Abstract
Locomotive syndrome (LS) is defined based on the Loco-Check, 25-question Geriatric Locomotive Function Scale (GLFS-25), 5-question Geriatric Locomotive Function Scale (GLFS-5), Stand-Up Test, Two-Step Test, or a total assessment (i.e., positive for one or more of the GLFS-25, Stand-Up Test, and Two-Step Test). Lumbar spine disease has been reported to be one of the most common musculoskeletal disorders leading to LS. We therefore conducted a systematic review via PubMed, Google Scholar, Cochrane Library, Web of Science, and MEDLINE, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A total of 26 studies were considered to be eligible for inclusion in this systematic review. The GLFS-25 showed an association with low back pain, sagittal spinopelvic malalignment, and lumbar spinal stenosis but not vertebral fracture. The GLFS-5 showed an association with low back pain and lumbar spinal stenosis. The Loco-Check and Two-Step Test showed an association with low back pain, sagittal spinopelvic malalignment, and lumbar spinal stenosis. The Stand-Up Test showed no association with lumbar spinal stenosis. The total assessment showed an association with low back pain and lumbar spinal stenosis. Furthermore, the GLFS-25, Two-Step Test, and total assessment were improved by spinal surgery for lumbar spinal stenosis. The current evidence concerning the relationship between LS and lumbar spine disease still seems insufficient, so further investigations are required on this topic.
Keywords: locomotive syndrome, Loco-Check, 25-question Geriatric Locomotive Function Scale, 5-question Geriatric Locomotive Function Scale, Two-Step Test, Stand-Up Test, lumbar spine
1. Introduction
The elderly population of Japan has continued to grow rapidly since the 1950s. According to the Ministry of Internal Affairs and Communications [1], people of ≥65 years of age numbered 4,110,000 in 1950 (5% of the population), 7,330,000 in 1970 (7% of the population), 14,930,000 in 1990 (12% of the population), 29,480,000 in 2010 (23% of the population), and 36,190,000 in 2020 (29% of the population). In 2007, the Japanese Orthopaedic Association (JOA) proposed the concept of locomotive syndrome (LS), which is defined as a state of reduced functional mobility due to musculoskeletal organ dysfunction [2,3]. LS occurs as the locomotive organs, such as bone (osteoporosis), joint and cartilage (osteoarthritis), muscle (sarcopenia), and intervertebral discs and nerves (spinal stenosis), deteriorate with aging [2,3].
The Loco-Check was designed as a simple self-administered check for LS that could be performed by individuals in the general population [2,3,4] (Figure 1). The Loco-Check includes seven items related to the activities of daily living (ADLs); the possible scores range from 0 to 7. Total scores of 0, 1, 2, and 3–7 points are to reflect non-LS, LS-1, LS-2, and LS-3, respectively. For healthcare professionals, the self-administered diagnostic tools for LS known as the 25-question Geriatric Locomotive Function Scale (GLFS-25) (Figure 2) and the 5-question Geriatric Locomotive Function Scale (GLFS-5) (Figure 3) were developed [5,6,7,8]. The GLFS-25 includes 25 items that are each graded on a 5-point scale (0–4 points) (possible scores range from 0 to 100). The domains covered by this scale include body pain (items 1–4), movement-related difficulty (items 5–7), usual care (items 8–11 and 14), social activities (items 12, 13, and 15–23), and cognition (items 24 and 25). Total scores of 0–6, 7–15, 16–23, and 24–100 are considered to reflect non-LS, LS-1, LS-2, and LS-3, respectively. The GLFS-5 is a 5-item version of the questionnaire and includes five items that are each graded on a 5-point scale (0–4 points) (possible scores range from 0 to 20). Total scores of 0–2, 3–5, 6–8, and 9–20 are considered to reflect non-LS, LS-1, LS-2, and LS-3, respectively.
Figure 1.
The Loco-Check. The Loco-Check includes seven items related to activities of daily living (ADLs); the possible scores range from 0 to 7. Total scores of 0, 1, 2, and 3–7 points are to reflect non-locomotive syndrome (LS), LS-1, LS-2, and LS-3, respectively.
Figure 2.
The 25-question Geriatric Locomotive Function Scale (GLFS-25). The GLFS-25 includes 25 items that are each graded on a 5-point scale (0–4 points) (possible scores range from 0 to 100). The domains covered by this scale include body pain (items 1–4), movement-related difficulty (items 5–7), usual care (items 8–11 and 14), social activities (items 12, 13, and 15–23), and cognition (items 24 and 25). Total scores of 0–6, 7–15, 16–23, and 24–100 are considered to reflect non-LS, LS-1, LS-2, and LS-3, respectively.
Figure 3.
The 5-question Geriatric Locomotive Function Scale (GLFS-5). The GLFS-5 is a 5-item version of the questionnaire and includes five items that are each graded on a 5-point scale (0–4 points) (possible scores range from 0 to 20). Total scores of 0–2, 3–5, 6–8, and 9–20 are considered to reflect non-locomotive syndrome (LS), LS-1, LS-2, and LS-3, respectively.
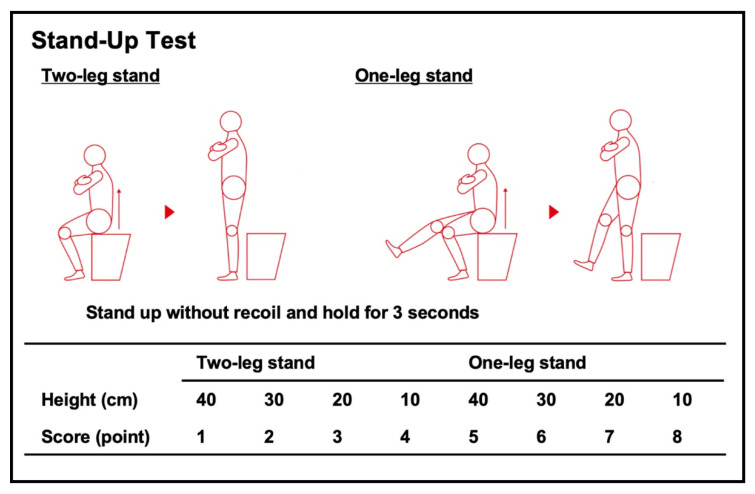
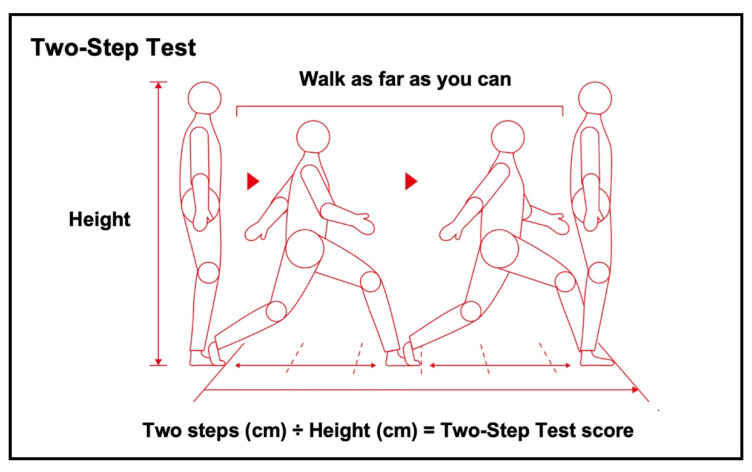
In addition, the JOA prescribed other official diagnostic tests, including the Stand-Up Test (Figure 4) and Two-Step Test (Figure 5) [9]. The Stand-Up Test evaluates lower limb strength according to stand—in a single-leg or double-leg stance— from four different heights (10, 20, 30, and 40 cm). The test is scored as 0–8, with the scores defined as follows: 0 (unable to stand); 1–4 (able to stand—using both legs—from 40, 30, 20, and 10 cm, respectively); and 5–8 (able to stand—using one leg—from 40, 30, 20, and 10 cm, respectively). Stand-Up Test scores of 0–1, 2, 3–4, and 5–8 points are equivalent to LS-3, LS-2, LS-1, and non-LS, respectively. The Two-Step Test evaluates walking ability. It is scored by normalizing the maximal length of two steps by the height. Two-Step Test scores <0.9, <1.1, <1.3, and ≥1.3 points correspond to LS-3, LS-2, LS-1 and non-LS, respectively. To prevent the demand for nursing care in the future, physical exercise is encouraged in patients with LS-1. To investigate musculoskeletal disorders that cause LS, orthopedic consultation is recommended for patients with LS-2. The utility of surgical intervention for LS-3 is an ongoing debate, but such an approach is thought to help improve the physical function.
