NIP with the rate of submitted nymphs in the tick-based risk metric rate of submitted infected nymphs is an improvement over the predictive value of just the rate of submitted nymphs. Moreover the inclusion of the percent of developed land further explains variability in Lyme disease incidence and improves model fit. We conducted chi-squared tests to assess whether the inclusion of predictors led to statistically significant improvements in model fit as measured by a reduction in the residual sum of squares. Compared to a null model, the rate of submitted infected nymphs improved model performance ( 2 = 12.874, p < 0.001). Inclusion of the percent of developed land in the county model further improved model fit without influencing the effect estimate for the rate of submitted infected nymphs ( 2 = 15.599, p < 0.001). Of the models tested, the rate of submitted infected nymphs along with the percent of developed land as a covariate at the county scale provided the best model fit for predicting Lyme disease incidence as measured by AIC (AIC = 1267, Table 3).
Table 3.
Model results comparing tick-based risk metric predictive value.
| Model parameters | (95% CI) | AIC | |
|---|---|---|---|
| Town spatial scale (n = 1859) | |||
| Rate of submitted nymphs | 1.200 (1.180, 1.221) | 10,711 | 0.598 |
| Nymphal infection prevalence | 0.988 (0.969, 1.007) | 10,263 | 0.598 |
| Rate of submitted infected nymphs | 1.187 (1.166, 1.208) | 9970 | 0.595 |
| Rate of submitted nymphs + degree developed | 1.017 (0.999, 1.036) | 7271 | 0.724 |
| Nymphal infection prevalence + degree developed | 0.985 (0.966, 1.004) | 6762 | 0.720 |
| Rate of submitted infected nymphs + degree developed | 1.021 (1.002, 1.041) | 6760 | 0.720 |
| County spatial scale (n = 88) | |||
| Rate of submitted nymphs | 1.050 (1.015, 1.087) | 1304 | 0.946 |
| Nymphal infection prevalence | 0.998 (0.976, 1.020) | 1294 | 0.944 |
| Rate of submitted infected nymphs | 1.050 (1.022, 1.078) | 1281 | 0.945 |
| Rate of submitted nymphs + degree developed | 1.051 (1.016, 1.088) | 1290 | 0.946 |
| Nymphal infection prevalence + degree developed | 0.998 (0.976, 1.020) | 1281 | 0.944 |
| Rate of submitted infected nymphs + degree developed | 1.051 (1.023, 1.079) | 1267 | 0.945 |
Fitted model values (predicted values) were strongly and positively correlated with observed values of Lyme disease incidence at the county scale (Table 3, s range from 0.945 to 0.946, p < 0.001; Fig. 7, Full Model). This indicates a signal between the rate of submitted (infected) nymphs with Lyme disease incidence regardless of potential spatiotemporal biases in passive tick or Lyme disease surveillance.
Fig. 7.
Model fits. Relationship of observed Lyme disease cases (red dots) and model predictions of Lyme disease cases (blue line). Predictions based on best fitting model by AIC the model including the rate of submitted infected nymphs and the degree of developed land use at the county spatial scale. (For interpretation of the references to color in the text, the reader is referred to the web version of this article.)
3.7. Spatiotemporal model validation, 2007 2017
By conducting leave-one-out temporal and spatial cross validations (LOOTCV and LOOSCV, respectively), we found the full model (RMSE = 40.91) performed better than either the LOOTCV model (RMSE = 73.27) or the LOOSCV model (RMSE = 136.70) (Fig. 7). The lower RMSE for the LOOTCV suggests that out of sample predictions (i.e. model predictions of a set of observations different than those that the model was fitted on) is better year-to-year than county-to-county. Models trained on data from certain counties (such as counties with more observations) may provide better predictions than models trained on data from others.
3.8. Conclusion
While Lyme disease has been endemic in Connecticut for over three decades, disease occurrence is still spreading geographically in other parts of the Eastern United States (Eisen and Eisen, 2018). We can learn from this Connecticut based research and employ the results in emergent areas facing a growing threat of Lyme disease (Stone et al., 2017). Results from this longitudinal analysis in an endemic setting suggest that the rate of submitted infected nymphs are highly predictive of Lyme disease incidence for each town or county. These metrics could be calculated from other passive surveillance datasets in emergent areas, but their accuracy in predicting Lyme disease occurrence would need to be evaluated. There are some very important caveats to passive tick surveillance programs, which were well accounted for in this study but can be difficult to achieve: tick identification being done by trained individuals and exclusion of ticks acquired while traveling out of county or state.
