Abstract
Depression and cognition are associated, but the role of depressive symptoms in lifestyle interventions to prevent dementia needs further study. We investigated the intervention effect on depressive symptoms and their associations with cognition in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER; NCT01041989), a two-year multidomain lifestyle trial. One thousand two-hundred and sixty individuals (60–77 years) at risk for dementia were randomised into a multidomain intervention (diet, exercise, cognitive training, and vascular/metabolic risk monitoring) or control group (regular health advice). Depressive symptoms (Zung scale) and cognition (modified Neuropsychological Test Battery) were evaluated at baseline, 12, and 24 months. One thousand one-hundred and twenty-five participants had baseline Zung data. Mean Zung score decreased 0.73 (SD 5.6) points in the intervention and 0.36 (5.6) points in the control group, with nonsignificant between-group difference (group × time coefficient −0.006, 95% CI −0.019 to 0.007). Overall, higher baseline Zung score was associated with less improvement in global cognition (−0.140, p = 0.005) and memory (−0.231, p = 0.005). Participants with clinically significant baseline depressive symptoms (Zung ≥ 40 points) had less intervention benefit to executive functioning (group × time × Zung −0.096, 95% CI −0.163 to −0.028). Change in Zung score was not associated with change in cognition. Clinically significant depressive symptoms warrant more attention when designing dementia-prevention interventions.
Keywords: clinical trial, cognition, dementia, depressive symptoms, prevention
1. Introduction
Cognitive impairment and dementia are major public health problems. Growing knowledge on modifiable risk factors has emphasised the importance of prevention [1]. The first World Health Organization (WHO) guidelines for risk reduction of cognitive decline and dementia mention, for instance, physical activity, smoking cessation, healthy diet, cognitive and social activities, and management of cardiovascular risk factors and depression [2]. Depression is an important risk factor for dementia [1], and depressive symptoms have been associated with poorer cognition in older age [3]. The link between late-life depression and cognition is complex, i.e., depressive symptoms may also represent prodromal symptoms of dementia, especially regarding vascular dementia [4,5].
Depression is closely connected with lifestyle factors. A Cochrane meta-analysis of randomised controlled trials (RCTs) in people with depression showed exercise to be moderately more effective than a control intervention for reducing depressive symptoms [6]. Physical activity was also protective against depression in a systematic review of observational studies [7]. A meta-analysis of observational studies showed that a healthy dietary pattern was associated with reduced risk of depression [8], and a Mediterranean diet with nuts intervention also reduced depression risk among type 2 diabetics [9]. Bene-ficial effects of lifestyle interventions on mood have additionally been observed in people with, e.g., chronic obstructive pulmonary disease, obesity, and psychotic disorders [10,11,12,13]. Regarding other interventions, an RCT among healthy older adults found that computerised cognitive training improved cognition but had no effect on depressive symptoms [14]. Most studies have investigated lifestyle factors separately, but fewer have considered more complex, multidomain lifestyle interventions. One multidomain trial targeting frailty reported that an intervention including physical activity, dietary supplementation, and cognitive training improved depressive symptoms [15].
In addition to being affected by lifestyle factors, depressive symptoms may impact an individual’s ability to adhere to healthy lifestyle changes and improve their cardiovascular and metabolic risk factors [16,17,18]. They may not necessarily hinder lifestyle imp-rovement, as shown for, e.g., cardiometabolic risk in people at risk for diabetes [19] or physical activity among sedentary people [20], but the role of depressive symptoms in multidomain lifestyle interventions aiming to preventing dementia has so far not been investigated.
Due to the multifactorial nature of dementia and disappointing results from many single-domain interventions for dementia risk reduction, the newest generation of prevention RCTs has been testing multidomain lifestyle interventions targeting multiple risk factors simultaneously [21]. Although no significant intervention benefits on either the primary cognitive outcome or depressive symptoms were reported in the Multidomain Alzheimer Preventive Trial (MAPT) and Prevention of Dementia by Intensive Vascular care (preDIVA) trials [22,23], the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial showed that multidomain lifestyle intervention was beneficial for cognition compared with regular health advice among older persons at risk for dementia [24]. It is important to study the effects of depressive symptoms in the context of dementia-prevention trials to see if they influence the intervention effects on cognition. In this study based on the FINGER trial, we investigated: (i) intervention effects on change in depressive symptoms (secondary outcome), (ii) whether depressive symptoms at baseline modified the previously reported intervention benefits of cognition (post hoc analysis), and (iii) whether the change in depressive symptoms were related to change in cognition (post hoc analysis).
2. Materials and Methods
2.1. The FINGER Trial Design and Participants
The FINGER trial protocol, recruitment process, and primary findings have been previously published [24,25,26]. In brief, FINGER was a two-year RCT conducted in six centers in Finland. Participants at risk for dementia were recruited from previous population-based surveys [27,28]. Eligibility criteria were age 60–77 years, Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) Dementia Risk Score [29] ≥6 points (range 0–15 points), and cognition at a mean level or slightly lower than expected for age (Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Word List memory learning score <20 points or Word List recall ≤75%, or Mini-Mental State Examination (MMSE) score ≤26 points) [30,31]. Exclusion criteria were diagnosed or suspected dementia, MMSE <20 points, conditions preventing cooperation or safe engagement in the intervention (based on interview with the study doctor, e.g., major depression; malignant tumor; symptomatic cardiovascular disease; and severe vision, hearing, or communicative impairment), and participation in another trial simultaneously.
Of the 2654 individuals screened for eligibility from 7 September 2009 to 24 November 2011, 1260 were randomised in a 1:1 ratio into a multidomain intervention or control group (computer-generated randomisation was done in blocks of four individuals at each site). Outcome assessors were blinded to group allocation and were not involved in intervention activities. The primary outcome was a change in global cognition (extended and modified version of the Neuropsychological Test Battery, mNTB) [32]. Secondary outcomes included cognitive domains (executive functioning, memory, and processing speed) and depressive symptoms.
