Abstract
Fifty-seven Salmonella enterica serotype Typhimurium (S. typhimurium) isolates were collected from human patients in two French hospitals, Hôpital Antoine Béclère (Clamart, France) and Hôpital Bicêtre (Le Kremlin-Bicêtre, France), between 1996 and 1997. Thirty of them (52 percent) were resistant to amino-, carbeni-, and ureidopenicillins, had reduced susceptibility to amoxicillin-clavulanic acid, were susceptible to cephalothin, and were resistant to sulfonamides, streptomycin, chloramphenicol, and tetracyclines. All these strains possessed a blaPSE-1-like gene and were of phage type DT104. Ten of them were studied in more detail, which revealed that blaPSE-1 is located on the variable region of a class 1 integron. This integron was found to be chromosomally located, as was another class 1 integron containing aadA2, a streptomycin-spectinomycin resistance gene. The reduced susceptibility to amoxicillin-clavulanic acid (and to ticarcillin-clavulanic acid) may result from the high level of hydrolysis of the β-lactam rather than to the clavulanic acid resistance properties of PSE-1 in these clonally related S. typhimurium isolates.
Many reports indicate that Salmonella enterica serotype Typhimurium (S. typhimurium) would be either the first or the second nontyphoid Salmonella species identified worldwide (14, 20). Many S. typhimurium isolates are resistant to multiple drugs and are most commonly resistant to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracyclines (8, 20, 31, 38, 42). β-Lactam resistance has often been reported in non-S. typhi Salmonella species, and many epidemiological studies indicate an increasing rate of resistance to aminopenicillins within the last 10 years (8, 17, 21, 28). The molecular mechanism of ampicillin resistance may be related to the presence of TEM-1 and TEM-2 β-lactamases or to extended-spectrum TEM derivatives (18, 32, 44). An additional decrease in susceptibility to amoxicillin-clavulanic acid has recently been analyzed in three S. typhimurium isolates from patients from Romania (7). Espinasse et al. (7) concluded that plasmid-mediated overproduction of a TEM-1-like β-lactamase occurs in these strains.
In two French university hospitals located in the suburbs of Paris, many S. typhimurium strains with an amino- and ureidopenicillin resistance pattern and reduced susceptibility to amoxicillin-clavulanic acid were isolated. The first aim of this study was to elucidate the molecular mechanism involved in this resistance phenotype. Theoretically, several mechanisms may explain this reduced susceptibility to amoxicillin-clavulanic acid, including a decrease in permeability to β-lactams, as is known for Escherichia coli (30), or the presence of a specific β-lactamase. Indeed, the overproduction of TEM-1 and TEM-2 or SHV-1 β-lactamases or the production of oxacillinases or IRT (inhibitor-resistant TEM derivative) β-lactamases has been reported in E. coli (7, 23, 35). Since our strains essentially gave positive results for β-lactamase production in a nitrocefin test in the laboratory, we set up a variety of experiments designed to elucidate the β-lactamase-related mechanism which was involved. In addition, a detailed epidemiological analysis of the 10 S. typhimurium strains was performed. None of the frequently identified mechanisms of amoxicillin-clavulanic resistance in members of the family Enterobactericeae was found. Instead, an integron-associated carbenicillinase gene was found in the clonally related S. typhimurium strains.
MATERIALS AND METHODS
Bacterial strains.
The S. typhimurium strains were isolated in 1996 and 1997 from French patients at two university hospitals, Hôpital Antoine Béclère (Clamart, France) and Hôpital Bicêtre (Le Kremlin-Bicêtre, France). Both hospitals are located in the southern suburbs of Paris. The isolates were identified with the API 20E system (bioMérieux, Marcy-l-Etoile, France), were serotyped with a slide agglutination kit (Sanofi-Diagnostics Pasteur, Marnes-la-Coquette, France), and were phage typed at the French National Center for Salmonellae (Institut Pasteur, Paris, France) by using a collection of 40 S. typhimurium-specific phages (1). E. coli DH10B, E. coli JM109 (Life Technologies, Gibco BRL, Paris, France), E. coli DH5α, and E. coli XL1-Blue MRF′Kan (Stratagene, Paris, France) were used as recipient strains for electroporation, mating-out assays, and cloning experiments. E. coli DH10B harboring pBR322 (38) was cultured for TEM-1 extract preparation and MIC determinations.
Susceptibility testing.
The susceptibilities of all S. typhimurium isolates to the following antibiotics were first determined by the disc agar diffusion method performed on Mueller-Hinton plates (Sanofi-Diagnostics Pasteur): amikacin, amoxicillin, amoxicillin-clavulanic acid, cephalothin, cefamandole, cefepime, cefoxitin, cefotaxime, ceftazidime, ceftriaxone, chloramphenicol, ciprofloxacin, gentamicin, kanamycin, imipenem, nalidixic acid, piperacillin, piperacillin-tazobactam, spectinomycin, streptomycin, sulfonamide, tetracycline, ticarcillin, tobramycin, trimethoprim-sulfamethoxazole.
The MICs of selected β-lactams were then determined by an agar dilution technique on Mueller-Hinton agar with a Steers multiple inoculator and an inoculum of 104 CFU per spot (22). All plates were incubated at 37°C for 18 h. The MICs of the β-lactams were determined alone or in combination with a fixed concentration of either 2 μg of clavulanic acid per ml or 4 μg of tazobactam per ml. The MICs of the following β-lactam antibiotics for 10 representative S. typhimurium isolates (strains 1 to 10), E. coli DH10B harboring either pLPO-1 (see below) or pBR322 (TEM-1), and the E. coli DH10B reference strain were determined: amoxicillin and ticarcillin (SmithKline Beecham, Nanterre, France); aztreonam and cefepime (Bristol-Myers Squibb, Paris La-Défense, France); ceftazidime (Glaxo, Paris, France); cefamandole, cephalothin, and moxalactam (Eli Lilly, Saint-Cloud, France); piperacillin and tazobactam (Lederle, Oullins, France); cefotaxime and cefpirome (Hoechst-Roussel, Paris, France); and cefoxitin and imipenem (Merck Sharp & Dohme-Chibret, Paris, France).
