Abstract
The in vitro and in vivo activities of T-3811ME, a novel des-F(6)-quinolone, were evaluated in comparison with those of some fluoroquinolones, including a newly developed one, trovafloxacin. T-3811, a free base of T-3811ME, showed a wide range of antimicrobial spectra, including activities against Chlamydia trachomatis, Mycoplasma pneumoniae, and Mycobacterium tuberculosis. In particular, T-3811 exhibited potent activity against various gram-positive cocci, with MICs at which 90% of the isolates are inhibited (MIC90s) of 0.025 to 6.25 μg/ml. T-3811 was the most active agent against methicillin-resistant Staphylococcus aureus and streptococci, including penicillin-resistant Streptococcus pneumoniae (PRSP). T-3811 also showed potent activity against quinolone-resistant gram-positive cocci with GyrA and ParC (GrlA) mutations. The activity of T-3811 against members of the family Enterobacteriaceae and nonfermentative gram-negative rods was comparable to that of trovafloxacin. In common with other fluoroquinolones, T-3811 was highly active against Haemophilus influenzae, Moraxella catarrhalis, and Legionella sp., with MIC90s of 0.0125 to 0.1 μg/ml. T-3811 showed a potent activity against anaerobic bacteria, such as Bacteroides fragilis and Clostridium difficile. T-3811 was the most active agent against C. trachomatis (MIC, 0.008 μg/ml) and M. pneumoniae (MIC90, 0.0313 μg/ml). The activity of T-3811 against M. tuberculosis (MIC90, 0.0625 μg/ml) was potent and superior to that of trovafloxacin. In experimental systemic infection with a GrlA mutant of S. aureus and experimental pneumonia with PRSP in mice, T-3811ME showed excellent therapeutic efficacy in oral and subcutaneous administrations.
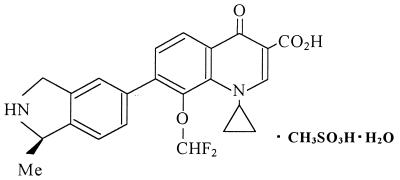
In our course of searching for a new quinolone with a high level of activity against gram-positive bacteria, including methicillin-resistant staphylococci, penicillin-resistant streptococci, and vancomycin-resistant enterococci, as well as activity against gram-negative bacteria, we have found that the 7-(2,3-dihydroisoindol-5-yl)-6(H)-quinolones have good antimicrobial activities, particularly against gram-positive bacteria, and are less toxic than the corresponding F(6) compounds in mice (8, 18, 22). T-3811ME {1-cyclopropyl-8-(difluoromethoxy)-7-[(1R)-(1-methyl-2,3-dihydro-1H-5-isoindolyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid methanesulfonate monohydrate} (Fig. 1) was chosen as a candidate for development from numerous synthesized compounds and is under development for both oral and parenteral administration.
FIG. 1.
Chemical structure of T-3811ME.
In this study, the in vitro and in vivo antibacterial activities of T-3811ME were mainly compared with those of ciprofloxacin, levofloxacin, and a novel naphthyridone compound, trovafloxacin (4, 14, 16, 24–26), against wide range of pathogens, including members of genera Mycobacterium, Mycoplasma, and Chlamydia.
MATERIALS AND METHODS
Antimicrobial agents.
The following agents were employed: T-3811ME (T-3811 methanesulfonate monohydrate), T-3811 (free base of T-3811ME), ciprofloxacin, trovafloxacin, and levofloxacin. T-3811, T-3811ME, and trovafloxacin were synthesized at the Research Laboratories of Toyama Chemical Co., Ltd. (Tokyo, Japan). Ciprofloxacin and levofloxacin were extracted from commercially available tablets. The purity of these two agents was above 99.8%, as measured by high-performance liquid chromatography. The rifampin (Sato Yakuhin Co., Ltd., Tokyo, Japan) and vancomycin (Shionogi Seiyaku Co., Ltd., Osaka, Japan) used in this study were commercial products.
Organisms.
A total of 1,160 clinical isolates used in this study were collected from various hospitals and research institutes in Japan from 1994 to 1997. The quinolone-resistant gram-positive cocci used in this study were as follows: S. aureus with a GyrA GrlA mutation, S. pneumoniae with a ParC mutation, and Enterococcus faecalis with a GyrA mutation. These strains were also obtained as clinical isolates from various hospitals in Japan. The nucleotide sequences of gyrA and parC (grlA) in the quinolone resistance determining regions of these quinolone-resistant strains were determined by the dideoxy chain termination method as previously reported (21). Chlamydia trachomatis D/UW-3/Cx was used for determination of antichlamydia activity.
Animals.
Male ICR mice (3.5 weeks old) were purchased from Japan SLC, Inc., (Shizuoka, Japan) and were assigned to the study after an acclimation period of 3 days. On the day of infection, the mice were randomly allocated into groups of 10.
In vitro studies.
T-3811, the free base of T-3811ME, was used for in vitro studies.
(i) Antibacterial activity.