The authors assert that all procedures contributing to this work comply with the et-hical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects were approved by the Coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (approval number 94/13/03/00/2009). Written informed consent was obtained from all subjects. The FINGER trial is registered with ClinicalTrials.gov (NCT01041989).
2.2. Intervention
The control group received regular health advice. All participants (control and intervention group) met the study nurse at screening, baseline, 6, 12, and 24 months (blood pressure, weight and BMI, and hip and waist circumference measurements), and the study physician at screening and 24 months (medical history and physical examination). At baseline, the study nurse gave all participants oral and written information and advice on healthy diet and physical, cognitive, and social activities beneficial for management of vascular risk factors and disability prevention. Blood samples were collected at baseline, 6, 12, and 24 months, and laboratory test results were mailed to all participants, with general written information about the clinical significance of measurements, and advice to contact primary health care if needed. The intervention group additionally received a multidomain intervention including all four intervention components, as described earlier [25]. Dietary intervention was based on the Finnish nutrition recommendations and comprised individual counselling (three sessions) and group meetings (6–9 sessions), led by study nutritionists. Physical exercise intervention comprised progressive muscle strength training (1–3 times/week), supervised by the study physiotherapists; independent aerobic exercise (2–5 times/week); and postural balance exercises. Cognitive training included 10 group sessions led by the study psychologists and individually tailored computer-based training sessions (2–3 times/week). Management of vascular and metabolic risk factors was based on national guidelines. The intervention group had additional meetings with the study nurse (at 3, 9, and 18 months) for motivational discussion and assessment of anthropometrics, and with the study physician (at 3, 6, and 12 months) for discussing their individual risk profile, including laboratory results and receiving personalised advice on vascular and metabolic factors. Contact with the participant’s own physician was recommended if a need for drug treatment or adjustment was identified. Intervention duration was two years, and the intervention was completed in February 2014.
2.3. Assessment of Depressive Symptoms
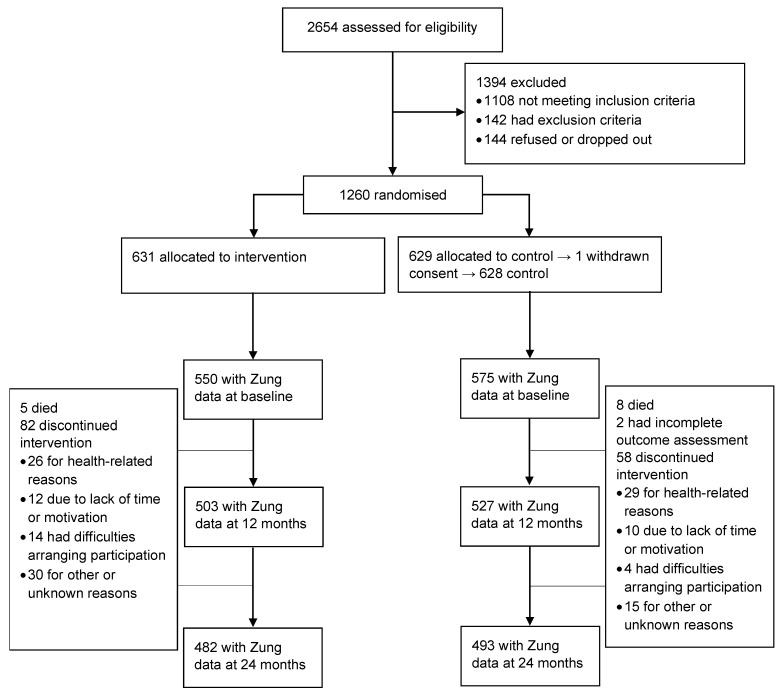
Depressive symptoms were evaluated at baseline, 12-month, and 24-month visits with the Zung Self-Rating Depression Scale (SDS), a 20-item questionnaire capturing the affective, psychological, and somatic symptoms associated with depression [33]. The scale has shown good validity and reliability [34,35]. Half of the items are phrased positively and half negatively, with items scored on a Likert scale ranging from 1 to 4. A total raw score ranging from 20 to 80 can be calculated, with a higher score indicating more severe depressive symptoms. An SDS index can be further calculated by dividing the total raw score by a maximum of 80. These two scores (raw and index) have led to some debate concerning cut-offs for clinically significant depressive symptoms [36]. Originally, a cut-off of 50 for the SDS index (representing a raw score of 40 points) was suggested [37]. In this study, we used this originally suggested cut-off of 40 points in the raw score. A CONSORT flow chart focusing on SDS data is provided as Figure 1.
Figure 1.
CONSORT flow chart of the study design.
2.4. Assessment of Cognition
Cognition was assessed for global cognition and cognitive domains with mNTB at baseline, 12-month, and 24-month visits [24]. A composite score for global cognition was calculated averaging 14 different tests standardised to z-scores using the total FINGER sample baseline mean and SD, with a higher score indicating better cognitive performance. As secondary cognitive outcomes, mNTB domain z-scores were calculated for executive functioning, memory, and processing speed, as previously described [24,25].
2.5. Other Factors
Participants’ annual study visits included anthropometric and blood pressure mea-surements; blood sampling; and questionnaires about sociodemographic factors, lifestyle, quality of life, and general health [24,25]. Self-reported antidepressant medication use was checked with the study doctor at screening and 24-month visit. Based on these data, we created a composite variable of antidepressant use at any timepoint vs. no antidepressant use. To account for the links between depressive symptoms and lifestyle, we calculated a healthy lifestyle change composite index for all participants, based on measures of diet, exercise frequency, and cardiovascular factors (based on the variables used in the FINRISK CVD risk equations) [38], as described earlier [39]. The index was calculated as the mean z-score change (with higher values indicating healthier change) if data were available on at least two of the three components.
