Abstract
The animal trypanosomiases are infections in a wide range of (domesticated) animals with any species of African trypanosome, such as Trypanosoma brucei, T. evansi, T. congolense, T. equiperdum and T. vivax. Symptoms differ between host and infective species and stage of infection and are treated with a small set of decades-old trypanocides. A complication is that not all trypanosome species are equally sensitive to all drugs and the reasons are at best partially understood. Here, we investigate whether drug transporters, mostly identified in T. b. brucei, determine the different drug sensitivities. We report that homologues of the aminopurine transporter TbAT1 and the aquaporin TbAQP2 are absent in T. congolense, while their introduction greatly sensitises this species to diamidine (pentamidine, diminazene) and melaminophenyl (melarsomine) drugs. Accumulation of these drugs in the transgenic lines was much more rapid. T. congolense is also inherently less sensitive to suramin than T. brucei, despite accumulating it faster. Expression of a proposed suramin transporter, located in T. brucei lysosomes, in T. congolense, did not alter its suramin sensitivity. We conclude that for several of the most important classes of trypanocides the presence of specific transporters, rather than drug targets, is the determining factor of drug efficacy.
Keywords: Trypanosoma congolense, Trypanosoma evansi, cymelarsan, pentamidine, suramin, diminazene, drug transporter, TbAT1, TbAQP2, MFST
1. Introduction
Animal African trypanosomiasis (AAT) is a diverse complex of diseases caused by African trypanosome species, including (but not limited to) Trypanosoma brucei brucei, T. congolense, T. vivax, T. evansi and T. equiperdum [1]. In sub-Saharan Africa, animal trypanosomiasis is mostly transmitted by tsetse flies and the etiological agents are mostly T. b. brucei, T. b. rhodesiense (Subgenus Trypanozoon), T. vivax (Subgenus Duttonella) and T. congolense (Subgenus Nannomonas). Trypanosomiasis has a severe impact on livestock rearing on the continent, with infections in ruminants, equines, pigs and dogs being common. Outside the tsetse belt, AAT is caused by T. vivax and T. evansi, which can be transmitted by other biting insects, as well as by T. equiperdum, which is sexually transmitted between horses, giving rise to a wasting disease called dourine [2]. T. vivax is a significant pathogen for a broad range of animals in South America and Africa [3,4,5] but has only very recently been reported for the first time in Asia [6]. In contrast, T. evansi has long been known to be an important veterinary infection and of all the pathogenic Trypanosoma spp. has the widest geographical distribution, from Africa to South America, Asia, Indonesia and the Philippines [7,8,9,10].
The total amount of damage done by AAT to (often developing) economies and to agricultural production is hard to tally up, but the yearly cost of bovine trypanosomiasis in Africa alone has been estimated as approximately US$ 5 billion [11]. Control of this disease spectrum is very difficult, considering the diversity of transmission modes and vectors, the enormous geographical spread, the lack of any vaccine and the challenges of diagnosis. In practice, chemotherapeutic treatment with veterinary trypanocides is usually the only option available but this has many challenges, also. All the available drugs are decades old, resistance to all drugs has been reported, the different species have different levels of sensitivity to the various drugs, several drugs are poorly tolerated by some of the important host species, some drugs, such as quinapyramine, induce cross-resistance to other drugs, and availability and quality can be poor [1]. Here we will focus on the innate differences in drug sensitivity of different AAT-causing trypanosome species, particularly Trypanozoon subgenus species T. b. brucei and T. evansi and Nannomonas species T. congolense. We recently highlighted that T. congolense is far less susceptible than T. b. brucei to the diamidine trypanocides diminazene and pentamidine and suggested that differences in drug accumulation may be responsible for this important difference. Suramin, used since 1920 for East African trypanosomiasis [12], is often the drug of choice to treat T. evansi infections in camels, known as surra, although melarsen oxide cysteamine (melarsomine, Cymelarsan, MelCy), introduced in the mid-1980s, is an efficacious alternative [13]. However, melarsomine and suramin are not considered effective against animal trypanosomiasis in sub-Saharan Africa [1]. Indeed, T. congolense and T. vivax are both believed not to be very susceptible to suramin in vivo [14], but little evidence can be found in the literature.
The action of anti-trypanosomiasis drugs has been predominantly investigated in T. b. brucei, which has always been considered a convenient model organism. For most of the traditional trypanocides, the activity and, frequently, the selectivity over human cells depends on the efficient uptake of the drugs by the parasite [15]. Pentamidine is taken up by the TbAT1/P2 aminopurine transporter [16,17] as well as by two additional transporters, initially named high affinity pentamidine transporter (HAPT1) and low affinity pentamidine transporter (LAPT1) [18]. HAPT1 was subsequently identified as an aquaglyceroporin, TbAQP2 [19,20,21], but the identity of the low-affinity carrier has not been elucidated. Diminazene, while a diamidine like pentamidine, is taken up solely by the TbAT1/P2 aminopurine transporter [22], as is the furamidine class of diamidines [23]. The lack of passage through TbAQP2 is explained by the short, inflexible linkers of diminazene and furamidine [20]. In contrast, the melaminophenyl arsenicals, melarsoprol and the derived veterinary product melarsomine, have been shown to be internalised by both TbAT1/P2 [24,25] and HAPT1/TbAQP2 [18,20,21]. The activity of eflornithine (α-difluoromethyl ornithine) is likewise dependent on a T. brucei carrier, amino acid transporter TbAAT6 [26,27,28]. Similarly, suramin is taken up specifically into T. b. brucei by receptor-mediated endocytosis, after binding to cell surface receptors, predominantly ISG75 [29,30], and isometamidium is efficiently taken up into trypanosomes by an as yet unidentified transporter [31,32]. Only the nitro compounds nifurtimox and fexinidazole are believed to enter trypanosomes by simple diffusion, although this likely only signifies that no serious work has as yet been done on the uptake of such compounds [33]. Interactions of nifurtimox with transporters at the blood–brain barrier have been reported [34,35] and a point mutation in a T. brucei transporter, Tb9.211.2900, has been reported in both nifurtimox-resistant and fexinidazole-resistant cells [36].
In view of the important role transporters have in determining the sensitivity of T. brucei to the current trypanocidal drugs, we here explore whether expression of genes involved in drug uptake in brucei-group trypanosomes would sensitise T. congolense to these compounds.
2. Results and Discussion
2.1. Sensitivity of Different Trypanosoma Species to Standard Trypanocides
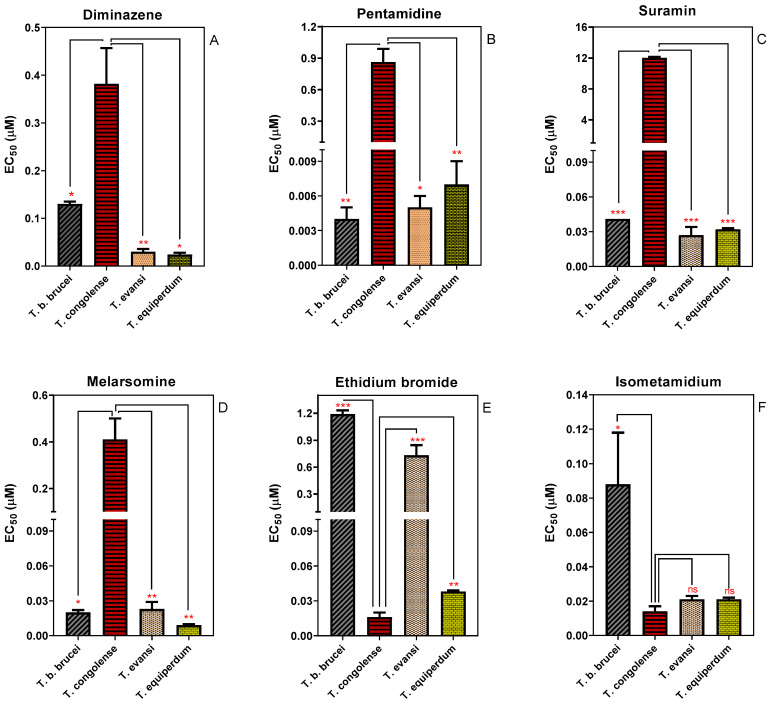
The Alamar Blue (resazurin) assay has been used to characterise the drug sensitivity profile of different Trypanosoma species [37,38,39,40]. We used the assay to determine and compare the in vitro sensitivity of four species of animal trypanosomes (Figure 1). T. congolense displayed a significantly (p ˂ 0.05) lower sensitivity to diminazene aceturate, pentamidine, melarsomine and suramin than any of the brucei group (Trypanozoon) trypanosomes, T. b. brucei, T. evansi and T. equiperdum. Specifically, the EC50 values of diminazene aceturate (a diamidine) and melarsomine (a melaminophenyl arsenical) in T. congolense were 3- and 20-fold higher than in T. brucei, respectively. Moreover, T. congolense was 200 times less sensitive to pentamidine and 296 times more resistant to suramin relative to T. brucei (Figure 1). However, T. congolense was significantly more sensitive to the phenanthridine compound ethidium bromide compared to the other species investigated here. Although T. congolense also trended towards higher sensitivity to the other phenanthridine compound, isometamidium, this did not reach statistical significance when compared with T. equiperdum and T. evansi. It has been widely reported that in brucei group trypanosomes drug sensitivity and resistance is often linked to the presence or absence of particular transport proteins, such as TbAT1, TbAQP2, TbMFST and TbAAT6, which mediate the uptake of diamidines and melaminophenyl arsenicals (TbAT1 and TbAQP2), suramin (TbMFST) and eflornithine (TbAAT6), respectively [26,29,41]. However, the now well-established models of drug transport and resistance do not seem to apply to all African trypanosome species, as recently shown for diminazene uptake and resistance in T. congolense [42]. We here investigate whether the relatively low sensitivity of T. congolense for diamidines, melaminophenyl arsenicals and suramin is linked to the absence of orthologues of TbAT1, TbAQP2 and TbMFST.