MICs were determined by the standard agar dilution method by the Japan Society of Chemotherapy (10, 11). Mueller-Hinton medium (Difco, Detroit, Mich.) was employed for aerobic bacteria. Mueller-Hinton medium was supplemented with 5% defibrinated sheep blood (Nippon Bio-Test Laboratories, Tokyo, Japan) and 5% Fildes enrichment (Difco) to support the growth of fastidious bacteria. GAM medium (Nissui Seiyaku Co., Ltd., Tokyo, Japan) was used for anaerobic bacteria. Almost all strains were tested at a final inoculum of 104 CFU/spot, with a few selected strains tested at an increased inoculum of 106 CFU/spot, by using a multipoint inoculator (Sakuma Seisakusyo, Tokyo, Japan). Aerobic bacteria were incubated at 37°C for 18 to 24 h in air, except for fastidious bacteria, which were incubated in an atmosphere enriched with 5% carbon dioxide. Anaerobic bacteria were incubated in an anaerobic cabinet (Forma scientific anaerobic system model) in an atmosphere of 10% hydrogen, 10% carbon dioxide, and 80% nitrogen. The MIC was defined as the lowest antibiotic concentration that prevented the visible growth of bacteria. For susceptibility testing of Legionella sp., Legionella medium (Difco) supplemented with Legionella enrichment broth (Difco) was used. MICs of the agents for Legionella sp. strains were determined by a broth microdilution procedure recommended by the Japan Society of Chemotherapy (13). The bacterial suspensions for inocula were prepared by 10-fold dilution of the precultures in which the strains were cultured for 3 days at 35°C, and 5 μl of each of the suspensions was inoculated into 96-well microplates (Corning, Corning, N.Y.) containing 100 μl of antibiotic solution. The microplates were incubated for 3 days at 35°C. The MIC was defined as the lowest antibiotic concentration which prevented visible growth.
Antimycobacterium activity was studied by the broth dilution method. The Mycobacterium tuberculosis and Mycobacterium avium complex samples used in this preliminary study comprised 10 and 8 clinical isolates, respectively. For susceptibility testing of Mycobacterium, Dubos broth medium (Difco) containing 5% glycerol (Bacto Glycerol; Difco) and 10% albumin (Difco) as a supplement was used. The bacterial suspensions for inocula were prepared by 10-fold dilution of the precultures in which the strains were cultured for 14 days at 37°C, and 5 μl of suspension was inoculated into 96-well microplates containing 100 μl of antibiotic solution. The microplates were incubated for 14 days at 37°C. The MIC was defined as the lowest antibiotic concentration that prevented visible growth.
(ii) Antimycoplasma activity.
A broth microdilution procedure was used according to the method of K. G. Whithear et al. (26). The medium used for MIC determination was PPLO broth (without CV; Difco) supplemented with 2.5% yeast extract (Oriental Yeast Co., Ltd., Tokyo, Japan) and 20% horse serum (donor horse serum; JRH Bioscience Co., Ltd., Tokyo, Japan), 0.5% glucose, 0.002% phenol red, and 500 μg of ampicillin per ml (Asahi Kasei Co., Ltd., Tokyo, Japan). Preculture was carried out with the same medium for 1 week. The cell suspension was prepared by twofold dilution of the precultures. Ten microliters of suspension was inoculated into 96-well microplates containing 90 μl of antibiotic solution. The MICs were determined after 5 days of incubation at 37°C. MIC determination was carried out by examining the change in color from red to yellow, because phenol red produced a yellow color (acidification) after cell growth resulting in consumption of glucose.
(iii) Antichlamydia activity.
The MICs for antichlamydia activity were determined by the twofold broth dilution method recommended by the Japan Society of Chemotherapy by using a microplate (12). One milliliter of HeLa 229 cell suspension (2.0 × 105 cells/ml) cultured in minimal essential medium (MEM) (Nissui Seiyaku Co., Ltd., Tokyo, Japan) containing 10% fetal calf serum as a supplement was incubated overnight at 37°C in an atmosphere enriched with 5% carbon dioxide. After the removal of the culture medium, C. trachomatis cells at 104 inclusion-forming units/well, suspended in sucrose-phosphate-glutamic acid medium, were inoculated into HeLa 229 cells. Chlamydia cells were absorbed by the centrifugation at 700 × g for 1 h. After the second removal of culture medium, the new MEM containing the agents was added. The microplates were incubated for 3 days at 37°C. The MIC was taken as the lowest concentration that inhibited the development of inclusion bodies.
In vivo therapeutic efficacy.
T-3811ME was used for the in vivo therapeutic efficacy test, experimental systemic infections, and experimental pneumonia.
(i) Experimental systemic infection.
S. aureus CR-3 (GrlA mutant) and S. aureus CRCP-9 (GrlA GyrA double mutant) were used for experimental systemic infections in mice. The bacterial cells, which were prepared from an overnight culture on heart infusion agar (HIA; Eiken Kagaku Co., Ltd., Tokyo, Japan) at 37°C, were suspended in saline to give a final concentration of approximately 108 CFU/ml. The inocula were obtained by 10-fold dilution in saline containing 5% gastric mucin (Difco). Intraperitoneal injection of 0.5 ml of 4.0 × 107 to 10.4 × 107 CFU/ml induced systemic infections. The agents for the therapy by oral administration were suspended and diluted in 0.5% methylcellulose SM-400 (Shin-Etsu Kagaku Co., Ltd., Tokyo, Japan). For therapy by subcutaneous administration, T-3811ME was dissolved and diluted in 5% mannitol. Ciprofloxacin and levofloxacin were dissolved in 0.1 N NaOH and diluted in saline. The diluted agents were administered orally or subcutaneously at 1 h after infection. The total number of surviving mice at day 7 postchallenge was recorded, the 50% effective dose (ED50) and confidence limits were determined, and the significance of differences between groups was estimated by the Probit method. The ED50s of T-3811ME were equivalent to those of T-3811.
(ii) Experimental pneumonia.
A clinically isolated S. pneumoniae strain, D-979 (PRSP), was used for experimental pneumonia in mice. The bacterial cells, which were prepared from overnight cultures on an HIA plate containing 5% sheep blood at 37°C, were suspended in brain heart infusion broth (BHIB; Eiken Kagaku Co., Ltd.) to give a final concentration of 108 CFU/ml. After 10-fold dilution in BHIB, this suspension was cultured at 37°C for 4 h with shaking. The inocula were obtained by 100-fold dilution in BHIB. Experimental pneumonia was induced in mice by intratracheal injection of 0.05 ml of this suspension while the mice were under ether anesthetization. Oral or subcutaneous administration three times a day (at intervals of 4 h) over 3 days was started 20 h after the infection. The total number of mice surviving at day 10 postchallenge was recorded, the ED50 and the confidence limits were determined, and the significance of differences between groups was estimated by the Probit method. The ED50s of T-3811ME were also equivalent to those of T-3811.