2.6. Statistical Analyses
We compared baseline characteristics between the intervention and control groups, and between people with and without baseline Zung score data using the χ2 test, t-test, or Mann–Whitney U test (rank-sum), as appropriate. Mixed-effects regression models with maximum likelihood estimation were used to analyze intervention effects on depressive symptoms (zero-skewness log-transformed Zung score) as a function of the randomisation group, time, and group × time interaction (unadjusted model). Analyses were further adjusted for age, sex, education, trial site, antidepressant use, and healthy lifestyle change index.
We applied a parallel latent growth curve model using a covariance structure analysis framework for examining the associations between Zung score (zero-skewness log-transformed) and cognition. The baseline level (latent intercept) and the change (latent slope) of both Zung score and cognition over the 2-year trial were estimated, and their associations were studied using structural equation modelling (SEM). Maximum likelihood estimation on all available data was used. Graphical presentation of the overall model is shown in Figure S1 in Supplementary material. Analyses were executed grouped based on intervention allocation and initiated with a theoretical full path model (model 0) where all parameters were estimated as unequal between intervention and control groups, as if there were two separate models. Parameters were constrained as equal one at a time, and each model was tested against the full model using the likelihood ratio test (details in Supplementary Methods in Supplementary material). When all constraints were allowed, the model treated the groups as equal (model 5). In more advanced models, some paths were further constrained to [0], and these were shown to be the best-fitting models for all cognitive variables (Supplementary Methods and Table S2 in Supplementary material). We present the results for the best-fitting model and for an intermediate model (model 2), to describe the relations between depressive symptoms and cognition also separately for the intervention and control groups. All SEM analyses were adjusted for age, sex, education, trial site, antidepressant use, and healthy lifestyle change index and were conducted using SPSS Amos (IBM, Chicago, IL, USA), version 25 [40].
We investigated the potential impact of clinically relevant depressive symptoms at baseline (Zung score ≥40 vs. <40 points) on the intervention effects on cognition, using mixed-effects regression model with the best model fit approach. Analyses were initiated with a theoretical full path model, including group × time, group × Zung, time × Zung, and group × time × Zung interactions. The best-fitting models were selected constraining parameters as equal between groups, and each model was compared with the full model using the likelihood ratio test. Bayesian information criterion (BIC) was used to select the best-fitting model. Analyses were adjusted for age, sex, education, trial site, antidepressant use, and healthy lifestyle change index, and were conducted using Stata software for Windows, version 14 (StataCorp, College Station, TX, USA).
3. Results
Baseline depressive symptom data were available for 1125 (89.3%) participants. Participants with missing Zung score data included more women and individuals living alone, had a higher body mass index, consumed alcohol less often and fish more often, and had poorer executive functioning compared with participants with available data (Table 1). Baseline demographic, vascular and lifestyle factors, medical history, and cognitive performance were not significantly different between the intervention and control groups (Table S1 in Supplementary material).
Table 1.
Comparison of baseline characteristics between people with and without Zung data at baseline.
| n (Available /Missing) | Zung Data Available | Zung Data Missing | p-Value 1 | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | 1125/134 | 69.2 (4.7) | 69.9 (4.9) | 0.13 |
| Sex: number of women | 1125/134 | 502 (45%) | 85 (63%) | <0.001 |
| Education (years) | 1124/133 | 10.0 (3.5) | 9.4 (3.1) | 0.14 |
| Married/cohabiting | 1125/133 | 848 (75%) | 86 (65%) | 0.008 |
| Vascular factors | ||||
| Systolic blood pressure (mmHg) | 1117/133 | 140 (16.4) | 141 (14.5) | 0.51 |
| Diastolic blood pressure (mmHg) | 1117/133 | 80 (9.5) | 81 (8.9) | 0.52 |
| Serum total cholesterol (mmol/L) | 1120/134 | 5.1 (1.0) | 5.2 (1.0) | 0.29 |
| Fasting plasma glucose (mmol/L) | 1122/134 | 6.1 (0.9) | 6.0 (1.0) | 0.23 |
| 2 h oral glucose tolerance test (mmol/L) | 972/112 | 7.0 (2.2) | 7.2 (2.3) | 0.39 |
| Body-Mass Index (kg/m2) | 1115/133 | 28.1 (4.7) | 29.1 (4.5) | 0.010 |
| Waist circumference (cm) | 1116/133 | 98.2 (12.5) | 99.1 (12.1) | 0.40 |
| Lifestyle factors | ||||
| Physically active ≥2/week | 1117/129 | 793 (71%) | 89 (69%) | 0.64 |
| Current smokers | 1123/131 | 104 (9%) | 10 (8%) | 0.54 |
| Alcohol drinking ≥1/week | 1120/131 | 515 (46%) | 41 (31%) | 0.001 |
| Fish intake ≥2/week | 1122/130 | 576 (51%) | 79 (61%) | 0.042 |
| Daily vegetable intake | 1124/132 | 698 (62%) | 77 (58%) | 0.40 |
| Self-reported medical conditions | ||||
| Hypertension | 1118/133 | 733 (66%) | 98 (74%) | 0.061 |
| Hypercholesterolemia | 1115/134 | 749 (67%) | 90 (67%) | 1.00 |
| Diabetes | 1119/134 | 147 (13%) | 21 (16%) | 0.42 |
| History of myocardial infarction | 1119/134 | 60 (5%) | 4 (3%) | 0.24 |
| History of stroke | 1116/134 | 59 (5%) | 9 (7%) | 0.49 |
| Antidepressant use | 1125/134 | 62 (6%) | 12 (9%) | 0.11 |
| Cognition | ||||
| Global cognition (mNTB) | 1124/134 | 0.003 (0.57) | −0.08 (0.60) | 0.099 |
| Executive functioning (mNTB) | 1123/134 | 0.002 (0.68) | −0.15 (0.66) | 0.017 |
| Memory (mNTB) | 1124/134 | 0.0005 (0.67) | −0.02 (0.71) | 0.52 |
| Processing speed (mNTB) | 1124/134 | 0.01 (0.81) | −0.11 (0.89) | 0.17 |
| Mini Mental State Examination | 1122/134 | 26.8 (2.0) | 26.6 (2.0) | 0.48 |
Note: Data are presented as mean (SD) for continuous variables and as n (%) for categorical variables. mNTB, modified Neuropsychological Test Battery. 1 p-values < 0.05 are in bold.