Figure 1.
Sensitivity of four species of animal trypanosomes to trypanocides. (A) Diminazene; (B) Pentamidine; (C) Suramin; (D) Cymelarsan; (E) Ethidium bromide; and (F) Isometamidium. Sensitivity is represented as EC50 averages of 3–5 independent determinations (mean ± SEM). * p < 0.05; ** p < 0.01; *** p < 0.001 by unpaired Student’s t-test.
As shown in Table 1, such orthologous proteins can readily be identified in T. evansi and T. equiperdum (species that are highly sensitive to these drugs (Figure 1)) but not in T. congolense. The TbAT1 orthologue in T. evansi was first amplified by Witola et al. [43] and is 99.35% identical to that of T. brucei (GenBank accession number AGT37292.1). BLAST searches using TbAT1 sequences in T. equiperdum genome found a gene (Genbank SCU70586.1) with approximately 99.8% identity. T. equiperdum AT1 has previously shown similar functional characteristics to TbAT1 [38,44]. BLAST searches with TbAQP2 found an orthologue with 100% identity in T. evansi, TevSTIB805.10.14910, annotated as TevAQP9. An identical AQP2 sequence was also found in T. equiperdum strain STIB818 [45].
Table 1.
Orthologues of T. b. brucei drug transporters, showing % identity by amino acid sequence.
| Species | Gene ID | T. brucei | ||
|---|---|---|---|---|
| AT1 | ||||
| Tb927.5.286b | ||||
| T. evansi | AGT37292.1 | 99.35 | ||
| T. equiperdum | SCU70586.1 | 99.78 | ||
| T. congolense | - | <75 | ||
| AQP2 | ||||
| Tb927.10.14170 | ||||
| T. evansi | TevSTIB805.10.14910 | 100 | ||
| T. equiperdum | not annotated | 100 1 | ||
| T. congolense | - | <75 | ||
| MFST | ||||
| Tb927.9.6360 2 | Tb927.9.6370 | Tb927.9.6380 | ||
| T. evansi | TevSTIB805.9.4540 | 99.58 | 89.15 | 96.87 |
| TevSTIB805.9.4550 | 89.79 | 98.30 | 90.43 | |
| TevSTIB805.9.4560 | 96.24 | 88.72 | 98.96 | |
| T. equiperdum | RHW70022.1 | 96.24 | 88.72 | 98.96 |
| RHW70658.1 | 88.30 | 97.23 | 90.21 | |
| RHW70215.1 | 96.45 | 88.94 | 99.16 | |
| T. congolense | - | <75 | <75 | <75 |
1 P. Buscher and N. van Reet, personal communication; 2 Gene ID identified by Alsford et al. [28] as being associated with suramin resistance after RNAi knockdown.
The T. brucei lysosomal MFST transporters exist as an array of three non-identical tandem copies on chromosome 9, each of which have protein orthologues in both T. evansi and T. equiperdum with at least 96% amino acid sequence identity (Table 1). However, no orthologue of TbAT1, TbAQP2 or TbMFST, or indeed any gene with >75% nucleotide sequence identity to either of them, was found in the T. congolense genome (nor in the T. vivax genome). We tested the hypothesis that the lack of these transporters renders T. congolense, at least, less susceptible to diamidines, melaminophenyl arsenicals and suramin by expressing each of these transporters separately in lab-adapted strain TcoIL3000.
2.2. Cloning of T. brucei Drug Transporters and Their Expression in T. congolense
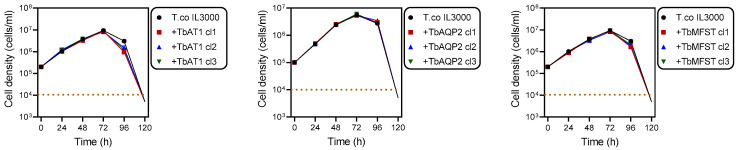
TbAT1, TbAQP2 or TbMFST were each amplified from T. brucei genomic DNA, purified and separately ligated into a digested pMPB-DP-012 plasmid or its SalI site-mutated derivative. Primers for the amplification of TbAT1 and TbMFST were designed to eliminate the stop codon and fuse the ORF to a C-terminal 6x haemagglutinin (HA) tag. The constructed plasmid was linearised with Asc1 and transfected into T. congolense and its correct integration verified by PCR. Positive clones were selected by adding 0.5 μg/mL blasticidin to the growth medium and characterised using drug sensitivity and uptake assays. From the blasticidin selection medium, multiple clonal lines were generated, and for each construct three clonal lines were used for subsequent experiments. The expression of these transporters had no effect on growth under standard in vitro conditions (Figure 2).
Figure 2.
Growth curves of T. congolense IL3000 and the same strain expressing TbAT1, TbAQP2 or TbMFST. The dotted line indicates the detection limit for trypanosome counting by haemocytometer counting.
2.3. Uptake of Drugs in Different Trypanosome Species—Influence of TbAT1 and TbAQP2
2.3.1. Pentamidine
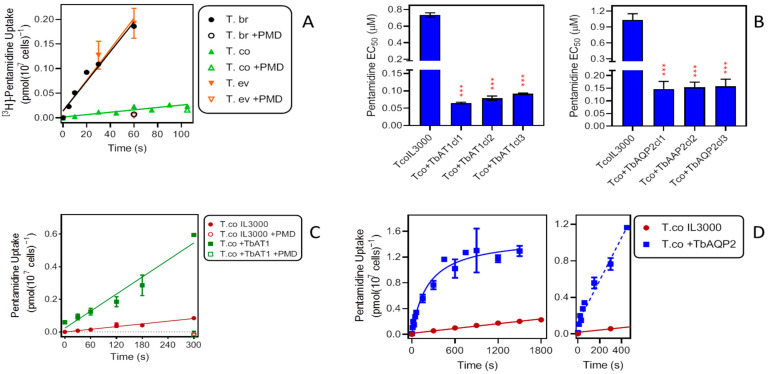
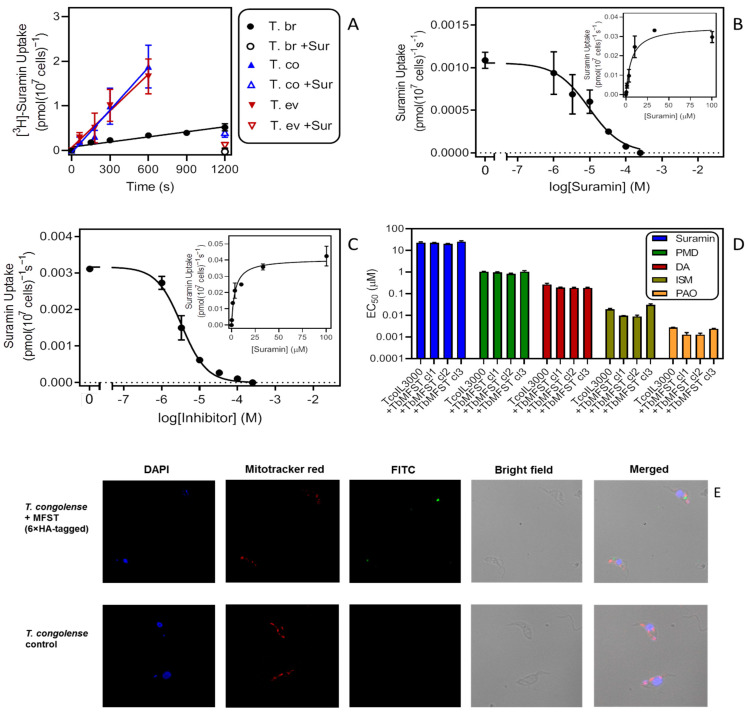
We have previously reported on the rate of pentamidine uptake in T. b. brucei. In T. b. brucei bloodstream forms isolated from infected Wistar rats, a rate of 0.0045 ± 0.0004 pmol(107 cells)−1s−1 was obtained for 25 nM [3H]-pentamidine [46]. Here, we found an average value of 0.0030 ± 0.0003 pmol(107 cells)−1s−1 (n = 4; Figure 3A) for the same strain and radiolabel concentration—slightly lower because the TbAT1/P2 transporter is more highly expressed in ex vivo cells [23]. These rates were compared with those observed in cultured bloodstream forms of T. evansi (0.0031 ± 0.0004 pmol(107 cells)−1s−1; n = 3) and T. congolense (0.00024 ± 0.00004 pmol(107 cells)−1s−1; n = 4), performed in parallel (Figure 3A). Whereas pentamidine uptake by T. evansi was not significantly different from that in T. b. brucei (p = 0.76; F-test comparing linear regression lines; Prism 9), the rate displayed by T. congolense was more than an order of magnitude lower (p < 0.0001).