Plasmid content, mating-out assays, curing experiments, and genomic DNA preparations.
The plasmid DNAs of 10 S. typhimurium isolates (strains 1 to 10) were tentatively extracted either with the Nucleobond AX kit (Macherey-Nagel, Hoerdt, France) or by the alkaline lysis procedure (34). The putative extracted plasmid DNA suspensions were electroporated into E. coli JM109, and recombinant bacteria were selected on Trypticase soy agar (TSA) plates containing either amoxicillin (100 μg/ml) or streptomycin (50 μg/ml).
Direct transfer of the amoxicillin resistance marker from the 10 S. typhimurium isolates into rifampin-resistant E. coli DH5α or E. coli JM109 strains obtained in vitro was attempted by liquid and solid mating-out assays by the filter mating technique at 37°C. Transconjugants were selected on TSA plates containing either rifampin (150 μg/ml; Sigma, Saint-Quentin Falavier, France) and amoxicillin (100 μg/ml) or streptomycin (50 μg/ml).
Curing experiments were performed by culturing S. typhimurium isolates in the presence of different concentrations of acriflavine (10 to 100 μg/ml) for 24 h to an exponential phase of growth, and these colonies (0.1 ml) were spread onto TSA plates (5). From each of the 10 S. typhimurium strains, single colonies were transferred either onto amoxicillin-containing TSA plates or onto amoxicillin-free TSA plates. One thousand isolates were tested in each curing experiment.
Genomic DNAs from the 10 selected S. typhimurium isolates were extracted as described previously (27).
RAPD fingerprinting.
(RAPD) analysis was performed as described by Williams et al. (45), with some modifications. The PCR mixture consisted of buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin), the four deoxynucleotide triphosphates (Boehringer Mannheim, Meylan, France) at a concentration of 400 μM each, 150 pmol of primer, about 1 μg of genomic DNA, and 2 U of Taq DNA polymerase (Perkin-Elmer Cetus) in a total volume of 50 μl. Primers B1 (5′-GTT TCG CTC C-3′), AP1 (5′-TCA CGA TGC A-3′), and ERIC-2 (5′-AAG TAA GTG ACT GGG GTG AGC G-3′) were used. Each sample was subjected to the first cycle of amplification (4 min at 94°C, 1 min at 36°C, and 2 min at 72°C) in a DNA thermal cycler 9600 (Perkin-Elmer Cetus). Each of the 35 subsequent cycles consisted of denaturation at 94°C for 1 min, annealing at 36°C for 1 min, and extension at 72°C for 2 min (for the last cycle, extension was at 72°C for 10 min). The amplified products were separated by electrophoresis in a 1.5% agarose gel (Sigma) and were visualized by UV transillumination following ethidium bromide staining. A 1-kb DNA ladder (Pharmacia Biotech) was used as a molecular size standard. The fingerprints were compared visually, and patterns were considered different when they differed by at least one amplification band, regardless of band intensity.
PFGE.
Plugs were prepared according to the instructions of Bio-Rad. Genomic DNA was digested either with XbaI or with SfiI at 37°C overnight. Electrophoresis through a 1% agarose gel in 0.5× TBE (Tris-borate-EDTA) buffer was performed with a CHEF DRIII apparatus (Bio-Rad). The following conditions for migration were chosen: 14°C, 6 V/cm, and a 120° switch angle. For XbaI a run time of 12 h followed by a run time of 12 h, with two linear switch ramps of 7 and 20 s and 5 to 24 s, respectively, was used. For SfiI a run time of 24 h with a linear switch ramp of 4 to 34 s was used. The ethidium bromide-stained gel was photographed (Polaroid) under UV illumination. A bacteriophage lambda DNA ladder (Bio-Rad) was used as a DNA molecular weight marker. The chromosomal fingerprints were compared by eye and were assigned to pulsed-field gel electrophoresis (PFGE) types and subtypes (40).
Isoelectric focusing and β-lactamase assays.
Cultures of the 10 S. typhimurium isolates were grown overnight at 37°C in 10 ml of Trypticase soy broth (TSB) containing amoxicillin at 100 μg/ml. One milliliter of each overnight culture was then grown for 3 h at 37°C in 10 ml of TSB without antibiotic. The bacterial suspensions were disrupted by sonification (twice for 30 s each time at 20 Hz [phospholyser Vibra Cell 300; Bioblock, Illkirch, France]) and were centrifuged (48,000 × g, 1 h, 4°C). The residual nucleic acids in the supernatant were precipitated with 7% 0.2 M spermin (Sigma) overnight at 4°C. This suspension was ultracentrifuged at 100,000 × g for 1 h at 4°C. The supernatant containing the enzyme extracts was subjected to analytical isoelectric focusing with a mini IEF 111 apparatus (Bio-Rad) with a polyacrylamide gel containing a gradient made up of ampholytes with a pH range of from 3 to 10 (Bio-Rad). Migration was performed with three consecutive voltages (100 V for 15 min, 200 V for 15 min, and 450 V for 1 h). The focused β-lactamases were detected by overlaying the gel with 1 mM nitrocefin (Oxoid, Paris, France) in a 50 mM phosphate buffer (pH 7.0). The pI values were determined and compared to those for β-lactamases whose pIs are known. From recombinant plasmid pLPO-1 (see Results section), the hydrolysis parameters for the extracted β-lactamase were determined as described previously (27). The 50% inhibitory concentrations (IC50s) of clavulanic acid and tazobactam, the affinity constant (Km), and Vmax values for amoxicillin and ticarcillin relative to the Vmax value for benzylpenicillin for PSE-1 were compared to those obtained for TEM-1.
The specific activities of the β-lactamases from the 10 selected S. typhimurium isolates and E. coli DH10B harboring either pBR322 (TEM-1) or pLPO-1 (PSE-1) were obtained as described previously (46). One unit of enzyme activity was defined as the activity which hydrolyzed 1 nmol of amoxicillin or ticarcillin per min per mg of protein. The total protein content was measured with the Bio-Rad DC Protein assay kit.