3.1. Intervention Effects on Change in Zung Score
There was no significant difference in Zung score change between the intervention and control groups (group × time interaction coefficient −0.005, 95% CI −0.018 to 0.007, p = 0.40). The result was similar after adjusting for age, sex, education, trial site, antidepressant use, and healthy lifestyle change index (coefficient −0.006, 95% CI −0.019 to 0.007, p = 0.35). The observed mean Zung score decreased 0.73 (SD 5.6) points in the intervention and 0.36 (SD 5.6) points in the control group.
3.2. Associations between Zung Score and Cognition
Associations of Zung score and cognition are presented in Table 2. Baseline Zung score was inversely associated with baseline cognition, i.e., more pronounced depressive symptoms were related to poorer global cognition (path coefficient −0.302, p < 0.001), exe-cutive functioning (−0.360, p < 0.001), processing speed (−0.540, p < 0.001), and memory (−0.177, p = 0.043).
Table 2.
Associations between cognition and the Zung depression score during the FINGER trial.
| Path | Path Coefficient (Standard Error) | p-Value |
|---|---|---|
| Global cognition | ||
| Baseline Zung and baseline cognition (b0) | ||
| Intervention 1 | −0.372 (0.099) | <0.001 |
| Control 1 | −0.244 (0.100) | 0.015 |
| Best model; combined 2 | −0.302 (0.070) | <0.001 |
| Baseline Zung and cognitive change (b1) | ||
| Intervention 1 | −0.142 (0.085) | 0.095 |
| Control 1 | −0.103 (0.087) | 0.235 |
| Best model; combined 2 | −0.140 (0.050) | 0.005 |
| Zung change and cognitive change (b3) | ||
| Intervention 1 | −0.124 (0.776) | 0.873 |
| Control 1 | −0.404 (0.773) | 0.601 |
| Best model; combined 2 | constrained to 0 | N/A |
| Executive functioning domain | ||
| Baseline Zung and baseline cognition (b0) | ||
| Intervention 1 | −0.393 (0.120) | 0.001 |
| Control 1 | −0.270 (0.122) | 0.027 |
| Best model; combined 3 | −0.360 (0.081) | <0.001 |
| Baseline Zung and cognitive change (b1) | ||
| Intervention 1 | −0.096 (0.113) | 0.396 |
| Control 1 | −0.038 (0.109) | 0.726 |
| Best model; combined 3 | constrained to 0 | N/A |
| Zung change and cognitive change (b3) | ||
| Intervention 1 | 0.593 (0.926) | 0.522 |
| Control 1 | −1.085 (0.915) | 0.235 |
| Best model/intervention 3 | constrained to 0 | N/A |
| Best model/control 3 | −1.371 (0.736) | 0.063 |
| Memory domain | ||
| Baseline Zung and baseline cognition (b0) | ||
| Intervention 1 | −0.218 (0.127) | 0.087 |
| Control 1 | −0.160 (0.120) | 0.185 |
| Best model; combined 4 | −0.177 (0.088) | 0.043 |
| Baseline Zung and cognitive change (b1) | ||
| Intervention 1 | −0.238 (0.130) | 0.068 |
| Control 1 | −0.166 (0.132) | 0.210 |
| Best model; combined 4 | −0.231 (0.083) | 0.005 |
| Zung change and cognitive change (b3) | ||
| Intervention 1 | −0.697 (1.108) | 0.529 |
| Control 1 | −0.296 (1.080) | 0.784 |
| Best model; combined 4 | constrained to 0 | N/A |
| Processing speed domain | ||
| Baseline Zung and baseline cognition (b0) | ||
| Intervention 1 | −0.648 (0.145) | <0.001 |
| Control 1 | −0.344 (0.147) | 0.019 |
| Best model; combined 4 | −0.540 (0.100) | <0.001 |
| Baseline Zung and cognitive change (b1) | ||
| Intervention 1 | −0.069 (0.112) | 0.535 |
| Control 1 | −0.120 (0.108) | 0.268 |
| Best model; combined 4 | constrained to 0 | N/A |
| Zung change and cognitive change (b3) | ||
| Intervention 1 | −1.051 (0.984) | 0.286 |
| Control 1 | 0.604 (0.851) | 0.478 |
| Best model; combined 4 | constrained to 0 | N/A |
Note: Path refers to Figure S1 and Table S2 in Supplementary material. Baseline refers to the latent intercept and change refers to the latent slope estimated with parallel growth curves. All models are adjusted for age, sex, education, trial site, antidepressant use, and healthy lifestyle change index. p-values < 0.05 are shown in bold. 1 Model 2 (see Supplementary Methods in Supplementary material for details), where measurement error covariances are estimated as equal between groups. 2 Model 6 (see Supplementary Methods in Supplementary material for details), where all parameters are estimated as equal and the path slope (Zung)-slope (cognition) is constrained to [0]. 3 Model 6 (see Supplementary Methods in Supplementary material for details), where the path slope (Zung)-slope (cognition) is constrained to [0] in the intervention group. 4 Model 7 (see Supplementary Methods in Supplementary material for details), where all parameters are estimated as equal and the path intercept (Zung)-slope (cognition) is constrained to [0].
In the intervention and control groups combined, a higher baseline Zung score was associated with less improvement in global cognition (path coefficient −0.140, p = 0.005) and memory (−0.231, p = 0.005) over two years (Table 2). No associations were found for the intervention or control groups separately, or with changes in other cognitive domains (Table 2).