Figure 3.
Effects of TbAT1 and TbAQP2 on pentamidine (PMD) uptake and sensitivity in T. congolense. (A) Uptake of 0.025 µM [3H]-pentamidine by bloodstream forms of T. b. brucei (n = 4), T. evansi (n = 3) and T. congolense (n = 4). Data shown are the average of three or four experiments combined; each experiment was performed in triplicate; lines are linear regressions calculated by Prism 9 (r2 = 0.97, 0.97 and 0.85, respectively), all with slope significantly non-zero (F test, p < 0.05) and not significantly non-linear (runs test, p > 0.3). +PMD, addition of 1 mM unlabelled pentamidine to the assay buffer. (B) Effects of expression of TbAT1 (left panel) or TbAQP2 (right panel) in T. congolense IL3000 (TcoIL3300) on sensitivity to pentamidine (PMD). Bars are the average ± SEM of three independent determinations, for three different clonal populations (cl1–cl3), arising from different transfections with the gene of interest. NS, not significantly different from untransfected control; *** p < 0.0001 by unpaired t-test. (C) Uptake of 0.025 µM [3H]-pentamidine by bloodstream forms of T. congolense IL3000 (untransfected and expressing TbAT1). The data shown are the average ± SEM of two experiments, each performed in triplicate. Lines were calculated by linear regression (r2 = 0.97 and 0.96, resp.) and not significantly non-linear (p > 0.3); slopes were significantly non-zero (p < 0.001). +PMD, addition of 1 mM unlabelled pentamidine to the assay buffer. (D) Uptake of 0.025 µM [3H]-pentamidine by bloodstream forms of T. congolense IL3000 (untransfected and expressing TbAQP2). The data shown are the average ± SEM of four experiments each performed in triplicate. Lines were calculated by linear regression (IL3000, r2 = 0.98) and non-linear regression (+TbAQP2). For the +TbAQP2 uptake, a linear regression was also performed over the interval 0–450 s (dashed line, r2 = 0.97, slope significantly non-zero (p < 0.0001), not significantly non-linear (p = 0.5).
Upon expression of TbAQP2 or TbAT1 in T. congolense IL3000, sensitivity to pentamidine was highly significantly increased in each of three independent clones. For +TbAT1 cells, the EC50 changed from 0.73 ± 0.03 µM in IL3000 control cells to an average of 0.078 ± 0.008 µM (p < 0.001). In a separate series of experiments the expression of TbAQP2 similarly reduced the EC50 from 1.03 ± 0.12 µM to 0.15 ± 0.003 µM (p < 0.001) (Figure 3B). Transport of 0.025 µM [3H]-pentamidine into the transgenic T. congolense was much increased. Transport in the +TbAT1 cells increased 6.3-fold, from 0.00028 ± 0.00003 pmol(107 cells)−1s−1 to 0.0017 ± 0.0002 pmol(107 cells)−1s−1 (p <0.0001) (Figure 3C). Pentamidine uptake was similarly increased upon expression of TbAQP2 in IL3000. Figure 3D shows an initial linear phase of uptake followed by a plateau after 450 s. Using linear regression over that initial phase to compare uptake of [3H]-pentamidine in control cells, it was found to be increased over 18-fold (0.0023 ± 0.0002 versus 0.00012 ± 0.00001 pmol(107 cells)−1s−1; p < 0.0001). It therefore appears that the rate of influx of pentamidine into T. congolense limits this parasite’s sensitivity to the drug and that expression of the known T. brucei pentamidine transporters both increases the uptake rate and the sensitivity to pentamidine. With either drug transporter expressed in T. congolense IL3000, both parameters remained somewhat below the observed level for T. brucei bloodstream forms, and it may be that full sensitivity would require the expression of both at the same time.
2.3.2. Diminazene Aceturate (DA)
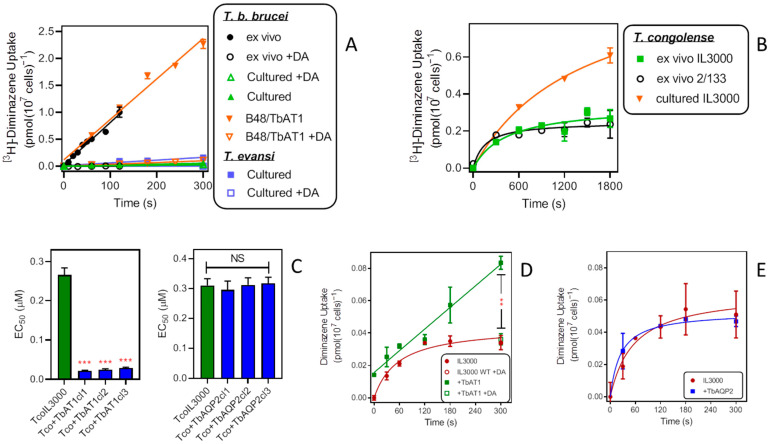
It has been previously established that uptake of diminazene is essentially mediated by TbAT1/P2 only in T. brucei bloodstream forms [22,39], T. evansi [43] and T. equiperdum [38]. However, the issues with the variable expression levels of TbAT1 [23] complicate a quantitative comparison between AAT trypanosome species. This is illustrated in Figure 4A: [3H]-diminazene uptake in wild-type T. b. brucei freshly isolated from a rat was rapid (0.0079 ± 0.0004 pmol(107 cells)−1s−1) and virtually identical to uptake in a T. b. brucei cell line stably expressing TbAT1 from vector pHD1336 [47], B48/TbAT1 (0.0075 ± 0.0006 pmol(107 cells)−1s−1; p = 0.71). In contrast, the same wild-type T. b. brucei grown in culture took up diminazene very poorly (0.00018 ± 0.00001 pmol(107 cells)−1s−1; p < 0.0001). In cultured T. evansi bloodstream, to the extent that a quantitative comparison is meaningful, the rate was similar though somewhat above the cultured T. b. brucei (0.00052 ± 0.00006 pmol(107 cells)−1s−1; p = 0.0039) (Figure 4A). T. congolense is known not to have a functional homolog of the TbAT1 transporter, with the gene known as TcoAT1 encoding a P1-type purine nucleoside transporter instead, the orthologue of TbNT10 [47]. Figure 4B shows that, in contrast to T. b. brucei, diminazene uptake from freshly isolated strains is not higher but in fact generally trends lower than with cultured IL3000 bloodstream forms. We have recently reported that [3H]-diminazene uptake in T. congolense is slow and low affinity, and that linearity of uptake is hard to achieve [42]. It is clear that under comparable conditions (ex vivo, in vitro culture) [3H]-diminazene uptake in T. b. brucei is much more robust than in T. congolense.
Figure 4.
Effects of TbAT1 and TbAQP2 on diminazene aceturate (DA) uptake and sensitivity by T. congolense. (A) Uptake of 0.1 µM [3H]-diminazene (DA) in cultured and ex vivo T. brucei and cultured T. evansi bloodstream forms. All lines were calculated by linear regression with r2 > 0.95. +DA indicates the addition of 1 mM DA to the assay buffer. In the absence of excess DA all slopes were significantly different from zero (p < 0.01) and not significantly non-linear (p > 0.3). All symbols are average ± SEM of triplicate determinations. (B) Uptake of 0.1 µM [3H]-DA in cultured and ex vivo T. congolense. Lines were calculated by non-linear regression. All symbols are averages ± SEM of triplicate determinations. (C) Sensitivity of T. congolense IL3000 (green bars) and TcoIL3000 expressing either TbAT1 or TbAQP2 to DA. Bars show averages ± SEM of three independent EC50 determinations. *** p < 0.001 by t-test; NS, not significant. (D) Uptake of 0.2 µM [3H]-DA in cultured IL3000 or the same cells expressing TbAT1, lines being calculated by non-linear and linear regression (r2 = 0.98), respectively. All symbols are averages ± SEM of triplicate determinations. ** p < 0.01. (E) Like D but with IL3000 expressing TbAQP2.