The use of passive surveillance to build predictive models for public health decision-making is limited, as it has been asserted that passive surveillance data are biased (Beck et al., 2014). However, tick submissions through passive surveillance were shown to predict Lyme disease cases at a town level in an emergent region in Canada (Ripoche et al., 2018). Moreover, a predictive model for Lyme disease based on passive surveillance data was successfully validated using active surveillance data in Canada (Soucy et al., 2018).
In this study we analyzed an eleven-year record of passive surveillance data with 23,432 reported Lyme disease cases and 26,116 tick submissions and found a strong relationship between the rate of submitted infected nymphs with Lyme disease incidence for each county over time. Our findings underscore the relevance of using passive surveillance based on ticks recovered from humans to guide informed decisions concerning prevention and treatment of tick-borne diseases.
Total numbers of I. scapularis submitted and/or tested for B. burgdorferi s.l. by life stage (nymph and adult female) for each year 1996 2017.
Temporal trends of tick-based risk metrics (rate of submitted nymphs, nymphal infection prevalence, and rate of submitted infected nymphs) and Lyme disease incidence across Connecticut. Here we report the coefficient estimate ( ) for year. Values under 1 support a decrease in each tick-based risk metric and Lyme disease incidence over time.
Generalized linear mixed effect models (family = Poisson, link = log) with year and county as crossed random effects. For each set of model parameters tested we compare: the coefficient ( ) estimate for the tick-based risk metric is given along with the 95% confidence interval; AIC is the Akaike Information Criterion for the model, lower is better; and Spearman’s rank correlation coefficient ( ) for the model-predicted and observed Lyme disease incidence are given. The models were conducted at two spatial scales, town and county. There were 1859 observations at the town spatial scale (169 towns and 11 years); and 88 observations at the county spatial scale (8 counties and 11 years).
Acknowledgements
We are grateful to the former and current staff at the CAES-TTL, Bonnie Hamid, Elizabeth Alves, Brenda Zolla, Jodie Correia, Michael Vasil, Saryn Kunajukr and Alex Diaz, as well as numerous other seasonal research assistants for technical help. We extend our appreciation to Starr-Hope Ertel and other officials at the Connecticut Department of Public Health for sharing data on physician and laboratory reported LD cases and for their continuous support of the CAES-TTL. We would like to thank Mark Delorey of the Centers for Disease Control and Prevention for help with the statistics and modeling efforts. We would like to thank Brianna Byrne for help with literature review. This paper is dedicated to the memory of Dr. Louis A. Magnarelli, the former director of the CAES, who passed away in July 2013. He was an outstanding research scientist, who made significant contribution to our knowledge of ticks and tick-associated diseases.
Funding
The Tick Testing Laboratory is funded by the State of Connecticut. This publication was supported in part by the cooperative agreement Number, U01 CK000509, funded by the Centers for Disease Control and Prevention. Its content is solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or Department of Health and Human Services.
Abbreviations:
- AIC
Akaike Information Criterion
- CAES
Connecticut Agricultural Experiment Station
- CDPH
Connecticut Department of Public Health
- DIN
density of infected nymphs
- DON
density of nymphs
- GLMER
Generalized Linear Mixed Effects Model
- LOO
leave-one-out
- NIP
nymph infection prevalence
- NLCD
National Land Cover Database
- PCR
polymerase chain reaction
- RMSE
root mean square error
- TTL
Tick Testing Laboratory
- US
United States
Footnotes
Availability of data and materials
The datasets generated and/or analyzed during the current study are mostly available online at: http://www.ct.gov/caes/cwp/view.asp?a=2837&q=378212&caesNav=|. More detailed information is also available from the CAES TTL on reasonable request by contacting the Tick.Testing.Laboratory@ct.gov.
References
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J, 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173, 403 409. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S, 2014. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1 51, https://www.jstatsoft.org/article/view/v067i01/0. [Google Scholar]
- Beck J, Böller M, Erhardt A, Schwanghart W, 2014. Spatial bias in the GBIF database and its effect on modeling species geographic distributions. Eco. Inf. 19, 10 15, https://app.dimensions.ai/details/publication/pub.1032771948. [Google Scholar]
- Burgdorfer W, Barbour A, Hayes S, Benach J, Grunwaldt E, Davis J, 1982. Lyme disease-a tick-borne spirochetosis?. Science 216, 1317 1319. [DOI] [PubMed] [Google Scholar]
- Butler BJ, 2017. Forests of Connecticut, 2016. Resource Update FS-130. U.S. Department of Agriculture, Forest Service, Northern Research Station, Newtown Square, PA, 10.2737/FS-RU-130, 4 p.. [DOI] [Google Scholar]
- Centers for Disease Control and Prevention, 2015. How Many People Get Lyme Disease?, https://www.cdc.gov/lyme/stats/humancases.html (accessed 10 December 2018).