Change in Zung score was not significantly associated with changes in global cognition or cognitive domains in either allocation group. A decrease in Zung score showed a trend for association with an increase in executive functioning in the control group (Table 2, path coefficient −1.371, p = 0.063).
Table 3 (also Table S3 in Supplementary material) shows associations between the dichotomous baseline Zung score (≥40 points, N = 243 vs. <40 points, N = 882 participants) and cognitive change. The full model fit best for executive functioning, whereas the least loaded models with only one interaction term (time × Zung) fit best for global cognition, memory, and processing speed (detailed in Table S4 in Supplementary material). The randomisation group × time × Zung interaction coefficient was −0.096 (95% CI −0.163 to −0.028, p = 0.005) for executive functioning, indicating less intervention benefit in this cognitive domain among participants with versus without clinically significant depressive symptoms. Among all participants irrespective of group allocation, Zung score ≥40 points were associated with less improvement in global cognition (time × Zung coefficient −0.035, 95% CI −0.062 to −0.008, p = 0.010) and memory (time × Zung coefficient −0.044, 95% CI −0.088 to −0.0001, p = 0.049) (Table 3).
Table 3.
The effect of clinically significant depressive symptoms at baseline on change in cognition (results of the best-fitting model).
| Global Cognition | Executive Functioning | Memory | Processing Speed | |
|---|---|---|---|---|
| Estimate (95 % CI), p-Value | ||||
| Baseline Zung and baseline cognition (“Zung”) |
−0.153 (−0.226–−0.079), p < 0.001 |
−0.140 (−0.267–−0.013), p = 0.031 |
−0.148 (−0.240–−0.055), p = 0.002 |
−0.148 (−0.257–−0.039), p = 0.008 |
| Baseline Zung and change in cognition (“Time × Zung”) |
−0.035 (−0.062–−0.008), p = 0.010 |
0.014 (−0.033–0.061), p = 0.562 |
−0.044 (−0.088–−0.0001), p = 0.049 |
−0.033 (−0.069–0.004), p = 0.081 |
| Impact on intervention effect (“Group × time × Zung”) |
N/A | −0.096 (−0.163–−0.028), p = 0.005 |
N/A | N/A |
Note: The best-fitting mixed-effects regression model is based on the lowest accepted Bayesian Information Criterion (BIC) shown in Table S4 in Supplementary material. Analyses are adjusted for age, sex, education, trial site, antidepressant use, and healthy lifestyle change index. Other parameters included in the best-fitting model: “Randomisation group”, “Time”, “Group × time”, and “Group × Zung” (see Table S3 in Supplementary material).
4. Discussion
In the FINGER multidomain lifestyle RCT, there was no difference in change in dep-ressive symptoms between the intervention and control groups. Overall, more pronounced depressive symptoms at baseline were associated with poorer baseline cognition (global and all domains) and less improvement in global cognition and memory over time, irrespective of intervention allocation. The participants with a baseline Zung score of ≥40 points (suggesting clinically relevant depressive symptoms) seemed to have smaller intervention-related benefits on executive functioning, compared with participants with a Zung score of <40 points.
Although the FINGER intervention had significant benefits on cognition in older adults at risk for dementia from the general population [24], the lack of effect on depressive symptoms was similar to the multidomain MAPT and preDIVA trials [22,23]. Due to its primary focus on early prevention of cognitive decline in at-risk individuals without substantial cognitive impairment, the FINGER trial excluded people with major depression. The mean baseline Zung score was relatively low (33.9 points), leaving less room for improvement compared with RCTs targeting people with depression. The focus on this specific target population, and the exclusion criteria, has probably contributed to the nonsignificant findings regarding intervention effects on depressive symptoms. In addition, in this study, people with missing Zung data included, e.g., more women, individuals living alone, and those with a higher BMI. These factors are also often associated with depressive symptoms, as well as an increased dementia risk [1,4]. Thus, the missing data may have led to an underestimation of the intervention effects on depressive symptoms and their impact on cognition. Previous studies have reported lifestyle-based interventions to be effective in reducing depressive symptoms [6,8,10,12,15]. However, these studies were not primarily designed for preventing cognitive decline or dementia and had different target populations and intervention designs compared with the FINGER.
Depressive symptoms have been associated with poorer cognition in several domains [3]. In line with this, we found that more pronounced depressive symptoms at baseline were related to less cognitive improvement over two years in the FINGER participants irrespective of group allocation. Given the small overall change in depressive symptoms during the trial, it is perhaps not surprising that this was not associated with a change in cognition.
Previous studies have shown that depressive symptoms do not necessarily hinder participants from benefitting from lifestyle interventions [19,20]. However, such studies focused on physical fitness and cardiovascular and diabetes outcomes [19,20], without considering cognition. Our finding, suggesting that clinically relevant depressive symptoms may affect intervention-related benefits to cognition, is particularly interesting in this context. This effect was observed for executive functioning but not global cognition or other cognitive domains. Executive functioning is often the first cognitive domain affected in preclinical Alzheimer’s disease [41] and is also commonly affected in depression [42]. Executive dysfunction is also common in vascular cognitive impairment [43]. There is a well-established association between depression and vascular dementia, although the direction of this association is uncertain [4,43]. Depressive symptomatology may decrease cognitive reserve [44] or may be a prodromal manifestation of dementia-related diseases [4,5], i.e., people with clinically significant depressive symptoms may be closer to cognitive disorder onset and may thus have less room for prevention. Furthermore, it was previously reported that depressive symptoms may affect adherence, dropout, and willingness to participate in intervention studies [16,18]. In the FINGER trial, more pronounced depressive symptoms were related to lower adherence to the physical exercise intervention [45].