RESULTS
Antibacterial activities.
The MICs at which 50% of the isolates are inhibited (MIC50s), MIC90s, and range of MICs of T-3811 and reference quinolones against various bacterial strains are shown in Table 1. T-3811 was most active of the agents tested against gram-positive isolates, with MIC90s of 0.025 to 6.25 μg/ml, except for methicillin-susceptible S. aureus (MSSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). The activity of T-3811 against MSSA and MRSE was comparable to that of trovafloxacin and was more potent than those of ciprofloxacin and levofloxacin. T-3811 was more active against ciprofloxacin-susceptible, methicillin-resistant S. aureus (MRSA), with a MIC90 of 0.025 μg/ml, than ciprofloxacin and levofloxacin. A higher concentration of T-3811 (MIC90, 6.25 μg/ml) was needed to inhibit ciprofloxacin-resistant MRSA, but 50% of such strains were inhibited by 1.56 μg of T-3811 per ml. T-3811 exhibited potent activity (MIC90, 0.05 μg/ml) against penicillin-intermediately-resistant and -resistant S. pneumoniae (PISP and PRSP, respectively). The antibacterial activity of T-3811 against streptococci, including PISP and PRSP strains (MIC90s, 0.05 to 0.10 μg/ml), was greater than those of ciprofloxacin (MIC90, 1.56 μg/ml) and trovafloxacin (MIC90, 0.10 to 0.39 μg/ml). T-3811 was also shown to be active against vancomycin-resistant enterococci (MIC90, 3.13 μg/ml). Against quinolone-resistant S. aureus with a GyrA GrlA double mutation, quinolone-resistant S. pneumoniae with a ParC mutation, and quinolone-resistant E. faecalis with a GyrA mutation, the antibacterial activity of T-3811 proved to be 16- to >64-fold greater than that of ciprofloxacin (Table 2). In particular, T-3811 showed eightfold-higher activity than trovafloxacin against quinolone-resistant MRSA with a double mutation of Ser84Leu and Glu88Lys in GyrA.
TABLE 1.
Antimicrobial activity of T-3811 and reference drugs against clinical isolates of gram-positive and gram-negative bacteria
| Organism (no. of strains) | Drug | MIC (μg/ml)a
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| MSSA (23) | T-3811 | 0.0125–0.05 | 0.025 | 0.025 |
| Ciprofloxacin | 0.10–1.56 | 0.39 | 0.78 | |
| Levofloxacin | 0.10–0.39 | 0.20 | 0.20 | |
| Trovafloxacin | 0.0125–0.05 | 0.025 | 0.025 | |
| Ciprofloxacin-susceptible MRSA (51) | T-3811 | 0.0125–0.05 | 0.025 | 0.025 |
| Ciprofloxacin | 0.10–1.56 | 0.78 | 1.56 | |
| Levofloxacin | 0.10–0.78 | 0.39 | 0.39 | |
| Trovafloxacin | 0.0125–0.10 | 0.05 | 0.05 | |
| Ciprofloxacin-resistant MRSA (26) | T-3811 | 0.10–6.25 | 1.56 | 6.25 |
| Ciprofloxacin | 6.25–>100 | 50 | >100 | |
| Levofloxacin | 3.13–50 | 12.5 | 25 | |
| Trovafloxacin | 0.39–12.5 | 1.56 | 12.5 | |
| MSSE (25) | T-3811 | 0.025–0.78 | 0.05 | 0.10 |
| Ciprofloxacin | 0.05–6.25 | 0.20 | 1.56 | |
| Levofloxacin | 0.10–3.13 | 0.20 | 0.78 | |
| Trovafloxacin | 0.025–1.56 | 0.05 | 0.20 | |
| MRSE (18) | T-3811 | 0.025–0.20 | 0.05 | 0.10 |
| Ciprofloxacin | 0.20–3.13 | 0.39 | 0.39 | |
| Levofloxacin | 0.20–3.13 | 0.20 | 0.39 | |
| Trovafloxacin | 0.05–1.56 | 0.05 | 0.10 | |
| Coagulase-negative staphylococci (25) | T-3811 | 0.025–1.56 | 0.05 | 0.78 |
| Ciprofloxacin | 0.05–6.25 | 0.39 | 6.25 | |
| Levofloxacin | 0.10–3.13 | 0.39 | 3.13 | |
| Trovafloxacin | 0.025–1.56 | 0.05 | 1.56 | |
| PSSP (24) | T-3811 | 0.025–0.05 | 0.05 | 0.05 |
| Ciprofloxacin | 0.39–1.56 | 0.78 | 1.56 | |
| Levofloxacin | 0.78–1.56 | 0.78 | 1.56 | |
| Trovafloxacin | 0.05–0.20 | 0.20 | 0.20 | |
| PISP and PRSP (23) | T-3811 | 0.025–0.05 | 0.025 | 0.05 |
| Ciprofloxacin | 0.78–3.13 | 0.78 | 1.56 | |
| Levofloxacin | 0.78–1.56 | 0.78 | 1.56 | |
| Trovafloxacin | 0.025–0.20 | 0.10 | 0.10 | |