Figure 4.
The Stand-Up Test. The Stand-Up Test evaluates lower limb strength according to stand—in a single-leg or double-leg stance—from 4 different heights (10, 20, 30, and 40 cm). The test is scored as 0–8, with the scores defined as follows: 0 (unable to stand); 1–4 (able to stand—using both legs—from 40, 30, 20, and 10 cm, respectively); and 5–8 (able to stand—using one leg—from 40, 30, 20, and 10 cm, respectively). Stand-Up Test scores of 0–1, 2, 3–4, and 5–8 points are equivalent to LS-3, LS-2, LS-1, and non-LS, respectively. The reproduction of this figure is permitted by the Japanese Orthopaedic Association (JOA) locomotive syndrome prevention awareness official website [9].
Figure 5.
The Two-Step Test. The Two-Step Test evaluates walking ability. It is scored by normalizing the maximal length of two steps by the height. Two-Step Test scores <0.9, <1.1, <1.3, and ≥1.3 points correspond to LS-3, LS-2, LS-1, and non-LS, respectively. The reproduction of this figure is permitted by the Japanese Orthopaedic Association (JOA) locomotive syndrome prevention awareness official website [9].
In the outpatient department of orthopedics, lumbar spine disease has been reported as an extremely common musculoskeletal disorder leading to LS [10,11,12]. For instance, 64.6–80.6% of community dwelling residents were reported as diagnosed with lumbar spondylosis [11,12]. Furthermore, 10.7–17.6% of community dwelling residents were reported to suffer from associated symptoms [10]. Therefore, clarifying the relationship between LS and lumbar spine disease is an urgent issue. We conducted a systematic review on the relationship between LS and lumbar spine disease.
2. Materials and Methods
We conducted the present systematic review, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [13]. All searches were conducted on 15 February 2022. We searched PubMed, Google Scholar, Cochrane Library, Web of Science, and MEDLINE for relevant English language peer-reviewed articles on the relationship between LS and lumbar spine disease. The following search phrase was used in PubMed: (locomotive syndrome [Title/Abstract]) AND (spine [Title/Abstract]). Other databases were carefully investigated by means of similar search strategies. Articles that were review articles, case reports (n < 3), commentary, editorial, insight articles, or proceedings were also reviewed. We excluded articles that did not mention the relationship between LS and lumbar spine disorders. According to previous reports [2,3,4,5,6,7,8,9], we defined LS according to the results of the Loco-Check, GLFS-25, GLFS-5, Stand-Up Test, Two-Step Test, or a total assessment (i.e., positive for one or more of the GLFS-25, Stand-Up Test, and Two-Step Test). We searched for unpublished or gray literature and screened websites, organizations, or reference lists of studies identified through the database search. Two researchers (T.K. and T.M.) independently assessed the paper selection. Any disagreements were discussed and resolved. The quality of the included studies was assessed based on the Newcastle–Ottawa Scale [14,15]. The following data were extracted: first author, publication year, study type, subject (i.e., number of patients, age, and sex), diagnostic test for LS, and clinical outcomes. Two researchers (T.K. and T.M.) independently assessed the quality of the included studies and extracted the data. Any disagreements were discussed and resolved.
3. Results
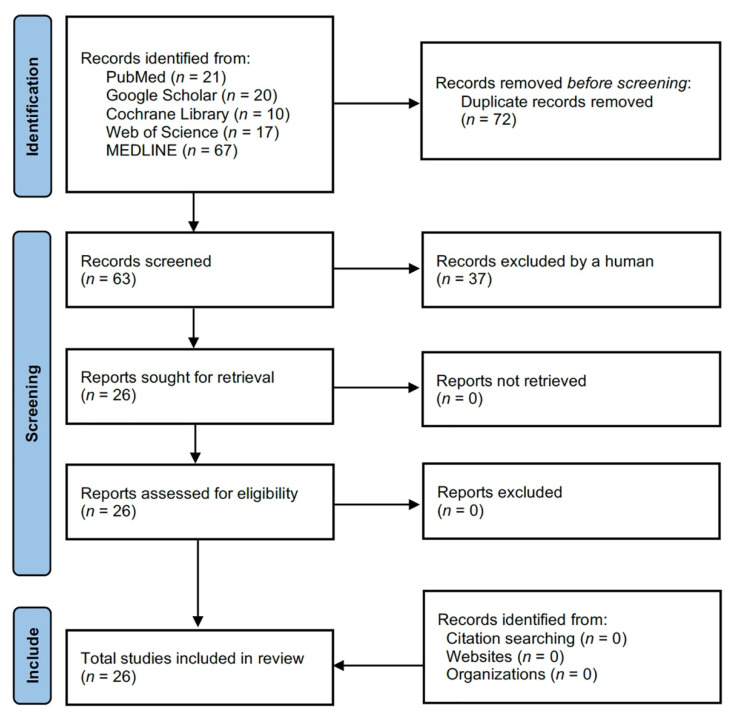
The initial database search identified 135 studies. After removing duplicates, 63 studies were screened. Finally, 26 studies (Table 1) were considered eligible for inclusion in this systematic review (Figure 6). The Newcastle–Ottawa Scale scores for the selected studies ranged from 5 to 9 (Table 2).
Table 1.