Dot blot and other hybridization experiments.
DNA-DNA hybridizations were performed as described by Sambrook et al. (34). Three microliters of total heat-denaturated DNA from a culture of each S. typhimurium isolate was placed on a nylon membrane (Hybond N+; Amersham, Les Ullis, France) that was lying on a Mueller-Hinton agar plate. Then, the membrane was air dried and the DNA was UV cross-linked for 2 min (UV cross-linker; Stratagene). Dot blot hybridizations were performed with the 354-bp ScaI fragment internal to blaPSE-1 (10), the 450-bp PstI-NotI fragment from recombinant plasmid pHUC37 for blaSHV-3 (24), or the 560-bp SspI-PstI fragment internal to blaTEM-1 from recombinant plasmid pBR322 (39).
Hybridizations were also performed with a gel containing EcoRV-restricted fragments of the genomic DNAs from the 10 S. typhimurium strains by using either the 354-bp ScaI fragment internal to blaPSE-1 (10), a PCR-amplified fragment (SulF [5′-CTT CGA TGA GAG CCG GCG GCG GC-3′] and SulB [5′-GCA AGG GGG AAA CCC GCG CC-3′]), giving an internal probe for a sulfonamide gene (sul1) (36), a 0.6-kb EcoRI fragment of plasmid pIZ-46 for IS200 hybridizations (37), or a PCR-amplified fragment (primer 1 [5′-GAG GGT AGC GGT GAC CAT CG-3′] and primer 2 [5′-ACT GAC TTG ATG ATC TCG CC-3′]), giving a 779-bp internal probe for the aadA2 gene (3).
The filters were incubated for 1 h at 42°C in a prehybridization solution containing 50 mM Tris-HCl (pH 7.5), 0.1 mg of salmon sperm DNA per ml, 5× Denhardt’s solution (0.1% Ficoll, 0.1% bovine serum albumin, 0.1% polyvinylpyrrolidone), 3× SSC (20× SSC is 3 M NaCl plus 0.3 M sodium citrate [pH 7]), and 30% formamide.
All probes were radiolabelled with [α32P]dATP by using a random-primer DNA labelling kit (Boehringer Mannheim, Meylan, France). Autoradiographies were performed by exposing the filters to Kodak films at −80°C with intensifying screens for 18 h.
PCR for blaPSE-1 and integron detection.
For each reaction, 2 μg of genomic DNA from each of the 10 S. typhimurium isolates was used. The PCR amplification for blaPSE-1 detection was performed with laboratory-designed primers (primers CARB-A [5′-GAA TGA CCA ATT TTA ACA ATC GC-3′] and CARB-B [5′-CGC TTT TAA TAC CAT CCG TGG-3′]). Primers for the detection of class 1 integrons were located in the 5′ conserved region (5′CS) and the 3′ conserved region (3′CS) encoding the disinfectant resistance gene qacEAd1 (5′CS, 5′-GGC ATC CAA GCA GCA AG-3′; 3′CS, 5′-AAG CAG ACT TGA CCT GA-3′) (16).
Cloning procedures and DNA sequencing.
The PCR fragments obtained with the 5′CS and 3′CS primers and the genomic DNAs of the S. typhimurium isolates as templates were ligated into the SrfI site of pCRScript Cam SK+ (Stratagene), as recommended by the manufacturer, giving rise to either pLPO-1 or pLPO-2 (see Results section). Recombinant plasmids were transformed into electrocompetent E. coli XL1-Blue MRF′Kan and were selected on TSA plates containing chloramphenicol (30 μg/ml). The sequences of both strands of the cloned DNA fragments were determined with an Applied Biosystems sequencer (ABI 311). The nucleotide sequence and the deduced protein sequence were analyzed with software available over the Internet (22a).
RESULTS
Epidemiological data.
During 1996 and 1997 a total of 57 S. typhimurium isolates were collected from hospitalized patients at the Hôpital Bicêtre and Hôpital Antoine Béclère, which are located in the suburbs of Paris. They represent 38% of all Salmonella species isolated during the period of time studied. The adult/child ratio of these strains was 11/46, and the stool specimen/other specimen ratio was 51/6.
Antibiotic susceptibility testing, dot blot hybridizations, and phage typing.
Among the 57 strains studied, 44 were resistant to aminopenicillins and carboxypenicillins (data not shown). In addition, 30 of these 44 isolates had reduced susceptibilities to amoxicillin-clavulanic acid; however, they remained susceptible to early cephalosporins such as cephalothin (data not shown). A β-lactamase extract was prepared from one of these isolates, S. typhimurium 1. The hydrolysis parameters that were determined suggested the presence of a carbenicillinase-type enzyme (data not shown). The dot blot hybridization results for total DNAs from the 30 S. typhimurium strains with reduced susceptibilities to amoxicillin-clavulanic acid were negative with blaSHV and blaTEM probes but were positive with a blaPSE-1 probe (data not shown). Of these 30 strains, 10 of them were further analyzed. The MICs for the 10 strains studied were identical (Table 1). Marked resistance to amoxicillin, ticarcillin, and piperacillin was noted, and this was reversed only partially for amoxicillin and ticarcillin in the presence of clavulanic acid and was reversed totally for piperacillin in the presence of either clavulanic acid or tazobactam (Table 1). The cephalothin MICs for the 10 S. typhimurium isolates remained low (Table 1). These results were different from those obtained for E. coli DH10B harboring multicopy plasmid pBR322 (TEM-1): clavulanic acid reduced sharply the amoxicillin and ticarcillin MICs for the strain (Table 1). Disc diffusion susceptibility assay results showed that the 30 blaPSE-1-positive S. typhimurium isolates were additionally resistant to chloramphenicol, streptomycin-spectinomycin, the tetracyclines, and the sulfonamides. Four of these 30 isolates were nalidixic acid resistant, including S. typhimurium 3, which was studied in further detail, but all isolates remained susceptible to ciprofloxacin and to all the other aminoglycosides. Phage typing of these blaPSE-1-positive S. typhimurium isolates identified them to be of DT104 phage type.