| Streptococcus pyogenes (39) | T-3811 | 0.0125–0.10 | 0.05 | 0.10 |
| Ciprofloxacin | 0.0125–1.56 | 0.39 | 1.56 | |
| Levofloxacin | 0.025–1.56 | 0.78 | 1.56 | |
| Trovafloxacin | 0.0125–0.39 | 0.10 | 0.39 | |
| Streptococcus agalactiae (21) | T-3811 | 0.05–0.20 | 0.10 | 0.10 |
| Ciprofloxacin | 0.39–1.56 | 0.78 | 1.56 | |
| Levofloxacin | 0.39–0.78 | 0.78 | 0.78 | |
| Trovafloxacin | 0.10–0.39 | 0.20 | 0.39 | |
| Enterococcus faecalis (31) | T-3811 | 0.20–6.25 | 0.39 | 3.13 |
| Ciprofloxacin | 0.39–25 | 1.56 | 25 | |
| Levofloxacin | 0.78–50 | 1.56 | 50 | |
| Trovafloxacin | 0.10–12.5 | 0.39 | 12.5 | |
| Enterococcus faecium (15) | T-3811 | 0.20–3.13 | 1.56 | 3.13 |
| Ciprofloxacin | 0.78–50 | 3.13 | 50 | |
| Levofloxacin | 1.56–25 | 3.13 | 25 | |
| Trovafloxacin | 0.20–6.25 | 1.56 | 6.25 | |
| Vancomycin-resistant enterococci (21 Enterococcus faecalis and 3 Enterococcus faecium) | T-3811 | ≦0.05–3.13 | 1.56 | 3.13 |
| Ciprofloxacin | 0.39–100 | 3.13 | 50 | |
| Levofloxacin | 0.78–50 | 3.13 | 50 | |
| Trovafloxacin | 0.10–25 | 1.56 | 25 | |
| Vancomycin | 6.25–400 | 200 | 400 | |
| Escherichia coli (43) | T-3811 | ≦0.00625–0.20 | 0.05 | 0.10 |
| Ciprofloxacin | ≦0.00625–0.20 | 0.0125 | 0.10 | |
| Levofloxacin | 0.0125–0.39 | 0.025 | 0.20 | |
| Trovafloxacin | ≦0.00625–0.39 | 0.025 | 0.10 | |
| Salmonella enteritidis (27) | T-3811 | 0.00625–0.10 | 0.10 | 0.10 |
| Ciprofloxacin | 0.00313–0.025 | 0.025 | 0.025 | |
| Levofloxacin | 0.00625–0.10 | 0.05 | 0.10 | |
| Trovafloxacin | 0.00625–0.05 | 0.05 | 0.05 | |
| Citrobacter freundii (50) | T-3811 | ≦0.00625–1.56 | 0.20 | 1.56 |
| Ciprofloxacin | ≦0.00625–1.56 | 0.05 | 0.20 | |
| Levofloxacin | ≦0.00625–1.56 | 0.05 | 0.20 | |
| Trovafloxacin | ≦0.00625–1.56 | 0.10 | 0.39 | |
| Haemophilus influenzae (50) | T-3811 | 0.00313–0.0125 | 0.00625 | 0.0125 |
| Ciprofloxacin | 0.00625–0.10 | 0.0125 | 0.025 | |
| Levofloxacin | 0.00625–0.10 | 0.0125 | 0.0125 | |
| Trovafloxacin | 0.00313–0.10 | 0.0125 | 0.025 | |
| Moraxella catarrhalis (50) | T-3811 | ≦0.00625–0.39 | 0.025 | 0.10 |
| Ciprofloxacin | 0.0125–0.39 | 0.05 | 0.39 | |
| Levofloxacin | 0.025–1.56 | 0.05 | 0.39 | |
| Trovafloxacin | ≦0.00625–0.39 | 0.05 | 0.20 | |
| Enterobacter cloacae (48) | T-3811 | 0.0125–0.39 | 0.10 | 0.20 |
| Ciprofloxacin | 0.00625–0.10 | 0.025 | 0.05 | |
| Levofloxacin | 0.0125–0.20 | 0.05 | 0.10 | |
| Trovafloxacin | 0.0125–0.39 | 0.05 | 0.10 | |
| Klebsiella pneumoniae (54) | T-3811 | 0.05–25 | 0.10 | 3.13 |
| Ciprofloxacin | 0.0125–50 | 0.05 | 1.56 | |
| Levofloxacin | 0.05–12.5 | 0.05 | 3.13 | |
| Trovafloxacin | 0.0125–50 | 0.10 | 3.13 | |
| Serratia marcescens (48) | T-3811 | 0.78–50 | 1.56 | 6.25 |
| Ciprofloxacin | 0.025–6.25 | 0.39 | 3.13 | |
| Levofloxacin | 0.10–12.5 | 0.78 | 3.13 | |
| Trovafloxacin | 0.20–100 | 0.78 | 6.25 | |
| Proteus mirabilis (50) | T-3811 | 0.10–6.25 | 0.20 | 0.78 |
| Ciprofloxacin | 0.0125–3.13 | 0.025 | 0.10 | |
| Levofloxacin | 0.025–3.13 | 0.05 | 0.20 | |
| Trovafloxacin | 0.10–12.5 | 0.20 | 0.78 | |
| Proteus vulgaris (50) | T-3811 | 0.05–1.56 | 0.39 | 0.78 |
| Ciprofloxacin | 0.0125–0.20 | 0.025 | 0.05 | |
| Levofloxacin | 0.0125–0.39 | 0.05 | 0.10 | |
| Trovafloxacin | 0.05–1.56 | 0.20 | 0.39 | |
| Providencia rettgeri (30) | T-3811 | 0.025–6.25 | 0.39 | 1.56 |
| Ciprofloxacin | ≦0.00625–0.39 | 0.025 | 0.20 | |
| Levofloxacin | 0.025–0.78 | 0.10 | 0.78 | |
| Trovafloxacin | 0.05–6.25 | 0.20 | 0.78 | |
| Morganella morganii (50) | T-3811 | ≦0.00625–6.25 | 0.39 | 0.78 |
| Ciprofloxacin | ≦0.00625–6.25 | 0.0125 | 0.05 | |
| Levofloxacin | 0.0125–6.25 | 0.05 | 0.20 | |
| Trovafloxacin | 0.025–6.25 | 0.20 | 1.56 | |