Summary of the results.
| Study | Design | Subject | LS | Outcome |
|---|---|---|---|---|
| Kasukawa et al., 2020 [16] | Cross-sectional study | 253 healthy volunteers (118 men, 135 women), age 60–88 years | Loco-Check | Low back pain |
| Sasaki et al., 2013 [17] | Cross-sectional study | 727 healthy volunteers (264 men, 463 women), age 56.6 ± 13.6 (21–87) years | Loco-Check | Low back pain |
| Iizuka et al., 2015 [18] | Cross-sectional study | 287 healthy volunteers (100 men, 187 women), age 64.7 ± 11.2 (40–89) years | GLFS-25 | Low back pain |
| Taniguchi et al., 2021 [19] | Cross-sectional study | 2077 healthy volunteers (730 men, 1347 women), age 68.3 ± 5.4 (30–74) years | GLFS-25 | Low back pain |
| Muramoto et al., 2012 [20] | Cross-sectional study | 358 healthy volunteers (128 men, 230 women), age 66.0 ± 10.0 (40–91) years | GLFS-25 | Low back pain |
| Muramoto et al., 2013 [21] | Cross-sectional study | 406 healthy volunteers (167 men, 239 women), age 68.8 ± 6.7 (60–88) years | GLFS-25 | Low back pain |
| Muramoto et al., 2014 [22] | Cross-sectional study | 217 healthy volunteers (217 women), age 68.2 ± 5.0 (60–79) years | GLFS-25 | Low back pain |
| Muramoto et al., 2016 [23] | Cross-sectional study | 125 healthy volunteers (125 women), age 66.2 ± 9.7 (40–88) years | GLFS-25 | Low back pain, sagittal spinopelvic alignment |
| Matsumoto et al., 2016 [24] | Cross-sectional study | 223 healthy volunteers (82 men, 141 women), age 73.6 ± 8.3 years | GLFS-5 | Low back pain, lumbar spinal stenosis |
| Fujita et al., 2019 [25] | Cross-sectional study | 357 patients scheduled to undergo primary surgery for lumbar spinal stenosis (201 men, 156 women), 73.3 ± 5.5 years |
Two-Step Test | Low back pain, sagittal spinopelvic alignment, lumbar spinal stenosis |
| Imagama 2017 [26] | Cross-sectional study | 523 healthy volunteers (240 men, 283 women), age 63.3 ± 10.0 years | Total assessment | Low back pain |
| Nishimura 2020 [27] | Cross-sectional study | 715 workers (579 men, 136 women), age 44.6 ± 10.0 (18–64) years | Total assessment | Low back pain |
| Chiba et al., 2016 [28] | Cross-sectional study | 647 healthy volunteers (247 men, 400 women), age 58.4 ± 11.0 years | GLFS-25 | Vertebral fracture, lumbar spinal stenosis |
| Machino et al., 2020 [29] | Cross-sectional study | 211 healthy volunteers (89 men, 122 women), age 64.0 ± 10.1 years | GLFS-25 | Sagittal spinopelvic alignment |
| Machino et al., 2020 [30] | Cross-sectional study | 448 healthy volunteers (184 men, 264 women), age 62.7 years | GLFS-25 | Sagittal spinopelvic alignment |
| Hirano et al., 2012 [31] | Cross-sectional study | 386 healthy volunteers (131 men, 233 women), age 67.6 ± 8.7 (50–91) years | Loco-Check | Sagittal spinopelvic alignment |
| Hirano et al., 2012 [32] | Cross-sectional study | 135 healthy volunteers (54 men, 81 women), 76.5 ± 4.7 (70–90) years | Loco-Check | Sagittal spinopelvic alignment |
| Hirano et al., 2013 [33] | Cross-sectional study | 187 healthy volunteers (187 women), age 68.0 ± 8.3 years | Loco-Check | Sagittal spinopelvic alignment |
| Hirano et al., 2012 [34] | Cross-sectional study | 105 healthy volunteers (105 men), age 69.5 ± 8.2 (50–90) years | Loco-Check | Sagittal spinopelvic alignment |
| Ohba et al., 2021 [35] | Retrospective cohort study | 40 patients with a diagnosis of adult spinal deformity who underwent spinal surgery (3 men, 37 women), age 72.6 ± 5.9 years | GLFS-25 | Sagittal spinopelvic alignment |
| Shigematsu et al., 2019 [36] | Case–control study | 28 patients with lumbar spinal stenosis who underwent spinal surgery (15 men, 13 women), age 73.7 ± 5.6 years 46 elderly persons (16 men, 30 women), age 73.9 ± 5.4 years |
Loco-Check | Lumbar spinal stenosis |
| Araki et al., 2021 [37] | Cross-sectional study | 82 patients with lumbar spinal stenosis who underwent decompression surgery (47 men, 35 women), age 73.4 ± 8.4 years | GLFS-25 | Lumbar spinal stenosis |
| Fujita et al., 2019 [38] | Cross-sectional study | 200 patients scheduled to undergo primary surgery for lumbar spinal stenosis (120 men, 80 women), age 73.2 years |
Total assessment | Lumbar spinal stenosis |
| Shimizu et al., 2021 [39] | Prospective cohort study | 101 patients scheduled to undergo primary surgery for lumbar spinal stenosis (46 men, 55 women), age 69.3 ± 8.1 years |
Total assessment | Surgery for lumbar spinal stenosis |
| Kato et al., 2020 [40] | Prospective cohort study | 257 patients who underwent surgery for degenerative diseases of the lumbar spine (209 men, 48 women), age 71.5 ± 6.9 years | Total assessment | Surgery for lumbar spinal stenosis |
| Fujita et al., 2020 [41] | Prospective cohort study | 166 patients scheduled to undergo primary surgery for lumbar spinal stenosis (95 men, 71 women), age 72.8 ± 5.5 years | Total assessment | Surgery for lumbar spinal stenosis |
LS: locomotive syndrome; GLFS-25: the 25-question Geriatric Locomotive Function Scale; GLFS-5: the 5-question Geriatric Locomotive Function Scale.
Figure 6.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIZMA) [13] flow chart of the paper selection.
Table 2.