TABLE 1.
MIC90sa of β-lactams for 10 selected S. typhimurium clinical isolates, E. coli DH10B harboring recombinant either plasmid pLPO-1 (blaPSE-1) or pBR322 (blaTEM-1), and an E. coli DH10B reference strain
| Antibiotic | MIC90 (μg/ml)
|
|||
|---|---|---|---|---|
| S. typhimurium isolates | E. coli DH10B (pLPO-1) | E. coli DH10B (pBR322) | E. coli DH10B | |
| Amoxicillin | 4,096 | 8,192 | 8,192 | 2 |
| Amoxicillin + Clab | 64 | 128 | 16 | 2 |
| Amoxicillin + Tazc | 32 | 128 | 32 | 2 |
| Ticarcillin | >8,192 | >8,192 | >8,192 | 2 |
| Ticarcillin + Cla | 512 | 512 | 64 | 1 |
| Ticarcillin + Taz | 256 | 512 | 256 | 1 |
| Piperacillin | 512 | 512 | 512 | 1 |
| Piperacillin + Cla | 8 | 8 | 2 | 1 |
| Piperacillin + Taz | 8 | 8 | 8 | 1 |
| Cephalothin | 8 | 8 | 512 | 4 |
| Cephalothin + Cla | 4 | 4 | 16 | 4 |
| Cephalothin + Taz | 4 | 4 | 16 | 4 |
| Cefamandole | 2 | 2 | 128 | 2 |
| Cefoxitin | 4 | 8 | 8 | 8 |
| Cefepime | <0.06 | 0.06 | 0.06 | 0.03 |
| Ceftazidime | 0.12 | 0.12 | 0.12 | 0.12 |
| Cefotaxime | 0.12 | 0.12 | 0.12 | 0.12 |
| Ceftriaxone | 0.06 | 0.06 | 0.06 | 0.03 |
| Imipenem | 0.12 | 0.12 | 0.06 | 0.06 |
| Aztreonam | 0.12 | 0.12 | 0.12 | 0.12 |
MIC90, MIC at which 90% of isolates are inhibited.
Cla, clavulanic acid at a fixed concentration of 2 μg/ml.
Taz, tazobactam at a fixed concentration of 4 μg/ml.
Mating-out assays, curing experiments, and plasmid analysis.
Mating-out assays and curing experiments were performed with each of the 10 S. typhimurium isolates. These assays were repeated eight times and remained unsuccessful.
Extraction of plasmids from S. typhimurium isolates followed by electroporation into E. coli and selection on streptomycin-containing plates failed to identify consistently any plasmid-containing amoxicillin- or streptomycin-resistant E. coli strains.
Isoelectric focusing and β-lactamase assays.
The 10 S. typhimurium isolates produced a similar β-lactamase of pI 5.7, which corresponded to PSE-1 (10). The IC50 for this β-lactamase was similar to that for TEM-1: for PSE-1 and TEM-1, the IC50s were 0.10 and 0.12 μM, respectively, for clavulanic acid and 0.032 and 0.023 μM, respectively, for tazobactam. Comparison of the kinetic parameters showed that the affinity of PSE-1 for amoxicillin and ticarcillin is lower than that of TEM-1 (Table 2). The Vmax/Km ratio for ticarcillin was higher for PSE-1 than for TEM-1 (Table 2). The specific activities of the β-lactamases from the 10 S. typhimurium isolates and E. coli harboring either pBR322 (TEM-1) or pLPO-1 (PSE-1; see below) were 1,024 ± 130, 3,300 ± 420, and 30,100 ± 600 nmol/min/mg of protein, respectively, when amoxicillin was used as the substrate. Similarly, the specific activities of the β-lactamases from S. typhimurium isolates, E. coli harboring pBR322, or E. coli harboring pLPO-1 when ticarcillin was used as substrate were 1,840 ± 20, 1,000 ± 35, and 36,700 ± 900 nmol/min/mg of protein, respectively.
TABLE 2.
Comparison of kinetic parameters for benzylpenicillin, amoxicillin, and ticarcillin for PSE-1 and TEM-1
| β-Lactamase | Benzylpenicillin
|
Amoxicillin
|
Ticarcillin
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Km (μM) | Relative Vmaxa | Relative Vmax/Kma | Km (μM) | Relative Vmax | Relative Vmax/Km | Km (μM) | Relative Vmax | Relative Vmax/Km | |
| PSE-1 | 30 | 100 | 100 | 68.4 | 49.6 | 22 | 47.4 | 54 | 34.5 |
| TEM-1 | 40 | 100 | 100 | 43.5 | 70 | 65 | 15 | 9.2 | 24.5 |
Relative Vmax and relative Vmax/Km ratio are expressed relative to the values for benzylpenicillin.
β-Lactamase gene identification and integron analysis.
By using blaPSE-1-specific primers, an 811-bp PCR fragment was obtained from genomic DNA from each of the 10 S. typhimurium isolates. Direct sequencing of the PCR product from S. typhimurium 1 revealed 100% identity with blaPSE-1 (10). This result, together with the pI data, indicated that the 10 selected strains produced an identical or very closely related carbenicillinase, PSE-1.
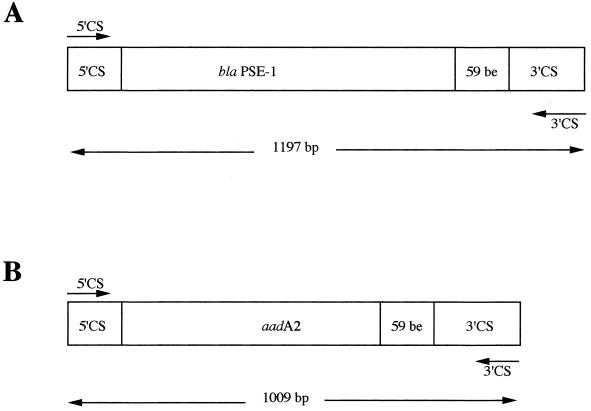
By using integron-specific PCR primers, two distinct amplicons of 1,197 and 1,009 bp were obtained from all 30 S. typhimurium genomic DNAs which were sequenced (Fig. 1). The larger amplicon encoded blaPSE-1 in association with gene cassette features (59-bp element) a core site (GTTRRY), and an inverse core site (RYYYAAC), which are typical of class 1 integrons. Cloning of the 1,197-bp amplicon into pCRScript gave recombinant plasmid pLPO-1. When it was expressed in E. coli DH10B, the β-lactam MICs for that strain were slightly higher compared to those for the S. typhimurium strains (Table 1). A 9.5-kb EcoRV restriction fragment of the genomic DNAs from all 30 S. typhimurium strains hybridized with a blaPSE-1 probe and with the 1,197-bp amplicon used as a probe (Fig. 2). A similar 1,197-bp PCR product was found in the 30 S. typhimurium isolates possessing blaPSE-1 (data not shown).