Expression of TbAT1 in T. congolense greatly increased sensitivity to diminazene in three independent clonal lines (p < 0.001), whereas the expression of TbAQP2 had no effect on diminazene sensitivity (Figure 4C). Uptake of 0.2 µM [3H]-diminazene was linear in +TbAT1 cells, in contrast to the quick plateauing consistently seen in untransfected IL3000 control cells, and was significantly increased by 300 s (p < 0.01) (Figure 4D). In the presence of 250 µM unlabelled diminazene aceturate, uptake in the +TbAT1 cells was identical in both cell lines, showing that the TbAT1-mediated flux was saturated by this ligand concentration but the endogenous T. congolense uptake system was not. These observations are consistent with previous observations of diminazene uptake in T. brucei and T. congolense [22,42]. In IL3000 cells expressing TbAQP2 there was no change in the rate of diminazene uptake (Figure 4E).
2.3.3. Suramin
The uptake of suramin, a large molecule that carries six negative charges, across the plasma membrane has long been speculated to involve endocytosis rather than a trans-membrane transporter [29,48]. Recently, it was described that suramin uptake involves binding to the invariant surface protein ISG75, followed by endocytic delivery to the lysosome [28,30]. We have also shown that reduced endocytic activity in T. brucei is associated with a similar reduction in the uptake of suramin but not of pentamidine [20].
Here, we monitored 0.2 µM [3H]-suramin uptake by bloodstream forms of T. b. brucei, T. evansi and T. congolense for a side-by-side evaluation. The result with T. b. brucei was consistent with our earlier report showing linear uptake of suramin over 20 min [30], with a rate of 0.00038 ± 0.00005 pmol(107 cells)−1s−1 (n = 4) (Figure 5A). Considering the insensitivity of T. congolense to suramin, we were surprised to find that this species accumulates suramin almost ten times faster than T. brucei, at 0.0032 ± 0.0003 pmol(107 cells)−1s−1 (p < 0.0001; n = 4). T. evansi displayed a rate (0.0027 ± 0.0002 pmol(107 cells)−1s−1, n = 3) that was not significantly different from the rate of T. congolense (p = 0.26). For each of the species, 0.2 µM suramin uptake was strongly inhibited by 100 µM unlabelled suramin, showing that the binding was specific and saturable.
Figure 5.
An investigation of suramin uptake and sensitivity in animal trypanosomes. (A) Uptake of 0.2 µM [3H]-suramin by bloodstream forms of T. b. brucei, T. evansi and T. congolense. The data shown are the averaged results (±SEM) of four (Tbb, Tco) or three (Tev) independent experiments each performed in triplicate. Lines were calculated using linear regression (Prism 9), yielding r2 of 0.93 (Tbb), 0.98 (Tev) and 0.97 (Tco). The lines were not significantly non-linear (runs test, p > 0.3) and the slopes significantly non-zero (p < 0.01). +Sur, addition of 100 µM unlabelled suramin in the assay buffer. (B) Uptake of 0.2 µM [3H]-suramin by T. b. brucei in the presence or absence of variable concentrations of unlabelled suramin. Incubation time was 600 s. Symbols show averages ± SEM of triplicate determinations; a representative experiment of three repeats is shown. Inset: conversion of the inhibition data to a Michaelis–Menten saturation plot. (C) Like frame B but with TcoIL3000. (D) Sensitivity of TcoIl3000 and three clones of Il3000 expressing TbMFST to suramin, pentamidine (PMD), diminazene aceturate (DA), isometamidium chloride (ISM) and phenylarsine oxide (PAO). Bars represent averages ± SEM of three independent determinations. Drug sensitivity was not significantly different in the untransfected control and the cells expressing TbMFST (p > 0.05). (E). Immunofluorescence microscopy of T. congolense IL3000 WT (control) and the same cells expressing TbMFST (Tb927.9.6360) coupled to a 6×HA tag. Cells were stained for DNA with DAPI, for their mitochondrion with mitotracker red and with FITC-coupled anti-HA antibodies.
The Km values measured for this uptake in the linear phase, presumably reflecting binding to ISG75, were not significantly different for T. b. brucei and T. congolense (5.87 ± 1.52 µM (n = 3) and 6.05 ± 2.39 µM (n = 2), respectively; p = 0.95, unpaired t-test) (Figure 5B,C). This seems to indicate that the interaction of suramin with the cell surface receptor was similar for these species. We hypothesised that a possible explanation for the disparate suramin sensitivity and transport rates could be the proposed escape route of suramin from the lysosome into the cytoplasm [28,29] via a major facilitator superfamily transporter (MFST) located in the lysosomal membrane, which, when knocked down by RNAi, is associated with suramin resistance in T. brucei. Our reasoning was that suramin appeared to be taken up and accumulate well in T. congolense but without the TbrMFST would remain in the lysosome. This would explain why T. congolense is highly resistant to the drug. Moreover, if we consider its multi-target actions in trypanosomes once it is present free in the cytosol [28,30], the difference between the trypanosome species is unlikely to be the result of a difference in a single intracellular drug target. Expression of the T. b. brucei MFST in T. congolense might therefore allow the efflux of suramin from the T. congolense lysosome into its cytosol, becoming sensitive. However, expression of 6xHA-tagged TbMFST had no effect on the sensitivity of IL3000 to any of the drugs tested: suramin, pentamidine, diminazene, isometamidium and phenylarsine oxide (PAO) (Figure 5D).
Unfortunately, this negative result is open to diverse interpretations, including non-expression or incorrect localization of TbMFST in T. congolense. To test for this possibility, we performed fluorescence microscopy with DAPI for DNA, MitoTracker Red for the mitochondrion and FITC-coupled anti-HA antibodies for the localization of TbMFST. Figure 5E shows that the anti-HA signal was limited to a distinct and defined cytosolic location that did not co-localize with the DNA stain and was not inside the mitochondrion. Instead the location of the dot-like signal, although not definitive, was compatible with association with the lysosome, which is positioned between the flagellar pocket and the nucleus. Lacking a known function or phenotype for TbMFST other than in suramin sensitivity, we were unable to come to a conclusion whether the low suramin sensitivity of T. congolense could be attributable to differences in the MFST protein or its localisation.
2.3.4. Melarsomine (Cymelarsan; MelCy)
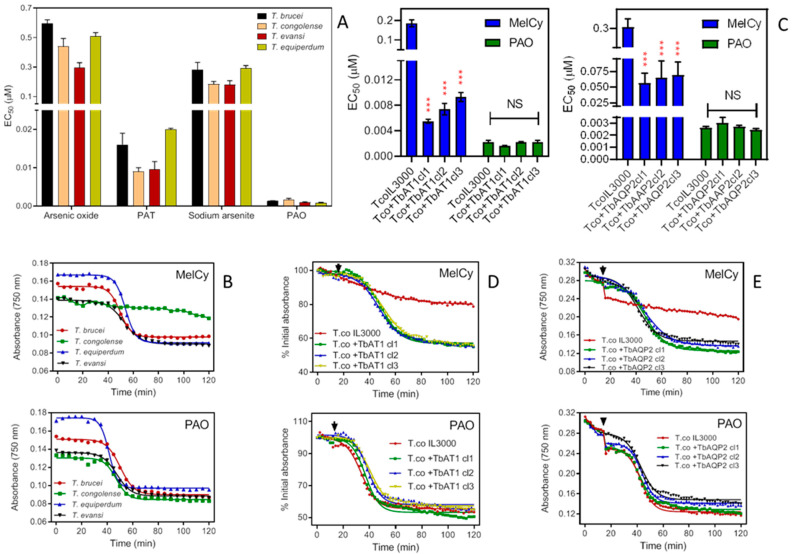
In T. brucei, the sensitivity of melaminophenyl arsenicals, such as melarsomine, depends on TbAT1 and TbAQP2, which transport them [19,21,24,49]. However, this does not apply to non-melaminophenyl arsenicals, such as phenylarsine oxide (PAO) [18]. The significant differences observed between the sensitivity of T. congolense and the brucei group to melarsomine prompted us to investigate sensitivity to non-melaminophenyl arsenicals in order to establish whether the difference is related to the melaminophenyl pharmacophore or to arsenic per se. T. congolense did not show any significant differences (one way Anova, Prism 9) in sensitivity to arsenicals other than those of the melaminophenyl class, including arsenic oxide, sodium arsenite and PAO, nor to the antimonial compound potassium antimony tartrate (PAT), screened using the Alamar Blue assay (Figure 6A). This clearly shows that the resistance to melarsomine is specific to melaminophenyl arsenicals and not to all arsenicals or heavy metals.