| Study | Selection | Comparability | Outcome/Exposure | Total Score |
|---|---|---|---|---|
| Kasukawa et al., 2020 [16] | ★★★★ | ★★ | 6 | |
| Sasaki et al., 2013 [17] | ★★★★ | ★★ | ★★ | 8 |
| Iizuka et al., 2015 [18] | ★★★★ | ★★ | ★★ | 8 |
| Taniguchi et al., 2021 [19] | ★★★★ | ★★ | 6 | |
| Muramoto et al., 2012 [20] | ★★★★ | ★★ | ★★ | 8 |
| Muramoto et al., 2013 [21] | ★★★ | ★★ | ★★ | 7 |
| Muramoto et al., 2014 [22] | ★★ | ★★ | ★★ | 6 |
| Muramoto et al., 2016 [23] | ★★ | ★★ | ★★ | 6 |
| Matsumoto et al., 2016 [24] | ★★★★ | ★★ | 6 | |
| Fujita et al., 2019 [25] | ★★★★★ | ★★ | ★★ | 9 |
| Imagama 2017 [26] | ★★★★ | ★★★ | 7 | |
| Nishimura 2020 [27] | ★★★ | ★★ | 5 | |
| Chiba et al., 2016 [28] | ★★★ | ★★ | ★★ | 7 |
| Machino et al., 2020 [29] | ★★★★ | ★★★ | 7 | |
| Machino et al., 2020 [30] | ★★★★★ | ★★★ | 8 | |
| Hirano et al., 2013 [31] | ★★★★ | ★★★ | 7 | |
| Hirano et al., 2012 [32] | ★★★★ | ★★ | ★★★ | 9 |
| Hirano et al., 2013 [33] | ★★★ | ★★ | ★★★ | 8 |
| Hirano et al., 2012 [34] | ★★★ | ★★ | ★★★ | 8 |
| Ohba et al., 2021 [35] | ★★★★ | ★★ | ★★★ | 9 |
| Shigematsu et al., 2019 [36] | ★★★ | ★★ | ★★ | 7 |
| Araki et al., 2021 [37] | ★★★ | ★★ | 5 | |
| Fujita et al., 2019 [38] | ★★★★ | ★★★ | 7 | |
| Shimizu et al., 2021 [39] | ★★★★ | ★★ | ★★ | 8 |
| Kato et al., 2020 [40] | ★★★★ | ★★ | ★★ | 8 |
| Fujita et al., 2020 [41] | ★★★★ | ★★ | ★★ | 8 |
Newcastle-Ottawa Scale for case-control studies: Selection (Maximum ★★★★)—(1) Is the case definition adequate? (2) Representativeness of the cases; (3) Selection of controls; (4) Definition of Controls. Comparability (Maximum ★★) 2013 (1) Confounding factors controlled. Exposure (Maximum ★★★)—(1) Ascertainment of exposure; (2) Same method of ascertainment for cases and controls; (3) Non-Response rate. Newcastle-Ottawa Scale for cohort studies: Selection (Maximum ★★★★)—(1) Representativeness of the exposed cohort; (2) Selection of the non-exposed cohort; (3) Ascertainment of exposure; (4) Demonstration that outcome of interest was not present at start of study. Comparability (Maximum ★★)—(1) Confounding factors controlled. Outcome (Maximum ★★★)—(1) Assessment of outcome; (2) Was follow-up long enough for outcomes to occur; (3) Adequacy of follow up of cohorts. Newcastle-Ottawa Scale adapted for cross-sectional studies: Selection (Maximum ★★★★★)—(1) Representativeness of the sample; (2) Sample size; (3) Non-respondents; (4) Ascertainment of the exposure. Comparability: (Maximum ★★)—(1) Confounding factors controlled. Outcome (Maximum ★★★)—(1) Assessment of outcome; (2) Statistical test.
In the included studies, we found that lumbar spine disease included low back pain, vertebral fracture, sagittal spinopelvic malalignment, and lumbar spinal stenosis.
3.1. LS and Low Back Pain
Although low back pain is multifactorial, it is one of the most commonly encountered symptoms related to lumbar spine diseases in daily practice, accounting for 12.9–15.8% of cases [10]. Low back pain was strongly related to disc degeneration [42]. The presence of low back pain showed an association with the Loco-Check [16,42], GLFS-25 [18,19,20,21,22,23], GLFS-5 [24], Two-Step Test [25], and total assessment [26,27] (Table 1). Furthermore, the degree of low back pain showed a positive association with the GLFS-25 score [20,21,22,23] and a negative association with Two-Step Test score [25].
3.2. LS and Vertebral Fracture
The prevalence of vertebral fractures is 11.8–13.8% in the general population [43]. Nevertheless, only one paper had previously investigated the relationship between LS and vertebral fracture; Chiba et al. [28] reported that the GLFS-25 showed no association with the presence of vertebral fracture (Table 1).
3.3. LS and Sagittal Spinopelvic Malalignment
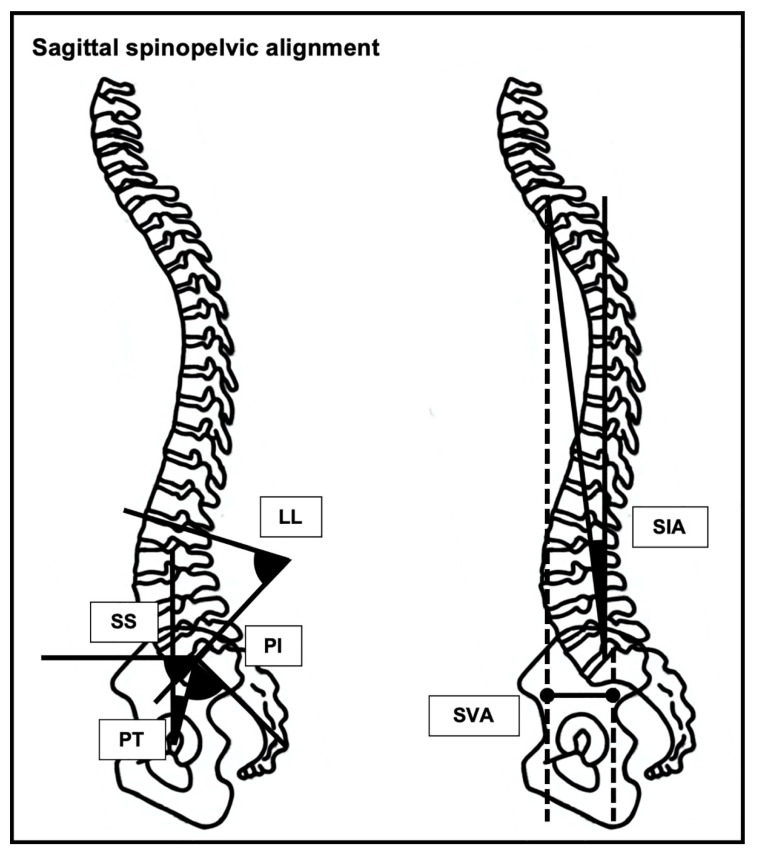
Various studies in the field of adult spinal deformity have described spinal sagittal imbalance as risk factor for a worsening in the quality of life [23,29,30,31,32,33,34,44,45,46]. Sagittal spinopelvic malalignment—flatback deformity (low pelvic tilt (PT), low sacral slope (SS), low lumbar lordosis (LL), and high pelvic incidence (PI)-LL mismatch) [46] and positive sagittal balance (high sagittal vertical axis (SVA), and high spinal inclination angle (SIA)) [47,48] (Figure 7)—showed an association with the Loco-Check [31,32,33,34], GLFS-25 [23,29,30,35,48], and Two-Step Test [25] (Table 1). There was no evidence concerning the relationship between the GLFS-5, Stand-Up Test, and total assessment and sagittal spinopelvic malalignment.
Figure 7.