| Pseudomonas aeruginosa (35) | T-3811 | 0.78–>100 | 1.56 | >100 |
| Ciprofloxacin | 0.10–>100 | 0.39 | 100 | |
| Levofloxacin | 0.39–>100 | 0.78 | >100 | |
| Trovafloxacin | 0.20–>100 | 0.78 | >100 | |
| Acinetobacter calcoaceticus (52) | T-3811 | ≦0.00625–0.78 | 0.025 | 0.05 |
| Ciprofloxacin | 0.05–6.25 | 0.10 | 0.78 | |
| Levofloxacin | 0.05–1.56 | 0.05 | 0.20 | |
| Trovafloxacin | ≦0.00625–0.39 | 0.025 | 0.05 | |
| Xanthomonas maltophilia (18) | T-3811 | 0.05–25 | 1.56 | 6.25 |
| Ciprofloxacin | 0.20–25 | 3.13 | 6.25 | |
| Levofloxacin | 0.20–12.5 | 1.56 | 3.13 | |
| Trovafloxacin | 0.05–6.25 | 0.78 | 1.56 | |
| Alcaligenes faecalis (18) | T-3811 | 0.05–100 | 6.25 | 50 |
| Ciprofloxacin | 0.78–50 | 1.56 | 25 | |
| Levofloxacin | 0.39–25 | 0.78 | 12.5 | |
| Trovafloxacin | 0.05–50 | 3.13 | 50 | |
| Burkholderia cepacia (18) | T-3811 | 0.78–100 | 3.13 | 100 |
| Ciprofloxacin | 0.20–100 | 0.78 | 100 | |
| Levofloxacin | 0.39–100 | 1.56 | 100 | |
| Trovafloxacin | 0.20–100 | 1.56 | 50 | |
| Peptostreptococcus asaccharolyticus (26) | T-3811 | 0.05–0.20 | 0.10 | 0.20 |
| Ciprofloxacin | 0.78–12.5 | 1.56 | 12.5 | |
| Levofloxacin | 3.13–25 | 3.13 | 12.5 | |
| Trovafloxacin | 0.39–1.56 | 0.39 | 1.56 | |
| Bacteroides fragilis (24) | T-3811 | 0.10–0.78 | 0.20 | 0.78 |
| Ciprofloxacin | 1.56–25 | 3.13 | 25 | |
| Levofloxacin | 0.78–50 | 1.56 | 6.25 | |
| Trovafloxacin | 0.20–3.13 | 0.20 | 1.56 | |
| Clostridium difficile (23) | T-3811 | 0.78–1.56 | 0.78 | 0.78 |
| Ciprofloxacin | 6.25–12.5 | 12.5 | 12.5 | |
| Levofloxacin | 3.13–6.25 | 3.13 | 6.25 | |
| Trovafloxacin | 0.39–1.56 | 0.78 | 1.56 | |
| Legionella sp. (15) | T-3811 | 0.002–0.0313 | 0.0156 | 0.0313 |
| Ciprofloxacin | 0.0039–0.0313 | 0.0078 | 0.0156 | |
| Levofloxacin | 0.0039–0.0156 | 0.0156 | 0.0156 | |
| Trovafloxacin | 0.002–0.0156 | 0.0039 | 0.0156 | |
| Mycoplasma pneumoniae (10) | T-3811 | 0.0156–0.0625 | 0.0313 | 0.0313 |
| Ciprofloxacin | 0.5–2 | 1 | 1 | |
| Levofloxacin | 0.5–1 | 0.5 | 1 | |
| Trovafloxacin | 0.125–0.25 | 0.25 | 0.25 | |
| Mycobacterium tuberculosis (10) | T-3811 | 0.0313–0.125 | 0.0625 | 0.0625 |
| Ciprofloxacin | 0.0625–0.25 | 0.125 | 0.125 | |
| Levofloxacin | 0.0625–0.125 | 0.125 | 0.125 | |
| Trovafloxacin | 0.5–2 | 1 | 1 | |
| Rifampin | 0.002–0.125 | 0.0039 | 0.0039 | |
| Mycobacterium avium complex (8) | T-3811 | 0.25–16 | 4 | 16 |
| Ciprofloxacin | 0.5–8 | 1 | 8 | |
| Levofloxacin | 1–8 | 2 | 8 | |
| Trovafloxacin | 2–>16 | 16 | >16 | |
| Rifampin | 0.0625–2 | 0.25 | 2 | |
50 and 90%, MIC50 and MIC90 respectively.
TABLE 2.
Antibacterial activity of T-3811 and reference drugs against quinolone-resistant MRSA, quinolone-resistant Streptococcus pneumoniae, and quinolone-resistant Enterococcus faecalis
| Strain | Amino acid substitution
|
MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| GyrA | GrlA or ParC | T-3811 | Ciprofloxacin | Levofloxacin | Trovafloxacin | |
| MRSA | ||||||
| F-2161 | Ser84Leu | Ser80Phe | ||||
| Glu88Lys | 1.56 | >100 | >100 | 12.5 | ||
| F-1664 | Ser84Leu | Glu84Lys | 0.78 | 100 | 50 | 0.78 |
| F-1953 | Glu88Lys | Ser80Phe | 0.39 | 12.5 | 3.13 | 0.78 |
| F-1659 | Glu88Gly | Ser80Tyr | 0.20 | 12.5 | 3.13 | 0.39 |
| Staphylococcus aureus FDA 209P | None | None | 0.025 | 0.10 | 0.10 | 0.025 |
| Streptococcus pneumoniae | ||||||
| D-1027 | NTa | Ser79Phe | 0.10 | 6.25 | 3.13 | 0.39 |
| D-1687 | NT | Asp83Tyr | 0.39 | 12.5 | 12.5 | 1.56 |
| IID 553 | None | None | 0.05 | 0.78 | 0.78 | 0.20 |
| Enterococcus faecalis | ||||||
| D-1495 | Ser84Ile | NT | 3.13 | 50 | 25 | 12.5 |
| D-1508 | Ser84Ile | NT | 6.25 | 50 | 25 | 25 |
| IID 682 | None | None | 0.20 | 0.39 | 0.78 | 0.20 |
NT, not tested.