The effect of baseline depressive symptoms on the intervention-related benefit to executive functioning was not observed for the continuous Zung score. This may suggest a “threshold effect” related to clinical significance. While the proposed clinical cut-off for the Zung scale has been debated [36], a cut-off of 39–40 is not uncommon in studies with older participants [46,47]. In this study, only 243 participants (21.6%) had a baseline Zung score of ≥40 points. Although we cannot fully confirm the clinical significance (or duration) of their depressive symptoms, our finding suggests that such individuals may require a different approach to prevent cognitive decline, e.g., more tailored support for adherence to a healthy lifestyle, or closer monitoring and early treatment of depression. Our analyses were adjusted for both healthy lifestyle changes (diet, exercise, and cardiovascular factors) and antidepressant medications, but the potential contribution of other lifestyle factors and medication-related factors (drug type, dose, and duration of treatment) could not be fully accounted for. It is also unclear if the observed effect is due to the depressive symptoms themselves or to an underlying dementia-related disease causing both depressive symptoms and poorer cognition. In the latter case, disease-modifying drugs may be needed, in addition to healthy lifestyle changes for preventing/delaying cognitive impairment.
The main strengths of this study include the large, longer-term population-based RCT with a thoroughly designed and conducted multidomain lifestyle intervention and comprehensive repeated measures of both cognition and depressive symptoms [24,25]. The Zung scale has been evaluated in community-based older populations and shown to have acceptable sensitivity and specificity [47].
The study has several limitations. Due to the primary focus of the trial, individuals with major depression were excluded, leaving less room to observe changes in depressive symptoms over time. Although the FINGER population is representative of the at-risk segment of the older Finnish general population without substantial cognitive impairment/dementia [26], as is common in RCTs, recruited individuals may have been more health-conscious and willing to adhere to the intervention than nonparticipants. Participants with missing data on the Zung scale had poorer baseline executive functioning, which may have diluted our findings. Due to the early prevention approach, dementia was not a feasible outcome after two years.
Analyses of the associations between depressive symptoms and cognition were post hoc, and the trial was not powered for three-way interactions. Our findings would thus need to be verified in other multidomain dementia prevention trials [48]. Future studies should also consider the potential impact of neuropathology (e.g., amyloid accumulation) on the interactions between depressive symptoms and cognition in the interventional context [49,50].
5. Conclusions
Depressive symptoms affect cognitive performance. If such symptoms are clinically significant, they may also influence the cognitive benefit from lifestyle interventions for dementia risk reduction. Although this finding requires further verification, future dementia-prevention trials may benefit from adapting specific intervention strategies for participants with clinically significant depressive symptoms.
Acknowledgments
We cordially thank all participants of the FINGER study and all members of the FINGER study group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11051449/s1, Figure S1: Graphical presentation of the growth curve model, Table S1: Baseline characteristics of the FINGER trial population with baseline Zung data, Supplementary Methods: Modelling and model selection of parallel process latent growth curves, Table S2: Model fit and model comparison for all nested parallel growth curve models of cognition and Zung score, Table S3: The effect of clinically significant depressive symptoms at baseline on change in cognition (results of the best-fitting model), Table S4: Assessment of the best-fitting model regarding the effects of baseline dichotomised Zung score using Bayesian Information Criterion (BIC).
Author Contributions
Conceptualization, T.N., R.A., T.H., T.L., J.L. (Jaana Lindström), T.P., H.S., T.S., J.T., M.K. and A.S.; Data curation, E.L.; Formal analysis, E.N. and E.L.; Funding acquisition, T.N., R.A., T.L., J.L. (Jaana Lindström), H.S., T.S., J.T., M.K. and A.S.; Methodology, E.N., J.L. (Jenni Lehtisalo), T.N., E.L. and A.S.; Project administration, J.L. (Jenni Lehtisalo), T.N., R.A., T.H., J.L. (Jaana Lindström), T.S. and J.T.; Supervision, J.L. (Jenni Lehtisalo), T.N. and M.K.; Writing—original draft, E.N., E.L. and A.S.; Writing—review and editing, E.N., J.L. (Jenni Lehtisalo), T.N., R.A., T.L., T.P., H.S., T.S., J.T. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Orion Research Foundation (Finland); Finnish Medical Foundation; Finnish Cultural Foundation; Juho Vainio Foundation (Finland); Jalmari and Rauha Ahokas Foundation (Finland); State research funding (EVO/VTR grants) from Oulu City Hospital and Kuopio University Hospital (Finland); Academy of Finland, grants 317465, 287490, 294061, and 319318; Finnish Social Insurance Institution; Finnish Ministry of Education and Culture; Alzheimer’s Research and Prevention Foundation (US); Alzheimerfonden (Sweden); Center for Innovative Medicine (CIMED) at Karolinska Institutet (Sweden); Region Stockholm ALF (Sweden); Knut and Alice Wallenberg Foundation (Sweden); Stiftelsen Stockholms Sjukhem (Sweden); Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse (Sweden); Swedish Research Council for Health, Working Life, and Welfare; European Research Council, grant 804371; EU Joint Programme - Neurodegenerative Disease Research; and Yrjö Jahnsson Foundation (Finland).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Coordinating Ethics Committee of the Hospital Dist-rict of Helsinki and Uusimaa (approval number 94/13/03/00/2009 and date 7 April 2009).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data used in this study are not publicly available due to ethical and legal reasons, but the data are available upon request. Those fulfilling the requirements for viewing confidential data as required by the Finnish legislation and the Finnish Institute for Health and Welfare are able to access the data after completion of a material transfer agreement. Requests may be directed to kirjaamo@thl.fi.