Sagittal spinopelvic alignment includes the pelvic incidence (PI), pelvic tilt (PT), sacral slope (SS), lumbar lordosis (LL), sagittal vertical axis (SVA), and spinal inclination angle (SIA). The PI is the angle between a line perpendicular to the sacral plate at its midpoint and a line connecting this point to the bi-coxo-femoral axis. The PT is the angle between a vertical line passing through the bi-coxo-femoral axis and a line joining the bi-coxo-femoral axis with the center of the upper sacral endplate. The SS is the angle between a tangent line to the superior endplate of S1 and the horizontal plane. The LL is the angle between the superior endplate of L1 and the upper sacral endplate. The SVA is the horizontal distance between a plumb line drawn from the center of C7 and a line drawn from the center of C7 to the posterior superior corner of S1. The SIA is the angle between the true vertical and a straight line from the tip of the T1 spinous process to that of S1.
3.4. LS and Lumbar Spinal Stenosis
Lumbar spinal stenosis is the most commonly encountered lumbar spine disorders in daily practice, accounting for 10.7–12.9% of cases [10]. Lumbar spinal stenosis showed an association with the Loco-Check [36], GLFS-25 [28,37], GLFS-5 [24], Two-Step Test [25], and total assessment [39] but not the Stand-Up Test [39] (Table 1). Furthermore, spinal surgery for lumbar spinal stenosis (i.e., posterior decompression or short segment spinal fusion surgeries) improves the GLFS-25 [39,40,41,48], Two-Step Test [39,40,41], and total assessment [39,40,41], but not the Stand-Up Test [39,40,41] (Table 1). There was no report regarding the effect of surgery for lumbar spine disorders on the Loco-Check and GLFS-5.
4. Discussion
This systematic review describes the available evidence concerning the relationship between LS and lumbar spine disease. In the included studies, we found that lumbar spine disease included low back pain, vertebral fracture, sagittal spinopelvic malalignment, and lumbar spinal stenosis. Our findings were that LS showed an overall association with low back pain, sagittal spinopelvic malalignment, and lumbar spinal stenosis but not vertebral fracture.
4.1. LS and Low Back Pain
Our findings are influenced by the fact that the questionnaires include items concerning low back pain or its related quality of life problems [42] (Figure 1)—item 2 (pain in the back), 3 (pain in the leg), 4 (pain on moving), 5 (getting up and lying down), 6 (getting up from a chair), 7 (walking around the house), 9 (difficulty putting on trousers), 10 (difficulty using the toilet), 11 (difficulty bathing), 12 (going up and down stairs), 13 (walking briskly), 15 (walking continuously), 16 (going outside), 17 (carrying 2 kg), 20 (doing heavy housework), 21 (playing sports), 24 (anxious about falling), and 25 (anxious about walking)—and that the stride length was negatively correlated with the degree of low back pain [18,23,49,50]. There is no evidence concerning the relationship between the Stand-Up Test and low back pain. More specifically, previous studies found that low back pain affected sit-to-stand movement [51,52], but its association with LS remains unknown. Further investigations on this topic are considered necessary.
4.2. LS and Vertebral Fracture
The health-related quality of life, which is evaluated using the SF-12 Physical Component Summary score, back pain, and physical function assessed using the one-leg standing, timed up-and-go, walking speed, 30-s chair stand test, and maximum grip strength evaluations showed a significant association with both the severity and number of vertebral fractures in older women [53,54,55]. These findings suggest that vertebral fractures may affect GLFS-25.
The inconsistency with our present findings can be explained by three reasons. First, the statistical method used is insufficient; previous authors analyzed this topic by gender, using the χ2 test despite the markedly low prevalence of vertebral fractures [28]. Second, the definition of vertebral fracture has varied among studies. Generally, ‘vertebral fracture’ was considered to be a compressive deformity wherein the height of the vertebra was >20% of the height of the adjacent uncompressed vertebra (20–25%, mild; >25–40%, moderate; >40%, severe) in lateral lumbar radiographs. Third, the relevance of our result regarding the relationship between LS and vertebral fracture is affected by the fact that only one retrospective study addressed this question. Further investigations are needed on the topic.
4.3. LS and Sagittal Spinopelvic Malalignment
Our results are consistent with previous reports, suggesting that spinopelvic malalignment may be a trigger for suspecting LS. Among the spinopelvic parameters, the SIA is reported to be the most relevant one for LS, and a SIA of ≥6° has a sensitivity of 52% and specificity of 87% for diagnosing LS-2 (GLFS-25 total score ≥16 points) [23].
The relationship between LS and lumbar flexibility remains unexplored. There is no evidence regarding the relationship between LS and the lumbo–pelvic complex [35,47,56]; individuals with a higher PI value are likely to have a higher LL value [35,47,56]. Furthermore, their lumbar facet joint is likely to have a more sagittal orientation [57,58], their lumbar facet joint contact force is likely to be lower in flexion–extension [59,60], and their anatomical acetabular anteversion angle is likely to be lower [35]. These conditions can be expected to lead individuals to greater use of their spine in ADLs (‘spine users’). Conversely, individuals with lower PI values can be expected to have lower LL values [35,47,56], their lumbar facet joint orientation is likely to be more coronal [57,58], their lumbar facet joint contact force in flexion–extension is likely to be higher [59,60], and their anatomical acetabular anteversion angle is likely to be higher [35]. These factors would lead to greater use of the hips in ADLs (‘hip users’). Further studies should be undertaken to validate these hypotheses.
4.4. LS and Lumbar Spinal Stenosis
Our findings may result from the hypothesis that the impairment of strength and balance in combination with gait disturbance with the pain status due to lumbar spinal stenosis affect scores of questionnaires (e.g., GLFS-25 item 3–25) and the Two-step Test [28,37,38,50]. In contrast, the Stand-Up Test predominantly measures the knee extension strength of the quadriceps femoris muscle, a parameter that stenosis does not directly affect at the most frequently responsible level (L4/5) [40,41].
Given the above, it is necessary to consider which LS test is the most useful in the field of spinal surgery. Preoperatively, the severity of LS based on the GLFS-25 and total assessment is almost the same [39,40,41]; in patients scheduled for primary surgery for the treatment of lumbar spinal stenosis, the prevalence of LS-1 and LS-2 assessed by GLFS-25 was 2.4–6.9% and 93.1–97.6%, respectively. Similarly, in patients scheduled to undergo primary surgery for lumbar spinal stenosis, the prevalence of LS-1 and LS-2 assessed by the total assessment were 1.8–5.0% and 95.0–98.2%, respectively. This indicates that the GLFS-25 is sufficient for the preoperative evaluation of severity of LS. Postoperatively, however, the GLFS-25 has a lower prevalence of LS-2 than the total assessment [39,40,41]. Among patients who received primary surgery for the treatment of lumbar spinal stenosis 1 year later, the prevalence of LS-1 and LS-2 assessed by GLFS-25 was 22.4–23.8% and 59.4–66.1%, respectively. In contrast, in patients who underwent primary surgery for lumbar spinal stenosis 1 year later, the prevalence of LS-1 and LS-2 assessed by the total assessment were 22.8–24.2% and 71.3–74.5%, respectively. This indicates that not only the GLFS-25 but also the total assessment (i.e., positive for one or more of the GLFS-25, Stand-Up Test, and Two-Step Test) is useful for accurately evaluating the improvement induced by spinal surgery. In other words, not only the GLFS-25 but also the Two-Step Test is useful for assessing the improvement induced by spinal surgery, as the Stand-Up Test showed no association with lumbar spinal stenosis [39]. Accordingly, the subjective and objective evaluations may differ, making it preferable to evaluate both in order to confirm the improvement induced by spinal surgery.