Figure 6.
Effects of arsenicals on various Trypanosoma species. (A) Sensitivity of four species of animal trypanosomes to arsenicals and an antimonial. Drug sensitivity is represented as EC50 averages of 3–5 independent Alamar Blue experiments (means ± standard error of mean). The differences in the EC50’s of arsenic oxide, sodium arsenite and phenylarsine oxide (PAO) and potassium antimony tartrate (PAT) between T. brucei, T. evansi and T. equiperdum are only marginal. (B) Effects of 3.3 µM melarsomine (upper frame) or 0.33 µM PAO (lower frame) on the absorbance of trypanosomes over time. Measurements of absorbance were carried out, taking readings every 5 min with a PHERAstar microplate reader at 750 nm, and presented as averages of a single experiment performed in triplicates. Upper frame: time to 50% lysis was 49.5 ± 0.4 min for T. b. brucei, 53.7 ± 0.19 min for T. equiperdum and 52.7 ± 0.6 min for T. evansi. Lines were calculated by non-linear regression using an equation for a sigmoid curve with variable slope (Prism 9). Lower frame: 50% lysis was at 50.2 ± 0.5 min, 47.0 ± 0.7 min, 41.5 ± 0.3 min and 47.6 ± 0.5 min for T. b. brucei, T. congolense, T. equiperdum and T. evansi, respectively. (C) Effects of melarsomine (MelCy) and PAO on TcoIL3000 and the same cells expressing either TbAT1 or TbAQP2. Bars represent the averages and SEMs of three independent experiments. *** p < 0.001; NS, not significant. (D) Like frame B, using IL3000 and IL3000 expressing TbAT1. The drug was added at 15 min (arrowheads); readings were taken every 2 min. Symbols are averages of triplicate determinations. (E) Like frame D except with IL3000 expressing TbAQP2.
The rate of lysis of Trypanosoma species incubated with melarsomine and PAO was measured as the decrease in cell absorbance over time [18,50], based on the reduction of cell motility and increased cell lysis, leading to reduced light scatter and absorbance in the cuvette. While PAO generally killed the four Trypanosoma species at a similar rate, melarsomine showed only a minimal effect on T. congolense but rapidly lysed T. brucei, T. evansi and T. equiperdum (Figure 6B).
Upon expression of either TbAT1 or TbAQP2, T. congolense became significantly more sensitive to melarsomine (p < 0.001) in three different clones for the heterologous expressions, whereas the EC50 value for PAO remained unchanged (p > 0.05) (Figure 6C). Expression of TbAT1 had the most profound effect on the melarsomine sensitivity of T. congolense, with an average 26.3-fold lower EC50 in the +TbAT1 clones compared to a 4.8-fold difference for the +TbAQP2 clones. These results were further confirmed using lysis experiments: even at the very high concentration of 10 µM, T. congolense IL3000 was not sensitive to melarsomine whereas absorbance declined to 50% in, on average, 33 min and 30 min for +TbAT1 and +TbAQP2 cells, respectively (Figure 6D,E).
3. Materials and Methods
3.1. Parasites and Cultures
The bloodstream form (BSF) T. b. brucei s427 [49], T. evansi AntTat 3/3 [51] and T. equiperdum [38] were cultured in HMI-9 medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% heat-inactivated foetal bovine serum (FBS (PAA Laboratories, Linz, Austria)), 14 µL/L β-mercaptoethanol (BDH, Dorset, UK) and 3.0 g/L NaHCO3 (Sigma-Aldrich, Gillingham, Dorset, UK) and adjusted to pH 7.4. These cell lines were maintained at 37 °C in a humidified, 5% CO2 environment.
The BSF T. congolense TcoIL3000 and derived cell lines were cultured in TCBSF3 medium without red blood cells. Dulbecco’s Minimum Essential Medium (MEM) was supplemented with 25 mM HEPES, 26 mM NaHCO3, 5.6 mM D-glucose, 1 mM sodium pyruvate, 40 μM adenosine, 100 μM hypoxanthine, 16.5 μM thymidine and 25 μM bathocuproinedisulfonic acid disodium salt. To this basal medium were added β-mercaptoethanol (0.0014% v/v), 1.6 mM glutamine, 100 units/mL penicillin/0.1 mg/mL streptomycin (Gibco), 20% goat serum (Gibco) and 5% Serum Plus (SAFC Biosciences) [52,53]. The BSF T. congolense IL3000 WT and its derived lines were cultured at 34 °C in a humidified, 5% CO2 environment.
3.2. Resazurin-Based Drug Sensitivity Assay
The resazurin-based (Alamar Blue) drug sensitivity assay was used to assess and compare the activity of standard trypanocides in the different cell lines investigated [52,54]. Briefly, 23 doubling dilutions of the test drug starting at 100 μM plus drug free control were prepared in 100 μL medium in a 96-well white plate (Greiner Bio-one, Frickenhausen, Germany). The cells were adjusted to 2 × 104 cells/mL for T. b. brucei and T. equiperdum; 4 × 104 cells/mL for T. evansi; and 5 × 105 cells/mL for T. congolense in the appropriate medium. Then, 100 μL of the adjusted cells was added to the wells containing drug dilutions and incubated for 48 h under respective culture conditions. Thereafter, 20 μL of 125 mg/mL resazurin sodium dye was added, and the plate was incubated for a further 24 h. The resorufin fluorescence in each well of the plate was determined and used to calculate the EC50 of the drug as described [42].
3.3. Growth Rate by Cell Count
A manual cell count of the cultures was carried out to determine the effect of expression of T. brucei transporters on the growth rate of T. congolense, as described [42]. Cell lines were counted and adjusted to the same density in 2 mL TCBSF3 medium and incubated in a 24-well plate. The density of each culture was determined by daily manual cell count using a Neubauer cell chamber and a phase-contrast light microscope (Zeiss).
3.4. Cell Absorbance Assay
The rate of drug-induced lysis of trypanosomes was measured as a decrease in absorbance over time [18,50]. Cells were washed in assay buffer (AB; 33 mM HEPES, 98 mM NaCl, 4.6 mM KCl, 0.5 mM CaCl2, 0.07 mM MgSO4, 5.8 mM NaH3PO4, 0.03 mM MgCl2, 23 mM NaHCO3, 14 mM D-glucose, pH 7.3) and resuspended to 107 cells/mL density in AB, after which 200 μL of the prepared cells was distributed into each well of a transparent-bottom black 96-well plate (Greiner Bio-one, Frickenhausen, Germany). Absorbance in each well was determined every 2 min using a microplate reader (PHERAstar or FLUORstar Optima (BMG Labtech, Durham, NC, USA)) set at 750 nm absorbance wavelength. At 15–20 min, 20 μL of either assay buffer or test drug at 10× the desired concentration in assay buffer was added to the respective wells and the measurements continued.
3.5. Immunofluorescence Microscopy
Immunofluorescence microscopy, carried out to visualise the localisation of the TbMFS transporter in T. congolenseTbMFT, was performed as described previously [55]. MitoTracker RedTM (Thermo Fisher Scientific, Hemel Hempstead, UK.) was added to the cell culture to a final concentration of 100 nM and incubated for 10 min. Culture containing approximately 2 × 106 cells was collected in an Eppendorf tube, washed in 1× PBS and applied to a 12-well glass slide (Menzel-Gläser, VWR, Lutterworth, Leicestershire, UK) treated with Poly-L-Lysine (Sigma-Aldrich). After the cells were fixed with 4% paraformaldehyde and washed twice in 1× PBS, the slide was treated with 1% triton X-100 (Thermo Fisher Scientific) in PBS for 10 min and then with 100 mM glycine for 20 min, followed by a wash with PBS. The slide was blocked for 1 h with 1% BSA/0.2% Tween-20 (Sigma-Aldrich) and then treated with the primary antibody (rabbit anti-HA (Sigma-Aldrich)) at a dilution of 1:1000 in blocking solution and incubated in a wet chamber for 1 h. The sample was washed three times with PBS; 1:2000 secondary antibody (goat anti-rabbit IgG FITC conjugate (Sigma-Aldrich)) in blocking solution was added and incubated in the dark for 1 h. The slide was then washed three times with PBS, treated with DAPI and covered with a cover slip [55]. The prepared slide was viewed under an Olympus IX71 DeltaVision Core System (Applied Precision, GE Healthcare, Amersham, UK) using the SoftWoRx suite 2.0 software (Applied Precision, GE). All images acquired were processed using Fiji software [56].