- Centers for Disease Control and Prevention, 2017. Reported Cases of Lyme Disease by State of Locality, 2006 2016, http://www.cdc.gov/lyme/stats/tables.html (accessed 10 December 2018).
- Clow KM, Finer R, Lumsden G, Jardine CM, 2018. Assessing the repeatability of tick dragging as a method for Ixodes scapularis surveillance. Vector Borne Zoonotic Dis. 18, 628 631. 10.1089/vbz.2018.2301. [DOI] [PubMed] [Google Scholar]
- Connally NP, Ginsberg HS, Mather TN, 2006. Assessing peridomestic entomological factors as predictors for Lyme disease. J. Vector Ecol. 31, 364 370, 10.3376/1081-1710(2006)31[364:APEFAP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cortinas R, Spomer SM, 2014. Occurrence and county-level distribution of ticks (Acari: Ixodoidea) in Nebraska using passive surveillance. J. Med. Entomol. 51, 352 359. 10.1603/ME13122. [DOI] [PubMed] [Google Scholar]
- Cromley EK, Cartter ML, Mrozinski RD, Ertel SH, 1998. Residential setting as a risk factor for Lyme disease in a hyperendemic region. Am. J. Epidemiol. 147, 472 477. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J, 1998. Reported distribution of Ixodes scapularis and Ixodes paciUcus (Acari: Ixodidae) in the United States. J. Med. Entomol. 35, 629 638. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc h G, Melton F, Hickling GJ, Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesman J, Fish D, 2012. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in Eastern United States. Am. J. Trop. Med. Hyg. 86, 320 327. 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden LA, Keirans JE, 1996. Nymphs of the Genus Ixodes (Acari: Ixodidae) of the United States: Taxonomy, Identification Key, Distribution, Hosts, and Medical/Veterinary Importance. Entomological Society of America. [Google Scholar]
- Eisenand Eisen, 2016. Eisen L, Eisen RJ, Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases, J. Med. Entomol. 53 (2016) 1050 1062, 10.1093/jme/tjw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 34, 295 309. 10.1016/j.pt.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB, 2016. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 53, 349 386. 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel SH, Nelson RS, Cartter ML, 2012. Effect of surveillance method on reported characteristics of Lyme disease, Connecticut, 1996 2007. Emerg. Infect. Dis. 18, 242. 10.3201/eid1802.101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI, 2011. ArcGIS Desktop: Release 10. Environmental Systems Research Institute, Redlands, CA. [Google Scholar]
- Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, Wormser GP, 1999. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am. J. Epidemiol. 149, 771 776. [DOI] [PubMed] [Google Scholar]
- Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, Hinckley AF, 2015. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J. Vector Ecol. 40, 198 201. 10.1111/jvec.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi S, Ogden NH, Leighton PA, Lindsay RL, Thivierge K, 2016. Analysis of the human population bitten by Ixodes scapularis ticks in Quebec, Canada: increasing risk of Lyme disease. Ticks Tick Borne Dis. 7, 1075 1081. 10.1016/j.ttbdis.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Gasmi S, Ogden NH, Ripoche M, Leighton PA, Lindsay RL, Nelder MP, Rees E, Bouchard C, Vrbova L, Rusk R, Russell C, 2019. Detection of municipalities at-risk of Lyme disease using passive surveillance of Ixodes scapularis as an early signal: a province-specific indicator in Canada. PLoS One 14 (2), e0212637. 10.1371/journal.pone.0212637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazumyan A, Schwartz JJ, Liveris D, Schwartz I, 1994. Sequence analysis of the ribosomal RNA operon of the Lyme disease spirochete, Borrelia burgdorferi. Gene 146, 57 65. [DOI] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS, 2014. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect Dis. 59, 676 681. 10.1093/infdis/jiv775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer C, Dewitz J, Yang L, Jin S, Danielson P, Xian G, Coulston J, Herold N, Wickham J, Megown K, 2015. Completion of the 2011 national land cover database for the conterminous United States representing a decade of land cover change information. Photo. Eng. Remote Sens. Off. J. Am. Soc. Photogram. Remote Sens. 81, 345 354. 10.14358/PERS.81.5.345. [DOI] [Google Scholar]