Further studies are needed to investigate whether the surgical improvement of LS can extend the healthy life expectancy of individuals with spinal disorders.
4.5. Limitations of This Systematic Review
This systematic review is not homogenous and has several limitations. Firstly, and most importantly, the majority of reports on this topic are from Japan, which may have introduced some publication bias. The concept of LS is an important point in aging societies, so reports from countries other than Japan are expected to accumulate in the future. Secondly, an English language bias may exist, with important data published in Japanese possibly being omitted. Thirdly, the information in this systematic review was limited to the studies included. Therefore, lumbar spine disease included only low back pain, vertebral fracture, sagittal spinopelvic malalignment, and lumbar spinal stenosis. Furthermore, we could not quantitatively evaluate the combined data of the eligible studies due to differences in data quality and research design. Due to these limitations, we concluded that the current evidence is still insufficient.
5. Conclusions
This systematic review described available evidence on the relationship between LS and lumbar spine disease (i.e., low back pain, vertebral fracture, sagittal spinopelvic malalignment, and lumbar spinal stenosis). The GLFS-25 showed an association with low back pain, sagittal spinopelvic malalignment, and lumbar spinal stenosis but not vertebral fracture. The GLFS-5 showed an association with low back pain and lumbar spinal stenosis. The Loco-Check and Two-Step Test showed an association with low back pain, sagittal spinopelvic malalignment, and lumbar spinal stenosis. The Stand-Up Test showed no association with lumbar spinal stenosis. The total assessment showed an association with low back pain and lumbar spinal stenosis. Furthermore, the GLFS-25, Two-Step Test, and total assessment were improved by spinal surgery for lumbar spinal stenosis
We delved into the detailed relationship between LS and lumbar spine disease via a systematic review and found that the current evidence was still insufficient to conduct a quantitative assessment. Further investigations are therefore warranted on this topic.
Author Contributions
Writing—original draft preparation, T.K.; writing—review and editing, T.M. and K.O., supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from JOA-Subsidized Science Project Research 2020-2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.The Ministry of Internal Affairs and Communications. [(accessed on 15 February 2022)]. Available online: https://www.mhlw.go.jp/toukei/list/81-1a.html.
- 2.Nakamura K. A “super-aged” society and the “locomotive syndrome”. J. Orthop. Sci. 2008;13:1–2. doi: 10.1007/s00776-007-1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura K., Ogata T. Locomotive syndrome: Definition and management. Clin. Rev. Bone Miner. Metab. 2016;14:56–67. doi: 10.1007/s12018-016-9208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi T., Morimoto T., Shimanoe C., Ono R., Otani K., Mawatari M. Development of a tool for screening the severity of locomotive syndrome by the loco-check. J. Orthop. Sci. 2021 doi: 10.1016/j.jos.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Seichi A., Hoshino Y., Doi T., Akai M., Tobimatsu Y., Iwaya T. Development of a screening tool for risk of locomotive syndrome in the elderly: The 25-question Geriatric Locomotive Function Scale. J. Orthop. Sci. 2012;17:163–172. doi: 10.1007/s00776-011-0193-5. [DOI] [PubMed] [Google Scholar]
- 6.Ohe T. The history of locomotive syndrome-3. Jpn. Orthop. Assoc. (JOA) News. 2020;122:6. (In Japanese) [Google Scholar]
- 7.Kobayashi T., Morimoto T., Shimanoe C., Ono R., Otani K., Mawatari M. Development of a simple screening tool based on the 5-question geriatric locomotive function scale for locomotive syndrome. J. Orthop. Sci. 2021 doi: 10.1016/j.jos.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T., Morimoto T., Shimanoe C., Ono R., Otani K., Mawatari M. The association of comorbidities with the 25-question geriatric locomotive function scale and the diagnosis of locomotive syndrome. J. Orthop. Sci. 2021 doi: 10.1016/j.jos.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 9.The Japanese Orthopedic Association Official Locomotive Syndrome Prevention Awareness Official Website. [(accessed on 15 February 2022)]. Available online: https://locomo-joa.jp.
- 10.Otani K., Takegami M., Fukumori N., Sekiguchi M., Onishi Y., Yamazaki S., Ono R., Otoshi K., Hayashino Y., Fukuhara S., et al. Locomotor dysfunction and risk of cardiovascular disease, quality of life, and medical costs: Design of the Locomotive Syndrome and Health Outcome in Aizu Cohort Study (LOHAS) and baseline characteristics of the study population. J. Orthop. Sci. 2012;17:261–271. doi: 10.1007/s00776-012-0200-5. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura N., Muraki S., Oka H., Mabuchi A., En-Yo Y., Yoshida M., Saika A., Yoshida H., Suzuki T., Yamamoto S., et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J. Bone Miner. Metab. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura N., Muraki S., Nakamura K., Tanaka S. Epidemiology of the locomotive syndrome: The research on osteoarthritis/osteoporosis against disability study 2005–2015. Mod. Rheumatol. 2017;27:1–7. doi: 10.1080/14397595.2016.1226471. [DOI] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Herzog R., Álvarez-Pasquin M.J., Díaz C., Del Barrio J.L., Estrada J.M., Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasukawa Y., Miyakoshi N., Hongo M., Ishikawa Y., Kudo D., Kimura R., Ono Y., Shimada Y. Locomotive syndrome is associated with health-related quality of life and low back pain in the elderly, including individuals more than 80 years old. Prog. Rehabil. Med. 2020;5:20200029. doi: 10.2490/prm.20200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki E., Ishibashi Y., Tsuda E., Ono A., Yamamoto Y., Inoue R., Takahashi I., Umeda T., Nakaji S. Evaluation of locomotive disability using loco-check: A cross-sectional study in the Japanese general population. J. Orthop. Sci. 2013;18:121–129. doi: 10.1007/s00776-012-0329-2. [DOI] [PubMed] [Google Scholar]
- 18.Iizuka Y., Iizuka H., Mieda T., Tajika T., Yamamoto A., Takagishi K. Population-based study of the association of osteoporosis and chronic musculoskeletal pain and locomotive syndrome: The Katashina study. J. Orthop. Sci. 2015;20:1085–1089. doi: 10.1007/s00776-015-0774-9. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M., Ikezoe T., Tsuboyama T., Tabara Y., Matsuda F., Ichihashi N. Prevalence and physical characteristics of locomotive syndrome stages as classified by the new criteria 2020 in older Japanese people: Results from the Nagahama study. BMC Geriatr. 2021;21:489. doi: 10.1186/s12877-021-02440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muramoto A., Imagama S., Ito Z., Hirano K., Ishiguro N., Hasegawa Y. Physical performance tests are useful for evaluating and monitoring the severity of locomotive syndrome. J. Orthop. Sci. 2012;17:782–788. doi: 10.1007/s00776-012-0283-z. [DOI] [PubMed] [Google Scholar]