3.6. Drug Uptake Assay
An uptake assay using radiolabelled trypanocides was carried out as described previously [57]. Radiolabelled ring-[3H]-DA was custom-made by PerkinElmer (CUST78468000MC; 60.7 Ci/mmol) and [3H]-pentamidine isethionate was custom-made by Amersham (TRQ40084; 3.26 TBq/mmol); [3H]-Suramin was supplied by American Radiolabeled Chemicals (ARC). Briefly, parasites were harvested washed twice by centrifugation in assay buffer before they were adjusted to a density of 1 × 108 cells/mL in AB. Then, 100 µL of AB containing 1 × 107 cells was mixed with the same volume of the radiolabelled drug in assay buffer, layered on top of an oil mix in microcentrifuge tubes and incubated for a pre-determined period. Uptake was stopped by the addition of high concentrations of ice-cold unlabelled substrate (stop solution) and immediate centrifugation of the cells through the oil layer. The microcentrifuge tubes were flash frozen in liquid nitrogen, and the bottom of each tube containing the cell pellet was cut off and transferred to a scintillation vial. Cell pellets were lysed with 2% SDS solution under slow agitation on a shaker for 30 min before the addition of 3 mL of scintillation fluid (Scintilogic U, Lablogic) into each vial and further incubation overnight in the dark. Radiation was measured in a 300SL (Hidex) scintillation counter (Hidex). Linear regression analysis using Prism (versions 8 and 9, GraphPad Software) allowed for the rate of uptake per unit time and other parameters, such as r2 and F-test, to be determined. For the determination of the Km, the radiolabelled permeant in the presence or absence of different concentrations of potential inhibitors reconstituted in assay buffer was placed on top of 250 μL oil mix in microcentrifuge tubes.
3.7. Genetic Manipulation of T. congolense
Information for the genes investigated in this study, such as the nucleotide sequences, synteny and similarity to other genes, was obtained from the GeneDB (genedb.org) and TritrypDB (tritrypdb.org/tritrypdb) genome databases. The CLC Genomics Workbench 7 software (CLC Bio, Qiagen) was used for the design of primers, construction of plasmids and alignment of nucleotide sequences.
A modified pRM481 plasmid [58] named pMPB-DP-012, which targets the tubulin locus of T. congolense and transcribes blasticidin S deaminase (BSD) and offers a 6×HA tag at the C-terminal [59], was kindly supplied by Prof. Michael Barrett, University of Glasgow. The open reading frame (ORF) of TbAQP2 was PCR-amplified from T. brucei genomic DNA and ligated into pMPB-DP-012 using SalI and BamH1, as described in [47,54] (primers detailed in Supplemental Table S1).
The SalI site in the pMPB-DP-012 plasmid was replaced with a BglII site using the Q5 Site-Directed Mutagenesis Kit (NEB) as per the manufacturer’s protocol. Mutation in the generated plasmid was confirmed using PCR and Sanger sequencing (Source BioScience, Nottingham, UK). The correctly mutated plasmid was digested with BglII and BamH1, followed by the insertion of amplified and purified TbAT1 or lysosomal TbMFST using the NEB Builder HiFi DNA assembly cloning kit (New England Biolabs, Hitchin, Herts, UK.
Each plasmid was verified by Sanger sequencing for correct integration of the insert, linearised with AscI (NEB, Hitchin, UK) and transfected into T. congolense IL3000. Transfection was carried out as described previously [60], after which 2–3 × 107 cells of T. congolense IL3000 resuspended in transfection buffer were mixed with 10 µg of digested plasmid and electroporated using an Amaxa Nucleofector with the Z-001 programme. The transfected cells were transferred into 25 mL pre-warmed TC-BSF3 medium and then distributed into 24-well plates by limited dilution (1:5 and 1:10), followed by incubation for 18 h at 34 °C and 5% CO2 to allow for recovery. Cells were selected by the addition of 0.5 µg/mL blasticidin antibiotic to the growth medium, and the presence of the construct was confirmed by PCR amplification from the genomic DNA.
4. Conclusions
In this study, we have investigated the differences in sensitivity to standard trypanocides among several important species responsible for animal African trypanosomiasis worldwide. We found that the sensitivity of T. b. brucei, T. evansi and T. equiperdum (all Trypanozoon subgenus) to these drugs was generally very similar, with the exception of T. b. brucei and T. evansi, which are substantially less sensitive to ethidium bromide than T. evansi. However, T. congolense (subgenus Nannomonas) was clearly less sensitive to several important trypanocides, including suramin, melarsomine and the diamidines pentamidine and diminazene. The fact that T. congolense lacks orthologues for the known drug transporters for melaminophenyl arsenicals and diamidines (TbAT1 and TbAQP2), as well as the orthologue for the suspected lysosomal suramin transporter MFST, appears to give a rationale for the large differences in drug efficacy. TbAT1 is one of at least a dozen T. brucei purine transporters [61] and is not essential (although purine uptake is), with the gene easily deleted [25] and defective mutant alleles reported in field isolates [39,62]. In T. congolense, purine nucleosides are salvaged by the transporter known as TcoAT1, which is the orthologue of TbNT10, a broad selectivity purine nucleoside transporter [47] and, presumably, by a number of nucleobase transporters that are found in the genome [63] but not yet characterised. TbAQP2 is not essential either, in vitro or in vivo [21,39,64]. Indeed, TbAQP2 is a mutant copy of the conserved TbAQP3 [19,20], which has at least one orthologue in T. congolense (TcIL3000_10_12040). The function of TbMFST is not known, but its knockdown by RNAi suggests that it is not essential [28].
Expression of each of these transport proteins in T. congolense did sensitise this species to the various drugs except suramin. It also increased uptake of [3H]-pentamidine, [3H]-diminazene and the rate of cell lysis caused by melarsomine. We propose that the presence of absence of the TbAT1 and TbAQP2 transporters in various trypanosome species is a major determining factor of the extent to which a given species is sensitive to diamidines and melaminophenyl arsenicals, separating the Trypanozoon subgenus from subgenus Nannomonas. For suramin, the issue is more complicated, due to the higher complexity of its uptake mechanism, and was not resolved by the expression of T. brucei MFST. As such, the paradox of rapid suramin uptake and low suramin sensitivity in T. congolense will require further research. This research also reemphasises the need for animal trypanocides to be tested on multiple species, from different sub-genera.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23052844/s1.
Author Contributions
Conceptualization, H.P.d.K.; methodology, M.A.U., G.D.C., M.J.N. and H.P.d.K.; formal analysis, M.A.U., G.D.C. and H.P.d.K.; investigation, M.A.U., G.D.C. and A.H.A.; writing—original draft preparation, M.A.U., G.D.C. and H.P.d.K.; writing—review and editing, H.P.d.K.; supervision, H.P.d.K. and M.J.N.; funding acquisition, M.A.U., G.D.C., M.J.N. and H.P.d.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a scholarship awarded to M.A.U. from the Petroleum Technology Development Fund of Nigeria and by a BBSRC Impact Accelerator Award (ref: BB/S506734/1); by a fellowship from the government of Saudi Arabia to M.J.N.; and by Science Without Borders with a scholarship to G.D.C. (206385/2014-5, CNPq, Brazil).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giordani F., Morrison L.J., Rowan T.G., De Koning H.P., Barrett M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology. 2016;143:1862–1889. doi: 10.1017/S0031182016001268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büscher P., Gonzatti M.I., Hébert L., Inoue N., Pascucci I., Schnaufer A., Suganuma K., Touratier L., Van Reet N. Equine trypanosomosis: Enigmas and diagnostic challenges. Parasit. Vectors. 2019;12:234. doi: 10.1186/s13071-019-3484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osório A.L., Madruga C.R., Desquesnes M., Soares C.O., Ribeiro L.R., Costa S.C. Trypanosoma (Duttonella) vivax: Its biology, epidemiology, pathogenesis, and introduction in the New World—A review. Mem. Inst. Oswaldo Cruz. 2008;103:1–13. doi: 10.1590/S0074-02762008000100001. [DOI] [PubMed] [Google Scholar]
- 4.Gonzatti M.I., Gonzalez-Baradat B., Aso P.M., Reyna-Bello A. Trypanosoma (Duttonella) vivax and trypanosomosis in Latin America: Secadera/huequera/cacho hueco. In: Magez S., Radwanska M., editors. Trypanosomes and Trypanosomiasis. Springer; Wien, Austria: 2014. pp. 261–285. [Google Scholar]