FIG. 1.
Schematic representation of the two PCR products obtained from S. typhimurium 1 with primers 5′CS and 3′CS. (A) Structure of the 1,197-bp PCR fragment encoding blaPSE-1. (B) Structure of the 1,009-bp PCR fragment encoding aadA2. The 59-bp element (59-be) is also represented.
FIG. 2.
EcoRV-restricted genomic DNAs of the 10 S. typhimurium isolates (lanes 1 to 10, respectively) hybridized with a 354-bp ScaI-restricted blaPSE-1 internal probe. Lane M, molecular size marker (in kilobases). Identical 9.5-kb positive hybridization bands were obtained for all S. typhimurium isolates.
The 1,009-bp amplicon encoded a streptomycin-spectinomycin resistance gene according to the resistance profile obtained after cloning with pCRScript (pLPO-2) and expression of pLPO-2 in E. coli (data not shown). Sequence analysis identified the aadA2 gene (Fig. 1). It was found in the 10 S. typhimurium isolates.
An internal probe for the sulfonamide gene hybridized to the 9.5-kb fragment resulting from EcoRV restriction of genomic DNAs from the S. typhimurium isolates. This internal probe also hybridized to the blaPSE-1 probe (data not shown). Moreover, EcoRV-restricted genomic DNAs from S. typhimurium isolates gave a second positive signal that corresponded to a 3.5-kb DNA fragment obtained after hybridization with the same sulfonamide probe (data not shown). The same 3.5-kb DNA fragment hybridized with a probe corresponding to the aadA2-containing 1,009-bp amplicon (data not shown).
Random PCR, PFGE, and IS200 hybridizations.
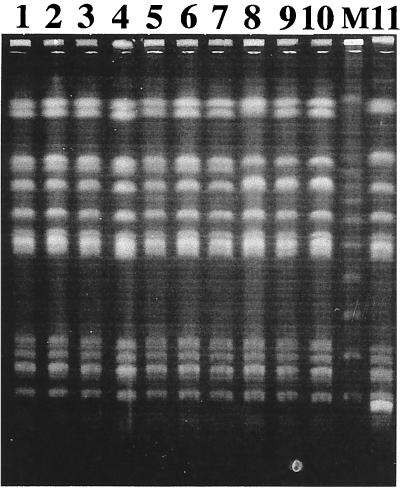
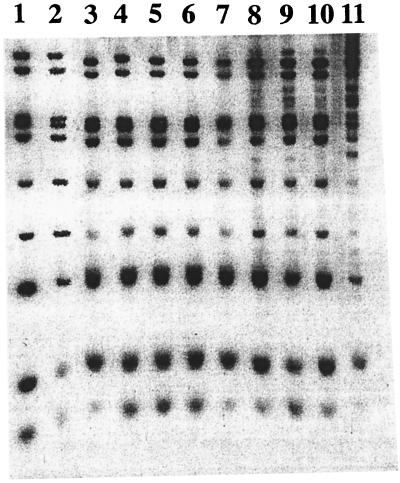
Analysis of the 10 S. typhimurium strains by random PCR gave identical and indistinguishable electrophoresis patterns, whichever primer was used (data not shown). PFGE of XbaI- and SfiI-restricted S. typhimurium DNAs indicated that the 10 S. typhimurium strains were very closely related (Fig. 3 and data not shown). Hybridization of EcoRV-digested genomic DNAs from the 10 S. typhimurium isolates with an internal IS200 probe gave banding patterns which confirmed the clonal relationship of these strains (Fig. 4).
FIG. 3.
PFGE of XbaI-digested genomic DNAs from 10 S. typhimurium strains. Lanes 1 to 10, S. typhimurium strains 1 to 10, respectively; lane 11, unrelated S. typhimurium strain; Lane M, bacteriophage lambda DNA ladder.
FIG. 4.
Restriction fragment length polymorphism analysis with IS200. Lanes 1 to 10, EcoRV-restricted genomic DNAs from the 10 S. typhimurium isolates, respectively; lane 11, an unrelated amoxicillin-susceptible S. typhimurium strain hybridized with an IS200 internal probe.
DISCUSSION
Our results indicate that among 57 S. typhimurium isolates, 44 (77%) were resistant to amoxicillin (and carboxypenicillins), which is an uncommonly high rate. Thirty of these 44 amoxicillin-resistant strains had reduced susceptibilities to amoxicillin-clavulanic acid. During a 22-month study, Kambal (13) analyzed the susceptibilities of 153 Salmonella isolates, of which 41% were of serogroup B and which mainly includes S. typhimurium species. All the ampicillin-resistant isolates were β-lactamase producers and had reduced susceptibilities to ampicillin-sulbactam. Similarly, 61% of the ampicillin-resistant Salmonella isolates reported by Ling et al. (17) showed similar reduced susceptibilities to ampicillin-sulbactam (17). Recently, Seyfarth et al. (38) detected ampicillin-resistant strains, and these accounted for 12% of S. typhimurium isolates from humans. In addition, all ampicillin-resistant clones had reduced susceptibilities to ampicillin-sulbactam. In the United Kingdom, epidemic S. typhimurium phage type DT104 strains of both animal and animal food origins have been reported to be resistant to multiple antibiotics (44). In a recent study conducted in the United States, S. typhimurium DT104 isolates resistant to ampicillin, chloramphenicol, sulfonamide, tetracycline, and streptomycin were found to be involved in an epidemic (8). However, no information concerning their amoxicillin-clavulanic acid susceptibilities was provided. These multiple-antibiotic-resistant strains represented 34% of S. typhimurium strains isolated in the United States in 1996. Identical resistance profiles were found among our 30 carbenicillinase-positive S. typhimurium strains. In addition, strain typing revealed the same DT104 phage type.