The activity of T-3811 against members of the family Enterobacteriaceae was similar to that observed for trovafloxacin, and T-3811 was generally one-half as active as ciprofloxacin, except against Proteus mirabilis, Proteus vulgaris, and Serratia marcescens. Against P. aeruginosa, the activity of T-3811 was similar to that of trovafloxacin. T-3811 was shown to be more active than ciprofloxacin and levofloxacin against Acinetobacter calcoaceticus. Some strains (MIC, ≧100 μg/ml) highly resistant to T-3811 were observed with P. aeruginosa and Burkholderia cepacia, nonfermentative gram-negative rods. In common with the other quinolones, T-3811 was highly active against Haemophilus influenzae (MIC90, 0.0125 μg/ml), Moraxella catarrhalis (MIC90, 0.10 μg/ml), and Legionella sp. (MIC90, 0.0313 μg/ml). Against gram-negative bacteria, the activity of T-3811 was generally comparable to that of trovafloxacin, although minor differences in the activity of each agent against individual species were seen.
T-3811 was found to be active against all of the strains of anaerobic bacteria studied (MIC90s of ≦0.78 μg/ml). T-3811 was 8- to 64-fold more active than ciprofloxacin and levofloxacin against Bacteroides fragilis, Peptostreptococcus sp., and Clostridium difficile.
The MIC90s of T-3811 for M. tuberculosis and M. avium complex were 0.0625 and 16 μg/ml, respectively. As shown in Table 1, the activity of T-3811 against Mycobacterium was comparable to those of ciprofloxacin and levofloxacin, and T-3811 was more active than trovafloxacin.
Antimycoplasma activity.
Against 10 strains of M. pneumoniae, the MIC90 of T-3811 was 0.0313 μg/ml. The activities of T-3811 proved to be 8- and 32-fold greater than those of trovafloxacin and ciprofloxacin, respectively (Table 1).
Antichlamydia activity.
Against C. trachomatis D/UW-3/Cx, the MIC of T-3811 was 0.008 μg/ml. The MICs of ciprofloxacin, levofloxacin, and trovafloxacin were 0.5, 0.25, and 0.063 μg/ml, respectively. The activities of T-3811 proved to be 8- and 64-fold greater than those of trovafloxacin and ciprofloxacin, respectively.
In vivo therapeutic efficacy.
As shown in Table 3, against experimental systemic infections in mice by S. aureus with GrlA and GrlA GyrA double mutations, the ED50s (equivalent of T-3811) of T-3811ME administered orally were 0.0189 and 0.622 mg/mouse, respectively. The therapeutic efficacies of T-3811ME against the GrlA mutant were 4.3-, 30.3-, and 70.4-fold superior to those of trovafloxacin, levofloxacin, and ciprofloxacin, respectively. Against GrlA GyrA double mutant-caused infection, T-3811ME was more potent than ciprofloxacin and levofloxacin and it had potency comparable to that of trovafloxacin. In subcutaneous administration, T-3811ME was also >13.3- and 86.6-fold more active than ciprofloxacin. As shown in Table 4, against PRSP D-979-caused pneumoniae in mice, the ED50s of T-3811ME administered orally and subcutaneously were 0.0278 and 0.0266 mg/mouse, respectively. The therapeutic efficacy of T-3811ME with oral administration was 18.9- to 19.3-fold greater than those of ciprofloxacin and levofloxacin, respectively, and equal to that of trovafloxacin. With subcutaneous administration, T-3811ME had activity 9.9- and 15.9-fold greater than those of ciprofloxacin and levofloxacin, respectively.
TABLE 3.
Therapeutic effect of T-3811ME and reference drugs on experimental systemic S. aureus infection in mice
| Drug route and strain | Inoculum (107 CFU/mouse) | Drug | MIC (μg/ml) | ED50 (mg/mouse) | 95% Confidence limit |
|---|---|---|---|---|---|
| Oral | |||||
| CR-3 (GrlA mutant) | 5.2 | T-3811ME | 0.063 | 0.0189a | 0.0138–0.0261a |
| Ciprofloxacin | 2 | 1.33 | 0.899–2.87 | ||
| Levofloxacin | 0.5 | 0.573 | 0.288–3.39 | ||
| Trovafloxacin | 0.125 | 0.0805 | 0.0519–0.129 | ||
| CRCP-9 (GrlA GyrA mutant) | 2.0 | T-3811ME | 0.5 | 0.622a | 0.447–0.883a |
| Ciprofloxacin | 8 | >4 | |||
| Levofloxacin | 4 | >4 | |||
| Trovafloxacin | 1 | 0.734 | 0.460–1.40 | ||
| Subcutaneous | |||||
| CR-3 (GrlA mutant) | 5.2 | T-3811ME | 0.063 | 0.00493a | 0.00347–0.00740a |
| Ciprofloxacin | 2 | 0.427 | 0.209–4.55 | ||
| Levofloxacin | 0.5 | 0.154 | 0.116–0.244 | ||
| CRCP-9 (GrlA GyrA mutant) | 2.0 | T-3811ME | 0.5 | 0.289a | 0.204–0.417a |
| Ciprofloxacin | 8 | >4 | |||
| Levofloxacin | 4 | 1.96 | 1.47–2.60 |
Equivalent to that of T-3811.