- 5.Fetene E., Leta S., Regassa F., Büscher P. Global distribution, host range and prevalence of Trypanosoma vivax: A systematic review and meta-analysis. Parasit. Vectors. 2021;14:80–100. doi: 10.1186/s13071-021-04584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asgari M.M., Rassouli M. First identification of Trypanosoma vivax among camels (Camelus dromedarius) in Yazd, central Iran, jointly with Trypanosoma evansi. Parasitol. Int. 2022;86:102450. doi: 10.1016/j.parint.2021.102450. [DOI] [PubMed] [Google Scholar]
- 7.Desquesnes M. Livestock Trypanosomoses and Their Vectors in Latin America. CIRAD-EMVT Publication, OIE; Paris, France: 2004. [(accessed on 2 March 2022)]. Available online: https://www.proquest.com/docview/214620829. [Google Scholar]
- 8.Desquesnes M., Dargantes A., Lai D.-H., Lun Z.-R., Holzmuller P., Jittapalapong S. Trypanosoma evansi and surra: A review and perspectives on transmission, epidemiology and control, impact, and zoonotic aspects. BioMed Res. Int. 2013;2013:321237. doi: 10.1155/2013/321237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elata A., Galon E.M., Moumouni P.F.A., Ybanez R.H.D., Mossaad E., Salces C.B., Bajenting G.P., Ybanez A.P., Xuan X., Inoue N., et al. First molecular detection and identification of Trypanosoma evansi in goats from Cebu, Philippines using a PCR-based assay. Vet. Parasitol. Reg. Stud. Rep. 2020;21:100414. doi: 10.1016/j.vprsr.2020.100414. [DOI] [PubMed] [Google Scholar]
- 10.Setiawan A., Nurcahyo W., Priyowidodo D., Budiati R.T., Susanti D.S.R. Genetic and parasitological identification of Trypanosoma evansi infecting cattle in South Sulawesi, Indonesia. Vet. World. 2021;14:113–119. doi: 10.14202/vetworld.2021.113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angara T.E., Ismail A., Ibrahim A. An overview on the economic impacts of animal trypanosomiasis. Glob. J. Res. Anal. 2012;3:275–276. doi: 10.15373/22778160/July2014/99. [DOI] [Google Scholar]
- 12.De Koning H.P. The drugs of sleeping sickness: Their mechanisms of action and resistance, and a brief history. Trop. Med. Infect. Dis. 2020;5:14. doi: 10.3390/tropicalmed5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinabo L.D.B. Pharmacology of existing drugs for animal trypanosomiasis. Acta Trop. 1993;54:169–183. doi: 10.1016/0001-706X(93)90091-O. [DOI] [PubMed] [Google Scholar]
- 14.Williamson J. Review of chemotherapeutic and chemoprophylactic agents. In: Mulligan H.W., editor. The African Trypanosomiases. George Allen and Unwin, Ltd.; London, UK: 1970. pp. 125–221. [Google Scholar]
- 15.Delespaux V., De Koning H.P. Transporters in antiparasitic drug development and resistance. In: Jäger T., Koch O., Flohe L., editors. Trypanosomatid Diseases: Molecular Routes to Drug Discovery. Wiley-Blackwell; Weinheim, Germany: 2013. pp. 335–349. [Google Scholar]
- 16.Carter N.S., Berger B.J., Fairlamb A.H. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 1995;270:28153–28157. doi: 10.1074/jbc.270.47.28153. [DOI] [PubMed] [Google Scholar]
- 17.De Koning H.P., Jarvis S.M. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by the P2 adenosine transporter and at least one novel, unrelated transporter. Acta Trop. 2001;80:245–250. doi: 10.1016/S0001-706X(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 18.Bridges D., Gould M.K., Nerima B., Mäser P., Burchmore R.J.S., De Koning H.P. Loss of the High Affinity Pentamidine Transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African Trypanosomes. Mol. Pharmacol. 2007;71:1098–1108. doi: 10.1124/mol.106.031351. [DOI] [PubMed] [Google Scholar]
- 19.Baker N., Glover L., Munday J.C., Aguinaga Andrés D., Barrett M.P., De Koning H.P., Horn D. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African Trypanosomes. Proc. Natl. Acad. Sci. USA. 2012;109:10996–11001. doi: 10.1073/pnas.1202885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alghamdi A.H., Munday J.C., Campagnaro G.D., Gurvic D., Svensson F., Okpara C.E., Kumar A., Quintana J., Martin Abril M.E., Milić P., et al. Positively selected modifications in the pore of TbAQP2 allow pentamidine to enter Trypanosoma brucei. eLife. 2020;9:e56416. doi: 10.7554/eLife.56416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munday J.C., Eze A.A., Baker N., Glover L., Clucas C., Aguinaga Andrés D., Natto M.J., Teka I.A., McDonald J., Lee R.S., et al. Trypanosoma brucei Aquaglyceroporin 2 is a high affinity transporter for pentamidine and melaminophenyl arsenic drugs and is the main genetic determinant of resistance to these drugs. J. Antimicrob. Chemother. 2014;69:651–663. doi: 10.1093/jac/dkt442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Koning H.P., Anderson L.F., Stewart M., Burchmore R.J.S., Wallace L.J.M., Barrett M.P. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter; additional insights in diamidine resistance in African Trypanosomes. Antimicrob. Agents Chemother. 2004;48:1515–1519. doi: 10.1128/AAC.48.5.1515-1519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward C.P., Wong P.E., Burchmore R.J., De Koning H.P., Barrett M.P. Trypanocidal furamidine analogues: Influence of pyridine nitrogens on trypanocidal activity, transport kinetics and resistance patterns. Antimicrob. Agents Chemother. 2011;55:2352–2361. doi: 10.1128/AAC.01551-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter N.S., Fairlamb A.H. Arsenical-resistant Trypanosomes lack an unusual adenosine transporter. Nature. 1993;361:173–176. doi: 10.1038/361173a0. [DOI] [PubMed] [Google Scholar]
- 25.Matovu E., Stewart M., Geiser F., Brun R., Mäser P., Wallace L.J.M., Burchmore R.J., Enyaru J.C.K., Barrett M.P., Kaminsky R., et al. The mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell. 2003;2:1003–1008. doi: 10.1128/EC.2.5.1003-1008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent I.M., Creek D., Watson D.G., Kamleh M.A., Woods D.J., Wong P.E., Burchmore R.J., Barrett M.P. A molecular mechanism for eflornithine resistance in African Trypanosomes. PLoS Pathog. 2010;6:e1001204. doi: 10.1371/journal.ppat.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumann Burkard G., Jutzi P., Roditi I. Genome-wide RNAi screens in bloodstream form Trypanosomes identify drug transporters. Mol. Biochem. Parasitol. 2011;175:91–94. doi: 10.1016/j.molbiopara.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Alsford S., Eckert S., Baker N., Glover L., Sanchez-Flores A., Leung K.F., Turner D.J., Field M.C., Berriman M., Horn D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoltner M., Horn D., De Koning H.P., Field M.C. Exploiting the Achilles’ heel of membrane trafficking in Trypanosomes. Curr. Opin. Microbiol. 2016;34:97–103. doi: 10.1016/j.mib.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoltner M., Campagnaro G.D., Taleva G., Burrell A., Cerone M., Leung K.-F., Achcar F., Horn D., Vaughan S., Gadelha C., et al. Suramin exposure alters cellular metabolism and mitochondrial energy production in African Trypanosomes. J. Biol. Chem. 2020;295:8331–8347. doi: 10.1074/jbc.RA120.012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkes J.M., Mulugeta W., Wells C., Peregrine A.S. Modulation of mitochondrial electrical potential: A candidate mechanism for drug resistance in African Trypanosomes. Biochem. J. 1997;326:755–761. doi: 10.1042/bj3260755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eze A.A., Gould M.K., Munday J.C., Tagoe D.N.A., Stelmanis V., Schnaufer A., De Koning H.P. Loss of mitochondrial membrane potential is a late adaptation of Trypanosoma brucei brucei to isometamidium preceded by mutations in the γ subunit of the F1F0-ATPase. PLoS Negl. Trop. Dis. 2016;10:e0004791. doi: 10.1371/journal.pntd.0004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kell D.B. The transporter-mediated cellular uptake and efflux of pharmaceutical drugs and biotechnology products: How and why phospholipid bilayer transport Is negligible in real biomembranes. Molecules. 2021;26:5629. doi: 10.3390/molecules26185629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeganathan S., Sanderson L., Dogruel M., Rodgers J., Croft S., Thomas S.A. The distribution of nifurtimox across the healthy and trypanosome-infected murine blood–brain and blood-CSF barriers. J. Pharmacol. Exp. Ther. 2011;336:506–515. doi: 10.1124/jpet.110.172981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson C.P., Dogruel M., Mihoreanu L., Begley D.J., Weksler B.B., Couraud P.O., Romero I.A., Thomas S.A. The transport of nifurtimox, an anti-trypanosomal drug, in an in vitro model of the human blood-brain barrier: Evidence for involvement of breast cancer resistance protein. Brain Res. 2012;1436:111–121. doi: 10.1016/j.brainres.2011.