Conflicts of Interest
Hilkka Soininen has served in the advisory boards of ACImmune and Novo Nordisk outside of this work. Other authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. World Health Organization; Geneva, Switzerland: 2019. [PubMed] [Google Scholar]
- 3.Shimada H., Park H., Makizako H., Doi T., Lee S., Suzuki T. Depressive symptoms and cognitive performance in older adults. J. Psychiatr. Res. 2014;57:149–156. doi: 10.1016/j.jpsychires.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Bennett S., Thomas A.J. Depression and dementia: Cause, consequence or coincidence? Maturitas. 2014;79:184–190. doi: 10.1016/j.maturitas.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Byers A.L., Yaffe K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooney G.M., Dwan K., Greig C.A., Lawlor D.A., Rimer J., Waugh F.R., McMurdo M., Mead G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013;9:CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammen G., Faulkner G. Physical Activity and the Prevention of Depression: A Systematic Review of Prospective Studies. Am. J. Prev. Med. 2013;45:649–657. doi: 10.1016/j.amepre.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Lv M.-R., Wei Y.-J., Sun L., Zhang J.-X., Zhang H.-G., Li B. Dietary patterns and depression risk: A meta-analysis. Psychiatry Res. 2017;253:373–382. doi: 10.1016/j.psychres.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Villegas A., Martínez-González M.A., Estruch R., Salas-Salvadó J., Corella D., Covas M.I., Arós F., Romaguera D., Gómez-Gracia E., Lapetra J., et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013;11:208. doi: 10.1186/1741-7015-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coventry P.A., Bower P., Keyworth C., Kenning C., Knopp J., Garrett C., Hind D., Malpass A., Dickens C. The Effect of Complex Interventions on Depression and Anxiety in Chronic Obstructive Pulmonary Disease: Systematic Review and Meta-Analysis. PLoS ONE. 2013;8:e60532. doi: 10.1371/journal.pone.0060532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruins J., Jörg F., Bruggeman R., Slooff C., Corpeleijn E., Pijnenborg M. The effects of lifestyle interventions on (long-term) weight management, cardiometabolic risk and depressive symptoms in people with psychotic disorders: A meta-analysis. PLoS ONE. 2014;9:e112276. doi: 10.1371/journal.pone.0112276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadden T.A. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: The Look AHEAD Trial. Diabetes Care. 2014;37:1544–1553. doi: 10.2337/dc13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cezaretto A., Ferreira S.R.G., Sharma S., Sadeghirad B., Kolahdooz F. Impact of lifestyle interventions on depressive symptoms in individuals at-risk of, or with, type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2016;26:649–662. doi: 10.1016/j.numecd.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Millán-Calenti J.C., Lorenzo T., Núñez-Naveira L., Buján A., Rodríguez-Villamil J.L., Maseda A. Efficacy of a computerized cognitive training application on cognition and depressive symptomatology in a group of healthy older adults: A randomized controlled trial. Arch. Gerontol. Geriatr. 2015;61:337–343. doi: 10.1016/j.archger.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Ng T.-P., Nyunt M.S.Z., Feng L., Feng L., Niti M., Tan B.Y., Chan G., Khoo S.A., Chan S.M., Yap P., et al. Multi-domains lifestyle interventions reduces depressive symptoms among frail and pre-frail older persons: Randomized controlled trial. J. Nutr. Health Aging. 2017;21:918–926. doi: 10.1007/s12603-016-0867-y. [DOI] [PubMed] [Google Scholar]
- 16.Mazzeschi C., Pazzagli C., Buratta L., Reboldi G.P., Battistini D., Piana N., Pippi R., Fatone C., De Feo P. Mutual interactions between depression/quality of life and adherence to a multidisciplinary lifestyle intervention in obesity. J. Clin. Endocrinol. Metab. 2012;97:E2261–E2265. doi: 10.1210/jc.2012-2364. [DOI] [PubMed] [Google Scholar]
- 17.Cezaretto A., Pakseresht M., Sharma S., Kolahdooz F., Siqueira-Catania A., Barros C.R., Ferreira S.R. Influence of depression on cardiometabolic responses to a lifestyle intervention in at-risk individuals. J. Affect. Disord. 2015;174:516–521. doi: 10.1016/j.jad.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Beishuizen C.R.L., Coley N., Moll van Charante E.P., van Gool W.A., Richard E., Andrieu S. Determinants of Dropout and Nonadherence in a Dementia Prevention Randomized Controlled Trial: The Prevention of Dementia by Intensive Vascular Care Trial. J. Am. Geriatr. Soc. 2017;65:1505–1513. doi: 10.1111/jgs.14834. [DOI] [PubMed] [Google Scholar]
- 19.Rautio N., Jokelainen J., Oksa H., Saaristo T., Moilanen L., Vanhala M., Peltonen M., Korpi-Hyövälti E., Tuomilehto J., Uusitupa M., et al. Do depressive symptoms have an impact on the effectiveness of lifestyle counseling in prevention of type 2 diabetes? One-year follow-up of FIN-D2D. Prim. Care Diabetes. 2014;8:43–47. doi: 10.1016/j.pcd.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Matthews M.M., Hsu F.-C., Walkup M.P., Barry L.C., Patel K.V., Blair S.N. Depressive symptoms and physical performance in the Lifestyle Interventions and Independence for Elders Pilot study. J. Am. Geriatr. Soc. 2011;59:495–500. doi: 10.1111/j.1532-5415.2011.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 22.Moll van Charante E.P., Richard E., Eurelings L.S., van Dalen J.W., Ligthart S.A., van Bussel E.F., Hoevenaar-Blom M.P., Vermeulen M., van Gool W.A. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): A cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 23.Andrieu S., Guyonnet S., Coley N., Cantet C., Bonnefoy M., Bordes S., Bories L., Cufi M.-N., Dantoine T., Dartigues J.-F., et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 24.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., Bäckman L., Hänninen T., Jula A., Laatikainen T., et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 25.Kivipelto M., Solomon A., Ahtiluoto S., Ngandu T., Lehtisalo J., Antikainen R., Bäckman L., Hänninen T., Jula A., Laatikainen T., et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): Study design and progress. Alzheimers Dement. 2013;9:657–665. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Ngandu T., Lehtisalo J., Levälahti E., Laatikainen T., Lindström J., Peltonen M., Solomon A., Ahtiluoto S., Antikainen R., Hänninen T., et al. Recruitment and baseline characteristics of participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)—A randomized controlled lifestyle trial. Int. J. Environ. Res. Public Health. 2014;11:9345–9360. doi: 10.3390/ijerph110909345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saaristo T., Peltonen M., Keinänen-Kiukaanniemi S., Vanhala M., Saltevo J., Niskanen L., Oksa H., Korpi-Hyövälti E., Tuomilehto J., FIN-D2D Study Group National type 2 diabetes prevention programme in Finland: FIN-D2D. Int. J. Circumpolar. Health. 2007;66:101–112. doi: 10.3402/ijch.v66i2.18239. [DOI] [PubMed] [Google Scholar]