None of these studies investigated the molecular mechanism that explains the β-lactam resistance profile. Hybridization experiments, PCR amplifications, and sequencing of the PCR products revealed that our S. typhimurium isolates possessed blaPSE-1. PSE-1 (also named CARB-2), which was first identified from Pseudomonas aeruginosa PU21-RPL11 (18), is a member of the carbenicillinase group 2c of the Bush functional classification (4, 10). This carbenicillinase has also been reported in some enterobacterial species, especially Proteus mirabilis, Acinetobacter calcoaceticus, and Alcaligenes xylosoxidans (6, 11, 12, 25). Medeiros et al. (19) found other carbenicillinases in Salmonella enteritidis. Comparison of the amino acid sequences among carbenicillinases shows that these class A enzymes share 34 to 99% amino acid identity (11). PSE-1, CARB-3, and PSE-4 differ by two amino acid residues, since the other carbenicillinases (PSE-3, GN79, and CARB-4) possess only 34 to 87% identity (11, 15).
Comparison of IC50s for TEM-1 and PSE-1 showed that these enzymes are similarly susceptible to inhibitors (26). However, the affinity of TEM-1 for amoxicillin (43.5 μM) is higher than that of PSE-1 (68.4 μM). These results were not able to explain the decreased susceptibilities of the S. typhimurium strains to amoxicillin-clavulanic that were observed. The Vmax/Km ratio for ticarcillin only was higher with PSE-1 than with TEM-1. Additionally, when amoxicillin (best when ticarcillin) was used as the substrate, the specific activity of the PSE-1 β-lactamase obtained from S. typhimurium isolates or E. coli harboring recombinant multicopy plasmid pLPO-1 was higher than that of TEM-1 when TEM-1 was expressed in E. coli from the multicopy vector pBR322. As found previously (35), the ticarcillin-clavulanic acid resistance of PSE-1-producing strains may be due to rapid hydrolysis of ticarcillin by PSE-1 rather than to the reduced susceptibility of the enzyme to inhibitors (35).
Plasmid analysis and electroporation experiments failed to give reproducible results. At the early stage of our work, we were able to isolate sporadically blaPSE-1-containing plasmids that varied in size and structure as a result of experiments in which the plasmids were electroporated into E. coli. We therefore cannot rule out the possibility that the chromosomally located blaPSE-1 gene in S. typhimurium is also a part of the chromosomally integrated plasmids that are excised at a low frequency and probably under certain conditions, as is the case for some transposons which become activated at a low frequency under stress conditions.
PCR experiments followed by sequencing and subsequent cloning of the PCR products showed that blaPSE-1 was located on a 1,197-bp amplicon, which is part of the class 1 integron, and class 1 integrons are the most prevalent integrons among clinical isolates (33). In this regard, the sulfonamide gene, which is usually associated with class 1 integrons, was located in the same blaPSE-1-positive 9.5-kb EcoRV fragment from genomic DNAs from the S. typhimurium isolates.
Besides carbenicillinase genes, the β-lactamase genes reported so far to be located in integrons encode class D (oxacillinases) or class B (blaIMP-1) enzymes (2, 9, 29). Interestingly, a recent study conducted with Albanian S. typhimurium isolates found integron-located and plasmid-mediated β-lactamase genes (43). However, oxacillinase genes were identified. Therefore, integron-located β-lactamase genes of at least Ambler class A and class D may explain the widespread amoxicillin resistance in S. typhimurium.
While this work was in progress, a Danish group reported on eight S. typhimurium isolates from pig herds. Those isolates carried the same blaPSE-1 gene located on the same PCR product of a class 1 integron. This resistance gene was previously identified to be part of transposon Tn21 (36, 47). These animal isolates were also ampicillin resistant, but neither a plasmid location nor a chromosomal location for the β-lactamase gene was reported (36). Moreover, no detailed data from an antibiotic susceptibility study were provided. A second PCR product of another class 1 integron carrying the aadA2 and sulfonamide genes was also identified in these S. typhimurium isolates (3, 37, 43). These results may indicate, when animal and human strains are closely related, the spread of S. typhimurium strains from animals to humans via food, as suggested before (36). Recently, the same two integrons have also been reported among multiple-drug-resistant S. typhimurium DT104 isolates from animals and humans from different parts of the world but not France (31). As opposed to our strains, these S. typhimurium strains were mostly ciprofloxacin resistant.
In our case, the sulfonamide gene was associated not only with a blaPSE-1-containing 9.5-kb EcoRV fragment but also with an aadA2-containing 3.5-kb EcoRV fragment from genomic DNAs from S. typhimurium isolates. Despite repeated attempts, no plasmid was recovered after electroporation into E. coli and selection on streptomycin-containing TSA plates, thus indicating that the aadA2 gene may not be associated with any putative chromosomally located plasmids in S. typhimurium, whereas blaPSE-1 is associated with chromosomally located plasmids in S. typhimurium.
This work identified a class 1 integron carrying blaPSE-1 as the molecular mechanism which may explain the reduced susceptibility to amoxicillin-clavulanic acid in S. typhimurium isolates of human origin. In most countries, the first-line antibiotic for the treatment of serious S. typhimurium infections is ampicillin, and the second-line agents include amoxicillin-clavulanic acid (14). The spread of this integron may lead to difficulties is the treatment of such infections when one takes into account the high incidence of resistance to other drugs such as chloramphenicol, sulfonamides, co-trimoxazole, and quinolones among S. typhimurium isolates. Finally, it would be interesting to compare our S. typhimurium DT104 strains with those recently identified in humans in the United States and in animals in Denmark to see whether they are clonally related. It would not be surprising to find that they are related since they have identical phage types and identical multiple antibiotic resistance patterns.