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wyllie S., Foth B.J., Kelner A., Sokolova A.Y., Berriman M., Fairlamb A.H. Nitroheterocyclic drug resistance mechanisms in Trypanosoma brucei. J. Antimicrob. Chemother. 2016;71:625–634. doi: 10.1093/jac/dkv376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Changtam C., De Koning H.P., Ibrahim H., Sajid S., Gould M.K., Suksamrarn A. Curcuminoid analogues with potent activity against Trypanosoma and Leishmania species. Eur. J. Med. Chem. 2010;45:941–956. doi: 10.1016/j.ejmech.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 38.Stewart M.L., Burchmore R.J.S., Clucas C., Hertz-Fowler C., Brook K., Tait A., McLeod A., Turner C.M.R., De Koning H.P., Wong P.E., et al. Multiple genetic mechanisms lead to the loss of functional TbAT1 expression in drug resistant Trypanosomes. Eukaryot. Cell. 2010;9:336–343. doi: 10.1128/EC.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graf F.E., Ludin P., Wenzler T., Kaiser M., Brun R., Pati Pyana P., Büscher P., De Koning H.P., Horn D., Mäser P. Aquaporin 2 mutations in Trypanosoma b. gambiense field isolates correlate with decreased susceptibility to pentamidine and melarsoprol. PLoS Negl. Trop. Dis. 2013;7:e2475. doi: 10.1371/journal.pntd.0002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fueyo González F.J., Ebiloma G.U., Izquierdo García C., Bruggeman V., Sánchez Villamañán J.M., Donachie A., Balogun E.O., Inaoka D.K., Shiba T., Harada S., et al. Conjugates of 2,4-dihydroxybenzoate and salicylhydroxamate and lipocations display potent anti-parasite effects by efficiently targeting the Trypanosoma brucei and Trypanosoma congolense mitochondrion. J. Med. Chem. 2017;60:1509–1522. doi: 10.1021/acs.jmedchem.6b01740. [DOI] [PubMed] [Google Scholar]
- 41.Munday J.C., Settimo L., De Koning H.P. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front. Pharmacol. 2015;6:32. doi: 10.3389/fphar.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carruthers L.V., Munday J.C., Ebiloma G.U., Steketee P., Jayaraman S., Campagnaro G.D., Ungogo M.A., Donnachie A., Lemgruber L., Rowan T.G., et al. Diminazene resistance in Trypanosoma congolense is not caused by reduced transport capacity but associated with reduced mitochondrial membrane potential. Mol. Microbiol. 2021;116:564–588. doi: 10.1111/mmi.14733. [DOI] [PubMed] [Google Scholar]
- 43.Witola W.H., Inoue N., Ohashi K., Onuma M. RNA-interference silencing of the adenosine transporter-1 gene in Trypanosoma evansi confers resistance to diminazene aceturate. Exp. Parasitol. 2004;107:47–57. doi: 10.1016/j.exppara.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Barrett M.P., Zhang Z.Q., Denise H., Baltz T. A diamidine-resistant Trypanosoma equiperdum clone contains a P2 purine transporter with reduced substrate affinity. Mol. Biochem. Parasitol. 1995;73:223–229. doi: 10.1016/0166-6851(95)00120-P. [DOI] [PubMed] [Google Scholar]
- 45.Büscher P., Van Reet N. (Institute of Tropical Medicine, Antwerp, Belgium). Personal communication. 2021.
- 46.De Koning H.P. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters. Implications for crossresistance with arsenicals. Mol. Pharmacol. 2001;59:586–592. doi: 10.1124/mol.59.3.586. [DOI] [PubMed] [Google Scholar]
- 47.Munday J.C., Rojas López K.E., Eze A.A., Delespaux V., Van Den Abbeele J., Rowan T., Barrett M.P., Morrison L.J., De Koning H.P. Functional expression of TcoAT1 reveals it to be a P1-type nucleoside transporter with no capacity for diminazene uptake. Int. J. Parasitol. Drugs Drug Resist. 2013;3:69–76. doi: 10.1016/j.ijpddr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delespaux V., De Koning H.P. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updat. 2007;10:30–50. doi: 10.1016/j.drup.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 49.De Koning H.P., MacLeod A., Barrett M.P., Cover B., Jarvis S.M. Further evidence for a link between melarsoprol resistance and P2 transporter function in African Trypanosomes. Mol. Biochem. Parasitol. 2000;106:181–185. doi: 10.1016/S0166-6851(99)00206-6. [DOI] [PubMed] [Google Scholar]
- 50.Fairlamb A.H., Carter N.S., Cunningham M., Smith K. Characterisation of melarsen-resistant Trypanosoma brucei brucei with respect to cross-resistance to other drugs and trypanothione metabolism. Mol. Biochem. Parasitol. 1992;53:213–222. doi: 10.1016/0166-6851(92)90023-D. [DOI] [PubMed] [Google Scholar]
- 51.Dean S., Gould M.K., Dewar C.E., Schnaufer A.C. Single point mutations in ATP synthase compensate for mitochondrial genome loss in Trypanosomes. Proc. Natl. Acad. Sci. USA. 2013;110:14741–14746. doi: 10.1073/pnas.1305404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coustou V., Guegan F., Plazolles N., Baltz T. Complete in vitro life cycle of Trypanosoma congolense: Development of genetic tools. PLoS Negl. Trop. Dis. 2010;4:e618. doi: 10.1371/journal.pntd.0000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giordani F., Khalaf A.I., Gillingwater K., Munday J.C., De Koning H.P., Suckling C.J., Barrett M.P., Scott F.J. Novel minor groove binders cure animal African trypanosomiasis in an in vivo mouse model. J. Med. Chem. 2019;62:3021–3035. doi: 10.1021/acs.jmedchem.8b01847. [DOI] [PubMed] [Google Scholar]
- 54.Gould M.K., Vu X.L., Seebeck T., De Koning H.P. Propidium iodide-based methods for monitoring drug action in the kinetoplastidae: Comparison with the Alamar Blue assay. Anal. Biochem. 2008;382:87–93. doi: 10.1016/j.ab.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 55.Stortz J.A., Serafim T.D., Alsford S., Wilkes J., Fernandez-Cortes F., Hamilton G., Briggs E., Lemgruber L., Horn D., Mottram J.C., et al. Genome-wide and protein kinase-focused RNAi screens reveal conserved and novel damage response pathways in Trypanosoma brucei. PLoS Pathog. 2017;13:e1006477. doi: 10.1371/journal.ppat.1006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campagnaro G.D., de Freitas Nascimento J., Girard R.B.M., Silber A.M., De Koning H.P. Cloning and characterisation of the Equilibrative Nucleoside Transporter family of Trypanosoma cruzi: Ultra-high affinity and selectivity to survive in the intracellular niche. Biochim. Biophys. Acta Gen. Subj. 2018;12:2750–2763. doi: 10.1016/j.bbagen.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Proudfoot C., McCulloch R. Distinct roles for two RAD51-related genes in Trypanosoma brucei antigenic variation. Nucleic Acids Res. 2005;33:6906–6919. doi: 10.1093/nar/gki996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giordani F., Paape D., Vincent I.M., Pountain A.W., Fernández-Cortés F., Rico E., Zhang N., Morrison L.J., Freund Y., Witty M.J., et al. Veterinary trypanocidal benzoxaboroles are peptidase-activated prodrugs. PLoS Pathog. 2020;16:e1008932. doi: 10.1371/journal.ppat.1008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Awuah-Mensah G., McDonald J., Steketee P.C., Autheman D., Whipple S., D’Archivio S., Brandt C., Clare S., Harcourt K., Wright G.J., et al. Reliable, scalable functional genetics in bloodstream-form Trypanosoma congolense in vitro and in vivo. PLoS Pathog. 2021;17:e1009224. doi: 10.1371/journal.ppat.1009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campagnaro G.D., De Koning H.P. Purine and pyrimidine transporters of pathogenic protozoa—Conduits for therapeutic agents. Med. Res. Rev. 2020;40:1679–1714. doi: 10.1002/med.21667. [DOI] [PubMed] [Google Scholar]
- 62.Munday J.C., Tagoe D.N.A., Eze A.A., Krezdorn J.A., Rojas López K.E., Alkhaldi A.A.M., McDonald F., Still J., Alzahrani K.J., Settimo L., et al. Functional analysis of drug resistance-associated mutations in the Trypanosoma brucei adenosine transporter 1 (TbAT1) and the proposal of a structural model for the protein. Mol. Microbiol. 2015;96:887–900. doi: 10.1111/mmi.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jackson A.P., Allison H.C., Barry J.D., Field M.C., Hertz-Fowler C., Berriman M. A Cell-surface phylome for African Trypanosomes. PLOS Negl. Trop. Dis. 2013;7:e2121. doi: 10.1371/journal.pntd.0002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeacock L., Baker N., Wiedemar N., Mäser P., Horn D. Aquaglyceroporin-null Trypanosomes display glycerol transport defects and respiratory-inhibitor sensitivity. PLoS Pathog. 2017;13:e1006307. doi: 10.1371/journal.ppat.1006307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.