- 28.Vartiainen E., Laatikainen T., Peltonen M., Juolevi A., Männistö S., Sundvall J., Jousilahti P., Salomaa V., Valsta L., Puska P. Thirty-five-year trends in cardiovascular risk factors in Finland. Int. J. Epidemiol. 2010;39:504–518. doi: 10.1093/ije/dyp330. [DOI] [PubMed] [Google Scholar]
- 29.Kivipelto M., Ngandu T., Laatikainen T., Winblad B., Soininen H., Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 30.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G., Mellits E.D., Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 31.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Harrison J., Minassian S.L., Jenkins L., Black R.S., Koller M., Grundman M. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch. Neurol. 2007;64:1323–1329. doi: 10.1001/archneur.64.9.1323. [DOI] [PubMed] [Google Scholar]
- 33.Zung W.W. A Self-Rating Depression Scale. Arch. Gen. Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 34.Biggs J.T., Wylie L.T., Ziegler V.E. Validity of the Zung Self Rating Depression Scale. Br. J. Psychiatry. 1978;132:381–385. doi: 10.1192/bjp.132.4.381. [DOI] [PubMed] [Google Scholar]
- 35.Gabrys J.B., Peters K. Reliability, discriminant and predictive validity of the Zung self-rating depression scale. Psychol. Rep. 1985;57:1091–1096. doi: 10.2466/pr0.1985.57.3f.1091. [DOI] [PubMed] [Google Scholar]
- 36.Dunstan D.A., Scott N. Assigning Clinical Significance and Symptom Severity Using the Zung Scales: Levels of Misclassification Arising from Confusion between Index and Raw Scores. Depress. Res. Treat. 2018;2018:9250972. doi: 10.1155/2018/9250972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zung W.W. From art to science. The diagnosis and treatment of depression. Arch. Gen. Psychiatry. 1973;29:328–337. doi: 10.1001/archpsyc.1973.04200030026004. [DOI] [PubMed] [Google Scholar]
- 38.Vartiainen E., Laatikainen T., Peltonen M., Puska P. Predicting Coronary Heart Disease and Stroke: The FINRISK Calculator. Glob. Heart. 2016;11:213–216. doi: 10.1016/j.gheart.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Sindi S., Solomon A., Kåreholt I., Hovatta I., Antikainen R., Hänninen T., Levälahti E., Laatikainen T., Lehtisalo J., Lindström J., et al. Telomere length change in a multidomain lifestyle intervention to prevent cognitive decline: A randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76:491–498. doi: 10.1093/gerona/glaa279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arbuckle J.L. Amos (Version 25) [Computer Program] IBM SPSS; Chicago, IL, USA: 2017. [Google Scholar]
- 41.Harrington M.G., Chiang J., Pogoda J.M., Gomez M., Thomas K., Deboard Marion S., Miller K.J., Siddarth P., Yi X., Zhou F., et al. Executive function changes before memory in preclinical Alzheimer’s pathology: A prospective, cross-sectional, case control study. PLoS ONE. 2013;8:e79378. doi: 10.1371/journal.pone.0079378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014;44:2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos G. Mechanisms and treatment of late-life depression. Transl. Psychiatry. 2019;9:188. doi: 10.1038/s41398-019-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butters M.A., Young J.B., Lopez O., Aizenstein H.J., Mulsant B.H., Reynolds C.F., III, DeKosky S.T., Becker J.T. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin. Neurosci. 2008;10:345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coley N., Ngandu T., Lehtisalo J., Soininen H., Vellas B., Richard E., Kivipelto M., Andrieu S., HATICE, FINGER, and MAPT/DSA groups Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimers Dement. 2019;15:729–741. doi: 10.1016/j.jalz.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Dunstan D.A., Scott N., Todd A.K. Screening for anxiety and depression: Reassessing the utility of the Zung scales. BMC Psychiatry. 2017;17:329. doi: 10.1186/s12888-017-1489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jokelainen J., Timonen M., Keinänen-Kiukaanniemi S., Härkönen P., Jurvelin H., Suija K. Validation of the Zung self-rating depression scale (SDS) in older adults. Scand. J. Prim. Health Care. 2019;37:353–357. doi: 10.1080/02813432.2019.1639923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kivipelto M., Mangialasche F., Snyder H.M., Allegri R., Andrieu S., Arai H., Baker L., Belleville S., Brodaty H., Brucki S.M., et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16:1078–1094. doi: 10.1002/alz.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gatchel J.R., Rabin J.S., Buckley R.F., Locascio J.J., Quiroz Y.T., Yang H.S., Vannini P., Amariglio R.E., Rentz D.M., Properzi M., et al. Longitudinal Association of Depression Symptoms With Cognition and Cortical Amyloid Among Community-Dwelling Older Adults. JAMA Netw. Open. 2019;8:e198964. doi: 10.1001/jamanetworkopen.2019.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrington K.D., Lim Y.Y., Gould E., Maruff P. Amyloid-beta and depression in healthy older adults: A systematic review. Aust. N. Z. J. Psychiatry. 2015;49:36–46. doi: 10.1177/0004867414557161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are not publicly available due to ethical and legal reasons, but the data are available upon request. Those fulfilling the requirements for viewing confidential data as required by the Finnish legislation and the Finnish Institute for Health and Welfare are able to access the data after completion of a material transfer agreement. Requests may be directed to kirjaamo@thl.fi.