- 21.Muramoto A., Imagama S., Ito Z., Hirano K., Tauchi R., Ishiguro N., Hasegawa Y. Threshold values of physical performance tests for locomotive syndrome. J. Orthop. Sci. 2013;18:618–626. doi: 10.1007/s00776-013-0382-5. [DOI] [PubMed] [Google Scholar]
- 22.Muramoto A., Imagama S., Ito Z., Hirano K., Tauchi R., Ishiguro N., Hasegawa Y. Waist circumference is associated with locomotive syndrome in elderly females. J. Orthop. Sci. 2014;19:612–619. doi: 10.1007/s00776-014-0559-6. [DOI] [PubMed] [Google Scholar]
- 23.Muramoto A., Imagama S., Ito Z., Hirano K., Ishiguro N., Hasegawa Y. Spinal sagittal balance substantially influences locomotive syndrome and physical performance in community-living middle-aged and elderly women. J. Orthop. Sci. 2016;21:216–221. doi: 10.1016/j.jos.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto H., Hagino H., Osaki M., Tanishima S., Tanimura C., Matsuura A., Makabe T. Gait variability analysed using an accelerometer is associated with locomotive syndrome among the general elderly population: The GAINA study. J. Orthop. Sci. 2016;21:354–360. doi: 10.1016/j.jos.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Fujita N., Sakurai A., Miyamoto A., Michikawa T., Otaka Y., Suzuki S., Tsuji O., Nagoshi N., Okada E., Yagi M., et al. Stride length of elderly patients with lumbar spinal stenosis: Multi-center study using the Two-Step test. J. Orthop. Sci. 2019;24:787–792. doi: 10.1016/j.jos.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Imagama S., Hasegawa Y., Ando K., Kobayashi K., Hida T., Ito K., Tsushima M., Nishida Y., Ishiguro N. Staged decrease of physical ability on the locomotive syndrome risk test is related to neuropathic pain, nociceptive pain, shoulder complaints, and quality of life in middle-aged and elderly people–The utility of the locomotive syndrome risk test. Mod. Rheumatol. 2017;27:1051–1056. doi: 10.1080/14397595.2017.1285856. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura A., Ohtsuki M., Kato T., Nagao R., Ito N., Kato K., Ogura T., Sudo A. Locomotive syndrome testing in young and middle adulthood. Mod. Rheumatol. 2020;30:178–183. doi: 10.1080/14397595.2018.1551176. [DOI] [PubMed] [Google Scholar]
- 28.Chiba D., Tsuda E., Wada K., Kumagai G., Sasaki E., Nawata A., Nakagomi S., Takahashi I., Nakaji S., Ishibashi Y. Lumbar spondylosis, lumbar spinal stenosis, knee pain, back muscle strength are associated with the locomotive syndrome: Rural population study in Japan. J. Orthop. Sci. 2016;21:366–372. doi: 10.1016/j.jos.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Machino M., Ando K., Kobayashi K., Nakashima H., Kanbara S., Ito S., Inoue T., Yamaguchi H., Koshimizu H., Seki T., et al. Influence of global spine sagittal balance and spinal degenerative changes on locomotive syndrome risk in a middle-age and elderly community-living population. BioMed Res. Int. 2020;2020:3274864. doi: 10.1155/2020/3274864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Machino M., Ando K., Kobayashi K., Nakashima H., Morozumi M., Tanaka S., Kanbara S., Ito S., Seki T., Ishizuka S., et al. Differences of lumbopelvic sagittal parameters among community-dwelling middle-age and elderly individuals: Relations with locomotor physical function. J. Clin. Neurosci. 2020;73:80–84. doi: 10.1016/j.jocn.2020.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Hirano K., Imagama S., Hasegawa Y., Ito Z., Muramoto A., Ishiguro N. The influence of locomotive syndrome on health-related quality of life in a community-living population. Mod. Rheumatol. 2013;23:939–944. doi: 10.3109/s10165-012-0770-2. [DOI] [PubMed] [Google Scholar]
- 32.Hirano K., Imagama S., Hasegawa Y., Wakao N., Muramoto A., Ishiguro N. Impact of spinal imbalance and back muscle strength on locomotive syndrome in community-living elderly people. J. Orthop. Sci. 2012;17:532–537. doi: 10.1007/s00776-012-0266-0. [DOI] [PubMed] [Google Scholar]
- 33.Hirano K., Imagama S., Hasegawa Y., Wakao N., Muramoto A., Ishiguro N. Impact of back muscle strength and aging on locomotive syndrome in community living Japanese women. Nagoya J. Med. Sci. 2013;75:47–55. [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano K., Imagama S., Hasegawa Y., Wakao N., Muramoto A., Ishiguro N. Effect of back muscle strength and sagittal spinal imbalance on locomotive syndrome in Japanese men. Orthopedics. 2012;35:e1073–e1078. doi: 10.3928/01477447-20120621-25. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T., Morimoto T., Yoshihara T., Sonohata M., Rivière C., Mawatari M. The relationship between pelvic incidence and anatomical acetabular anteversion in female Japanese patients with hip osteoarthritis: A retrospective iconographic study. Surg. Radiol. Anat. 2021;43:1141–1147. doi: 10.1007/s00276-021-02710-z. [DOI] [PubMed] [Google Scholar]
- 36.Shigematsu H., Tanaka M., Kawasaki S., Iwata E., Masuda K., Morimoto Y., Yamamoto Y., Tanaka Y. Loco-check presents a useful tool to determine health-related quality of life in elderly people with lumbar spinal stenosis. J. Orthop. Sci. 2019;24:715–719. doi: 10.1016/j.jos.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Araki M., Nonoshita H., Kitano S., Shigematsu H., Tanaka M., Kawasaki S., Suga Y., Yamamoto Y., Tanaka Y. The critical cutoff point of the Zurich Claudication Questionnaire and the Japanese Orthopaedic Association score indicating locomotive syndrome in patients with lumbar spinal canal stenosis. J. Orthop. Sci. 2021;26:290–294. doi: 10.1016/j.jos.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Fujita N., Sakurai A., Miyamoto A., Michikawa T., Tsuji O., Nagoshi N., Okada E., Yagi M., Otaka Y., Tsuji T., et al. Lumbar spinal canal stenosis leads to locomotive syndrome in elderly patients. J. Orthop. Sci. 2019;24:19–23. doi: 10.1016/j.jos.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu T., Kato S., Demura S., Shinmura K., Yokogawa N., Kurokawa Y., Yonezawa N., Oku N., Kitagawa R., Handa M., et al. The efficacy of surgical treatment on locomotive syndrome and physical function in patients with lumbar spinal canal stenosis. J. Orthop. Sci. 2021;26:327–331. doi: 10.1016/j.jos.