ACKNOWLEDGMENTS
This work was partially funded by grants from the Ministère de la Recherche et de l’Enseignement (UPRES, JE 2227) and from the Institut SmithKline Beecham (La Défense, France).
We are very grateful to Roger Labia for help with the determination of the preliminary β-lactamase parameters and to P. A. D. Grimont for phage typing and J. Casadesus for providing plasmid pIZ46.
REFERENCES
- 1.Anderson E S, Ward L R, De Saxe M J, De Sa J D. Bacteriophage typing designations of Salmonella typhimurium. J Hyg (London) 1977;78:297–300. doi: 10.1017/s0022172400056187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, Kato N, Ohta M. A novel integron-like element carrying the metallo-β-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–1615. doi: 10.1128/aac.39.7.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bito A, Susani M. Revised analysis of aadA2 gene of plasmid pSa. Antimicrob Agents Chemother. 1994;38:1172–1175. doi: 10.1128/aac.38.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta S, Pal A, Basu S, Banerjee P C. Involvement of a 70-kb plasmid of the epidemic Shigella dysenteriae type (Dt66) strains in drug-resistance, lipopolysaccharide synthesis and virulence. Microb Drug Resist. 1997;3:351–357. doi: 10.1089/mdr.1997.3.351. [DOI] [PubMed] [Google Scholar]
- 6.Decré D, Arlet G, Bergogne-Bérézin E, Philippon A. Identification of a carbenicillin-hydrolyzing β-lactamase in Alcaligenes denitrificans subsp. xylosoxydans. Antimicrob Agents Chemother. 1995;39:771–774. doi: 10.1128/AAC.39.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinasse F, Gheorghiu R, Poiata A, Labia R, Nicolas-Chanoine M H. Reduced susceptibility to co-amoxiclav in Escherichia coli, Salmonella typhimurium and Klebsiella pneumoniae isolated in Romania between 1985 and 1993. J Antimicrob Chemother. 1997;39:103–106. doi: 10.1093/jac/39.1.103. [DOI] [PubMed] [Google Scholar]
- 8.Glynn M K, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo F. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. doi: 10.1056/NEJM199805073381901. [DOI] [PubMed] [Google Scholar]
- 9.Hall R M. Mobile gene cassettes and integrons moving antibiotic resistance genes in gram-negative bacteria. Ciba Found Symp. 1997;207:192–205. doi: 10.1002/9780470515358.ch12. [DOI] [PubMed] [Google Scholar]
- 10.Huovinen P, Jacoby G A. Sequence of PSE-1 β-lactamase. Antimicrob Agents Chemother. 1991;35:2428–2430. doi: 10.1128/aac.35.11.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito Y, Hirano T. Carbenicillin-hydrolysing penicillinase mediated by a plasmid of Proteus mirabilis and its relationship to the PSE-type enzymes of Pseudomonas aeruginosa. J Appl Microbiol. 1997;83:175–180. doi: 10.1046/j.1365-2672.1997.00203.x. [DOI] [PubMed] [Google Scholar]
- 12.Joly-Guillou M L, Vallée E, Bergogne-Bérézin E, Philippon A. Distribution of β-lactamases and phenotype analysis in clinical strains of Acinetobacter calcoaceticus. J Antimicrob Chemother. 1988;22:597–604. doi: 10.1093/jac/22.5.597. [DOI] [PubMed] [Google Scholar]
- 13.Kambal A M. Antimicrobial susceptibility and serogroups of Salmonella isolates from Riyadh, Saudi Arabia. Int J Antimicrob Chemother. 1996;7:265–269. doi: 10.1016/s0924-8579(96)00336-6. [DOI] [PubMed] [Google Scholar]
- 14.Kariuki S, Gilks C, Corkill J, Kimari J, Benea A, Waiyaki P, Hart C A. Multi-drug resistant non-typhi salmonellae in Kenya. J Antimicrob Chemother. 1996;38:425–434. doi: 10.1093/jac/38.3.425. [DOI] [PubMed] [Google Scholar]
- 15.Lenfant F, Petit A, Labia R, Maveyraud L, Samama J P, Masson J M. Site-directed mutagenesis of β-lactamase TEM-1. Investigating the potential role of specific residues on the activity of Pseudomonas-specific enzymes. Eur J Biochem. 1993;217:939–946. doi: 10.1111/j.1432-1033.1993.tb18324.x. [DOI] [PubMed] [Google Scholar]
- 16.Lévesque C, Piché L, Larose C, Roy P H. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–191. doi: 10.1128/aac.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling J M, Zhou G M, Woo T H, French G L. Antimicrobial susceptibilities and β-lactamase production of Hong Kong isolates of gastro-enteric salmonellae and Salmonella typhi. J Antimicrob Chemother. 1991;28:877–885. doi: 10.1093/jac/28.6.877. [DOI] [PubMed] [Google Scholar]
- 18.Matthew M. Plasmid-mediated β-lactamases of gram-negative bacteria: properties and distribution. J Antimicrob Chemother. 1979;5:349–358. doi: 10.1093/jac/5.4.349. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros A A, Hedges R, Jacoby G A. Spread of a “Pseudomonas-specific” β-lactamase to plasmids of enterobacteria. J Bacteriol. 1982;149:700–707. doi: 10.1128/jb.149.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz P, Diaz M D, Rodriguez-Creixems M, Cercenado E, Pelaez T, Bouza E. Antimicrobial resistance of Salmonella isolates in a Spanish hospital. Antimicrob Agents Chemother. 1993;37:1200–1202. doi: 10.1128/aac.37.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray B E. Resistance of Shigella, Salmonella and other selected enteric pathogens to antimicrobial agents. Rev Infect Dis. 1986;8:S172–S181. doi: 10.1093/clinids/8.supplement_2.s172. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 22a.National Institutes of Health. 1998. [Online.] http://www.ncbi.nlm.:nih.gov. [2 March 1999, date last accessed.]