- Johnson JL, Ginsberg HS, Zhioua E, Whitworth UG, Markowski D, Hyland KE, Hu R, 2004. Passive tick surveillance, dog seropositivity, and incidence of human Lyme disease. Vector Borne Zoonotic Dis. 4, 137 142. 10.1089/1530366041210710. [DOI] [PubMed] [Google Scholar]
- Jordan R, Egizi A, 2019. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006 2016. PLoS One 14 (2), e0211778. 10.1371/journal.pone.0211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR, 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol. 26, 435 448. 10.1093/jmedent/26.5.435. [DOI] [PubMed] [Google Scholar]
- Killilea ME, Swei A, Lane RS, Briggs CJ, Ostfeld RS, 2008. Spatial dynamics of Lyme disease: a review. EcoHealth 5, 167 195. 10.1007/s10393-0080171-3. [DOI] [PubMed] [Google Scholar]
- Koffi JK, Leighton PA, Pelcat Y, Trudel L, Lindsay LR, Milord F, Ogden NH, 2012. Passive surveillance for I. scapularis ticks: enhanced analysis for early detection of emerging Lyme disease risk. J. Med. Entomol. 49, 400 409. 10.1603/ME11210. [DOI] [PubMed] [Google Scholar]
- Magnarelli LA, Anderson JF, Cartter ML, 1993. Geographic distribution of white-tailed deer with ticks and antibodies to Borrelia burgdorferi in Connecticut, Yale. J. Biol. Med. 66, 19. [PMC free article] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT, 1996. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol. 144, 1066 1069. [DOI] [PubMed] [Google Scholar]
- Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J, 1991. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am. J. Epidemiol. 133, 1105 1113. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Voss- brinck CR, 2006. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, Northeastern United States. Emerg. Infect. Dis. 12, 468 474. 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder MP, Russell C, Lindsay LR, Dhar B, Patel SN, Johnson S, Moore S, Kristjanson E, Li Y, Ralevski F, 2014. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS One 9, e105358. 10.1371/journal.pone.0105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS, 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005 2010. Emerg. Infect. Dis. 21, 1625 1631. 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York City Department of Health and Mental Hygiene, 2018. 2017 DOHMH advisory 14: Tick-borne Disease Advisory, https://www1.nyc.gov/assets/doh/downloads/pdf/han/advisory/tick-borne-disease-advisory14.pdf.
- Nicholson MC, Mather TN, 1996. Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysis. J. Med. Entomol. 33, 711 720. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Trudel L, Artsob H, Barker IK, Beauchamp G, Charron DF, Drebot MA, Galloway TD, O handley R, Thompson RA, Lindsay LR, 2006. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J. Med. Entomol. 43, 600 609. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Bouchard C, Kurtenbach K, Margos G, Lindsay LR, Trudel L, Nguon S, Milord F, 2010. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ. Health Perspect. 118, 909 914. 10.1289/ehp.0901766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld RS, Hazler KR, Cepeda OM, 1996. Temporal and spatial dynamics of Ixodes scapularis (Acari: Ixodidae) in a rural landscape. J. Med. Entomol. 33, 90 95. [DOI] [PubMed] [Google Scholar]
- Pardanani N, Mather TN, 2004. Lack of spatial autocorrelation in Une-scale distributions of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 41, 861 864. 10.1603/0022-2585-41.5.861. [DOI] [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA, 2012. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am. J. Trop. Med. Hyg. 86, 1062 1071. 10.4269/ajtmh.2012.11-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D, Telford S, Spielman A, Barthold S, 1990. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction. J. Clin. Microbiol. 28, 566 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinski M, Kokas J, Hukey K, Kogut S, Lee J, Backenson P, 2014. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J. Med. Entomol. 51, 226 236. 10.1603/ME13101. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment For Statistical Computing, http://www.r-project.org/.