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Kato S., Kurokawa Y., Kabata T., Demura S., Matsubara H., Kajino Y., Okamoto Y., Kimura H., Shinmura K., Igarashi K., et al. Improvement of locomotive syndrome with surgical treatment in patients with degenerative diseases in the lumbar spine and lower extremities: A prospective cohort study. BMC Musculoskelet. Disord. 2020;21:515. doi: 10.1186/s12891-020-03547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita N., Michikawa T., Miyamoto A., Sakurai A., Otaka Y., Suzuki S., Tsuji O., Nagoshi N., Okada E., Yagi M., et al. Lumbar spinal surgery improves locomotive syndrome in elderly patients with lumbar spinal canal stenosis: A multicenter prospective study. J. Orthop. Sci. 2020;25:213–218. doi: 10.1016/j.jos.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Ohtori S., Ito T., Yamashita M., Murata Y., Morinaga T., Hirayama J., Kinoshita T., Ataka H., Koshi T., Sekikawa T., et al. Evaluation of low back pain using the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire for lumbar spinal disease in a multicenter study: Differences in scores based on age, sex, and type of disease. J. Orthop. Sci. 2010;15:86–91. doi: 10.1007/s00776-009-1426-8. [DOI] [PubMed] [Google Scholar]
- 43.Waterloo S., Ahmed L.A., Center J.R., Eisman J.A., Morseth B., Nguyen N.D., Nguyen T., Sogaard A.J., Emaus N. Prevalence of vertebral fractures in women and men in the population-based Tromsø Study. BMC Musculoskelet. Disord. 2012;13:3. doi: 10.1186/1471-2474-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tominaga R., Kurita N., Kokubun Y., Nikaido T., Sekiguchi M., Otani K., Iwabuchi M., Shirado O., Fukuhara S., Konno S.I. Dose-response relationship between spino-pelvic alignment determined by sagittal modifiers and back pain-specific quality of life. Eur. Spine J. 2021;30:3019–3027. doi: 10.1007/s00586-021-06965-3. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K., Otani K., Tominaga R., Kokubun Y., Sekiguchi M., Fukuma S., Kamitani T., Nikaido T., Kato K., Kobayashi H., et al. Sagittal imbalance and symptoms of depression in adults: Locomotive Syndrome and Health Outcomes in the Aizu Cohort Study (LOHAS) Eur. Spine J. 2021;30:2450–2456. doi: 10.1007/s00586-020-06660-9. [DOI] [PubMed] [Google Scholar]
- 46.Schwab F., Ungar B., Blondel B., Buchowski J., Coe J., Deinlein D., DeWald C., Mehdian H., Shaffrey C., Tribus C., et al. Scoliosis Research Society-Schwab adult spinal deformity classification: A validation study. Spine. 2012;37:1077–1082. doi: 10.1097/BRS.0b013e31823e15e2. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi T., Morimoto T., Yoshihara T., Sonohata M., Rivière C., Mawatari M. The significant relationship among the factors of pelvic incidence, standing lumbar lordosis, and lumbar flexibility in Japanese patients with hip osteoarthritis: A descriptive radiographic study. Orthop. Trauma Surg. Res. 2021:103123. doi: 10.1016/j.otsr.2021.103123. [DOI] [PubMed] [Google Scholar]
- 48.Ohba T., Oba H., Koyama K., Oda K., Tanaka N., Fujita K., Haro H. Locomotive syndrome: Prevalence, surgical outcomes, and physical performance of patients treated to correct adult spinal deformity. J. Orthop. Sci. 2021;26:678–683. doi: 10.1016/j.jos.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Castro-Méndez A., Requelo-Rodríguez I., Pabón-Carrasco M., González-Elena M.L., Ponce-Blandón J.A., Palomo-Toucedo I.C. A case-control study of the effects of chronic low back pain in spatiotemporal gait parameters. Sensors. 2021;21:5247. doi: 10.3390/s21155247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akahane M., Maeyashiki A., Tanaka Y., Imamura T. The impact of musculoskeletal diseases on the presence of locomotive syndrome. Mod. Rheumatol. 2019;29:151–156. doi: 10.1080/14397595.2018.1452173. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs J.V., Yaguchi C., Kaida C., Irei M., Naka M., Henry S.M., Fujiwara K. Effects of experimentally induced low back pain on the sit-to-stand movement and electroencephalographic contingent negative variation. Exp. Brain Res. 2011;215:123–134. doi: 10.1007/s00221-011-2880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moissenet F., Rose-Dulcina K., Armand S., Genevay S. A systematic review of movement and muscular activity biomarkers to discriminate non-specific chronic low back pain patients from an asymptomatic population. Sci. Rep. 2021;11:5850. doi: 10.1038/s41598-021-84034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanfélix-Genovés J., Hurtado I., Sanfélix-Gimeno G., Reig-Molla B., Peiró S. Impact of osteoporosis and vertebral fractures on quality-of-life. A population-based study in Valencia, Spain (The FRAVO Study) Health Qual. Life Outcomes. 2011;9:20. doi: 10.1186/1477-7525-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanghelle B., Bentzen H., Giangregorio L., Pripp A.H., Bergland A. Associations between health-related quality of life, physical function and pain in older women with osteoporosis and vertebral fracture. BMC Geriatr. 2019;19:298. doi: 10.1186/s12877-019-1268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson L., Sundh D., Nilsson M., Mellström D., Lorentzon M. Vertebral fractures and their association with health-related quality of life, back pain and physical function in older women. Osteoporos. Int. 2018;29:89–99. doi: 10.1007/s00198-017-4296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivière C., Lazennec J.Y., Van Der Straeten C., Auvinet E., Cobb J., Muirhead-Allwood S. The influence of spine-hip relations on total hip replacement: A systematic review. Orthop. Traumatol. Surg. Res. 2017;103:559–568. doi: 10.1016/j.otsr.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Jentzsch T., Geiger J., Bouaicha S., Slankamenac K., Nguyen-Kim T.D., Werner C.M. Increased pelvic incidence may lead to arthritis and sagittal orientation of the facet joints at the lower lumbar spine. BMC Med. Imaging. 2013;13:34. doi: 10.1186/1471-2342-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinberg D.S., Liu R.W., Xie K.K., Morris W.Z., Gebhart J.J., Gordon Z.L. Increased and decreased pelvic incidence, sagittal facet joint orientations are associated with lumbar spine osteoarthritis in a large cadaveric collection. Int. Orthop. 2017;41:1593–1600. doi: 10.1007/s00264-017-3426-1. [DOI] [PubMed] [Google Scholar]
- 59.Liu X., Huang Z., Zhou R., Zhu Q., Ji W., Long Y., Wang J. The effects of orientation of lumbar facet joints on the facet joint contact forces: An in vitro biomechanical study. Spine. 2018;43:E216–E220. doi: 10.1097/BRS.0000000000002290. [DOI] [PubMed] [Google Scholar]
- 60.Kim H.J., Chun H.J., Lee H.M., Kang K.T., Lee C.K., Chang B.S., Yeom J.S. The biomechanical influence of the facet joint orientation and the facet tropism in the lumbar spine. Spine J. 2013;13:1301–1308. doi: 10.1016/j.spinee.2013.06.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.