- 23.Nicolas M H. Inhibitor-resistant β-lactamases. J Antimicrob Chemother. 1997;40:1–3. doi: 10.1093/jac/40.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Nicolas M H, Jarlier V, Honoré N, Philippon A, Cole S T. Molecular characterization of the gene encoding SHV-3 β-lactamase responsible for transferable cefotaxime resistance in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1989;33:2096–2100. doi: 10.1128/aac.33.12.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul G, Joly-Guillou M L, Bergogne-Berezin E, Névot P, Philippon A. Novel carbenicillin-hydrolyzing β-lactamase (CARB-5) from Acinetobacter calcoaticus var. anitratus. FEMS Microbiol Lett. 1989;59:45–50. doi: 10.1111/j.1574-6968.1989.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 26.Payne D J, Cramp R, Winstanley D J, Knowles D J. Comparative activity of clavulanic acid, sulbactam and tazobactam against clinically important β-lactamases. Antimicrob Agents Chemother. 1994;38:767–772. doi: 10.1128/aac.38.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philippon L N, Naas T, Bouthors A T, Barakett V, Nordmann P. OXA-18, a class D clavulanic-acid inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos J M, Ales J M, Cuenca-Estrella M, Fernandez-Roblas R, Soriano F. Changes in susceptibility of Salmonella enteriditidis, Salmonella typhimurium, and Salmonella virchow to six antimicrobial agents in a Spanish hospital, 1980–1994. Eur J Clin Microbiol Infect Dis. 1996;15:85–88. doi: 10.1007/BF01586193. [DOI] [PubMed] [Google Scholar]
- 29.Recchia G D, Hall R M. Gene casettes: a new class of mobile elements. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 30.Reguera J A, Baquero F, Perez-Diaz J C, Martinez J L. Factors determining resistance to β-lactam combined with β-lactamase inhibitors in Escherichia coli. J Antimicrob Chemother. 1991;27:569–573. doi: 10.1093/jac/27.5.569. [DOI] [PubMed] [Google Scholar]
- 31.Ridley A, Threlfall E J. Molecular epidemiology of antibiotic resistance genes in multiresistant epidemic Salmonella typhimurium DT104. Microb Drug Resist. 1998;4:113–118. doi: 10.1089/mdr.1998.4.113. [DOI] [PubMed] [Google Scholar]
- 32.Rossi A, Lopardo H, Woloj M, Picandet A M, Marino M, Galds M, Radice M, Gutkind G. Non-typhoid Salmonella spp. resistant to cefotaxime. J Antimicrob Chemother. 1995;36:697–702. doi: 10.1093/jac/36.4.697. [DOI] [PubMed] [Google Scholar]
- 33.Sallen B, Rajoharison A, Desvarenne S, Mabilat C. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb Drug Resist. 1995;1:195–202. doi: 10.1089/mdr.1995.1.195. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J E, Fritsch F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sanders C C, Iaconis J P, Bodey G P, Samonis G. Resistance to ticarcillin-potassium clavulanate among clinical isolates of the family Enterobacteriaceae: role of PSE-1 β-lactamase and high levels of TEM-1 and SHV-1 and problems with false susceptibility in disk diffusion tests. Antimicrob Agents Chemother. 1988;32:1365–1369. doi: 10.1128/aac.32.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandvang D, Aarestrup F M, Jensen L B. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica typhimurium DT104. FEMS Microbiol Lett. 1997;157:177–181. doi: 10.1111/j.1574-6968.1997.tb12770.x. [DOI] [PubMed] [Google Scholar]
- 37.Schiaffino A, Beuzon C R, Uzzau S, Leori G, Cappuccinelli P, Casadesus J, Rubino S. Strain typing with IS200 fingerprints in Salmonella aborturovis. Appl Environ Microbiol. 1996;62:2375–2380. doi: 10.1128/aem.62.7.2375-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seyfarth A M, Wegener H C, Frimodt-Moller N. Antimicrobial resistance in Salmonella enterica subsp. enterica serovar typhimurium from humans and production animals. J Antimicrob Chemother. 1997;40:67–75. doi: 10.1093/jac/40.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Sutcliffe J G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci USA. 1978;75:3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulse-field gel electrophoresis criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tolmasky M E, Crosa J H. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa-9) in the multiresistance transposon Tn1331. Plasmid. 1993;29:31–40. doi: 10.1006/plas.1993.1004. [DOI] [PubMed] [Google Scholar]
- 42.Tosini F, Visca P, Luzzi I, Dionisi A M, Pezzella C, Petrucca A, Carattoli A. Class 1 integron-borne multiple-antibiotic resistance carried by IncFI and IncL/M plasmids in Salmonella enterica serotype Typhimurium. Antimicrob Agents Chemother. 1998;42:3053–3058. doi: 10.1128/aac.42.12.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vatopoulos A C, Mainas E, Balis E, Threlfall E J, Kanelopoulou M, Kalapothaki V, Malamou-Lada H, Legakis N J. Molecular epidemiology of ampicillin-resistant clinical isolates of Salmonella enteritidis. J Clin Microbiol. 1994;32:1322–1325. doi: 10.1128/jcm.32.5.1322-1325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wall P G, Morgan D, Lamden K, Ryan M, Griffin M, Threlfall E J, Ward L R, Rowe B. A case control study of infection with an epidemic strain of multiresistant Salmonella typhimurium DT104 in England and Wales. Commun Dis Rep CDR Rev. 1994;4:R130–R135. [PubMed] [Google Scholar]
- 45.Williams J G, Kubelik A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Livermore D M. Chromosomal β-lactamase expression and resistance of β-lactams antibiotics in Proteus vulgaris and Morganella morganii. Antimicrob Agents Chemother. 1988;32:1385–1391. doi: 10.1128/aac.32.9.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zühlsdorf M T, Wiedemann B. Tn21-specific structures in gram-negative bacteria from clinical isolates. Antimicrob Agents Chemother. 1992;36:1915–1921. doi: 10.1128/aac.36.9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]