TABLE 4.
Therapeutic effect of T-3811ME and reference drugs on experimental pneumonia (PRSP D-979) in mice
| Drug route | Inoculum (CFU/mouse) | Drug | MIC (μg/ml) | ED50 (mg/mouse) | 95% Confidence limit |
|---|---|---|---|---|---|
| Oral | 5.5 × 104 | T-3811ME | 0.032 | 0.0278a | 0.0180–0.0479a |
| Ciprofloxacin | 1 | 0.525 | 0.331–1.618 | ||
| Levofloxacin | 1 | 0.537 | 0.365–1.113 | ||
| Trovafloxacin | 0.063 | 0.0372 | 0.0261–0.0600 | ||
| Subcutaneous | 6.9 × 103 | T-3811ME | 0.032 | 0.0266a | 0.0195–0.0398a |
| Ciprofloxacin | 1 | 0.422 | 0.282–0.898 | ||
| Levofloxacin | 1 | 0.264 | 0.181–0.400 |
Equivalent to that of T-3811.
DISCUSSION
In this study, the in vitro and in vivo activities of T-3811ME were compared with those of ciprofloxacin, levofloxacin, and trovafloxacin. T-3811, the free base of T-3811ME, showed a broad spectrum of activity, including such organisms as C. trachomatis, M. pneumoniae, and M. tuberculosis. T-3811 also exhibited potent antimicrobial activity. In particular, T-3811 was found to be more active than ciprofloxacin and levofloxacin against gram-positive cocci, such as S. pneumoniae, Streptococcus pyogenes, MSSA, and MRSA. In addition, the activity of T-3811 against penicillin-intermediately-resistant and -resistant S. pneumoniae was more potent than that of trovafloxacin, which has previously been shown to possess improved activity against gram-positive bacteria (1, 2, 5, 6, 15, 20). Against quinolone-resistant gram-positive bacteria, the antibacterial activity of T-3811 proved to be 16- to >64-fold greater than that of ciprofloxacin. This potent activity of T-3811 against gram-positive bacteria was also recognized in in vivo therapeutic studies, such as experimental systemic infections in mice by S. aureus with GrlA GyrA double mutations and experimental pneumonia caused by PRSP.
Gram-positive bacteria are rapidly becoming the most important pathogens in nosocomial infections. In recent years, attention and concern have been focused on gram-positive bacteria such as S. aureus, S. pneumoniae, and E. faecalis (3, 9). The potent activity of T-3811 against gram-positive cocci, including quinolone- and β-lactam-resistant strains, has been considered to represent a substantial advantage over the activities of currently available quinolones.
In gram-negative bacteria, the activity of T-3811 against members of the family Enterobacteriaceae and P. aeruginosa was similar to that observed for trovafloxacin. T-3811 was shown to be more active than ciprofloxacin against A. calcoaceticus, a pathogen implicated in lower respiratory tract infection (17). As well as other fluoroquinolones (5–7, 19), T-3811 was highly active against common respiratory pathogens of gram-negative bacteria, such as M. catarrhalis and H. influenzae. Against the anaerobic bacteria, C. trachomatis and M. pneumoniae, the activity of T-3811 proved to be 8- to 64-fold greater than those of ciprofloxacin and levofloxacin, respectively.
In conclusion, T-3811ME is characterized by improved activity against gram-positive bacteria, while retaining most of its efficacy against gram negative bacteria. T-3811, the free base of T-3811ME, was highly active against the most important pathogens of community-acquired respiratory tract infections, including penicillin-resistant pneumococci, H. influenzae, and M. catarrhalis, as well as atypical organisms, such as M. pneumoniae. T-3811 was more active than trovafloxacin against streptococci, M. pneumoniae, and Chlamydia. Further advantages were the good activities observed against anaerobes and M. tuberculosis.
ACKNOWLEDGMENT
We express our thanks to Matsuhisa Inoue, Kitasato University School of Medicine, for providing us with vancomycin-resistant enterococcal strains.