- Rand PW, Lacombe EH, Dearborn R, Cahill B, Elias S, Lubelczyk CB, Beckett GA, Smith RP Jr., 2007. Passive surveillance in Maine, an area emergent for tick-borne diseases. J. Med. Entomol. 44, 1118 1129. 10.1093/jmedent/44.6.1118. [DOI] [PubMed] [Google Scholar]
- Ripoche M, Gasmi S, Adam-Poupart A, Koffi JK, Lindsay LR, Ludwig A, Milord F, Ogden NH, Thivierge K, Leighton PA, 2018. Passive tick surveillance provides an accurate early signal of emerging Lyme disease risk and human cases in Southern Canada. J. Med. Entomol. 55, 1016 1026. 10.1093/jme/tjy030. [DOI] [PubMed] [Google Scholar]
- Rossi C, Stromdahl E, Rohrbeck P, Olsen C, DeFraites R, 2015. Characterizing the relationship between tick bites and Lyme disease in active component us armed forces in the Eastern United States. MSMR 22, 2 10. [PubMed] [Google Scholar]
- Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ, 2017. Surveillance for Lyme disease United States, 2008 2015. MMWR Surveill. Summ. 66, 1 12. 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton TJ, 2015. Passive Tick Surveillance for Ixodes scapularis and the Incidence of Lyme Disease in Connecticut. Master’s thesis. The University of Connecticut, Storrs, https://opencommons.uconn.edu/gs_theses/719/. [Google Scholar]
- Soucy JPR, Slatculescu AM, Nyiraneza C, Ogden NH, Leighton PA, Kerr JT, Kulkarni MA, 2018. High-resolution ecological niche modeling of Ixodes scapularis ticks based on passive surveillance data at the northern frontier of Lyme disease emergence in North America. Vector Borne Zoonotic Dis. 18, 235 242. 10.1089/vbz.2017.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StaXord KC, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA, 1998. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J. Clin. Microbiol. 36, 1240 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM, 1977. An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheumat.: OX. J. Am. Coll. Rheumatol. 20, 7 17. [DOI] [PubMed] [Google Scholar]
- Stone BL, Tourand Y, Brissette CA, 2017. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis. 17, 619 629. 10.1089/vbz.2017.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl EY, Evans SR, O Brien JJ, Gutierrez AG, 2001. Prevalence of infection in ticks submitted to the human tick test kit program of the U.S. Army Center for Health Promotion and Preventive Medicine. J. Med. Entomol. 38, 67 74. 10.1603/0022-2585-38.1.67. [DOI] [PubMed] [Google Scholar]
- The Community Health Foundation, 2007. Community Health Data Scan for Connecticut Executive Summary, http://www.ct.gov/oha/lib/oha/equitycommission/documents/execsummary.pdf.
- U.S. Census Bureau, 2017. U.S. Census Bureau, https://www.census.gov/.
- Waddell LA, Greig J, Mascarenhas M, Harding S, Lindsay R, Ogden N, 2016. The accuracy of diagnostic tests for Lyme disease in humans, a systematic review and meta-analysis of North American research. PLoS One 11, e0168613, 10.1371/journal.pone.0168613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller LA, Goodwin BJ, Wilson ML, Ostfeld RS, Marshall SL, Hayes EB, 2007. Spatio-temporal patterns in county-level incidence and reporting of Lyme disease in the Northeastern United States, 1990 2000. Env. Ecol. Stat. 14, 83. 10.1007/s10651-006-0002-z. [DOI] [Google Scholar]
- Wharton EH, Widmann RH, Alerich CL, Barnett CJ, Lister AJ, Lister TW, Smith D, Borman F, 2004. The Forests of Connecticut, https://www.fs.fed.us/ne/newtown_square/publications/resource_bulletins/pdfs/2004/ne_rb160.pdf. [Google Scholar]
- Williams SC, StaXord KC III, Molaei G, Linske MA, 2018. Integrated control of nymphal Ixodes scapularis: effectiveness of white-tailed deer reduction, the entomopathogenic fungus Metarhizium anisopliae, and fipronil-based rodent bait boxes. Vector Borne Zoonotic Dis. 18, 55 64. 10.1089/vbz.2017.2146. [DOI] [PubMed] [Google Scholar]
- Wilson ML, 1998. Distribution and abundance of Ixodes scapularis (Acari: Ixodidae) in North America: ecological processes and spatial analysis. J. Med. Entomol. 35, 446 457. 10.1093/jmedent/35.4.446. [DOI] [PubMed] [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, Rich SM, 2016. Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vector Borne Zoonotic Dis. 16, 520 527. 10.1089/vbz.2015.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Pearson P, Dykstra E, Andrews ES, Rich SM, 2018. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 10.1089/vbz.2018.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]