REFERENCES
- 1.Brightly K E, Gootz T D. The chemistry and biological profile of trovafloxacin. J Antimicrob Chemother. 1997;39(Suppl. B):1–14. doi: 10.1093/jac/39.suppl_2.1. [DOI] [PubMed] [Google Scholar]
- 2.Child J, Andrews J, Boswell F, Brenwarld N, Wise R. The in-vitro activity of CP-99219, a new naphthyridone antimicrobial agent: a comparison with fluoroquinolone agents. J Antimicrob Chemother. 1995;35:869–876. doi: 10.1093/jac/35.6.869. [DOI] [PubMed] [Google Scholar]
- 3.Daschner F D, Kropec A. Glycopeptides in the treatment of staphylococcal infections. Eur J Clin Microbiol. 1995;14(Suppl. 1):12–17. [PubMed] [Google Scholar]
- 4.Edelstein P H, Edelstein M A C, Ren J, Polzer R, Gladue R P. Activity of trovafloxacin (CP-99,219) against Legionella isolates: in vitro activity, intracellular accumulation and killing in macrophages, and pharmacokinetics and treatment of guinea pigs with L. pneumophila pneumonia. Antimicrob Agents Chemother. 1996;40:314–319. doi: 10.1128/aac.40.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliopoulos G M, Klimm K, Eliopoulos C T, Ferraro M J, Moellering R C., Jr In vitro activity of CP-99,219, a new fluoroquinolone, against clinical isolates of gram-positive bacteria. Antimicrob Agents Chemother. 1993;37:366–370. doi: 10.1128/aac.37.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felmingham D, Robbins M J, Ingley K, Mathias I, Bhogal H, Leakey A. In-vitro activity of trovafloxacin, a new fluoroquinolone, against recent clinical isolates. J Antimicrob Chemother. 1997;39(Suppl. B):43–50. doi: 10.1093/jac/39.suppl_2.43. [DOI] [PubMed] [Google Scholar]
- 7.Gootz T D, Brightly K E. Fluoroquinolone antibacterials: SAR, mechanism of action, resistance and clinical aspects. Med Res Rev. 1996;16:433–486. doi: 10.1002/(SICI)1098-1128(199609)16:5<433::AID-MED3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K, Todo Y, Hamamoto S, Ojima K, Yamada M, Kito T, Takahata M, Watanabe Y, Narita H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. T-3811, a novel des-F(6)-quinolone: synthesis and in vitro activity of 7-(isoindolin-5-yl) derivatives, abstr. F158; p. 173. [Google Scholar]
- 9.Hooper D C, Wolfson J S, editors. Quinolone antimicrobial agents. 2nd ed. Washington, D.C: American Society for Microbiology; 1993. [Google Scholar]
- 10.Japan Society of Chemotherapy. Method for the determination of minimum inhibitory concentration (MIC) of anaerobic bacteria by agar dilution method. Chemotherapy (Tokyo) 1979;27:559–560. [Google Scholar]
- 11.Japan Society of Chemotherapy. Method for the determination of minimum inhibitory concentration (MIC) of aerobic bacteria by agar dilution method. Chemotherapy (Tokyo) 1981;29:76–79. [Google Scholar]
- 12.Japan Society of Chemotherapy. Method for the determination of minimum inhibitory concentration (MIC) of Chlamydia. The report of committee of the determination of minimum inhibitory concentration (MIC) of Chlamydia. Chemotherapy (Tokyo) 1989;37:1–10. [Google Scholar]
- 13.Japan Society of Chemotherapy. Method for the determination of minimum inhibitory concentration (MIC) by micro-broth dilution method. Chemotherapy (Tokyo) 1992;40:184–189. [Google Scholar]
- 14.Kenny G E, Cartwright F D. Susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to a new quinolone, trovafloxacin (CP-99,219) Antimicrob Agents Chemother. 1996;40:1048–1049. doi: 10.1128/aac.40.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klugman K, Wasas A. In vitro activity of the fluoroquinolone trovafloxacin against penicillin-sensitive and penicillin-resistant Streptococcus pneumoniae. J Antimicrob Chemother. 1995;36:873–874. doi: 10.1093/jac/36.5.873. [DOI] [PubMed] [Google Scholar]
- 16.Knapp J S, Neal S W, Parekh M C, Rice R J. In vitro activity of a new fluoroquinolone, CP-99,219, against strains of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1995;39:987–989. doi: 10.1128/aac.39.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosmidis J, Koratzanis G. Emergence of resistant bacterial strains during treatment of infections in the respiratory tract. Scand J Infect Dis. 1986;49:135–139. [PubMed] [Google Scholar]
- 18.Nagai A, Takahata M, Miyazaki M, Kawamura Y, Kodama T, Todo Y, Watanabe Y, Narita H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. T-3811, a novel des-F(6)-quinolone: toxicological evaluation, abstr. F162; p. 173. [Google Scholar]
- 19.Neu H C, Chin N-X. In vitro activity of the new fluoroquinolone CP-99,219. Antimicrob Agents Chemother. 1994;38:2615–2622. doi: 10.1128/aac.38.11.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankuch G A, Jacob M R, Applebaum P C. Activity of CP-99,219 compared with DU-6859a, ciprofloxacin, ofloxacin, levofloxacin, lomefloxacin, tosufloxacin, sparfloxacin, and grepafloxacin against penicillin-susceptible and -resistant pneumococci. J Antimicrob Chemother. 1995;35:230–232. doi: 10.1093/jac/35.1.230. [DOI] [PubMed] [Google Scholar]
- 21.Takahata M, Yonezawa M, Kurose S, Futakuchi N, Matsubara N, Watanabe Y, Narita H. Mutation in gyrA and grlA genes of quinolone-resistant clinical isolates of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;38:543–546. doi: 10.1093/jac/38.3.543. [DOI] [PubMed] [Google Scholar]
- 22.Takahata M, Mitsuyama J, Yamashiro Y, Yonezawa M, Araki H, Yamada H, Todo Y, Minami S, Watanabe Y, Narita H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. T-3811, a novel des-F(6)-quinolone: in vitro and in vivo antimicrobial activities, abstr. F159; p. 173. [Google Scholar]
- 23.Takahata M, Mitsuyama J, Yamashiro Y, Araki H, Yamada H, Hayakawa H, Todo Y, Minami S, Watanabe Y, Narita H. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. T-3811, a novel des-F(6)-quinolone: study of pharmacokinetics in animals, abstr. F160; p. 173. [Google Scholar]
- 24.Thomson K S, Chartrand S A, Sanders C C, Block S L. Trovafloxacin, a new fluoroquinolone with potent activity against Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:478–480. doi: 10.1128/aac.41.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wexler H M, Molitoris E, Molitoris D, Finegold S M. In vitro activities of trovafloxacin against 557 strains of anaerobic bacteria. Antimicrob Agents Chemother. 1996;40:2232–2235. doi: 10.1128/aac.40.9.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whithear K G, Bowtell D D, Hughes K L. Evaluation and use of a micro-broth dilution procedure for testing sensitivity of fermentative avian mycoplasmas to antibiotics. Avian Dis. 1983;27:937–949. [PubMed] [Google Scholar]